Abstract
Overexpression of the centrosome-associated serine/threonine kinase Aurora Kinase A (AURKA) has been demonstrated in both advanced prostate cancer and high-grade prostatic intraepithelial neoplasia lesions. The single-nucleotide polymorphism T91A (Phe31Ile) has been implicated in AURKA overexpression and has been suggested as a low-penetrance susceptibility allele in multiple human cancers, including prostate cancer. We studied the transcriptional consequences of the AURKA Ile31 allele in 28 commercial normal prostate tissue RNA samples (median age, 27 years). Significant overexpression of AURKA was demonstrated in homozygous and heterozygous AURKA Ile31 prostate RNA (2.07-fold and 1.93-fold, respectively; P < .05). Expression levels of 1509 genes differentiated between samples homozygous for Phe31 alleles and samples homozygous for Ile31 alleles (P = .05). Gene Ontology classification revealed overrepresentation of cell cycle arrest, ubiquitin cycle, antiapoptosis, and angiogenesis-related genes. When these hypothesis-generating results were subjected to more stringent statistical criteria, overexpression of a novel transcript of the natural killer tumor recognition sequence (NKTR) gene was revealed and validated in homozygous Ile31 samples (2.6-fold; P < .05). In summary, our data suggest an association between the AURKA Ile31 allele and an altered transcriptome in normal non-neoplastic prostates.
Keywords: Aurora Kinase A, overexpression, cancer, prostate, NKTR
Introduction
Prostate cancer is the most commonly diagnosed neoplasia among men in the western world. Although genetic predisposition to prostate cancer has been well established, identification of alterations in highly penetrant genes in only a small proportion of patients suggests a role for multiple low-penetrance susceptibility alleles in disease risk [1].
We have previously demonstrated an association between significant overexpression of the Aurora Kinase A (AURKA; MIM 603072) gene, located on chromosome 20q13.2, and prostate cancer and metastases [2]. AURKA encodes a centrosome-related serine/threonine kinase and is frequently amplified and/or overexpressed in additional human cancers and cell lines, including those of the breast, ovary, bladder, pancreas, colon [3], and soft tissue [4]. Increased AURKA expression has also been reported in premalignant breast [5] and prostate [6] lesions. Forced AURKA overexpression in mammalian cell lines induces centrosomal amplification, impaired chromosomal segregation, aneuploidy, and transformation [7–9], suggesting an association between enhanced AURKA expression and molecular mechanisms underlying tumorigenesis.
A single-nucleotide polymorphism (SNP), T91A, resulting in Phe31Ile amino acid substitution has been implicated in AURKA overexpression. AURKA Phe31Ile allelic variant frequencies vary among different ethnic populations worldwide. The frequency of AURKA Ile31 in healthy populations is highest in Asians, followed by Hispanics, Caucasians, and African Americans (0.62, 0.33, 0.21, and 0.13, respectively) [10–13]. None of these populations deviated from allele frequencies expected from the Hardy-Weinberg equilibrium. The Ile31 variant is preferentially amplified, is associated with the degree of aneuploidy in human tumors, and has a more potent transforming capacity when compared to AURKA Phe31, inducing cell growth in vitro and enhancing tumorigenicity in nude mice [14]. Cumulative evidence suggests that AURKA 91A (Ile31) is a low-penetrance tumor-susceptibility allele, predisposing both homozygous and heterozygous carriers to an increased risk of developing multiple human cancers, including prostate cancer [10–12,15–17]. The significance and impact of this variant in non-neoplastic human tissues has not been reported.
Because complex molecular changes associated with tumorigenesis may hinder the study of AURKA Ile31 overexpression, we hypothesize that studying cellular transcriptome in nonneoplastic tissues will provide an improved model for exploring the influence of this genetic variant on the prostate.
Materials and Methods
Commercial Prostate RNA and Tumor Models
Twenty-eight RNA samples from donor prostate tissues classified as normal prostate were purchased: 27 samples from donors of Asian descent (BioChain Institute, Inc., Hayward, CA) and 1 sample from a donor of Caucasian descent (Ambion, Inc., Austin, TX). The age range of donors was 20 to 71 years (average, 35 years; median, 27 years). The ages of the donors are presented in Table W1. All purchased human tissue samples were collected with informed consent from the donors and their relatives.
DNA and RNA were also generated from three human prostate cancer xenografts: WISH-PC14 and LuCaP35 (human androgen-dependent prostate-specific antigen-secreting prostatic adenocarcinoma xenografts that have been described previously) [2,18] and WM2.C (a prostatic adenocarcinoma xenograft established and provided by Z. Eshhar of the Weizmann Institute, Rehovot, Israel).
Genotyping
The 28 RNA samples were genotyped for AURKA T91A (Phe31Ile) and G169A (Val57Ile) sequence alterations. cDNA was prepared using random primers in accordance with the manufacturer's instructions (Invitrogen Life Technologies, Carlsbad, CA). All polymerase chain reaction (PCR) primer pair sequences were determined based on the reported transcribed sequence of AURKA (Table 1A). PCRs were performed with StartFast Taq Polymerase in accordance with the manufacturer's instructions (Roche Diagnostics, Mannheim, Germany) using a Biometra PCR system (Biometra GmbH, Go" ttingen, Germany). cDNA was amplified to generate a 427-bp amplicon using an AURKA Ex2-Ex5-specific primer pair (Table 1A). PCR products were digested with XapI (Fermentas Life Sciences, Hanover, MD) in accordance with the manufacturer's recommendations and were analyzed by 4% agarose gel electrophoresis. The wild-type AURKA 91T allele was digested to 276- and 151-bp fragments, whereas in the presence of 91A alteration, the 151-bp fragment was further digested to 87- and 64-bp fragments. For confirmation, PCR products were sequenced using BigDye Terminator Chemistry (Applied Biosystems, Foster City, CA) and analyzed using an automated ABI Prism 310 Genetic Analyzer (Applied Biosystems). The G169A (Val57Ile) status of these samples was determined according to the sequence obtained. To confirm the homogeneity of homozygous cDNA fragments, we further performed denaturing high-performance liquid chromatography (DHPLC) analysis using a WAVE apparatus (Transgenomic, Inc., Omaha, NE), as previously described [19].
