Abstract
Increases in the levels and/or activity of nonreceptor tyrosine kinases c-Src and c-Yes are often associated with colorectal carcinogenesis. The physiological consequences of increased c-Yes activity during the early and late stages of tumorigenesis, in addition to the degree of redundancy between c-Yes and c-Src in colorectal cancer cells, remain elusive. To study the consequences of increases in c-Yes levels and activity in later stages of colorectal carcinogenesis, we developed human colorectal cancer cell lines in which c-Yes levels and activity can be inducibly increased by a tightly controlled expression of wild-type c-Yes or by constitutively active mutants of c-Yes, c-YesY537F, and c-YesΔt6aa. c-Yes induction resulted in increased cell motility but did not promote proliferation either in vitro or in vivo. These results suggest that in later stages of colorectal carcinogenesis, elevations in c-Yes levels/activity may promote cancer spread and metastasis rather than tumor growth.
Keywords: c-Yes, c-Src, colon cancer, proliferation, motility
Introduction
The Src family of nonreceptor tyrosine kinases (SFK) consists of nine members (c-Src, c-Yes, Fyn, Lck, Lyn, Hck, Fgr, Blk, and Yrk). Three of them—c-Src, c-Yes, and Fyn—are ubiquitously expressed, whereas the expression of others is more restricted, mainly to cells of hematopoietic origin [1]. c-Src and c-Yes are proteins with molecular masses of 60 and 62 kDa, respectively. They have been reported to regulate many signaling pathways involved in the control of cell proliferation, survival, differentiation, adhesion, and motility [1]. In addition to tyrosine kinase activity, c-Src and c-Yes can also act as scaffolds for protein-protein interactions. Both kinase-dependent and kinase-independent functions of c-Src and c-Yes are regulated by multiple mechanisms and contribute to a broad spectrum of cellular processes in a variety of cell types [1]. Abnormal levels and/or catalytic activities of c-Src and c-Yes have been found in a number of human tumors, including lung, breast, ovarian, gastric, and colorectal tumors [2,3].
c-Src has been reported to be upregulated in > 70% of colorectal cancers, and levels of this protein increase in the course of tumor development, being highest in metastatic malignancies [4–7]. Upregulation of c-Yes is less frequent and has been reported in about 50% of colorectal carcinomas [8]. Importantly, however, an elevated expression of the c-yes gene has been linked to malignancy of human colorectal carcinoma by parametric clustering of quantitative expression data [9], and, in the case of liver metastases, patients with increased c-Yes activity had a shorter survival time when compared with patients without c-Yes activation [10].
Although c-Src and c-Yes share significant sequence and structural homology, they serve not only redundant but also distinct functions, and consequences of their activation may differ in diverse cellular backgrounds [11]. For example, activation of c-Yes is known to be associated with the formation of tight junctions in canine kidney epithelial cells, whereas activation of c-Src is believed to be involved in the dissociation of these structures [12].
A few investigations have analyzed the consequences of increases in c-Src levels and activity on the growth of human colorectal cancer cells [13–15]. Unfortunately, there are no complementary mechanistic studies on the consequences of c-Yes upregulation. Therefore, considering strong evidence correlating c-Yes deregulation to the pathology of colorectal cancer, there is a pressing need to elucidate the functions of c-Yes in colorectal cancer cells. Moreover, published reports suggest that c-Yes and c-Src may be differentially regulated and may perform different functions in this cellular background [16].
Here we describe the construction of colorectal cancer cell lines in which the levels and activity of c-Yes can be increased by a tightly controlled inducible expression of wild-type (wt) c-Yes or by constitutively active mutants of c-Yes. Functional studies using these cell lines indicate that in more advanced stages of colorectal carcinogenesis, increases in c-Yes level and activity promote motility rather than cell proliferation and tumor growth. These results may have important clinical implications with respect to the substantial development and clinical testing of SFK inhibitors as anticancer drugs [17]. Furthermore, the results complement our previous studies on the consequences of c-Src upregulation on colorectal cancer cells [15].
Materials and Methods
Plasmid Vectors
Super module vectors pSMVLuc-wtYes, pSMVLuc-Yes537F, and pSMVLuc-YesΔt6aa are identical to the previously described pSMVLuc-Src527F plasmid [15], except that they contain wtc-Yes, c-YesY537F, or c-YesΔt6aa, respectively, instead of the c-SrcY527F mutant. They were generated by AseI-based fusion of the pN1pβactin-rtTA2SM2-IRES-EGFP plasmid [18] and Dox-inducible modules derived from pBILuc-wtYes, pBILuc-Yes537F, and pBILuc-YesΔt6aa plasmids. The pBILuc-wtYes, pBILuc-Yes537F, and pBILuc-YesΔt6aa vectors are identical to the previously described pBILuc-Src527F plasmid [18], except that the coding sequence for the c-SrcY527F mutant has been replaced with coding sequences for wtc-Yes, c-YesY537F, or c-YesΔt6aa, respectively.
