Abstract
In the present work, the antitumor effect of fastuosain, a cysteine proteinase from Bromelia fastuosa, was investigated. In the intravenous model of lung colonization in C57Bl/6 mice, fastuosain and bromelain injected intraperitoneally were protective, and very few nodules of B16F10-Nex2 melanoma cells were detected. Tumor cells treated with fastuosain showed reduced expression of CD44 and decreased invasion through Matrigel, lost their cytoplasmic extensions and substrate adherence, and became round and detached, forming strongly bound cell clusters in suspension. Peritoneal cells recruited and activated by fastuosain treatment (mainly monocytic cells and lymphocytes) migrated to the lung, where pulmonary melanoma metastases grew. Adoptive transference of peritoneal cells recruited by fastuosain had no protective effect against lung metastases in recipient mice. Treatment of green fluorescent protein-chimeric animals with fastuosain did not change the number of cells that migrated to the lung, compared to PBS-injected control mice, but the number of positive major histocompatibility complex class II cells increased with fastuosain treatment. Murine antibodies against fastuosain, bromelain, and cathepsins B and L cross-reacted in ELISA and recognized surface and cytoplasmic components expressed on B16F10-Nex2 cells. Anti-fastuosain antibodies were cytotoxic/lytic to B16F10-Nex2 cells. Antitumor effects of fastuosain involve mainly the direct effect of the enzyme and elicitation of protective antibodies.
Keywords: Fastuosain, bromelain, B16F10-Nex2 tumor cells, cathepsins B/L, protective antibodies
Introduction
Endogenous cysteine proteinases that are expressed and regulated in defined microenvironments, with complementary and often contradictory responses, functionally depend on the biological system examined and generally stimulate angiogenesis and tumor growth. When exogenously administered, cysteine proteinases may have several pharmacological properties and, contrariwise, display antitumor activities.
Fastuosain is a novel cysteine proteinase from Bromelia fastuosa with an estimated molecular mass of 25 kDa. It has been purified from the “gravatá” fruit from southeastern Brazil. The fruit is edible, and its juice is popularly used in the treatment of bronchitis and asthma. It has been functionally characterized, cloned, and sequenced by Cabral et al. [1]. This enzyme is similar to bromelain, with a 79.4% sequence homology of identical amino acids. It is also partially homologous to papain (43% identity) and to mammalian cathepsins L and B (37.8% and 22.8% identities, respectively). The sequence QGS(Q)CGSCWAF is 100% conserved in fastuosain, papain, and cathepsins B and L.
Bromelain has antiedematous, anti-inflammatory, anti-thrombotic, and fibrinolytic activities, as demonstrated in vitro and in vivo. It is effective after oral administration and, therefore, has become a popular phytotherapeutic drug [2]. Presently, we compared fastuosain and bromelain, focusing on their antitumor activities. Bromelain showed antimetastatic efficacy and inhibited platelet aggregation, growth, and invasiveness of tumor cells [3,4].
Multienzyme mixtures, comprising cysteine proteinases such as bromelain and papain and also trypsin and chymotrypsin, have been used mainly because of the safety and efficacy of oral administration. Most proteins given orally result in tolerance, but long-term exposure to bromelain leads to a high anti-bromelain antibody response [5]. These antibodies did not affect the proteolytic activity of bromelain. The protective effect of papain, alone or together with trypsin and chymotrypsin, against murine melanoma has been reported [6,7].
In the present work, we investigated the effect of fastuosain administration on B16F10-Nex2 growth in vitro and in vivo. Our results show that fastuosain has antitumor activity and that this property is due to a direct effect of fastuosain on tumor cells and also to antibodies to fastuosain that cross-react with cathepsins B and L. These antibodies promoted direct cell cytotoxicity and lysis mediated by complement. The cellular immune response raised by active and inactive fastuosain did not effectively contribute to antitumor protective effect.
Materials and Methods
Proteases
Bromelain (B-4882; Sigma Chemical Co., St. Louis, MO) was freshly prepared as a 10- to 20-mg/ml stock solution in PBS. The protease fastuosain was purified as previously described [1]. Briefly, the juicy pulp of unripe fruits from B. fastuosa, popularly known as gravatá, was stirred in a blender containing chilly buffer A (70 mM acetate buffer, pH 4.0, containing 2 mM EDTA). The extract was filtered through cheesecloth and plastic sieve, and centrifuged at 5000g for 40 minutes at 4°C. Ion exchange chromatography was performed on an SP-Sepharose column (Pharmacia, Piscataway, NJ) equilibrated previously with buffer A and eluted with a convex gradient of buffer B (70 mM acetate buffer, pH 4.0, containing 0–2.0 M NaCl). Fractions containing proteolytic activity were pooled, concentrated, and filtered by gel chromatography in a Sephadex G-50 column (Pharmacia) using buffer B. A 15% SDS-PAGE stained with silver nitrate showed a single protein band with an estimated Mr of 25 kDa.
Animals
Male C57Bl/6 mice, 6 to 8 weeks old (20–25 g), were obtained from the Center for Development of Experimental Models, Universidade Federal de São Paulo (São Paulo, SP, Brazil). Male C57Bl/6-GFP transgenic mice, 3 weeks old (20–23 g), were bred and maintained at the Animal Facility of Gonçalo Moniz Research Center/FIOCRUZ. All animals were kept in isolators in rooms with controlled temperature (22 ± 2°C) and humidity (55 ± 10%), and continuous air renovation. Animals were housed in a 12-hour light/12-hour dark cycle and were provided autoclaved rodent diet and water ad libitum. All experiments were carried out in accordance with prior approval from the local animal ethics committee.
Cells and Cell Culture
The B16F10 murine melanoma cell line was originally obtained from the Ludwig Institute for Cancer Research, São Paulo branch. At the Experimental Oncology Unit (UNONEX), we isolated several sublines from the original cell line, and the melanotic subline used in this work, B16F10-Nex2, is characterized by low immunogenicity and moderate virulence. It forms lethal subcutaneous tumors, but pulmonary nodules are formed only when injected endovenously. Cells were cultivated in RPMI 1640 medium (pH 7.2; Invitrogen, Carlsbad, CA) supplemented with 10 mM Hepes, 24 mM sodium bicarbonate, 40 mg/ml gentamycin, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% fetal calf serum, all from Invitrogen. Cells were maintained in culture flasks at 37°C in a humidified atmosphere with 5% CO2, and cell detachment was performed using a PBS-EDTA (1 mM) solution.
MTT Assay for Cell Viability
B16F10-Nex2 tumor cells were seeded at 103 per well into 96-well plates (Corning Costar Co., New York, NY) 12 hours previously and incubated with serially diluted proteolytic enzymes up to a final volume of 180 µl (in complete RPMI medium). At different time points, 20 µl of PBS containing 5 mg/ml MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma Chemical Co.] was added to each well. After 4 hours, 50 µl/well of acid SDS solution (SDS 10%, HCl 0.84%) was added and incubated overnight for the solubilization of the formazan crystals generated. Absorbance was read at 570 nm with a microplate reader (MCC/340; Titertek Multiscan, Flow Laboratory, Rockville, MD).