Table 1.
Primers and Reaction Conditions.
| (A) RT-PCR Analysis | ||||
| Cycling Conditions (°C; sec) | ||||
| Gene (GO No.) | Primers | Denaturation | Annealing | Elongation |
| AURKA Ex2-Ex5 (3213196) | F: 5′-GGACCGATCTAAAGAAAACTGC-3′ | 95; 20 | 60; 20 | 72; 30 |
| R: 5′-CTTTCCTTTACCCAGAGGGCG-3′ | ||||
| AURKA Ex2-Ex3 (3213196) | F: 5′-GGACCGATCTAAAGAAAACTGC-3′ | 95; 20 | 63; 20 | 72; 30 |
| R: 5′-CAAGACCCGCTGAGCCTGGCC-3′ | ||||
| NKTR Ex6-Int6a (5923890) | F: 5′-CTCAAACATGACAGAGCGTTCC-3′ | 95; 20 | 60; 20 | 72; 20 |
| R: 5′-AAAGAGAGAGAGAGCCTTAGAG-3′ | ||||
| NKTR Ex6-Ex8a (5923890) | F: 5′-CTCAAACATGACAGAGCGTTCC-3′ | 95; 20 | 60; 20 | 72; 20 |
| R: 5′-CCAGAAATAACCAGTCCAAAGAC-3′ | ||||
| NKTR Ex1-Int6b (5923890) | F: 5′-CCAGCCAGCTCTTGCCGCCA-3′ | 95; 20 | 60; 20 | 72; 40 |
| R: 5′-CCACCAGAAGATGTACAGGAAC-3′ | ||||
| NKTR Ex1-Ex8b (5923890) | F: 5′-CCAGCCAGCTCTTGCCGCCA-3′ | 95; 20 | 62; 20 | 72; 40 |
| R: 5′-GCTTGCAGCATCGGTCTTCAG-3′ | ||||
| GAPDH (83641890) | F: 5′-CCAGAACATCATCCCTGC-3′ | 95; 20 | 60; 20 | 72; 20 |
| R: 5′-GGAAGGCCATGCCAGTGAGC-3′ | ||||
| (B) Quantitative RT-PCR Analysis | ||||
| Cycling Conditions (°C; sec) | ||||
| Gene (GO No.) | Primers | Annealing | Elongation | Acquisition |
| AURKA Ex2-Ex5 (3213196) | F: 5′-GGACCGATCTAAAGAAAACTGC-3′ | 63; 10 | 72; 11 | 84; 5 |
| R: 5′-CTTTCCTTTACCCAGAGGGCG-3′ | ||||
| CDKN1C (4557440) | F: 5′-CGCGGCGATCAAGAAGCTG-3′ | 68; 4 | 72; 10 | 92; 5 |
| R: 5′-CCTCGGGGCTCTTTGGGCTC-3′ | ||||
| BAX (34335114) | F: 5′-CCAGCAAACTGGTGCTCAAGG-3′ | 64; 10 | 72; 10 | 87; 5 |
| R: 5′-CAACCACCCTGGTCTTGGATC-3′ | ||||
| NKTR Ex6-Int6a (5923890) | F: 5′-CTCAAACATGACAGAGCGTTCC-3′ | 58; 10 | 72; 10 | 79; 5 |
| R: 5′-AAAGAGAGAGAGAGCCTTAGAG-3′ | ||||
| NPC1 (89242153) | F: 5′-TGGGCGCGATATTTCTGGTG-3′ | 68; 5 | 72; 9 | 87; 5 |
| R: 5′-CTCCACGCGGCTGCCTTTC-3′ | ||||
Quantitative Real-Time PCR
Real-time polymerase chain reaction (RT-PCR) analyses were performed to determine the expression of AURKA, natural killer tumor recognition sequence (NKTR; MIM 161565), cyclin-dependent kinase inhibitor 1C (CDKN1C; MIM 600856), BCL2-associated X protein (BAX; MIM 600040), and Niemann-Pick disease type C1 (NPC1; MIM 607623) genes in normal prostate RNA samples. All primers were designed to include the exon-intron junction to avoid possible contamination of genomic DNA. Amplifications were carried out using LightCycler (Roche Biochemicals, Mannheim, Germany), as described previously [4]. Briefly, a total reaction volume of 10 µl contained FastStart Master mix (Roche Biochemicals, Mannheim,Germany), 3mMMgCl2, and 0.5 µM of each primer (see Table 1B for primer sequences and reaction conditions). Fluorescence quantification was calculated using LightCycler software 3.01 (Roche Biochemicals). The expression of AURKA, NKTR, CDKN1C, and BAX genes was normalized as described previously [4] using NPC1 expression levels.
Affymetrix GeneChip Expression Analysis
RNA samples from normal prostate tissue—three samples homozygous for the AURKA Phe31 allele and five samples homozygous for the AURKA Ile31 allele—were tested using Affymetrix HG-U133 set microarrays (Affymetrix, Inc., Santa Clara, CA), as described previously [2].