Cell Culture, Transfections, and Selection of Stable Cell Lines
LS174T, DLD-1, HT29, LoVo, WiDr, and Caco cells were grown in RPMI 1640 medium. HCT116 cells were grown in McCoy's 5A medium. SW480, SW620, and Int407 cells were grown in DMEM. All media (Gibco/Invitrogen, Paisley, UK) were supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 µg/ml streptomycin.
The transfection and selection of stable cell lines suitable for the Dox-inducible expression of wtc-Yes, c-YesY537F, c-YesΔt6aa, and luciferase were performed as described previously [19].
Doxycycline Treatments
Doxycycline (Dox) was obtained from Clontech (Palo Alto, CA; cat. no. 8634-1) and stored at -20°C as a 2-mg/ml stock in ddH2O.
Cells were plated out at 1 × 106 cells/3 ml of medium per well in six-well plates (Costar 3516; Corning, Corning, NY). After 20 to 24 hours, the medium was replaced with 3 ml of fresh medium with or without 2 µg/ml Dox. Detached and adherent cells were counted 24 to 30 hours later using a Coulter particle counter Z1 (Beckman Coulter, High Wycombe, UK). Alternatively, the cells were dissolved directly in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA; cat. no. 161-0737) or cell lysis buffer (Cell Signaling, Danvers, MA; cat. no. 9803), and lysates were analyzed by SDS-PAGE and Western blot analysis.
Four-day ± Dox growth curves were determined after seeding cells at 4 × 105/3 ml of medium per well in six-well plates. After 24 hours (day 1), the medium was replaced with 3 ml of fresh medium with or without 0.5 µg/ml Dox, and the cells were counted daily for a period of 3 days using a Coulter particle counter.
Cell Cycle Analysis
Cell cycle analysis was performed as previously described [15].
“Wound-Healing” Motility Assay
For wound-healing assays, cells were seeded at 1 × 106 cells/1.5 ml of medium per well in 24-well plates (Multiwell 353047; Becton Dickinson, Franklin Lakes, NJ). After 24 hours, the medium was replaced with 1.5 ml of fresh medium with or without 0.5 µg/ml Dox. After a further 2 hours, the medium was removed, and “wounds” were made by scratching cell monolayers with P200 automatic pipette tips. To remove cellular debris, the cells were washed twice with tissue-culture medium with or without 0.5 µg/ml Dox. Subsequently, 1.5 ml of L-15 (Leibovitz) medium (Gibco/Invitrogen) with or without 0.5 µg/ml Dox and supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 mg/ml streptomycin was placed in each well. The closure of wounds by cell migration was monitored at 37°C for 48 hours, with photographs taken every 10 minutes. The cells were observed under an inverted microscope (Zeiss Axiovert 200M; Carl Zeiss, Welwyn Garden City, UK) equipped with an Achroplan × 10 objective with NA 0.25 (Carl Zeiss). Images were recorded with a cooled slow-scanning charge-coupled device camera (CoolSnapHQ; Roper Scientific, Harlow, UK) controlled by the image processing program Metamorph (Universal Imaging, West Chester, PA).
Antibodies, Gel Electrophoresis, and Western Blot Analysis
SDS-PAGE was performed using 10% polyacrylamide gels. Proteins were blotted onto polyvinylidene fluoride membranes (Perkin-Elmer, Boston, MA) and incubated with the appropriate primary antibody and horseradish peroxidase-coupled anti-mouse or anti-rabbit secondary IgG (Dako, Glostrup, Denmark). The following primary antibodies were used: monoclonal mouse anti-Yes, clone 1B7 (Wako, Osaka, Japan; cat. no. 013-14261); monoclonal mouse anti-Src, clone GD11 (Upstate, Chandlers Ford, UK; cat. no. 05-184); monoclonal mouse anti-Fyn, clone Fyn301 (Wako; cat. no. 019-14241); polyclonal rabbit anti-Lck (Cell Signaling; cat. no. 2752); monoclonal mouse anti-phosphotyrosine, clone 4G10 (Upstate; cat. no. 05-321); and monoclonal mouse anti-actin (Sigma, Poole, UK; cat. no. A-4700).
