Abstract
We studied the putative significance of angiotensin I-converting enzyme (ACE) in colorectal cancer (CRC) biology. Local expression of ACE was investigated by quantitative reverse transcription-polymerase chain reaction and by immunohistochemistry in CRCs and adenomas. ACE insertion (I)/deletion (D) polymorphism was studied in 141 CRC patients and 189 controls. ACE mRNA was upregulated in CRCs compared to corresponding nonlesional tissues (2.5-fold; P = .009). ACE protein was more commonly expressed in adenomas [17 (81%)] and cancer epithelial cells [22 (100%)] than in corresponding non-neoplastic crypt and surface epithelium [2 (10%) and 2 (9%), respectively]. Thirty-seven CRC patients (26%) carried II genotype, 69 (49%) carried ID genotype, and 35 (25%) carried DD genotype. The distribution of the genotypes did not differ from that of controls. Female CRC patients more commonly carried the ID genotype and less frequently the II and DD genotypes compared with male patients (P = .033). Men heterozygous or homozygous for the D-allele had larger tumors compared to carriers of the II genotype (P < .01). Women homozygous for the D-allele lived longer than carriers of the ID and II genotypes. Our study shows that ACE is differentially expressed in CRCs and that gene polymorphism is associated with gender-specific differences in primary tumor size and patient survival.
Keywords: Colorectal cancer, angiotensin-converting enzyme, polymorphism, gender, adenoma
Introduction
Angiotensin I-converting enzyme (ACE; CD143) is a type I cell surface zinc metallopeptidase that is expressed by many cell types of various organs and tissues, including vascular endothelial cells, epithelial cells of the small intestine, kidney tubular cells, mononuclear cells, and fibroblasts [1]. ACE cleaves C-terminal dipeptides from oligopeptide substrates with an unhindered C-terminus. It generates angiotensin II, the major effector of the renin-angiotensin system, and cleaves angiotensin 1–9 into angiotensin 1–7. There is mounting evidence that ACE participates locally in the pathology of carcinomas [1,2]. ACE is differentially expressed in several malignancies [1] and influences tumor cell proliferation, tumor cell migration, angiogenesis, and metastatic behavior [2–4]. Inhibition of ACE activity influences tumor growth and angiogenesis in vitro and in vivo in animal models [5–10]. Epidemiological studies have provided evidence that ACE inhibitors may decrease the risk and mortality rate of cancer [11,12]. ACE inhibitors are currently under consideration as “novel” antineoplastic treatment and cancer prevention strategies [2,12]. A polymorphism in the ACE gene, consisting of the insertion (I) or the deletion (D) of a 287-bp DNA fragment in intron 16, accounts for 20% to 50% of the variance in ACE expression or activity in blood and tissues among individuals [13–15]. Recently, we have shown that ACE is expressed locally in gastric cancer [16] and that I/D gene polymorphism influences metastatic behavior [17]. Patients with DD genotype had a greater number of lymph node metastases and an advanced Union International Contre le Cancer (UICC) tumor stage compared with carriers of ID or II genotype [17]. Furthermore, a retrospective study provided evidence that long-term ACE medication decreases the risk of developing colorectal cancer (CRC) [18]. Intrigued by these observations, we aimed to further substantiate the putative significance of ACE by investigating its local expression in colorectal adenomas and CRCs and by correlating its gene polymorphism with CRC pathology.
Materials and Methods
Patient Populations and Samples
Samples from 141 CRC patients and 21 patients with colorectal adenomas operated on between 2001 and 2006 were retrieved from the archive of the Department of Pathology (Table 1). Tissue samples used in the present study were obtained from patients who had undergone either polypectomy or right/left-sided hemicolectomy, as well as from 189 control patients without CRC, as described previously (Table 1) [17]. This study was carried out in accordance with the guidelines of the Ethics Committee of the University Hospital Berlin, and the patients gave their informed consent before their inclusion in the study. Data were encoded to ensure patient protection.
Table 1.
Patient Characteristics.