Bioinformatics Data Analysis
Bioinformatics analysis was performed as previously reported [20]. Statistical algorithm, implanted in Affymetrix Suite Version 5.0 software (MAS5; Affymetrix, Inc.), generated a signal value (which designates a relative measure of the abundance of a transcript), a detection P value (which indicates the reliability of the transcript's detection call), and a detection call (present, absent, or marginal) for each transcript on a microarray. Detection calls were calculated, based on the detection P value, as follows: probe sets with P > .06 were designated as absent; probe sets with .06 > P > .04 were designated as marginal; and probe sets with P < .04 were designated as present. Signal values were normalized both per gene and per entire microarray by dividing each signal by the median of the gene and by the median of the microarray. Normalized data were then subjected to filtering, leaving 19,097 probe sets of 56,000 probes presented on the microarray that were present in at least four of eight tested arrays. Genes that distinguished between the AURKA Phe31 and AURKA Ile31 groups were delineated from 19,097 transcripts, based on two different statistical approaches using GeneSpring version 7 software (Silicon Genetics, Redwood City, CA): one-way analysis of variance (ANOVA) with P = .05 and one-way ANOVA with multiple correction restriction analysis using the Benjamini and Hochberg false discovery rate (FDR) algorithm, with P = .05 as cutoff for statistical significance.
Genes that differentiated between the two groups of Phe31- and Ile31-homozygous RNA were subjected to annotation analysis. Functional classification in Gene Ontology (GO) was examined to find annotation categories that are overrepresented compared to their representation in the array. Annotation analysis was preformed using “David” and Expression Analysis Systematic Explorer (EASE) software application (http://apps1.niaid.nih.gov/david/). Fisher's exact test was applied to choose categories that were significantly overrepresented (P < .05). Statistical P values were calculated using SPSS software version 12 (SPSS, Chicago, IL).
Expression of NKTR Transcript Variants in RNA from Human Tissues
cDNA from commercial normal RNA of brain, placenta, muscle, skeletal muscle, lung, kidney, colon, adipose, white blood cells, and prostate were prepared as described above. RT-PCRs were performed using StartFast Taq Polymerase (Roche Diagnostics) as described above, using three pairs of NKTR primers (NKTR Ex1-Int6b, NKTR Ex6-Int6a, and NKTR Ex6-Ex8a) and GAPDH primers (primer sequences and reaction conditions are detailed in Table 1A). PCR fragments were separated by 2% agarose gel electrophoresis.
Results
Genotyping Commercial RNA Samples for AURKA Phe31Ile Sequence Alteration
Two nonsynonymous SNPs have been identified in the AURKA gene: T91A (Phe31Ile) and G169A (Val57Ile). Genotyping for T91A (Phe31Ile) was supplemented by genotyping for G169A (Val57Ile) to rule out the possible influence of the latter on the overexpression of AURKA.
Of the 28 RNA samples, 3 were Phe31-homozygous (two were Val57-homozygous and one was Val57/Ile57-homozygous), 5 were homozygous for both Ile31 and Val57 alleles, and 20 were Phe31/Ile31-heterozygous (11 were Val57/Val57-heterozygous and 9 were Val57/Ile57-heterozygous). The small number of homozygous samples is a recognized limitation; however, all commercial prostate RNA samples available at the time of the study were purchased, and additional samples were not available. The ages of the three donors homozygous for the wild-type Phe31 allele were 23, 69, and 71 years. The age range of the five donors homozygous for the Ile31 allele was 26 to 65 years (average, 36 years; median, 27 years). The age range of the 20 heterozygous Phe31/Ile31 donors was 20 to 76 years (average, 32 years; median, 26 years). The lifetime risk for prostate cancer is 17% to 20%. Given the donor ages, it is of note that the prostate tissue classified herein as normal might have eventually undergone malignant change in some of the donors.
DHPLC analysis performed on eight commercial homozygous RNA samples (three Phe31 samples and five Ile31 samples) displayed DHPLC patterns identical to chromatogram patterns obtained from individual subclones of each AURKA variant (data not shown), further confirming their homozygosity and the homogeneity of these RNA samples.
AURKA Is Overexpressed in Normal Homozygous and Heterozygous Ile31 Prostate RNA
Expression levels of AURKA in normal prostate tissues were examined using quantitative RT-PCR analysis of AURKA Ex2-Ex5 amplification. In this experiment, RNA samples of Ile31-homozygous (n = 5), Phe31-homozygous (n = 3), and Phe31/Ile31-heterozygous (n = 20) genotypes were compared. AURKA overexpression was demonstrated in Ile31-homozygous and Phe31/Ile31-heterozygous RNA samples (2.07-fold and 1.93-fold, respectively) compared to Phe31-homozygousRNA samples (relative expression values of 1.30 ± 0.27, 1.21 ± 0.37, and 0.63 ± 0.10, respectively; P < .05; Figure 1). No significant difference was found between the Ile31/Ile31 and Phe31/Ile31 genotype groups.
Figure 1.
Quantitative RT-PCR analysis of the AURKA gene in normal prostate RNA samples homozygous for AURKA Phe31, heterozygous for Phe31/Ile31, and homozygous for Ile31 alleles. Values represent mRNA expression levels that were obtained from 10 ng of total RNA divided by NPC1 mRNA values (cDNA obtained from 1 µg of total RNA). Relative expression units were calculated by a standard curve using LightCycler 5.1 software (Roche Applied Bioscience). n, number of RNA samples tested.
AURKA Is Overexpressed in Human Prostate Cancer Xenografts Homozygous for Both AURKA Ile31 and Phe31 Alleles
The WISH-PC14 xenograft, which was found to be homozygous for the AURKA Ile31 allele, demonstrated significant overexpression (8.5-fold) of AURKA compared to RNA from normal AURKA Phe31-homozygous prostates. The WM2.C and LuCaP35 xenografts, both homozygous for the Phe31 allele, also overexpressed AURKA (9.3-fold and 5.2-fold, respectively) compared to RNA from normal AURKA Phe31-homozygous prostates (data not shown).