Tumor Xenografts
LS174T Luc-wtYes 17 and LS174T Luc-Yes537F 29 cells were grown as subcutaneous xenografts in 8-week-old female Balb/c-NUDE nude mice (Paterson Institute for Cancer Research, Manchester, UK) following the injection of 1 × 107 cells in 0.1 ml of Optimem (Gibco/Invitrogen; cat. no. 51985-026). Mice were housed in an individually ventilated caging system on a 12-hour light/dark environment maintained at constant temperature and humidity. The mice were fed a standard diet (controls) or a Dox-containing diet (625 mg/kg TD 01306; Harlan-Teklad, Madison, WI), as described [20]. Tumor volume (length × width2/2) was measured every 2 days. When tumors approached the legal maximum tumor volume, the mice were sacrificed. Following dissection, the tumors were halved. One half was snap frozen for subsequent luciferase reporter assay. The other half, to be used subsequently for immunochemistry analysis, was immediately placed in 4% formalin. After 24 hours, 4% formalin was removed and replaced with 70% ethanol. All procedures were carried out in accordance with UK Coordinating Committee on Cancer Research guidelines by approved protocol (Home Office Project license no. 40-2746).
Immunohistochemistry
Immunohistochemistry was performed using monoclonal mouse antibody against human Ki67 antigen (Dako; cat. no. M7240), and monoclonal mouse IgG1 against Aspergillus niger glucose oxidase (Dako; cat. no. X0931) was used as a negative control. Tumor samples were imbedded in Paraplast wax (McCormick Scientific, St. Louis, MO; cat. no. 501006) using Histolette II cassettes (Simport Plastics, Bernard-Pilon Beloeil, Canada; cat. no. M493). They were sliced using a microtome (4-µm sections) and mounted onto superfrosted microscope slides (76 × 26 mm; Menzel-Glasser, Braunschweig, Germany). [The microscope slides were immersed for 5 minutes in a 2% solution of (3-aminopropyl)triethoxysilane (Sigma; cat. no. A3648) in acetone and dried at 45°C for 2 hours before mounting cut paraffin sections.] For dewaxing and rehydration, slides were bathed in xylene (twice, 5 minutes each), 100% ethanol (four times, 1 minute each), 90% ethanol (for 1 minute), 70% ethanol (for 1 minute), and tap water (for 1 minute). They were then placed in 10 mM citric acid-NaOH (pH 6.0), heated in microwave for 25 minutes, allowed to cool down to room temperature (RT; ∼ 15 minutes), and bathed in tap water. To deactivate endogenous peroxidase within the sections, the slides were immersed for 30 minutes in PBS containing 0.3% hydrogen peroxide. The slides were placed in humidified Immuno Slide staining trays (Raymond a Lamb, Eastbourne, UK; cat. no. E103.2). Mounted sections were “outlined” using ImmEdge pen (Vector Laboratories, Burlingame, CA; cat. no. H-4000) to prevent the spill of the solutions to be used later, and each section was covered with a droplet of PBS. Subsequently, the sections were treated with the following solutions (100 µl of each solution was used per section): casein solution (Vector Laboratories; cat. no. SP-5020) in PBS for 1 hour at RT; low-cross buffer (Candor Bioscience, Weissensberg, Germany; cat. no. 100050) for 30 minutes at RT; primary antibody solution (1:25 dilution of Ki67 antibody in casein solution in PBS; a 1:1000 dilution of IgG against glucose oxidase from A. niger was used in negative controls) overnight at 4°C; PBS twice for 5 minutes at RT; secondary antibody solution [1:1000 dilution of anti-mouse IgG antibody (from Vectastain ABC mouse IgG kit; Vector Laboratories; cat. no. PK4002) in PBS] for 30 minutes at RT; PBS twice for 5 minutes at RT; Vectastain ABC reagent (from Vectastain ABC mouse IgG kit) for 30 minutes at RT; PBS twice for 5 minutes at RT; and DAB solution (Vector Laboratories; cat. no. SK4100) for 10 minutes at RT (this is the final “visualization” step). Sections were then counterstained with hematoxylin, dehydrated in increasing concentrations of ethanol [70%, 90%, and 100% (3×), 1 minute each], and then immersed in xylene for 5 minutes. This was followed by the mounting of glass coverslips and microscopic analysis.
Results
Characterization of c-Yes, c-Src, Fyn, and Lck Levels in Colorectal Cancer Cell Lines
We analyzed the protein levels of the three ubiquitously expressed members of the Src family (c-Yes, c-Src, and Fyn) in a panel of nine colorectal cancer cell lines. Considering that it has been previously reported that abnormal expression of Lck can sometimes occur during colorectal carcinogenesis [21], we also determined the expression levels of this member of the Src family. As illustrated in Figure 1, diverse but significant levels of c-Yes, c-Src, and Fyn could be detected in all colorectal cancer cell lines tested. They were usually higher than the levels observed in the nontransformed human intestinal epithelial cell line Int407. Lck was abundant in SW620 cells and detectable in LoVo and SW480 cells. No detectable expression of Lck was seen in the other cell lines analyzed. These results complement previously published studies on SFK expression in colorectal cancer cell lines [22].