Flow Cytometry Analysis of Adhesion Molecules on the B16F10-Nex2 Cell Surface after Enzyme Treatment
B16F10-Nex2 tumor cells (106 cells/well in 24-well plates) were treated with serially diluted fastuosain or bromelain up to a final volume of 1 ml of serum-free RPMI medium for 1 hour at 37°C. After enzyme removal by PBS washings, cells were resuspended in PBS containing 10% BSA, incubated for 10 minutes on ice, and washed with PBS. After the addition of 1 mg of fluorescein isothiocyanate (FITC)-conjugated antibodies against mouse adhesion molecules (CD44 or ICAM-1; BD Biosciences, San Jose, CA) diluted in 50 µl of fluorescence-activated cell sorting (FACS) buffer (PBS containing 1% BSA), cells were incubated on ice protected from light for 1 hour, washed thrice with PBS, and resuspended in 2% cold paraformaldehyde (wt/vol). Fluorescence was measured on a FACScan flow cytometer (BD Biosciences), and data were analyzed by CellQuest software (Becton Dickinson, San Jose, CA). For analysis of adhesion molecule reexpression on tumor cell surface after enzyme treatment, B16F10-Nex2 cells were treated with fastuosain, washed with serum-free RPMI, plated on a six-well plate (Corning Costar Co.), and incubated in complete RPMI medium at 37°C and 5% CO2 for different time periods. Cells were then harvested, and 106 cells were labeled with anti-CD44 or anti-ICAM-1 as described.
Matrigel Invasion Assay
The effect of protease treatment on B16F10-Nex2 cell invasion was observed in Transwell chambers (3-µm pore size, 12-mm polycarbonate filters; Corning Costar Co.) coated with Matrigel (Basement Membrane Matrix; Becton Dickinson). Diluted cold Matrigel (50 µg, or 56 µl of a stock solution previously diluted 1:3, vol/vol, in serum-free RPMI) was added to the upper 24-well Transwell chamber and incubated for 30 minutes at 37°C for gel formation. Lower chambers were filled with 200 µl of a 10-µg/ml laminin-1 solution, diluted in serum-free RPMI medium, as a haptotactic substrate. Tumor cells (2 x 105 ml-1) were treated with fastuosain, bromelain (10 and 5 µg/ml), or 1 µg of anti-CD44 monoclonal antibody (mAb; BD Biosciences) in serum-free RPMI mediumfor 1 hour at 37°C and 5% CO2. After incubation, cells were washed and resuspended in 200 µl of serum-free RPMI, added to the upper Transwell compartment containing polymerized Matrigel, and incubated for 5 hours at 37°C and 5% CO2. Cells unable to invade the Matrigel were scraped off mechanically from the upper chamber with a cotton swab. After PBS washings, cells underneath the membrane filter were fixed in paraformaldehyde (3.7%) for 15 minutes and stained with 0.1% toluidine blue solution for 2 minutes at 37°C; after washing with tap water, the filters were incubated with 200 µl of 1% SDS solution for 1 hour at 37°C. This solution was transferred to a 96-well ELISA plate, and absorbance was measured at 600 nm in a microplate reader (MCC/340; Titertek Multiscan).
Obtention of C57Bl/6-GFP-Chimeric Mice
C57Bl/6 mice were lethally irradiated with 600 rad. Adult male hemizygous green fluorescent protein (GFP) donor transgenic mice were killed by cervical dislocation under deep anesthesia, and bone marrow was obtained by flushing the femur and tibia with serum-free RPMI medium. Cells were washed, counted, and resuspended in sterile saline at a concentration of 108 cells/ml. Two hundred microliters of the suspension was administrated intravenously into each irradiated C57Bl/6 recipient animal. Chimeric mice were allowed to recover from manipulation and to have their marrow engrafted for 1 month before an intravenous injection of B16F10-Nex2 cells. For control, flow cytometry was performed with some animals to check the presence of GFP cells in chimeric bone marrow.
Experimental Tumor Metastasis Assay In Vivo
C57Bl/6 wild-type or GFP-chimeric mice were injected intravenously into lateral tail veins with 100 µl of PBS containing 5 x 105 B16F10-Nex2 viable cells. Intraperitoneal injections of fastuosain (60 µg/animal) or PBS (control group) were started on the same day and repeated three times a week for 21 days. After the administration of anesthesia, the animals were killed by cervical dislocation, their lungs were collected and formalin-fixed, and pulmonary nodules were counted with the aid of an inverted microscope.
Following the counting of nodules, lungs were dehydrated and embedded in paraffin by routine methods for immuno-histopathological analysis. Paraffin sections of GFP-chimeric animals (5 µm) were stained with anti-GFP antibodies conjugated with Alexa-Fluor 488 (Molecular Probes, Carlsbad, CA). For quantification of major histocompatibility complex (MHC) class II-expressing cells, lung sections were stained using a biotin-conjugated mAb (BD Biosciences), followed by incubation with streptavidin conjugated with Alexa 568 (Molecular Probes). Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; VectaShield Hard Set mounting medium with DAPI H-1500; Vector Laboratories, Burlingame, CA). Fluorescent cells were observed in a BX61 microscope with an epifluorescence system plus grid to enhance fluorescence resolution (OptiGrid Structured Light Imaging System; Thales Optem, Inc., Fairport, NY) using appropriate filters (Olympus, Tokyo, Japan). Images were captured using a color digital video SPOT flex camera (15.2, 64 Mp, Shifting Pixel; Diagnostic Instruments, Inc., Sterling Heights, MI).
Flow Cytometry of Total Peritoneal Cells and Macrophages Isolated from Fastuosain-Treated Animals
Twenty male C57Bl/6 mice were intraperitoneally treated with fastuosain or with fastuosain treated with E-64, a cysteine protease inhibitor, to inhibit enzyme catalytic activity. The treatment consisted of 60-µg injections of active or inactivated enzyme three times a week for 21 days. Peritoneal cells were then collected with cold serum-free RPMI, and part of these cells were submitted to 3-hour adhesion on a Petri dish for obtention of a macrophage-enriched fraction. Non-adherent cells were removed by serum-free medium washings. To detach macrophages from the plates, they were placed on ice for 15 minutes, followed by cold PBS washings. After counting, total peritoneal and macrophage-enriched cells were incubated with FITC-conjugated, phycoerythrin-conjugated, or unlabeled mAbs for 1 hour on ice. When an unlabeled antibody was used, this step was followed by a second incubation with an anti-mouse Ig conjugated with a fluorochrome (FITC or phycoerythrin) for 30 minutes. All samples were fixed with 0.4% paraformaldehyde before analysis on a FACScan Flow cytometer (BD Biosciences). F4/80 expression was used to gate macrophage cells. The mAbs used were antibodies to CD11b (Mac-1), CD1d, CD3e, CD54 (ICAM-1), CD119 [interferon (IFN) γ receptor], CD11c, CD25, CD4, CD80 (B7), CD86 (B7.2), CD90 (Thy-1), CD178 (Fas-L), MHC I, MHC II, NK 1.1, CD8a, CD44, and Vβ8.2 TCR (BD Biosciences).
Production of Polyclonal Monospecific Murine Antibodies against Fastuosain, Papain, and Bromelain: Purification of Protease-Specific IgG and Detection By ELISA
Male Balb/c mice, 6 to 8 weeks old, were treated intraperitoneally with four doses of 250 µg/100 µl fastuosain, bromelain, or papain, or with the same volume of PBS (control animals) once a week. One week after the last injection, serum was collected, and antibodies were precipitated by saturated ammonium sulfate solution. To purify specific protease antibodies, tresyl-agarose resin (Supelco, Bellefonte, PA) was coupled with fastuosain or bromelain, and purified antibodies were affinity-chromatographed. IgG protease-specific fraction was isolated using a Protein G column (Hi-Trap Protein G affinity column; Amersham Biosciences, Piscataway, NJ). Silver-stained 12% SDS-PAGE was performed to verify the purity of antibody preparation.