| I/D Genotype | |||||
| Total | II | ID | DD | P | |
| Controls | |||||
| Patients [n (%)] | 189 | 41 (22) | 95 (49) | 53 (28) | |
| Age in years [mean ± SD] | 67.7 ± 6.1 | 69.2 ± 6.2 | 67.9 ± 6.4 | 65.8 ± 5.0 | ns |
| Gender [n (%)] | |||||
| Men | 75 (40) | 17 (23) | 38 (51) | 20 (27) | ns |
| Women | 114 (60) | 24 (21) | 57 (50) | 33 (29) | |
| Colon cancer patients | |||||
| Patients [n (%)] | 141 | 37 (26) | 69 (49) | 35 (25) | ns |
| Age in years [mean ± SD] | 66.7 ± 12.4 | 66.1 ± 13.0 | 67.9 ± 10.7 | 65.2 ± 14.7 | ns |
| Gender [n (%)] | |||||
| Men | 83 (59) | 26 (31) | 33 (40) | 24 (29) | .033 |
| Women | 58 (41) | 11 (19) | 36 (62) | 11 (19) | |
| Localization [n (%)] | |||||
| Colon | 47 (33) | 18 (38) | 21 (45) | 8 (17) | ns |
| Sigma | 26 (18) | 5 (19) | 13 (50) | 8 (31) | |
| Rectum | 68 (48) | 14 (21) | 35 (52) | 19 (28) | |
| T-category [n (%)] | |||||
| pT1 | 10 (7) | 2 (20) | 6 (60) | 2 (20) | ns |
| pT2 | 17 (12) | 2 (12) | 11 (65) | 4 (24) | |
| pT3 | 99 (70) | 30 (30) | 44 (44) | 25 (25) | |
| pT4 | 15 (11) | 3 (20) | 8 (53) | 4 (27) | |
| Tumor diameter (mm) [mean ± SD] | |||||
| All | 47.4 ± 23.0 | 42.4 ± 12.0 | 50.8 ± 29.1 | 46.03 ± 16.9 | ns |
| Men | 47.0 ± 19.5 | 40.2 ± 12.3 | 52.3 ± 23.9 | 47.1 ± 17.3 | .058 |
| Women | 47.9 ± 27.5 | 47.6 ± 10.2 | 49.3 ± 33.5 | 43.7 ± 16.7 | ns |
| Number of lymph nodes [mean ± SD] | |||||
| Studied | 15.9 ± 5.8 | 15.7 ± 4.6 | 16.1 ± 6.2 | 15.5 ± 6.4 | ns |
| With metastases | 2.5 ± 4.1 | 2.5 ± 3.3 | 2.0 ± 4.1 | 3.3 ± 4.9 | |
| Men | 2.5 ± 3.7 | 2.9 ± 3.7 | 1.6 ± 2.9 | 3.3 ± 4.7 | |
| Women | 2.4 ± 4.7 | 1.7 ± 2.1 | 2.3 ± 5.0 | 3.5 ± 5.7 | |
| N-category [n (%)] | |||||
| pN0 | 69 (49) | 16 (23) | 38 (55) | 15 (22) | ns |
| pN1 | 37 (26) | 10 (27) | 19 (51) | 8 (22) | |
| pN2 | 35 (25) | 11 (31) | 12 (35) | 12 (35) | |
| M-category [n (%)] | |||||
| pM0 | 113 (80) | 31 (27) | 56 (50) | 26 (23) | ns |
| pM1 | 28 (20) | 6 (21) | 13 (46) | 9 (32) | |
| UICC tumor stage [n (%)] | |||||
| IA | 20 (14) | 3 (15) | 12 (60) | 5 (25) | ns |
| IB | 36 (25) | 10 (28) | 18 (50) | 8 (22) | |
| II | 3 (2) | 0 | 3 (100) | 0 | |
| IIIA | 4 (3) | 0 | 3 (75) | 1 (35) | |
| IIIB | 24 (17) | 9 (38) | 12 (50) | 3 (13) | |
| IIIC | 26 (18) | 9 (35) | 8 (31) | 9 (35) | |
| IV | 28 (20) | 6 (21) | 13 (46) | 9 (32) | |
ns, statistically not significant.
Histology and Immunohistochemistry
For histology, tissue samples from all patients were fixed in 10% neutralized formalin and embedded in paraffin. Deparaffinized sections were stained using hematoxylin and eosin. Tumor-Node-Metastasis stage was determined according to UICC guidelines and was based on histologic confirmation [19]. For immunohistochemical studies, samples from a series of 22 consecutive patients with CRC and 21 randomly chosen patients with colorectal adenomas were used. Immunostaining was performed as described elsewhere, using monoclonal antibodies directed against ACE (clone CG2, 1:50; Dianova, Hamburg, Germany) [16]. Omission of primary antibodies served as negative control.
Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
To measure ACE mRNA expression levels, total RNA was extracted from frozen tissues using Trizol reagent (Invitrogen, Karlsruhe, Germany), followed by the RNeasy Kit (Qiagen, Hilden, Germany) for mRNA preparation, according to the manufacturer's instructions. cDNA was synthesized from 1 µg of RNA using Omniscript Reverse Transcriptase (Qiagen), and quantitative real-time RT-PCR was performed using LightCycler (Roche Diagnostics, Mannheim, Germany). A 20-µl reaction mixture consisted of 10 µl of Quantitect SYBR Green MasterMix (Qiagen), 2 µl of cDNA, and 1 µM of specific primers for ACE (forward: CTCAAGTACTTCCAGCCAGTC; reverse: GCAGAATCTTGCTGGTCTCTG; product, 371 bp) or β-actin (forward: CATGTACGTTGCTATCCAGGC; reverse: CTCCTTAATGTCACGCACGAT; product, 250 bp). The initial denaturation and activation of Taq polymerase at 95°C for 15 minutes were followed by 40 cycles with denaturation at 94°C for 15 seconds, annealing at 62°C for 20 seconds, and elongation at 72°C for 20 seconds (ACE) or 15 seconds (β-actin), followed by a melting curve analysis between 65°C and 95°C to verify the absence of primer artifacts. Only samples without primer artifacts were included in the analyses. Specific initial template mRNA amounts were calculated as described above from a standard curve obtained by serial dilution of known copy numbers of corresponding cloned PCR fragments. cDNA contents were normalized for any variability in RNA amounts or for integrity by calculating the ACE/β-actin ratio.