Identification of Genes That May Be Associated with AURKA Ile31 Overexpression
To identify novel genes and genetic pathways associated with the Ile31 allele, we used the Affymetrix HG-U133 set microarrays to compare global gene expression in normal prostates homozygous for the Phe31 (n = 3) and Ile31 (n = 5) alleles. Of the 56,000 probes presented on the microarray, the 19,097 transcripts passed the first filtration present in at least four of eight experimental arrays. We then applied one-way ANOVA for each of the genes separately. Genes totaling 1509 were distinguished between the two different genotype groups (homozygous for either AURKA Phe31 or AURKA Ile31), whereas only 955 genes were expected to pass this test by chance alone. This analysis provided an estimation of how distinct these two groups of prostate tissues are.
A Novel Splice Variant of the NKTR Gene Is Overexpressed in Normal Homozygous AURKA Ile31 Prostate RNA
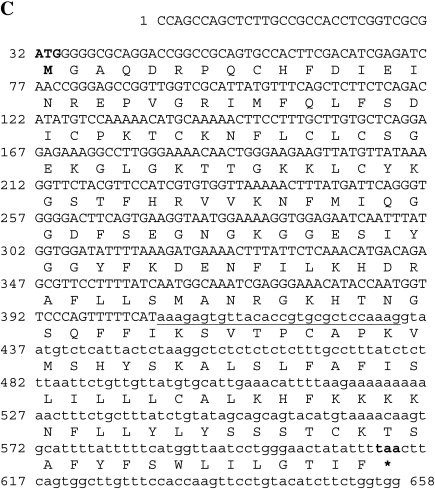
One-way ANOVA, followed by multiple correction restriction analysis using the Benjamini and Hochberg FDR algorithm, detected a single transcript, the NKTR overexpressed in normal prostate tissue homozygous for AURKA Ile31 compared to normal prostate tissue homozygous for AURKA Phe31 (Figure 2A). Interestingly, the overexpressed Affymetrix probe set for the NKTR transcript hybridized to sequences in intron 6 of the NKTR gene. Amplification of the NKTR region spanning from a primer at exon 6 to a primer at intron 6 (primers Ex6F and Int6aR; Table 1A) revealed a 138-bp cDNA fragment, including 64 bp of intron 6, with a unique open reading frame (ORF; Figure 3A). The full ORF fragment of this transcript was identified in a spectrum of human tissues using specific primers located at exon 1 (Ex1F) and intron 6 (Int6bR), upstream of the NKTR ATG codon and downstream of the putative stop codon of this novel NKTR transcript, respectively (658 bp; Figure 3A), with greater abundance in brain, lung, adipose, and prostate RNA. This alternative splice variant includes a (5′) 28-bp sequence from intron 6 that has been previously reported in an alternative NKTR transcript that includes only the 28 bp of intron 6 [21] (Figure 3B). The newly described and previously published splice variants share the same 5′ splice site in intron 6. The full putative ORF of this novel splice transcript is presented in Figure 3C. It is worth noting that the transcript containing the 28 bp of intron 6 alone, which was also presented as a probe set on the Affymetrix microarray, was not overexpressed.
Figure 2.
Significant overexpression of the novel NKTR transcript in prostate RNA samples homozygous for the AURKA Ile31 compared to prostate RNA samples homozygous for AURKA Phe31. (A) Affymetrix-normalized expression values of probe set 231235_at following multiple correction restriction analysis using the Benjamini and Hochberg FDR algorithm (P = .05). (B) Relative expression of the novel NKTR transcript using quantitative RT-PCR with primer pair Ex6F-Int6aR to generate a 138-bp fragment from NKTR exon 6 to intron 6. Values represent mRNA levels of expression that were obtained from 10 ng of total RNA divided by the values of the expression levels of the NPC1 gene (P < .005). Relative expression units were calculated by standard curves using LightCycler 5.1 software (Roche Applied Bioscience). Pr, prostate RNA sample number.
Figure 3.
Expression analysis and gene structure of the novel NKTR transcript that is overexpressed in normal homozygous AURKA Ile31 prostates. (A) Expression of the NKTR transcript in a variety of human tissues. RT-PCR amplification of the 658-bp full ORF transcript spanning from exon 1 (upstream of the ATG codon) to intron 6 (downstream of the putative stop codon; upper panel). The expression pattern of the 138-bp-short fragment spanning from exon 6 to intron 6, which is presented on the microarray (middle panel) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (lower panel), is also demonstrated. RTPCR was performed using human commercial whole brain RNA (lane 1 from left), placenta (lane 2), muscle (lane 3), skeletal muscle (lane 4), lung (lane 5), kidney (lane 6), colon (lane 7), adipose (lane 8), white blood cells (lane 9), and prostate (lane 10). RT-PCR without cDNA was used as a negative control (lane 11). Lane 12 contains a 100-bp ladder. (B) The structure of the NKTR gene (upper row, according to Ensembl gene report for ENSG00000114857) and the two alternative splice variants that contain sequences from intron 6. The novel alternative splice site variant (lower row) shares the (5′) 28-bp sequence of intron 6 with the transcript previously reported by Rinfret and Anderson [21], which contains only the 28 bp of intron 6 (middle row). The coding sequence in intron 6 of both alternative splice variants start at the same 5′ base. Lines and boxes represent introns and exons, respectively. Full box, coding sequences; empty box, noncoding sequences; ex, exon; int, intron. (C) Sequence of the novel splice site NKTR variant, including an ORF of 193 amino acids. The ATG and putative stop codon are in boldface. Intronic sequences are in lower case. The first 28 bp that originated from intron 6, identical to those previously published [21], are underlined.