The binding specificity of purified antibodies was verified by ELISA. Proteases (100 pg/100 µl) were added on 96-well plates (ELISA Hi Binding Plate; Nunc, Roskilde, Denmark) and incubated overnight at 4°C in 50 mM carbonate-bicarbonate buffer (pH 9.6). Unbound antigen was removed by washings with PBS containing 0.05% Tween 20 (PBS-T). After blocking for 5 hours with PBS containing 5% fat-free powder milk and 2% BSA, plates were washed with PBS-T; anti-fastuosain, anti-papain, and anti-bromelain purified antibodies, or commercial anti-cathepsin B antibodies (Calbiochem, Darmstadt, Germany) serially diluted in reaction buffer (PBS containing 2% fat-free powder milk and 1% BSA) were added. After overnight incubation at 4°C, plates were washed with PBS-T, and HRP-conjugated anti-mouse antibody (ICN Biomedicals, Inc., Irvine, CA) was added (diluted 1:1000 in reaction buffer). After 5 hours of incubation at 4°C, the reaction was revealed with a specific substrate (orthophenylenediamine) and stopped with 4 N H2SO4. Absorbance was read at 490 nm with a microplate reader (MCC/340; Titertek Multiscan).
Determination of the Reactivity of Antiproteases with B16F10-Nex2 Cells By Flow Cytometry
The reactivity of anti-fastuosain, anti-bromelain, anti-cathepsin B, and anti-cathepsin D antibodies with B16F10-Nex2 melanoma cells was verified by flow cytometry. B16F10-Nex2 cells (106) were permeabilized or not with PBS containing 0.05% saponin. The cells were then incubated for 1 hour on ice with 1 µg of purified polyclonal IgG against proteolytic enzymes; or with commercial mAbs anti-CD44 (BD Biosciences), anti-cathepsin B, or anti-cathepsin D (Calbiochem); or yet with an irrelevant mAb anti-IL-5 (BD Biosciences). Polyclonal antibodies and mAbs were diluted in 50 µl of FACS buffer (PBS containing 1% BSA and 1% normal mouse serum). After washings with PBS, cells were incubated for 1 hour with FITC-conjugated secondary antibodies and resuspended in 2% cold paraformaldehyde (wt/vol). Analyses were performed on a FACScan flow cytometer (BD Biosciences). As negative control, FITC-conjugated secondary antibodies were used without primary antibody binding. Data were analyzed with CellQuest software.
Confocal Microscopy with Anti-Fastuosain Purified Antibodies
B16F10-Nex2 cells were seeded on sterilized round coverslips (Fisher Scientific Co., Pittsburgh, PA) inserted into 24-well plates and allowed to reach 80% confluency. Cells were then fixed with cold paraformaldehyde (3.7%) and permeabilized with PBS containing 0.5% saponin. Coverslips were washed with saponin buffer (PBS containing 0.05% saponin and 1% BSA); incubated with purified anti-fastuosain, anti-cathepsin B, and anti-IL-5 antibodies diluted in this buffer for 1 hour on ice; and washed twice with saponin buffer. FITC-conjugated anti-mouse Ig antibody (BD Biosciences) and phalloidin (Molecular Probes), diluted in saponin buffer, were incubated for 1 hour on ice. Slides were washed in PBS and distilled water, and mounted using Vectashield (Vector Laboratories) in the presence of 10 µg/ml DAPI. They were examined with laser-scanning fluorescence confocal microscopy (Bio-Rad MRC 1024/UV System; Bio-Rad, Hercules, CA) equipped with a transmitted light detector for Nomarski differential interference contrast. Images were collected with a 40x 1.2/water immersion PlanApo objective (Carl Zeiss MicroImaging, Inc., Thornwood, NY), Kalman averaging at least 20 frames using a 2-mm iris (pinhole) [8]. As negative control, FITC-conjugated secondary antibody was used without primary antibody binding.
Adoptive Transfer of Total Peritoneal Cells from C57Bl/6-GFP Transgenic Mice Treated with Fastuosain to B16F10-Nex2-Inoculated C57Bl/6 Mice
C57Bl/6-GFP transgenic mice were treated intraperitoneally with 60 µg/100 µl fastuosain or with the same volume of PBS (control group) three times a week for 21 days. After treatment, total peritoneal cells were collected with 5 ml of cold PBS and centrifuged for 5 minutes at 1500 rpm. The cell pellet of one mouse was suspended in 0.5 ml of PBS and inoculated intraperitoneally in one C57Bl/6 mouse (total peritoneal cells of a mouse transferred to a new mouse). On the same day, 5 x 105 B16F10-Nex2 cells (in 100 µl of PBS) were injected intravenously into the lateral tail veins. After 21 days, the animals were sacrificed, and their lungs were formalin-fixed. After counting pulmonary nodules with the aid of an inverted microscope, the lungs were dehydrated and embedded in paraffin by routine methods. Paraffin sections of 5 µm were stained with anti-GFP antibodies conjugated with Alexa-Fluor 488 (Molecular Probes) and analyzed as described for chimeric animals.
Results
While describing the effects of fastuosain on melanoma cells, some experiments included bromelain because the two enzymes share amino acid identities as high as 79.4%. Both enzymes were tested in vitro and in vivo.
Effect of Cysteine Proteinases on Tumor Cell Metastasis Using the Intravenous Route
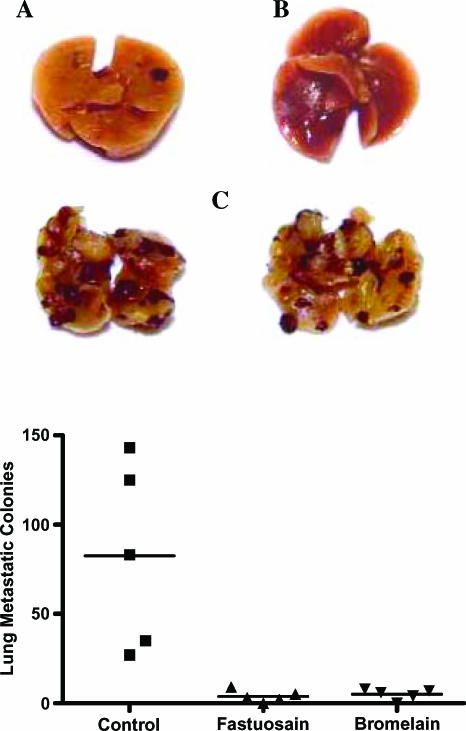
Intravenous injection of B16F10-Nex2 melanoma cells in C57Bl/6 mice is followed by significant lung colonization after 21 days. Intraperitoneal treatment with fastuosain or bromelain (60 µg/ml) starting on the same day of intravenous challenge with tumor cells greatly reduced the number of lung metastatic nodules (Figure 1). Furthermore, no metastatic tumor nodules were found in the myocardium, brain, kidney, liver, and spleen. Apparently, a number of injections are needed to keep an optimal level of fastuosain in the circulation to achieve antitumor protective effect.
Figure 1.
Inhibitory effects of enzyme treatment on pulmonary metastasis in C57Bl/6 mice intravenously injected with B16F10-Nex2 melanoma cells (1 x 105 Fastuosain and bromelain (60 µg) were administered intraperitoneally every other day for 21 days. Mice were killed on day 21 after tumor cell challenge, and lungs were fixed in formaldehyde (10%). Pulmonary nodules were counted using an inverted microscope. Photographs of lung metastatic colonies in the fastuosain group (A), bromelain group (B), and untreated control group (C) are shown.