Determination of ACE Genotype
Genomic DNA was purified from non-neoplastic tissue specimens using the EZNA Tissue DNA Mini Kit (PEQLAB Biotechnologie GmbH, Elangen, Germany). DNA was dissolved at 100 ng/µl in 10 mM Tris-HCl and 1 mM EDTA, pH 8.0. The ACE genotype of patients and healthy controls was determined by PCR according to Yoshida et al. [20]. A typical 50-µl reaction mixture consisted of 25 µl of HotStarTaq Master Mix (Qiagen), 100 ng of genomic DNA, 250 pmol of each primer (ACE-US: 5′-CTggAgACCACTCCCATCCTTTCT; ACE-DS: 5′-gATgTggCCATCAC-ATTCgTCAgAT), and 5% (vol/vol) DMSO. An initial 15-minute denaturation at 95°C was followed by 40 cycles of 1 minute at 64°C, 1 minute at 72°C, and 0.6 minute at 94°C. Amplified ACE gene fragments were separated on 1.6% agarose gels and visualized by ethidium bromide staining. D-alleles or I-alleles were identified by the presence of 190-bp or 490-bp fragments, respectively. The ID genotype commonly shows a double band at 490 bp and a single band at 190 bp [21]. An independent PCR analysis was carried out for each sample.
Statistical Analysis
Statistical analysis was carried out with SPSS software, Version 14.01 (Chicago, IL). Values are expressed as mean ± standard deviation (SD). The independence of qualitative outcomes was tested using Pearson's chi-square test and Fisher's exact test, where appropriate. One-way analysis of variance and t-test were used for the comparison of group means. The dependency of patient survival on ACE genotype was evaluated using the Kaplan-Meier method and was compared using log-rank test. P < .05 (two-tailed) was considered “significant.”
Results
Expression of ACE in Nonlesional Colon Mucosa, Adenomas, and Colorectal Carcinomas
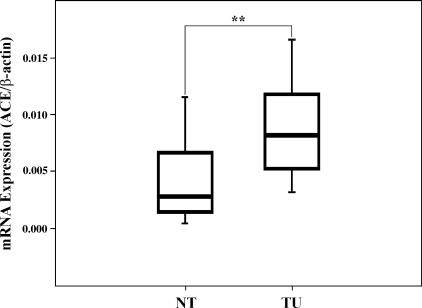
The local expression of ACE in CRCs and corresponding nonlesional tissues was studied at the mRNA and protein levels. As shown in Figure 1, ACE mRNA was highly significantly upregulated in colon carcinomas compared to corresponding nonlesional tissues (2.5-fold; P = .009).
Figure 1.
Expression of ACE mRNA in colon carcinoma and nonlesional tissues. ACE expression was measured by quantitative real-time RT-PCR and normalized against β-actin. Box boundaries: 25th and 75th percentiles; solid line: median; whiskers: lowest and highest nonoutlier values. **P < .01. TU, tumor; NT, nontumorous mucosa.
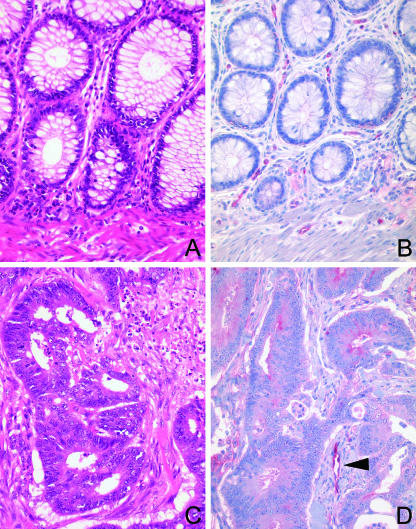
Immunohistochemistry was performed on paraffin-embedded tissue sections from 22 of 141 patients (see below) with CRC and from 21 patients with colorectal adenomas. We examined the expression of ACE in the non-tumorous crypt and surface epithelium, endothelial cells, and carcinomas. ACE was significantly more commonly expressed in CRC cells [22 (100%)] than in non-neoplastic crypt and surface epithelium [2 (9%)]. Immunostaining was usually moderate to strong, and was confined to the apical membrane of tumor cells [19 (86%)] and/or the cytoplasm [21 (95%)] (Figure 2). ACE was already expressed by neoplastic cells of colorectal adenomas [17 (81%)]. However, in adenomas, expression was usually weak and mainly localized in the cytoplasm [17 (81%)], and less commonly at the apical membrane [9 (43%)]. ACE was found in endothelial cells of the tumor vessels of all (100%) patients (Figure 2).