Quantitative RT-PCR, using primer pairs Ex6F and Int6aR (Table 1B) to amplify the 138-bp fragment, confirmed a 2.6 overexpression of this specific NKTR splice variant in RNA samples homozygous for the Ile31 allele compared to samples homozygous for Phe31 (n = 5 and n = 3, 1.46 ± 0.31 and 0.57 ± 0.58, respectively; t-test, P < .05; Figure 2B).
A Potentially Pro-Oncogenic Expression Pattern Was Detected in Normal Homozygous AURKA Ile31 Prostate RNA
The classification of genes based on GO terms is a powerful bioinformatics tool for expression microarray analysis, and GO overrepresentation analysis allows the identification of families of genes that may play significant molecular and biologic roles. EASE software [22,23] was used to annotate the 1509 genes differentially expressed in prostate RNA samples homozygous for either the AURKA Ile31 or the AURKA Phe31 variant. Of the 1509 genes identified by ANOVA, the GO database provided annotation for 644 (42.7%) genes of biologic processes (Figure W1), 679 (45.0%) genes of molecular processes, and 572 (37.9%) genes of cellular processes. These annotations revealed statistically significant gene expression variations in multiple categories. In the cellular process genes category, overall changes in the metabolism of DNA, RNA, and proteins were demonstrated. Statistically significant differences were observed in ribosomal subunits, splicosomes, ribonuclear-related and translation initiation complexes, as well as in nucleoplasm categories (data not shown). Information regarding genes reported in Figure W1 is presented in Table W2 (http://apps1.niaid.nih.gov/david/).
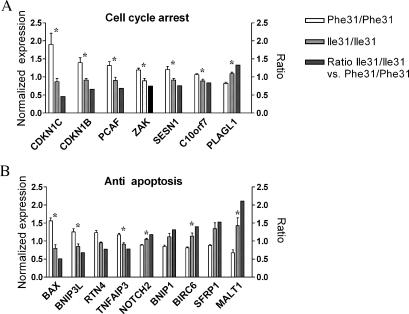
Overrepresented categories of biologic processes included cell cycle arrest, ubiquitin cycle, antiapoptosis, angiogenesis, establishment and/or maintenance of chromatin architecture, and cell migration (Figure W1). Figure 4 presents two of these annotation categories with the expression levels of all genes in each category in both the homozygous AURKA Phe31 group and the homozygous Ile31 group. For each gene in these categories, the ratio of the expression level in prostate RNA samples homozygous for AURKA Ile31 to the expression level in prostate RNA samples homozygous for AURKA Phe31 was determined. In the cell cycle arrest category, we detected six genes (CDKN1C, CDKN1B, PCAF, ZAK, SENSN2, and C10orf7) that were significantly downregulated and one gene (PLAGL1) that was significantly upregulated in AURKA Ile31 samples compared to AURKA Phe31 samples (Figure 4A).
Figure 4.
Comparison of normalized gene expression values that are differentially expressed in normal prostates homozygous for either AURKA Phe31 or Ile31. The expression values of two molecular annotations are presented: cell cycle arrest (A) and antiapoptosis (B). Values on the two left bars represent the average of normalized mRNA expression obtained from each group homozygous for the AURKA Ile31 allele (n = 5; middle bar) and for the Phe31 allele (n = 3; left bar). The ratio between groups (right bar) was calculated as the mean in AURKA Ile31 samples divided by the mean in AURKA Phe31 samples. *Genes whose expression levels were significantly changed between the two AURKA genotype groups of normal prostate RNA samples (t-test, P < .05).
Given the role of CDKN1C, also termed p57, as a potent inhibitor of several G1 cyclin/cyclin-dependent kinase complexes and as a negative regulator of cell proliferation [24,25], this gene was chosen for validation by quantitative RNA expression analysis. Quantitative RT-PCR confirmed a 13.5-fold downregulation of CDKN1C in Ile31 homozygote versus Phe31 homozygote RNA samples derived from normal prostates (n = 5 and n = 2, 1 ± 0.7 and 13.5 ± 0.7, respectively; t-test, P < .05).
The expression changes seen in genes included in the antiapoptosis annotation category are demonstrated in Figure 4B. Four genes were downregulated in AURKA Ile31 tissues compared to normal prostate tissues homozygous for AURKA Phe31, and changes in three of them (BAX, BNIP3L, and TNFAIP3) were statistically significant. Similarly, the expression of five genes was upregulated in the group of homozygous Ile31 RNA samples. Changes in three genes (NOTCH2, BIRC6, and MALT1) were statistically significant, suggesting that alterations in apoptosis may take place in normal prostates homozygous for the AURKA Ile31 allele. The proapoptotic BAX gene [26] was chosen for quantitative RT-PCR validation, which confirmed the 1.8-fold downregulation of this gene in homozygous Ile31 samples, compared to homozygous Phe31 prostate RNA samples (n = 5 and n = 3, 1.3 ± 0.36 and 2.4 ± 0.9, respectively; t-test, P = .057).
Discussion
Centrosome defects and deregulation of mitotic apparatus, chromosomal instability, and aneuploidy have been implicated in the pathway of events leading to prostate cancer [27], suggesting a link between regulators of mitosis and tumor initiation. The overexpression of the mitosis-regulating AURKA demonstrated in prostate tumors in human and mouse models [2,8,28], together with the demonstration of elevated AURKA expression in high-grade prostatic intraepithelial neoplasia (PIN) and, to a lesser degree, in normal cells in cancer-containing prostates, suggests that AURKA overexpression may play an early role in prostate tumorigenesis [6]. Functional analyses have implicated the AURKA SNP, T91A (Phe31Ile), in malignant transformation, and substantial epidemiological evidence supports the AURKA 91A variant as a low-penetrance cancer-susceptibility allele [14,16]. In the present study, we investigated the biologic consequences of the AURKA Ile31 allelic variant and changes in overall gene expression profile in human prostate.