Effect on Tumor Cell Growth In Vitro
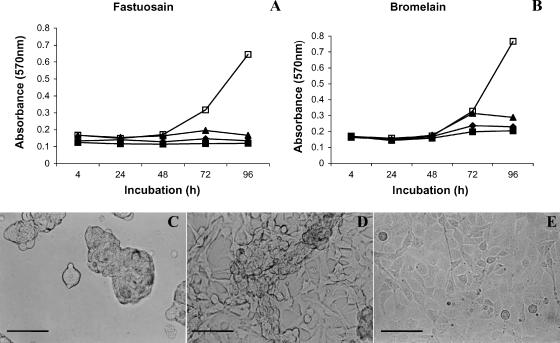
Tumor cell growth was evaluated in vitro in a time-dependent manner. The cell viability of fastuosain-treated B16F10-Nex2 was measured by MTT assay. After 72 hours of treatment, the inhibitory effect of fastuosain (2.5–10 µg/ml) was significant, as shown in Figure 2A. B16F10-Nex2 melanoma cells were also quite sensitive to bromelain treatment (Figure 2B). After 96 hours of treatment with fastuosain and bromelain, the growth of B16F10-Nex2 melanoma cells was completely inhibited. By examining cell morphology, it was observed that fastuosain—more than bromelain—caused cell detachment and formation of clusters and aggregates that still contained live cells within (Figure 2, C and D). These cells were not stained by trypan blue and could return to normal morphology after removal of the enzyme (data not shown). The enzyme seemed to affect intercellular and substrate adhesion, with no direct killing of tumor cells.
Figure 2.
Direct effect of enzyme treatment on B16F10-Nex2 cells. Cells were plated at 1 x 103 into 96-well plates in quadruplicates and treated with increasing concentrations of fastuosain (A) and bromelain (B). The growth of melanoma cells was measured at various time periods using the MTT method: (■) 10 µg/ml; (◆) 5 µg/ml; (▲) 2.5 µg/ml; (□) control cells. Alterations in cell morphology were observed after 48 hours of fastuosain (C) and bromelain (D) treatment (10 µg/ml). (E) Untreated tumor cells. Scale bars, 20 µm.
CD44 Expression
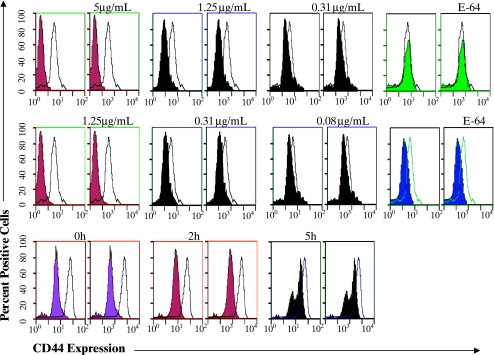
Fastuosain could influence the expression of surface molecules on B16F10-Nex2 cells, and the first component examined was CD44, owing to its role in tumor cell adherence. The expression of CD44 on B16F10 melanoma cells has also been described as a target of bromelain [9]. After treatment with fastuosain at 5 µg/ml, no CD44 expression was detected on the cell surface (Figure 3A). Removal of CD44 was dose-dependent and was not observed when fastuosain had its catalytic activity abolished by E-64. A similar response was obtained with bromelain (Figure 3B). Reexpression of CD44 molecule on the cell surface after fastuosain removal was also examined. After fastuosain treatment (10 µg/ml for 1 hour), the enzyme was removed by washing, and CD44 levels were measured immediately, after 2 hours, and after 5 hours. Five hours after fastuosain removal, 40% of the CD44 molecule was reexpressed on B16F10 Nex2 cells. Reexpression of CD44 was heterogeneous, and at least two populations were seen with different CD44 contents after 5 hours.
Figure 3.
Effect of fastuosain (A and C) and bromelain (B) treatment (1 hour) of B16F10-Nex2 cells on CD44 expression. Open peaks represent CD44 expression on untreated tumor cells; solid curves show CD44 expression after enzyme treatment at indicated concentrations. E-64 is a specific inhibitor of cysteine proteinases. The time course of CD44 reexpression on fastuosain-treated B16F10-Nex2 cells (10 µg/ml for 1 hour) after fastuosain removal is shown in (C). Data shown are representative of four independent experiments.
Effect of Fastuosain on Tumor Cell Invasion
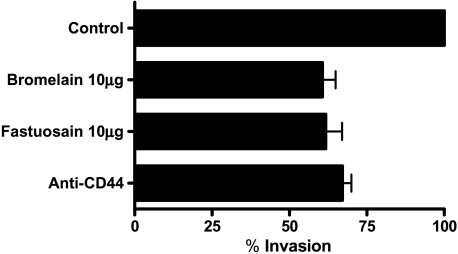
Tumor cell invasion of connective tissues and basal membrane is usually tested in vitro using Matrigel, a product of a murine sarcoma cell line. The test using Transwell chambers can measure both migration of cells and penetration through Matrigel and across a membrane, using laminin as a haptotactic stimulus. After the fastuosain and bromelain treatment (10 µg/ml) of B16F10-Nex2 melanoma cells, a significant reduction in cell invasion through the reconstituted basal membrane was observed (60% invasion compared to controls without treatment). Incubation of tumor cells with mAb anti-CD44 was also effective in reducing the invasion rate (about 70% invasion), compared to untreated controls (Figure 4). In a previous test of cell migration in the absence of Matrigel, treatment with either fastuosain or bromelain did not change the migration rate, which, therefore, does not seem to depend on CD44 (data not shown). The same was observed with the addition of anti-CD44 antibody.
Figure 4.
Effect of fastuosain and bromelain treatment on B16F10-Nex2 cell invasion of Matrigel in vitro. Treatment with fastuosain or bromelain (10 µg/ml, 1 hour) inhibited B16F10-Nex2 cell invasion. Incubation with anti-CD44 mAbs promotes a similar inhibition of the cell invasion process. The effect is expressed as percentages relative to controls (invasion by cells treated with saline). Standard deviations from the means of three experiments are shown.
Recruitment and Surface Antigen Expression on Peritoneal Cells in Mice Injected Intraperitoneally with Fastuosain
The effect of proteolytic enzymes on the cell surface expression of molecules involved in lymphocyte adhesion and activation has been reported in vitro [10,11]. Presently, we investigated the recruitment and expression of surface antigens on peritoneal cells after the intraperitoneal injection of active fastuosain (60 µg) and fastuosain inactivated by E-64. The protocol of fastuosain administration was the same as used before in a lung colonization model. After 21 days, peritoneal cells were harvested, washed, and labeled with antibodies to CD119, CD11b, CD11c, CD1d, CD25, CD4, CD8a, CD54, CD80, CD86, CD3e, CD44, MHC I, MHC II, NK 1.1, CD90, or CD178 following analysis by flow cytometry (FACS) (Table 1). Antigen expression as a function of cell recruitment and activation by fastuosain was clearly stimulated for CD178, CD3, CD86, CD25, and CD11b. Generally, labeling of peritoneal cells was comparable in both systems of active and inhibited fastuosain administration, with some exceptions, notably CD80, which was less expressed in the active fastuosain-injected group. We focused next on macrophages that represent a critical link between innate and adaptive immunity and are important modulators of tumor growth.
Table 1.