Figure 2.
Expression of ACE in CRC. The distribution and expression pattern of ACE in non-neoplastic colon mucosa (A and B) and colorectal carcinomas (C and D) was investigated by immunohistochemistry. ACE was found in (B and D) endothelial cells and (D) tumor epithelial cells. Hematoxylin and eosin (A and C); monoclonal anti-ACE antibody (B and D); hematoxylin counterstain. Original magnification, x400.
ACE Gene Polymorphism
ACE gene polymorphism was studied in 141 CRC patients and was compared with that of 189 individuals without cancer (Figure 3). The control group had been published previously [17]. Table 1 summarizes the clinical characteristics. The mean age of colon cancer patients (including 83 men and 58 women) was 66.7 ± 12.4 years, and the mean age of control patients (including 75 men and 114 women) was 67.7 ± 6.1 years. Although the control group included significantly more women than the CRC group, gene polymorphism was not associated with patient gender in the control population. Thirty-seven (26%) of 141 patients with CRC had the II genotype, 69 (49%) had the ID genotype, and 35 (25%) had the DD genotype. The distribution of the ACE genotypes did not differ significantly from the control group or from the distribution predicted by the Hardy-Weinberg equilibrium (Table 1).
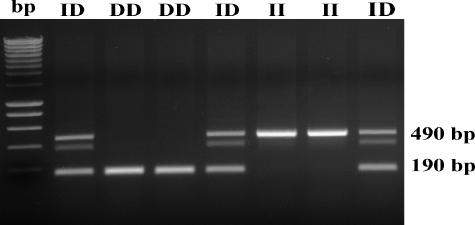
Figure 3.
Determination of ACE genotypes by PCR amplification. Amplified ACE gene fragments were separated on 1.6% agarose gels and visualized by ethidium bromide staining. D-alleles or I-alleles were identified by the presence of 190-bp or 490-bp fragments, respectively. bp, molecular-weight markers.
Univariate analyses showed that the ACE genotypes were associated with patient gender in the CRC group. Women more commonly carried the ID genotype and less frequently the II and DD genotypes compared with men (P = .033). Furthermore, in men, the mean metric tumor diameter was higher in carriers of the ID and DD genotypes. However, this did not reach statistical significance. Therefore, we dichotomized male CRC patients into carriers homozygous for the II-allele and carriers heterozygous or homozygous for the D-allele (Table 2). This demonstrated that the D-allele was associated with a significantly higher mean metric tumor diameter than homozygosity for the II-allele (P < .01; Table 2).
Table 2.
Mean Metric Tumor Size of CRC in Men and Women Dichotomized into Carriers with and without the D-Allele.
| Gender | I/D Genotype | n (%) | Mean ± SD (mm) | P |
| Men | II | 26 (31) | 40.2 ± 12.2 | < .01 |
| ID + DD | 57 (69) | 50.1 ± 21.4 | ||
| Women | II | 11 (19) | 47.6 ± 10.2 | ns |
| ID + DD | 47 (81) | 47.9 ± 30.3 |
No gender-dependent or gender-independent correlation was found between tumor location (colon versus sigma versus rectum), depth of local tumor invasion (T-category), nodal spread (N-category), distant metastases (M-category), UICC tumor stage, and ACE genotype.
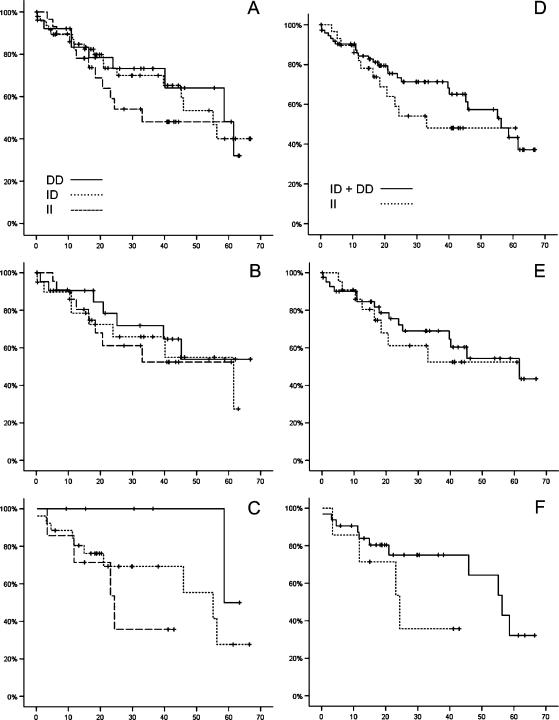
Finally, we studied the influence of the ACE genotypes on patient survival (Table 3). Follow-up data were available from 104 patients (65 men, 39 women). Overall patient survival correlated, although not significantly, with the ACE I/D gene polymorphism. Carriers homozygous for the I-allele lived shorter (mean survival, 38.04 µ 4.75 months) than carriers of the D-allele (46.37 ± 4.90 months; Figure 4, A–C). Interestingly, this difference was mainly related to female gender. Women with the DD genotype had the best prognosis (61.02 ± 1.69 months) compared with women carrying the ID genotype (43.53 ± 5.27 months) or women carrying the II genotype (25.99 ± 5.64 months; Figure 4C). After the dichotomization of the patient population into carriers homozygous for the II-allele and carriers heterozygous (ID) or homozygous for the D-allele (Figure 4, D–F), the correlation between ACE polymorphism and patient survival was still apparent for women (25.99 ± 5.64 vs 47.21 ± 4.53 months; Figure 4F).