Quantitative RT-PCR analysis of RNA from normal prostatic tissue demonstrated that the Ile31/Ile31 and Phe31/Ile31 AURKA genotypes were associated with significant AURKA overexpression, although not to the degree detected in an advanced prostate cancer xenograft [2]. Interestingly, AURKA, independent of its genotype (Ile31 or Phe31), was significantly overexpressed in a small number of prostate tumor xenografts compared to normal prostate tissues. To our knowledge, this is the first demonstration of Ile31-related overexpression of AURKA in normal prostate tissue.
Despite substantial evidence supporting an association between enhanced AURKA expression and genetic instability, induced overexpression of AURKA in primary mouse embryonic fibroblasts did not promote colony formation [29], suggesting that additional alterations (mutations and alterations in expression) cooperate with AURKA overexpression to induce tumorigenesis. To identify genes that may be related to the AURKA Ile31/Ile31 genotype, global prostate RNA expression profiles from homozygous Phe31 (n = 3) and Ile31 (n = 5) samples were analyzed using Affymetrix microarray. Genes whose expression levels differentiated between these two genotypes were categorized by GO, which revealed gene families implicated in processes underlying carcinogenesis, such as cell cycle arrest, ubiquitin cycle, antiapoptosis, angiogenesis, establishment and/or maintenance of chromatin architecture, and cell migration.
Disruption of the normal cell cycle is a critical step in cancer development. Because the major regulatory events leading to cell proliferation and differentiation occur within the G1 phase of the cell cycle, attention has been focused on altered expression patterns of G1 cyclins, cyclin-dependent kinases, and the Cip/Kip family of cyclin-dependent kinase inhibitors, including CDKN1C [30]. Decreased CDKN1C expression has been associated with pancreatic [31], hepatocelluar [32], ovarian [33], and bladder [34] carcinomas, and the tumor-suppressive activities of this inhibitor of cell cycle progression have been established in experimental models [35,36]. Interestingly, we demonstrated significantly decreased CDKN1C expression in AURKA Ile31/Ile31 normal prostates. Additionally, we demonstrated downregulation of the proapoptotic BAX gene in RNA from AURKA Ile31/Ile31 normal prostates. Because dysfunctional apoptotic programming and suppression of apoptosis have also been implicated in prostate cancer development and progression [30], our data further suggest that the AURKA Ile31/Ile31 genotype influences a network of interacting genes and pathways, possibly inducing an imbalance in signals promoting and inhibiting cellular proliferation and apoptosis, respectively; compromising the fidelity of cell cycle progression; and setting the stage for potential proliferation and tumorigenesis.
We consider the results of the hypothesis-generating microarray experiment due to the availability of only a small number of homozygous samples. However, when using stringent statistical criteria (FDR), we identified a specific transcript, the NKTR variant, that differentiated between AURKA Phe31/Phe31 and Ile31/Ile31 prostate RNA. Although the identification of only one discriminating transcript may be the result of the small number of samples, it emphasizes the significance of NKTR transcript overexpression in AURKA Ile31 homozygotes. The NKTR gene, mapped to human chromosome 3p23-p21 [37], encodes for the NKTR, a 150,000-Da protein, with a unique amino acid structure consisting of a 58-amino-acid hydrophobic amino terminus followed by a cyclophilin-related domain [38,39]. NKTRs are principally expressed and functional on the surface of natural killer (NK) cells, a subpopulation of white blood cells (large granular lymphocytes) that are essential effectors of antitumor immune responses in vivo [38,40–42]. NKTR plays a central role in NK-mediated tumor recognition and lysis [43]. Detection of the overexpression of this novel NKTR variant in normal AURKA Ile31/Ile31 prostates imposes a number of interesting questions. Possibly, concomitant expression of an NK protein both in NK effector cells and in Ile31-homozygous prostate cells, which are potential premalignant candidate targets of NK-mediated tumor recognition and lysis, might enable these target cells to escape recognition and NK-mediated cytotoxicity. A similar example has been recently reported in a melanoma model in relation to CD66a expression by both NK cells and melanoma cells in humans [44]. The interaction between these cells, through the homotypic protein, was demonstrated to result in NK cell inhibition, thus providing a possibly novel mechanism of major histocompatibility complex-independent inhibitory mechanism of cytotoxicity. Chromosome 3p deletion has been commonly demonstrated in human cancers, including prostate cancer [45–47]. Nevertheless, our preliminary analysis of NKTR expression levels in three prostate xenografts does not reveal significant NKTR overexpression, nor does it suggest 3p deletion (data not shown).
In summary, we report the first demonstration of AURKA gene overexpression related to the AURKA Ile31 variant in normal prostates homozygous or heterozygous for this genetic change. The identification of altered gene expression profiles in Ile31-homozygote prostates, including genes related to mechanisms of carcinogenesis, suggests that the AURKA Ile31 variant may be involved in the disruption of normal prostate transcriptome and may increase the risk for prostate tumorigenesis. Overexpression of the novel NKTR splice variant revealed in AURKA Ile31 carriers should be further studied to explore the relationship between NKTR and AURKA expression.
Supplementary Material
Acknowledgements
We thank Dani Bercovich for his expert assistance with DHPLC. We thank Merav Kedmi and Nicola Mabjeesh for their advice and support.
Abbreviations
- AURKA
Aurora Kinase A
- PIN
prostatic intraepithelial neoplasia
- NKTR
natural killer tumor recognition sequence
Footnotes
This work was supported by the M.K. Humanitarian Fund and the Wolfson Foundation.