Effect of Intraperitoneal Fastuosain Treatment on the Expression of Cell Surface Antigens, as Analyzed By FACS.
| Antigen | Fastuosain | Inactive Fastuosain |
| CD178 | ++ | +++ |
| CD90 | + | + |
| CD8 | + | + |
| CD4 | - | + |
| NK 1.1 | ++ | + |
| MHC II | + | + |
| MHC I | ++ | + |
| CD44 | ++ | + |
| CD3 | ++ | +++ |
| CD86 | ++++ | ++++ |
| CD80 | - | +++ |
| CD54 | + | + |
| CD25 | ++++ | ++++ |
| CD1d | - | + |
| CD11c | + | ++ |
| CD11b | ++ | ++ |
| CD119 | - | + |
Total peritoneal cells from animals treated with fastuosain ive form or with inhibited catalytic activity (E-64 treatment) were examined.
Changes in the mean fluorescence intensity of cells, compared to the control group injected with PBS, are shown as follows: (-) lower expression; (+) equal to control; (++) increased expression, 1.5 times the antigen expression in controls; (+++) increased expression, 2 times the antigen expression in controls; (++++) increased expression, > 2 times the antigen expression in controls.
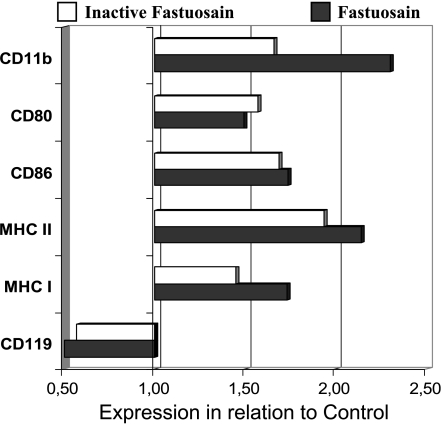
Total adherent cells were double-stained with anti-F4/80 (a pan macrophage marker), anti-CD119 (IFN-γ-R), anti-CD11b (Mac-1), anti-CD80, anti-CD86, anti-MHC I, and anti-MHC II antibodies (Figure 5). Both treatments with fastuosain or inactive fastuosain promoted a significant increase in the expression of those antigens, with the exception of CD119, which was less expressed than in the PBS-treated control group (Figure 5). This result suggests that the fastuosain protein (active/inactive) activated macrophages and that proinflammatory response, including IFN-γ production, led to downregulation of the respective receptor.
Figure 5.
Effect of intraperitoneal fastuosain treatment on the expression of surface antigens on F4/80+ macrophages. Peritoneal adherent cells from animals treated with fastuosain in the native form or with abolished catalytic activity (by E-64) were analyzed by flow cytometry (FACS). Changes in the mean fluorescence intensity of cells in relation to the control group (injected with PBS) are shown.
Adoptive Transference of Peritoneal Cells from GFP Animals to Wild-Type C57Bl/6 Mice
This experiment aimed at determining whether total peritoneal cells from animals that went through the complete protocol of fastuosain administration could protect naïve animals challenged intravenously with B16F10-Nex2 cells. The fastuosain treatment protocol was therefore reproduced in GFP animals, and, after 21 days, green peritoneal cells were transferred to the peritoneum of naïve mice simultaneously with intravenous injection of tumor cells. After 21 days of adoptive cell transference and challenge with tumor cells, a slight reduction in lung metastatic colonies was observed in relation to the control group that received peritoneal cells treated with saline (data not shown), but the difference was not statistically significant. Twenty-one days after adoptive transference, the number of green peritoneal cells in the lung was rather reduced but was still detectable and was similar in the fastuosain and control groups (not shown). It was evident that peritoneal cells migrated to the lung, where metastatic nodules developed but, alone, were not able to protect mice against melanoma growth.
Lung Metastatic Colonization in Fastuosain-Treated GFP-Chimeric Animals
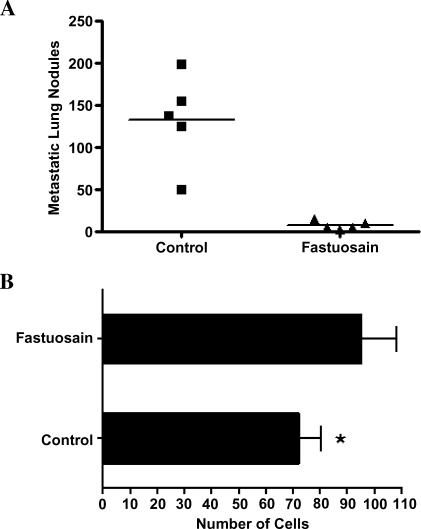
One month after the irradiation of wild-type C57Bl/6 mice and the reconstitution of the bone marrow of these animals with GFP bone marrow cells, chimeric mice were intravenously injected with melanoma cells and treated with fastuosain (intraperitoneally) using the same protocol as above. After 21 days, chimeric animals were sacrificed and lung tumor colonies were counted. The result was identical to that using wild-type C57Bl/6 mouse, with full protection being obtained on fastuosain treatment (Figure 6A).
Figure 6.
Fastuosain treatment (intraperitoneally, for 21 days) of GFP-chimeric C57Bl/6 mice injected intravenously with B16F10-Nex2 melanoma cells. GFP-chimeric mice were treated with fastuosain and challenged intravenously with B16F10-Nex2 cells (105). (A) Metastatic lung nodules; (B) double-labeled (GFP+, MHC class II+) cell migration to the lungs of chimeric mice. *P < .05.
Immunofluorescence staining using anti-GFP antibodies demonstrated intense migration of bone marrow-derived green cells to the lung parenchyma. No significant alteration in GFP cell migration was observed in animals treated with fastuosain compared to the saline-treated group (data not shown). However, the number of MHC class II-positive cells in the lung increased after fastuosain treatment (about 43%; Figure 6B).
Assays Using Anti-Fastuosain Polyclonal Antibodies
In addition to the direct cytotoxic activity of fastuosain on melanoma cells, induction of anti-fastuosain antibodies could also play a role in antitumor protection. Furthermore, considering the sequence similarities of cysteine proteinases, particularly between fastuosain and bromelain (79.4% identity), the cross-reactivity of antibodies to these enzymes was also examined.
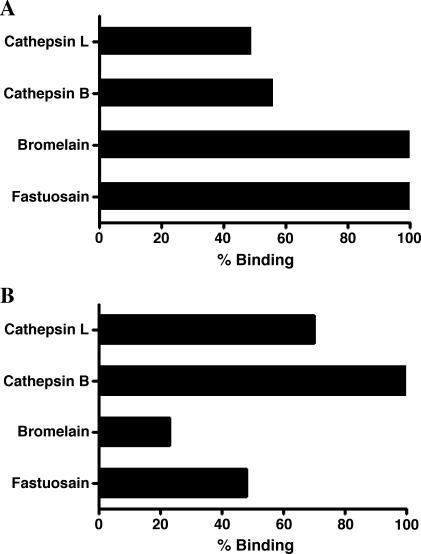
Anti-fastuosain antibodies were able to recognize bromelain with 100% binding in comparison to the homologous recognition of fastuosain (Figure 7A). Cross-reactivity with the animal cysteine proteinases cathepsins B and L was observed with 56% and 49% binding, respectively. In agreement with shared epitopes, anti-cathepsin B antibodies recognized cathepsin L (70% binding) and also reacted with fastuosain and bromelain (48% and 23% binding, respectively; Figure 7B).
Figure 7.