Table 3.
Mean Survival of Patients with CRC, By ACE Genotype.
| Gender | n (%) | I/D Genotype | Survival (Months) [Mean ± SD] | P |
| Whole study population | 29 (28) | II | 38.04 ± 4.75 | .508 |
| 48 (46) | ID | 45.29 ± 3.94 | ||
| 27 (26) | DD | 46.37 ± 4.90 | ||
| 75 (72) | ID + DD | 46.13 ± 3.13 | .250* | |
| Men | 22 (34) | II | 40.06 ± 5.54 | .704 |
| 22 (34) | ID | 47.95 ± 5.63 | ||
| 21 (32) | DD | 42.53 ± 5.86 | ||
| 43 (66) | ID + DD | 45.96 ± 4.20 | .587* | |
| Women | 7 (18) | II | 25.99 ± 5.64 | .141 |
| 26 (67) | ID | 43.53 ± 5.27 | ||
| 6 (21) | DD | 61.02 ± 1.69 | ||
| 32 (82) | ID + DD | 47.21 ± 4.53 | .141* |
II versus ID + DD.
Figure 4.
Kaplan-Meier survival curves for the ACE I/D gene polymorphism in CRC patients. Patient survival is shown for the whole study population in (A) and (D), for men in (B) and (E), and for women in (C) and (F) across all genotypes (A–C), or for the dichotomization of the patient population into carriers homozygous for the II-allele and carriers heterozygous (ID) or homozygous for the D-allele (D–F). Gender difference was noted. Women with the DD genotype lived longer than women with the ID or II genotype. The x-axis denotes survival time (months), and the y-axis denotes survival probability.
Discussion
CRC affects approximately 6% of the population and is the second leading cause of cancer-related deaths in the United States and Europe. In 2003, approximately 150,000 new cases of CRC were diagnosed in the United States, and 57,100 individuals died from this disease. Until today, most cases are detected in advanced stages, in which curative treatment is not possible and chemotherapy remains the only, but unsatisfying, option. Thus, improving early diagnosis and finding new treatment strategies still are of paramount importance in CRC [22–24].
Recently, we have shown that ACE is expressed locally in gastric cancer [16] and that the I/D gene polymorphism of the ACE gene influences tumor development [25] and metastatic behavior [17]. Furthermore, the retrospective study provided evidence that long-term ACE medication decreases the risk of developing CRC [18]. Intrigued by these findings, we aimed to further substantiate the putative significance of ACE in gastrointestinal cancer biology by investigating its local expression in CRC and the correlation between ACE gene polymorphism and CRC progression.
We believe that we are the first to show that CRCs differentially express ACE in tumor cells at the mRNA and protein levels, whereas non-neoplastic surface and crypt epithelium rarely express any ACE. Interestingly, ACE was primarily localized at the apical membrane and in the cytoplasm of tumor cells, and not at the basolateral surface. This leads to the conjecture that ACE might be involved in autocrine or paracrine tumor cell homeostasis rather than tumor cell invasion (e.g., matrix degradation and remodeling). ACE is a type I integral membrane protein with a catalytic site exposed to the extracellular surface. Considerable amounts of ACE are expressed by epithelial cells of the small intestine, but also in the chief cells of the gastric foveolar epithelium [16,26–29], where it may play a role in the metabolism of gastrointestinal hormones and regulatory peptides [30,31]. Being a relatively nonspecific enzyme, ACE cleaves a number of synthetic and naturally occurring substrates. Thus, differential upregulation of ACE in CRCs may potentially influence tumor cell biology. In addition, ACE was also strongly expressed in endothelial cells of tumor vessels, leading us to suggest that it may be additionally involved in the neoangiogenesis of CRC.