References
- 1.Schaid DJ. The complex genetic epidemiology of prostate cancer. Hum Mol Genet. 2004;13:R103–R121. doi: 10.1093/hmg/ddh072. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Shira A, Pinthus JH, Rozovsky U, Goldstein M, Sellers WR, Yaron Y, Eshhar Z, Orr-Urtreger A. Multiple genes in human 20q13 chromosomal region are involved in an advanced prostate cancer xenograft. Cancer Res. 2002;62:6803–6807. [PubMed] [Google Scholar]
- 3.Marumoto T, Zhang D, Saya H. Aurora-A—a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein M, Meller I, Issakov J, Orr-Urtreger A. Novel genes implicated in embryonal, alveolar, and pleomorphic rhabdomyosarcoma: a cytogenetic and molecular analysis of primary tumors. Neoplasia. 2006;8:332–343. doi: 10.1593/neo.05829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoque A, Carter J, Xia W, Hung MC, Sahin AA, Sen S, Lippman SM. Loss of aurora A/STK15/BTAK overexpression correlates with transition of in situ to invasive ductal carcinoma of the breast. Cancer Epidemiol Biomark Prev. 2003;12:1518–1522. [PubMed] [Google Scholar]
- 6.Buschhorn HM, Klein RR, Chambers SM, Hardy MC, Green S, Bearss D, Nagle RB. Aurora-A over-expression in high-grade PIN lesions and prostate cancer. Prostate. 2005;64:341–346. doi: 10.1002/pros.20247. [DOI] [PubMed] [Google Scholar]
- 7.Sen S, Zhou H, White RA. A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene. 1997;14:2195–2200. doi: 10.1038/sj.onc.1201065. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 10.Miao X, Sun T, Wang Y, Zhang X, Tan W, Lin D. Functional STK15 Phe31Ile polymorphism is associated with the occurrence and advanced disease status of esophageal squamous cell carcinoma. Cancer Res. 2004;64:2680–2683. doi: 10.1158/0008-5472.can-04-0651. [DOI] [PubMed] [Google Scholar]
- 11.Sun T, Miao X, Wang J, Tan W, Zhou Y, Yu C, Lin D. Functional Phe31Ile polymorphism in Aurora A and risk of breast carcinoma. Carcinogenesis. 2004;25:2225–2230. doi: 10.1093/carcin/bgh244. [DOI] [PubMed] [Google Scholar]
- 12.Dicioccio RA, Song H, Waterfall C, Kimura MT, Nagase H, McGuire V, Hogdall E, Shah MN, Luben RN, Easton DF, et al. STK15 polymorphisms and association with risk of invasive ovarian cancer. Cancer Epidemiol Biomark Prev. 2004;13:1589–1594. [PubMed] [Google Scholar]
- 13.Gu J, Gong Y, Huang M, Lu C, Spitz MR, Wu X. Polymorphisms of STK15 (Aurora-A) gene and lung cancer risk in Caucasians. Carcinogenesis. 2007;28:350–355. doi: 10.1093/carcin/bgl149. [DOI] [PubMed] [Google Scholar]
- 14.Ewart-Toland A, Briassouli P, de Koning JP, Mao JH, Yuan J, Chan F, MacCarthy-Morrogh L, Ponder BA, Nagase H, Burn J, et al. Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nat Genet. 2003;34:403–412. doi: 10.1038/ng1220. [DOI] [PubMed] [Google Scholar]
- 15.Cox DG, Hankinson SE, Hunter DJ. Polymorphisms of the AURKA (STK15/Aurora kinase) gene and breast cancer risk (United States) Cancer Causes Control. 2006;17:81–83. doi: 10.1007/s10552-005-0429-9. [DOI] [PubMed] [Google Scholar]
- 16.Ewart-Toland A, Dai Q, Gao YT, Nagase H, Dunlop MG, Farrington SM, Barnetson RA, Anton-Culver H, Peel D, Ziogas A, et al. Aurora-A/STK15 T + 91A is a general low penetrance cancer susceptibility gene: a meta-analysis of multiple cancer types. Carcinogenesis. 2005;26:1368–1373. doi: 10.1093/carcin/bgi085. [DOI] [PubMed] [Google Scholar]
- 17.Hienonen T, Salovaara R, Mecklin JP, Jarvinen H, Karhu A, Aaltonen LA. Preferential amplification of AURKA 91A (Ile31) in familial colorectal cancers. Int J Cancer. 2006;118:505–508. doi: 10.1002/ijc.21344. [DOI] [PubMed] [Google Scholar]
- 18.Corey E, Quinn JE, Buhler KR, Nelson PS, Macoska JA, True LD, Vessella RL. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate. 2003;55:239–246. doi: 10.1002/pros.10198. [DOI] [PubMed] [Google Scholar]
- 19.Orr-Urtreger A, Bar-Shira A, Bercovich D, Matarasso N, Rozovsky U, Rosner S, Soloviov S, Rennert G, Kadouri L, Hubert A, et al. RNASEL mutation screening and association study in Ashkenazi and non-Ashkenazi prostate cancer patients. Cancer Epidemiol Biomark Prev. 2006;15:474–479. doi: 10.1158/1055-9965.EPI-05-0606. [DOI] [PubMed] [Google Scholar]
- 20.Kedmi M, Orr-Urtreger A. Differential brain transcriptome of beta4 nAChR subunit-deficient mice: is it the effect of the null mutation or the background strain? Physiol Genomics. 2007;28:213–222. doi: 10.1152/physiolgenomics.00155.2006. [DOI] [PubMed] [Google Scholar]
- 21.Rinfret A, Anderson SK. IL-2 regulates the expression of the NK-TR gene via an alternate RNA splicing mechanism. Mol Immunol. 1993;30:1307–1313. doi: 10.1016/0161-5890(93)90047-f. [DOI] [PubMed] [Google Scholar]
- 22.Dennis C. Draft guidelines ease restrictions on use of genome sequence data. Nature. 2003;421:877–878. doi: 10.1038/421877a. [DOI] [PubMed] [Google Scholar]