Anti-fastuosain (A) and anti-cathepsin B (B) affinity-purified antibodies cross-react with bromelain and fastuosain and with cathepsins B and L. Antibody binding was determined by ELISA, taking the homologous system as 100% reactivity.
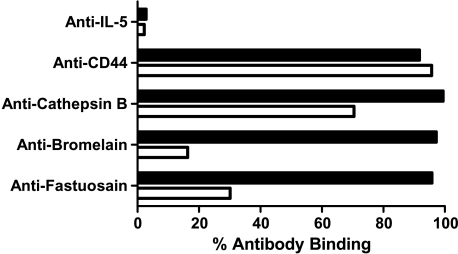
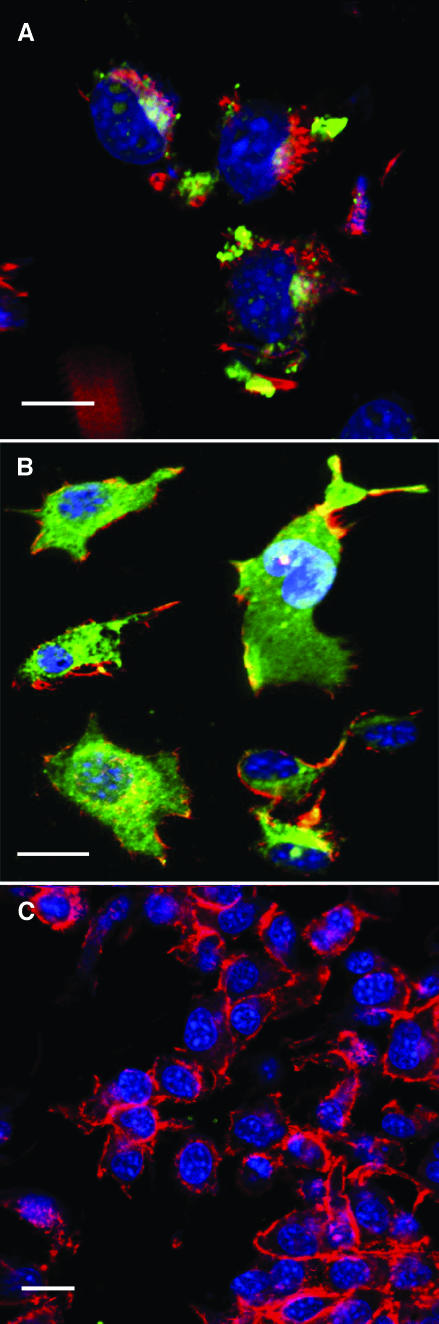
Aiming to verify whether anti-fastuosain antibodies were able to recognize antigens expressed on B16F10-Nex2 melanoma cells, we performed a flow cytometry analysis using B16F10-Nex2 cells permeabilized and nonpermeabilized with saponin. With nonpermeabilized cells, fastuosain antibodies recognized surface components, with 30% binding taking the reactivity of anti-CD44 antibodies with melanoma cells as 100%. Anti-bromelain antibodies bound with 17% intensity (Figure 8). We also observed a high binding of anti-cathepsin B antibody on the tumor cell surface, confirming that this enzyme is expressed on the cell surface as reported by Sloane et al. [12]. After tumor cell permeabilization, a great increase in antibody binding, including anti-fastuosain, anti-bromelain, and anti-cathepsin B antibodies, was observed (Figure 8). Confocal microscopy of saponin-permeabilized cells showed intense anti-fastuosain antibody reactivity that was concentrated in certain areas but also spread to the cytoplasm. Labeling with anti-cathepsin B was more discrete in some defined regions (Figure 9).
Figure 8.
Percent flow cytometry analysis of antibody binding to B16F10-Nex2 cells, permeabilized (black bars) and nonpermeabilized (white bars) with saponin. Data show the percentages of cells labeled from 104 cells as examined by FACS.
Figure 9.
Confocal microscopy of B16F10-Nex2 cells stained with anti-cathepsin B antibodies (A); anti-fastuosain antibodies (B); and anti-IL-5 anti-bodies (negative control) (C). Positive reactions labeled with green fluorescence. Actin is red-labeled with phalloidin, and nuclei are blue-stained with DAPI. Scale bars, 20µm.
Antitumor Activity of Anti-Fastuosain Antibodies
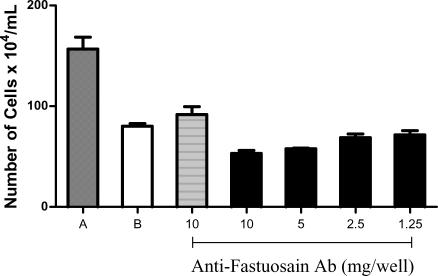
Because anti-fastuosain antibodies reacted with the surface and cytoplasmic components of B16F10-Nex2 cells and also cross-reacted with cathepsins B and L, we determined the direct cytotoxicity of these antibodies on melanoma cells with and without complement. Anti-fastuosain antibodies reduced by half the viability of tumor cells similarly with the activity of complement (1:80). The association of anti-fastuosain antibodies and complement further increased cytotoxicity, with reduction of cell viability to 30% (Figure 10).
Figure 10.
Anti-fastuosain antibody cytotoxicity on B16F10-Nex2 cells. B16F10-Nex2 cells were incubated with increasing concentrations of anti-fastuosain antibodies (1.25–10 µg/well) with or without diluted complement (1:80). After 12 hours, cell viability was measured by trypan blue. (A) Control cells without complement and antibody; (B) cells incubated with complement diluted 1:80. Black bars, antibody titration with complement; hatched bars, antibody at 10 µg/well without complement.
Discussion
Exogenously added proteinases may protect against tumor growth, in contrast with endogenous proteinases that, in many cases, have been associated with cancer development. Increased expression of cathepsin B, for instance, correlates with tumor progression and metastatic potential [12–14]. Increased cathepsin L in primary melanoma suggests early metastatic property [15]. The use of cysteine proteinase inhibitors reduced the invasibility of B16 murine melanoma in vitro [16], and deficiency in cathepsins B, L, and S reduced the tumor growth of pancreatic RT2 cells and affected angiogenesis [17].
In contrast, administration of proteolytic enzymes (trypsin, chymotrypsin, and papain) using different protocols was highly effective against subcutaneously growing Lewis lung carcinoma cells in C57Bl/6 mice [18]. In the syngeneic B16 melanoma model, proteolytic enzymes (a mixture of trypsin, chymotrypsin, and papain) inhibited the growth of primary tumors, and metastasis was curtailed. This effect was associated with a decreased expression of CD44 and CD54 in tumor cells [19]. Much attention has been given to cysteine proteinases, particularly to bromelain from Ananas comosus, which has anti-inflammatory, antithrombotic, and antifibrinolytic properties. Because bromelain inhibits platelet aggregation, it affects embolization and, thus, has antimetastatic activity, but it also reduced the expression ofCD44 in Molt4/8 leukemia and SK-Mel28 human melanoma cells [20].
Fastuosain, a cysteine proteinase, is much related to bromelain, sharing 79.4% identical amino acids. Bromelain has been reported to suppress the growth, invasion, and lung metastasis of B16F10 murine melanoma cells [9]. The mechanisms involved in this protection are poorly understood. Presently, we investigated the antitumor activity of fastuosain in comparison with bromelain and determined the basis of protective effect using the lung colonization model of murine melanoma B16F10-Nex2 and the intraperitoneal administration of the enzyme in its active and inactive forms.