To further substantiate the putative impact of ACE on CRC biology, we then investigated the correlation of the ACE I/D gene polymorphism with various tumor characteristics. The polymorphism in the ACE gene accounts for 20% to 50% of the variance in ACE expression or activity in blood and tissues among individuals and influences its pathophysiological function in diseases such as hypertension [32], atherosclerotic cardiovascular complications [33], and diabetic nephropathy [34]. The distribution of different ACE gene alleles in our entire CRC population did not differ from the distribution in the general population, indicating that the overall risk of developing CRC is not linked to a specific ACE genotype and, hence, enzyme expression or activity. However, the distribution of individual genotypes among men and women was significantly different, suggesting that the ACE genotype may have a gender-specific impact on CRC cancer risk or progression. This is in line with a recent study published by Reyes-Engel et al. [35], who found significant ACE genotype-dependent differences in the serum levels of angiotensin I and angiotensin 1–7. The gender-dependent influence of ACE on tumor biology was further substantiated by correlating tumor size with the ACE genotypes. Men carrying the D-allele had significantly larger tumors than men homozygous for the I-allele. A similar observation was made in premenopausal women with breast cancer. Female carriers of the D-allele had significantly larger breast cancers than carriers of the I-allele [36]. The absolute size of a primary tumor depends on tumor cell proliferation and neoangiogenesis. Both may be influenced by ACE because tumor cells and tumor vessels express ACE. Interestingly, we did not find a correlation between ACE genotype and tumor size in our female patients, who (different from the study on breast cancer published by Yaren et al. [36]) were primarily postmenopausal in our series. However, a correlation was found when ACE gene polymorphism was correlated with patient survival, and, here, women with the DD genotype had the best prognosis. These seemingly contradictory observations may be related to the hormonal modulation of ACE activity. In postmenopausal women, the DD genotype is associated with endothelial dysfunction, which may also influence CRC vasculature and tumor growth [37,38]. This might further explain why the DD genotype is underrepresented in our female patients compared withmaleCRCpatients. In postmenopausal women, the DD genotype seems to protect from the tumor progression of CRC.
Although ACE gene polymorphism was not linked to the T-category, N-category, or M-category, it correlated with patient survival, indicating that ACE may be involved in disease progression. However, due the small number—particularly of women–in our series, we were unable to demonstrate statistical significance. Therefore, future validation studies should focus on the female patient population.
Different from observations made previously in gastric cancer patients, we were unable to find any correlation between the ACE genotypes and nodal spread in CRC. This probably stems from the overall lower number of lymph node metastases observed in CRC. The CRC patients in our present series had a considerably lower number of lymph node metastases (mean, 2.5 ± 4.1; median, 1.0) than the gastric cancer patients (mean, 6.9 ± 9.0; median, 4.0) studied previously [17].
Our observations may also provide putative explanations to the findings of a retrospective study where ACE medication was associated with a significantly reduced risk of developing CRC [18]. It is now widely accepted that CRCs frequently arise from preneoplastic lesions (i.e., adenomas)—through activation of oncogenes (k-ras) and inactivation of tumor-suppressor genes (APC, p53, and DCC)—and DNA mismatch repair genes [39]. Therefore, we finally studied the expression of ACE in colorectal adenomas. ACE was expressed in colorectal adenomas showing expression patterns similar to those in CRC. Thus, based on our observations and the findings made in a retrospective study, we hypothesize that ACE contributes to the progression of colorectal adenomas to CRC by influencing local tumor growth and neoangiogenesis. ACE inhibitors might interfere with this effect, and further studies into this topic are warranted.
Footnotes
This work was supported by grants from the Wilhelm Sander-Stiftung.