- 23.Hosack DA, Dennis G, Jr, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- 26.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 27.Pihan GA, Purohit A, Wallace J, Malhotra R, Liotta L, Doxsey SJ. Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res. 2001;61:2212–2219. [PubMed] [Google Scholar]
- 28.Lee EC, Frolov A, Li R, Ayala G, Greenberg NM. Targeting Aurora kinases for the treatment of prostate cancer. Cancer Res. 2006;66:4996–5002. doi: 10.1158/0008-5472.CAN-05-2796. [DOI] [PubMed] [Google Scholar]
- 29.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 30.Bruckheimer EM, Kyprianou N. Apoptosis in prostate carcinogenesis. A growth regulator and a therapeutic target. Cell Tissue Res. 2000;301:153–162. doi: 10.1007/s004410000196. [DOI] [PubMed] [Google Scholar]
- 31.Sato N, Matsubayashi H, Abe T, Fukushima N, Goggins M. Epigenetic down-regulation of CDKN1C/p57KIP2 in pancreatic ductal neoplasms identified by gene expression profiling. Clin Cancer Res. 2005;11:4681–4688. doi: 10.1158/1078-0432.CCR-04-2471. [DOI] [PubMed] [Google Scholar]
- 32.Ito Y, Takeda T, Sakon M, Tsujimoto M, Monden M, Matsuura N. Expression of p57/Kip2 protein in hepatocellular carcinoma. Oncology. 2001;61:221–225. doi: 10.1159/000055378. [DOI] [PubMed] [Google Scholar]
- 33.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Expression of p57kip2 and its clinical relevance in epithelial ovarian tumors. Anticancer Res. 2002;22:3191–3196. [PubMed] [Google Scholar]
- 34.Oya M, Schulz WA. Decreased expression of p57(KIP2) mRNA in human bladder cancer. Br J Cancer. 2000;83:626–631. doi: 10.1054/bjoc.2000.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe H, Pan ZQ, Schreiber-Agus N, DePinho RA, Hurwitz J, Xiong Y. Suppression of cell transformation by the cyclindependent kinase inhibitor p57KIP2 requires binding to proliferating cell nuclear antigen. Proc Natl Acad Sci USA. 1998;95:1392–1397. doi: 10.1073/pnas.95.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsugu A, Sakai K, Dirks PB, Jung S, Weksberg R, Fei YL, Mondal S, Ivanchuk S, Ackerley C, Hamel PA, et al. Expression of p57(KIP2) potently blocks the growth of human astrocytomas and induces cell senescence. Am J Pathol. 2000;157:919–932. doi: 10.1016/S0002-9440(10)64605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young HA, Jenkins NA, Copeland NG, Simek S, Lerman MI, Zbar B, Glenn G, Ortaldo JR, Anderson SK. Localization of a novel natural killer triggering receptor locus to human chromosome 3p23-p21 and mouse chromosome 9. Genomics. 1993;16:548–549. doi: 10.1006/geno.1993.1229. [DOI] [PubMed] [Google Scholar]
- 38.Frey JL, Bino T, Kantor RR, Segal DM, Giardina SL, Roder J, Anderson S, Ortaldo JR. Mechanism of target cell recognition by natural killer cells: characterization of a novel triggering molecule restricted to CD3- large granular lymphocytes. J Exp Med. 1991;174:1527–1536. doi: 10.1084/jem.174.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson SK, Gallinger S, Roder J, Frey J, Young HA, Ortaldo JR. A cyclophilin-related protein involved in the function of natural killer cells. Proc Natl Acad Sci USA. 1993;90:542–546. doi: 10.1073/pnas.90.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mocellin S, Rossi CR, Nitti D. Cancer vaccine development: on the way to break immune tolerance to malignant cells. Exp Cell Res. 2004;299:267–278. doi: 10.1016/j.yexcr.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Giardina SL, Anderson SK, Sayers TJ, Chambers WH, Palumbo GA, Young HA, Ortaldo JR. Selective loss of NK cytotoxicity in antisense NK-TR1 rat LGL cell lines. Abrogation of antibody-independent tumor and virus-infected target cell killing. J Immunol. 1995;154:80–87. [PubMed] [Google Scholar]
- 44.Markel G, Lieberman N, Katz G, Arnon TI, Lotem M, Drize O, Blumberg RS, Bar-Haim E, Mader R, Eisenbach L, et al. CD66a interactions between human melanoma and NK cells: a novel class I MHCindependent inhibitory mechanism of cytotoxicity. J Immunol. 2002;168:2803–2810. doi: 10.4049/jimmunol.168.6.2803. [DOI] [PubMed] [Google Scholar]
- 45.Hesson LB, Cooper WN, Latif F. Evaluation of the 3p21.3 tumour-suppressor gene cluster. Oncogene. 2007 doi: 10.1038/sj.onc.1210547. (advance online publication, May 28, 2007) [DOI] [PubMed] [Google Scholar]
- 46.Bashyam MD, Bair R, Kim YH, Wang P, Hernandez-Boussard T, Karikari CA, Tibshirani R, Maitra A, Pollack JR. Array-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancer. Neoplasia. 2005;7:556–562. doi: 10.1593/neo.04586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf M, Mousses S, Hautaniemi S, Karhu R, Huusko P, Allinen M, Elkahloun A, Monni O, Chen Y, Kallioniemi A, et al. High-resolution analysis of gene copy number alterations in human prostate cancer using CGH on cDNA microarrays: impact of copy number on gene expression. Neoplasia. 2004;6:240–247. doi: 10.1593/neo.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.