Fastuosain, in a protocol of intraperitoneal injections along with the tumor cell challenge in C57Bl/6 mice, largely inhibited lung colonization, and this effect matched that of bromelain. A direct effect of both enzymes on tumor cells was suggested. Although they inhibited the growth of B16F10-Nex2 cells in the same concentration range, fastuosain seemed more active in detaching cultured tumor cells in vitro, which formed clusters of viable cells in suspension. Fastuosain also reduced the expression of CD44 on tumor cells at concentrations four-fold higher than that of bromelain, and melanoma cells formed two subpopulations with different levels of reexpression of CD44 after removal of the enzyme. One of them almost achieved the same expression of this adhesive molecule as in original tumor cells after 5 hours of incubation. Although CD44 is an important component of tumor cells in their matrix-invasive capacity, it is clear from the Matrigel invasion experiment that its complete removal from the cell surface or blocking by anti-CD44 antibody inhibited only up to 30% to 40% of cell invasion. Other components are therefore important in melanoma cell invasion. Administration of cysteine proteinases in vivo may, however, reduce CD44 expression in cells of the immune system. Oral bromelain therapy in breast cancer patients, for instance, although possessing antitumor activity, reduced the expression of CD44 on patient lymphocytes [21].
Because the antitumor effect of fastuosain was obtained by a series of intraperitoneal injections, we investigated the immune cells recruited and their capacity of migration to the lungs and eventual antitumor activity. It is interesting to note that the inactive form of fastuosain recruited a very similar cell population to the peritoneum compared with the active enzyme, with a few exceptions suggested by the different expression of surface antigens. These cells were mainly lymphocytes and cells of monocytic lineage. Some of the main antigens expressed as induced by inactive fastuosain were CD3, CD80, CD86, CD25, CD11c, CD11b, and CD178. When F4/80+ macrophages were selected from recruited cells, besides CD11b, CD80, and CD86, MHC classes I and II showed also increased expression. Noticeable was the decreased expression of CD119 (IFN-γ-R) in cells recruited by both active and inactive fastuosain. This could be explained by the internalization of the receptor after binding to IFN-γ [22], and it is an indication of macrophage activation in vivo promoted by fastuosain. With bone marrow and peritoneal resident macrophages, however, fastuosain did not stimulate nitric oxide production in IFN-γ-preactivated cells (data not shown). In contrast, bromelain increased nitrite accumulation in RAW 264-7 murine macrophage cells in the presence of IFN-γ, but not lipopolysaccharide alone [23].
Using the adoptive transference of peritoneal cells from GFP mice treated with fastuosain to wild-type C57Bl/6 mice, we showed that green cells migrated to the lungs of mice challenged with melanoma cells but were not able to control metastatic growth. Using GFP-chimeric mice with green bone marrow cells, we found that these cells migrated to the lungs with tumor-growing cells but in equal numbers, when comparing fastuosain-treated and PBS-treated mice. Fastuosain-treated mice had 40% more MHC class II-expressing green cells, but their role in antitumor protection is doubtful. Cross-priming of tumor antigens might, however, occur, and possible T-CD4+ lymphocyte proliferation could be a source of IFN-γ that is directly cytotoxic to melanoma cells [24]. It seems, however, that full protection is only achieved in the course of fastuosain administration. This situation is opposite to that of mice with spontaneous regression/complete resistance, in which resistance is completely transferable to recipient mice through splenocytes, bone marrow cells, or enriched peritoneal macrophages [25].
The lack of clear evidence that a cellular immune response was the key antitumor mechanism in mice treated with fastuosain led us to investigate the possibility of an effective antibody response that could be protective in addition to direct effects of the cysteine proteinase. Owing to certain similarities in the sequences of plant and mammalian cysteine proteinases, antibodies raised against fastuosain and cathepsin B were shown to cross-react with cathepsins B and L, fastuosain, and bromelain. Considering that B16F10 murine melanoma cells express high levels of cathepsin B [26,27], anti-fastuosain, anti-bromelain, and anti-cathepsin B antibodies were tested for binding to tumor cells. Based on anti-CD44 binding to B16F10-Nex2 cells, which was taken as 100% binding efficiency, anti-cathepsin B and anti-fastuosain antibodies bound with 70% and 30% efficiency, respectively. Anti-bromelain antibodies were the least reactive. Surprisingly, all antibodies were highly reactive with saponin-permeabilized cells. Besides cathepsin B, other internal targets for antibody reactivity could be cathepsins L and S, which have been identified in B16F10-Nex2 cells [28] (T. Paschoalin, unpublished results). Anti-fastuosain antibodies strongly reacted with the cytoplasm of melanoma cells, compared to anti-cathepsin B antibodies, which reacted on several defined regions of the cell. Fastuosain used as immunogen for antibody production gave a single band in silver-stained SDS-PAGE that is presumably free of contaminants.
The reactivity of anti-fastuosain antibodies with melanoma cells, presumably cross-reacting with cathepsin B, is consistent with the association of the latter to tumor cell membrane [29]. Membrane-associated cathepsin B is important for the activation of receptor-bound pro-urokinase plasminogen activator (pro-uPA) that is important for Matrigel invasion [30]. Caveolin-1 mediates the expression and localization of cathepsin B, pro-uPA, and their cell surface receptors p11 and uPAR, respectively [31].
Membrane cathepsin B apparently is a target for antifastuosain antibody binding. Such reactivity reduces by half the growth of B16F10-Nex2 cells. This cytotoxicity increases in the presence of complement. We believe, therefore, that the antitumor activity of fastuosain against melanoma cells is mainly due to the direct effects of the enzyme on tumor cells and on proangiogenic endothelial cell extensions, as shown in vitro (data not shown), but also due to cytotoxic cross-reactive antibodies raised by fastuosain immunization. Cell immunity can have a role (which seems to be a minor one) in the protection induced by fastuosain in the lung colonization melanoma model.
Acknowledgements
We thank the Department of Biophysics, Universidade Federal de São Paulo, for facilities made available for the present study.
Footnotes
The present work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo.
E.G.R., L.R.T., R.A.M., and R.R.S. are career fellows of the National Council for Scientific and Technological Development.