References
- 1.Bauvois B. Transmembrane proteases in cell growth and invasion: new contributors to angiogenesis? Oncogene. 2004;23:317–329. doi: 10.1038/sj.onc.1207124. [DOI] [PubMed] [Google Scholar]
- 2.Abali H, Gullu IH, Engin H, Haznedaroglu IC, Erman M, Tekuzman G. Old antihypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists. Med Hypotheses. 2002;59:344–348. doi: 10.1016/s0306-9877(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 3.Yoshiji H, Kuriyama S, Fukui H. Perindopril: possible use in cancer therapy. Anticancer Drugs. 2002;13:221–228. doi: 10.1097/00001813-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Yoshiji H, Kuriyama S, Fukui H. Angiotensin-I-converting enzyme inhibitors may be an alternative anti-angiogenic strategy in the treatment of liver fibrosis and hepatocellular carcinoma. Possible role of vascular endothelial growth factor. Tumour Biol. 2002;23:348–356. doi: 10.1159/000069792. [DOI] [PubMed] [Google Scholar]
- 5.Fujita M, Hayashi I, Yamashina S, Itoman M, Majima M. Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys Res Commun. 2002;294:441–447. doi: 10.1016/S0006-291X(02)00496-5. [DOI] [PubMed] [Google Scholar]
- 6.Volpert OV, Ward WF, Lingen MW, Chesler L, Solt DB, Johnson MD, Molteni A, Polverini PJ, Bouck NP. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J Clin Invest. 1996;98:671–679. doi: 10.1172/JCI118838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wysocki PJ, Kwiatkowska EP, Kazimierczak U, Suchorska W, Kowalczyk DW, Mackiewicz A. Captopril, an angiotensin-converting enzyme inhibitor, promotes growth of immunogenic tumors in mice. Clin Cancer Res. 2006;12:4095–4102. doi: 10.1158/1078-0432.CCR-05-2489. [DOI] [PubMed] [Google Scholar]
- 8.Yasumaru M, Tsuji S, Tsujii M, Irie T, Komori M, Kimura A, Nishida T, Kakiuchi Y, Kawai N, Murata H, et al. Inhibition of angiotensin II activity enhanced the antitumor effect of cyclooxygenase-2 inhibitors via insulin-like growth factor I receptor pathway. Cancer Res. 2003;63:6726–6734. [PubMed] [Google Scholar]
- 9.Yoshiji H, Kuriyama S, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factor. Clin Cancer Res. 2001;7:1073–1078. [PubMed] [Google Scholar]
- 10.Yoshiji H, Yoshii J, Ikenaka Y, Noguchi R, Yanase K, Tsujinoue H, Imazu H, Fukui H. Suppression of the renin-angiotensin system attenuates vascular endothelial growth factor-mediated tumor development and angiogenesis in murine hepatocellular carcinoma cells. Int J Oncol. 2002;20:1227–1231. [PubMed] [Google Scholar]
- 11.Jick H, Jick S, Derby LE, Vasilakis C, Myers MW, Meier CR. Calcium-channel blockers and risk of cancer. Lancet. 1997;349:525–528. doi: 10.1016/S0140-6736(97)80084-0. [DOI] [PubMed] [Google Scholar]
- 12.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL, Robertson JW. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 13.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, Soubrier F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet. 1992;51:197–205. [PMC free article] [PubMed] [Google Scholar]
- 15.Villard E, Tiret L, Visvikis S, Rakotovao R, Cambien F, Soubrier F. Identification of new polymorphisms of the angiotensin I-converting enzyme (ACE) gene, and study of their relationship to plasma ACE levels by two-QTL segregation-linkage analysis. Am J Hum Genet. 1996;58:1268–1278. [PMC free article] [PubMed] [Google Scholar]
- 16.Carl-McGrath S, Lendeckel U, Ebert M, Wolter AB, Roessner A, Röcken C. The ectopeptidases CD10, CD13, CD26, and CD143 are upregulated in gastric cancer. Int J Oncol. 2004;25:1223–1232. [PubMed] [Google Scholar]
- 17.Röcken C, Lendeckel U, Dierkes J, Westphal S, Carl-McGrath S, Peters B, Krüger S, Malfertheiner P, Roessner A, Ebert MP. The number of lymph node metastases in gastric cancer correlates with the angiotensin I-converting enzyme gene insertion/deletion polymorphism. Clin Cancer Res. 2005;11:2526–2530. doi: 10.1158/1078-0432.CCR-04-1922. [DOI] [PubMed] [Google Scholar]
- 18.Lang L. ACE inhibitors may reduce esophageal cancer incidence. Gastroenterology. 2006;131:343–344. [Google Scholar]
- 19.Wittekind C, Sobin LH. TNM Classification of Malignant Tumours. 6th ed. New York: Wiley-Liss, Inc.; 2002. [Google Scholar]
- 20.Yoshida H, Mitarai T, Kawamura T, Kitajima T, Miyazaki Y, Nagasawa R, Kawaguchi Y, Kubo H, Ichikawa I, Sakai O. Role of the deletion of polymorphism of the angiotensin converting enzyme gene in the progression and therapeutic responsiveness of IgA nephropathy. J Clin Invest. 1995;96:2162–2169. doi: 10.1172/JCI118270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medeiros R, Vasconcelos A, Costa S, Pinto D, Lobo F, Morais A, Oliveira J, Lopes C. Linkage of angiotensin I-converting enzyme gene insertion/deletion polymorphism to the progression of human prostate cancer. J Pathol. 2004;202:330–335. doi: 10.1002/path.1529. [DOI] [PubMed] [Google Scholar]
- 22.Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiewlich D, Zhang J, Gross C, Xia W, Larsen B, Cobb RR, Biroc S, Gu JM, Sato T, Light DR, et al. Anti-EphA2 antibodies decrease EphA2 protein levels in murine CT26 colorectal and human MDA-231 breast tumors but do not inhibit tumor growth. Neoplasia. 2006;8:18–30. doi: 10.1593/neo.05544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Zhang Q, Zhang Y, Wang S, Ding Y. Lentivirus-mediated silencing of Tiam1 gene influences multiple functions of a human colorectal cancer cell line. Neoplasia. 2006;8:917–924. doi: 10.1593/neo.06364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebert MP, Lendeckel U, Westphal S, Dierkes J, Glas J, Folwaczny C, Roessner A, Stolte M, Malfertheiner P, Röcken C. The angiotensin I-converting enzyme gene insertion/deletion polymorphism is linked to early gastric cancer. Cancer Epidemiol Biomark Prev. 2005;14:2987–2989. doi: 10.1158/1055-9965.EPI-05-0411. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi R, Sun XY, Walsh JH. Angiotensin-converting enzyme in the rabbit stomach wall. Identification in the membrane fraction by affinity purification. Gastroenterology. 1991;100:25–32. doi: 10.1016/0016-5085(91)90578-9. [DOI] [PubMed] [Google Scholar]
- 27.Laliberte F, Laliberte MF, Nonotte I, Bali JP, Chevillard C. Angiotensin I converting enzyme in gastric mucosa of the rabbit: localization by autoradiography, immunofluorescence, and immunoelectron microscopy. J Histochem Cytochem. 1991;39:1519–1529. doi: 10.1177/39.11.1655876. [DOI] [PubMed] [Google Scholar]
- 28.Nonotte I, Laliberte MF, Duperray C, Hollande F, Bali JP, Laliberte F, Chevillard C. Expression of angiotensin I converting enzyme mRNA in rabbit gastric epithelial cells. Mol Cell Endocrinol. 1993;92:167–174. doi: 10.1016/0303-7207(93)90004-4. [DOI] [PubMed] [Google Scholar]
- 29.Nonotte I, Laliberte MF, Remy-Heintz N, Laliberte F, Chevillard C. Expression of angiotensin I-converting enzyme in the human gastric HGT-1 cell line. Regul Pept. 1995;59:379–387. doi: 10.1016/0167-0115(95)00090-x. [DOI] [PubMed] [Google Scholar]
- 30.Lendeckel U, Kähne T, Riemann D, Neubert K, Arndt M, Reinhold D. Review: the role of membrane peptidases in immune functions. Adv Exp Med Biol. 2000;477:1–24. doi: 10.1007/0-306-46826-3_1. [DOI] [PubMed] [Google Scholar]
- 31.Turner AJ, Hooper NM, Kenny AJ. Mammalian Ectoenzymes. Amsterdam: Elsevier; 1987. Metabolism of neuropeptides; pp. 211–248. [Google Scholar]
- 32.Abbud ZA, Wilson AC, Cosgrove NM, Kostis JB. Angiotensinconverting enzyme gene polymorphism in systemic hypertension. Am J Cardiol. 1998;81:244–246. doi: 10.1016/s0002-9149(97)00876-x. [DOI] [PubMed] [Google Scholar]
- 33.Bedir A, Arik N, Adam B, Kilinc K, Gumus T, Guner E. Angiotensin converting enzyme gene polymorphism and activity in Turkish patients with essential hypertension. Am J Hypertens. 1999;12:1038–1043. doi: 10.1016/s0895-7061(99)00096-5. [DOI] [PubMed] [Google Scholar]
- 34.Boright AP, Paterson AD, Mirea L, Bull SB, Mowjoodi A, Scherer SW, Zinman B. Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics Study. Diabetes. 2005;54:1238–1244. doi: 10.2337/diabetes.54.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes-Engel A, Morcillo L, Aranda FJ, Ruiz M, Gaitan MJ, Mayor-Olea A, Aranda P, Ferrario CM. Influence of gender and genetic variability on plasma angiotensin peptides. JRAAS J Renin-Angiotensin-Aldosterone Syst. 2006;7:92–97. doi: 10.3317/jraas.2006.015. [DOI] [PubMed] [Google Scholar]
- 36.Yaren A, Turgut S, Kursunluoglu R, Oztop I, Turgut G, Kelten C, Erdem E. Association between the polymorphism of the angiotensin-converting enzyme gene and tumor size of breast cancer in premenopausal patients. Tohoku J Exp Med. 2006;210:109–116. doi: 10.1620/tjem.210.109. [DOI] [PubMed] [Google Scholar]
- 37.Methot J, Hamelin BA, Arsenault M, Bogaty P, Plante S, Poirier P. The ACE-DD genotype is associated with endothelial dysfunction in postmenopausal women. Menopause. 2006;13:959–966. doi: 10.1097/01.gme.0000243576.09065.93. [DOI] [PubMed] [Google Scholar]
- 38.Wassmann K, Ghiassi A, Wassmann S, Bohm M, Nickenig G. AT1 receptor antagonism improves endothelial dysfunction in postmenopausal women. Maturitas. 2006;53:176–183. doi: 10.1016/j.maturitas.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Thorstensen L, Lind GE, Lovig T, Diep CB, Meling GI, Rognum TO, Lothe RA. Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia. 2005;7:99–108. doi: 10.1593/neo.04448. [DOI] [PMC free article] [PubMed] [Google Scholar]