References
- 1.Cabral H, Leopoldino AM, Tajara EH, Greene LJ, Faca VM, Mateus RP, Ceron CR, Judice WAD, Juliano L, Bonilla-Rodriguez GO. Preliminary functional characterization, cloning and primary sequence of Fastuosain, a cysteine peptidase isolated from fruits of Bromelia fastuosa. Protein Pept Lett. 2006;13:83–89. doi: 10.2174/092986606774502072. [DOI] [PubMed] [Google Scholar]
- 2.Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Cell Mol Life Sci. 2001;58:1234–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metzig C, Grabowska E, Eckert K, Maurer HR. Bromelain proteases reduce human platelet aggregation in vitro, adhesion to bovine endothelial cells and thrombus formation in rat vessels in vivo. In Vivo. 1999;13:7–12. [PubMed] [Google Scholar]
- 4.Tysnes BB, Maurer HR, Porwol T, Probst B, Bjerkvig R, Hoover F. Bromelain reversibly inhibits invasive properties of glioma cells. Neoplasia. 2001;3:469–479. doi: 10.1038/sj.neo.7900196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hale LP, Fitzhugh DJ, Staats HF. Oral immunogenicity of the plant proteinase bromelain. Int Immunopharmacol. 2006;6:2038–2046. doi: 10.1016/j.intimp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Bellelli A, Mattioni M, Rusconi V, Sezzi ML, Bellellei L. Inhibition of tumor growth, invasion and metastasis in papain-immunized mice. Invasion Metastasis. 1990;10:142–169. [PubMed] [Google Scholar]
- 7.Wald M, Zavadova E, Pouckova P, Zadinova M, Boubelik M. Polyenzyme preparation Wobe-Mugos inhibits growth of solid tumors and development of experimental metastases in mice. Life Sci. 1998;62:PL43–PL48. [PubMed] [Google Scholar]
- 8.Barros HC, Verbisck NV, DaSilva S, Araguth MF, Mortara RA. Distribution of epitopes of Trypanosoma cruzi amastigotes during the intracellular life cycle within mammalian cells. J Eukaryot Microbiol. 1997;44:332–344. doi: 10.1111/j.1550-7408.1997.tb05675.x. [DOI] [PubMed] [Google Scholar]
- 9.Grabowska E, Eckert K, Fichtner I, Schulze-Forster K, Maurer HR. Bromelain proteases suppress growth, invasion and lung metastasis of B16F10 mouse melanoma cells. Int J Oncol. 1997;11:243–248. doi: 10.3892/ijo.11.2.243. [DOI] [PubMed] [Google Scholar]
- 10.Hale LP, Haynes BF. Bromelain treatment of human T cells removes CD44, CD45RA, E2/MIC2, CD6, CD7, CD8, and Leu 8/LAM1 surface molecules and markedly enhances CD2-mediated T cell activation. J Immunol. 1992;149:3809–3816. [PubMed] [Google Scholar]
- 11.Hale LP, Greer PK, Sempowski GD. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin Immunol. 2002;104:183–190. doi: 10.1006/clim.2002.5254. [DOI] [PubMed] [Google Scholar]
- 12.Sloane BF, Rozhin J, Krepela E, Zlegler G, Sameni M. The malignant phenotype and cysteine proteinases. Biomed Biochim Acta. 1991;50:549–554. [PubMed] [Google Scholar]
- 13.Kageshita T, Yoshii A, Kimura T, Maruo K, Ono T, Himeno M. Biochemical and immunohistochemical analysis of cathepsin B, H, L and D in human melanotic tumors. Arch Dermatol Res. 1995;287:266–272. doi: 10.1007/BF01105077. [DOI] [PubMed] [Google Scholar]
- 14.Frohlich E, Schlagenhauff B, Mohrle M, Weber E, Klessen C, Rassner G. Activity, expression, and transcription rate of the cathepsins B, D, H and L in cutaneous malignant melanoma. Cancer. 2001;91:972–982. [PubMed] [Google Scholar]
- 15.Stabuc B, Mrevlje Z, Markovic J, Stabuc-Silih M. Expression and prognostic significance of Cathepsin L in early cutaneous malignant melanoma. Neoplasma. 2006;53:259–262. [PubMed] [Google Scholar]
- 16.Server N, Filipic M, Brzin J, Lah TT. Effect of cysteine proteinase inhibitors on murine B16 melanoma cell invasion in vitro. Biol Chem. 2002;383:839–842. doi: 10.1515/BC.2002.088. [DOI] [PubMed] [Google Scholar]
- 17.Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wald M, Olejar T, Pouckova P, Zadinova M. Proteinases reduce metastatic dissemination and increase survival time in C57BL/6 mice with the Lewis lung carcinoma. Life Sci. 1998;63:PL237–PL243. doi: 10.1016/s0024-3205(98)00425-1. [DOI] [PubMed] [Google Scholar]
- 19.Wald M, Olejar T, Sebkova V, Zadinova M, Boubelik M, Pouckova P. Mixture of trypsin, chymotrypsin and papain reduces formation of metastases and extends survival time of C57BL/6 mice with syngeneic melanoma B16. Cancer Chemother Pharmacol. 2001;47:S16–S22. doi: 10.1007/s002800170004. [DOI] [PubMed] [Google Scholar]
- 20.Harrach T, Gebauer F, Eckert K, Kunke R, Maurer HR. Bromelain proteinases modulate the CD44 expression on human Molt 4/8 leukemia and SkMel-28 melanoma cells in vitro. Int J Oncol. 1994;5:485–488. doi: 10.3892/ijo.5.3.485. [DOI] [PubMed] [Google Scholar]
- 21.Eckert K, Grabowska E, Stange R, Schneider U, Eschmann K, Maurer HR. Effects of oral bromelain administration on the impaired immunocytotoxicity of mononuclear cells from mammary tumor patients. Oncol Rep. 1999;6:1191–1199. doi: 10.3892/or.6.6.1191. [DOI] [PubMed] [Google Scholar]
- 22.Bader T, Wietzerbin J. Modulation of murine and human interferon-gamma receptor expression by their ligands or phorbol ester. Cytokine. 1994;6:70–78. doi: 10.1016/1043-4666(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 23.Engwerda CR, Andrew D, Murphy M, Mynott T. Bromelain activates murine macrophages and natural killer cells in vitro. Cell Immunol. 2001;210:5–10. doi: 10.1006/cimm.2001.1793. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues EG, Garofalo AS, Travassos LR. Endogenous accumulation of IFN-gamma in IFN-gamma-R(-/-) mice increases resistance to B16F10-Nex2 murine melanoma: a model for direct IFN-gamma anti-tumor cytoxicity in vitro and in vivo. Cytokines Cell Mol Ther. 2002;7:107–116. doi: 10.1080/13684730310000121. [DOI] [PubMed] [Google Scholar]
- 25.Hicks AM, Riedlinger G, Willingham MC, Alexander-Miller MA, Von Kap-Herr C, Pettenati MJ, Sanders AM, Weir HM, Du W, Kim J, et al. Transferable anticancer innate immunity in spontaneous regression/complete resistance mice. Proc Natl Acad Sci USA. 2006;103:7753–7758. doi: 10.1073/pnas.0602382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian F, Frankfater A, Steiner DF, Bajkowski AS, Chan SJ. Characterization of multiple cathepsin B mRNAs in murine B16a melanoma. Anticancer Res. 1991;11:1445–1451. [PubMed] [Google Scholar]
- 27.Moin K, Cao L, Day NA, Koblinski JE, Sloane BF. Tumor cell membrane cathepsin B. Biol Chem. 1998;379:1093–1099. doi: 10.1515/bchm.1998.379.8-9.1093. [DOI] [PubMed] [Google Scholar]
- 28.Freitas ZF, Rodrigues EG, Oliveira V, Carmona AK, Travassos LR. Melanoma heterogeneity: differential, invasive, metastatic properties and profiles of cathepsin B, D and L activities in subclones of the B16F10-NEX2 cell line. Melanoma Res. 2004;14:333–344. doi: 10.1097/00008390-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Frosch BA, Berquin I, Emmert-Buck MR, Moin K, Sloane BF. Molecular regulation, membrane association and secretion of tumor cathepsin B. APMIS. 1999;107:28–37. doi: 10.1111/j.1699-0463.1999.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi H, Moniwa N, Sugimura M, Shinohara H, Ohi H, Terao T. Effects of membrane-associated cathepsin B on the activation of receptor-bound prourokinase and subsequent invasion of reconstituted basement membranes. Biochim Biophys Acta. 1993;1178:55–62. doi: 10.1016/0167-4889(93)90109-3. [DOI] [PubMed] [Google Scholar]
- 31.Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J Cell Sci. 2005;118:1493–1503. doi: 10.1242/jcs.02278. [DOI] [PubMed] [Google Scholar]