Figure 1.
Expression levels of selected Src family kinases in human colorectal cancer cell lines. Western blot analysis comparing the endogenous protein levels of c-Yes, c-Src, Fyn, and Lck in nine colorectal cancer cell lines (HCT116, LoVo, DLD-1, HT-29, Caco-2, LS174T, WiDr, SW480, and SW620). Int407 is a nontransformed human intestinal epithelial cell line. Data are representative of three independent experiments.
Generation of Colorectal Cancer Cell Lines for Dox-Regulated Expression of wtc-Yes and Constitutively Active Mutants of c-Yes
Considering the fact that LS174T cells displayed the lowest level of endogenous c-Yes out of all the colorectal cancer cell lines analyzed (Figure 1), we have chosen this cell line as a primary model in which to investigate the consequences of increases in the levels and activity of this protein. HCT116 cells have been selected as a secondary model because we have previously performed extensive studies on the consequences of c-Src upregulation on the growth of this colorectal cancer cell line [15]. The use of HCT116 cells enabled a direct comparison between the effects of c-Yes and c-Src in the same cellular background.
Based on structural similarities between c-Yes and the more extensively studied c-Src kinase, we designed two mutants of c-Yes, c-YesY537F and c-YesΔt6aa. In the c-YesY537F mutant, the tyrosine residue at position 537 has been substituted by phenylalanine. In the c-YesΔt6aa mutant, six C-terminal amino acids directly following tyrosine-537 have been removed. When phosphorylated, tyrosine-537 is believed to be essential for keeping the protein in an inactive closed conformation. Analogous mutations in c-Src resulted in a constitutively active open conformation of the protein [23,24]; thus, we expected c-YesY537F and c-YesΔt6aa mutants to be constitutively active.
The coding sequences of both wtc-Yes and generated mutants of c-Yes have been integrated with the components of the recently described rapid single-step nonviral system for inducible gene expression to construct vectors suitable for a simultaneous Dox-dependent expression of c-Yes protein (wild type or mutant) and firefly luciferase serving as a reporter [15]. The vectors have been subsequently used to generate LS174T and HCT116 cell lines capable of an inducible expression of luciferase and wtc-Yes, c-YesY537F, or c-YesΔt6aa in the presence of Dox (Figures 2A and W1A). Cell clones suitable for inducible expression of only the luciferase reporter have also been developed to serve as controls. All the cell clones selected for further investigations displayed growth properties similar to their respective parental cell line in the absence of induction (Figures 2C and W1C). Importantly, inducible expression of c-YesY537F and c-YesΔt6aa mutants resulted in highly increased intracellular phosphotyrosine levels, confirming that these mutants were indeed constitutively active as predicted (Figures 2B and W1B).
Figure 2.
Properties of LS174T clones engineered for inducible expression of wtc-Yes, c-YesY537F, or c-YesΔt6aa. (A) The upper panel shows Western blot analyses illustrating the expression of indicated c-Yes constructs 24 hours following Dox-triggered induction in engineered LS174T Luc-wtYes, LS174T Luc-YesY537F, and LS174T Luc-YesΔt6aa cell lines. Parental wild-type LS174T cells and LS174 Luc-only B20 cells have been included as controls. The lower panel directly compares endogenous c-Yes levels in selected colon cancer cell lines with the levels of c-Yes observed in some of the engineered LS174T clones in the presence and in the absence of induction. Data are representative of three independent repeat experiments. (B) Western blot analysis of total tyrosine phosphorylation in representative LS174T Luc-wtYes, LS174T Luc-YesY537F, and LS174T Luc-YesΔt6aa clones after 24 hours of growth in the presence or in the absence of Dox. Parental wild-type LS174T cells and LS174 Luc-only B20 cells have been included as controls. Data are representative of three independent repeat experiments. (C) Growth characteristics of parental wild-type LS174T cells, LS174T Luc-only control cells, and selected LS174T Luc-wtYes, LS174T Luc-YesY537F, and LS174T Luc-YesΔt6aa clones in the absence of Dox. Growth curves were determined after seeding 1 × 105 cells/well in six-well plates. Data are representative of three independent experiments performed in triplicate.
Inducible Increases in c-Yes Levels and Activity Do Not Promote the Proliferation of LS174T and HCT116 Cells In Vitro
We examined the consequences of the induction of wtc-Yes, c-YesY537F, and c-YesΔt6aa on the growth of LS174T and HCT116 cells. As shown in Figures 3 and W2, we were unable to detect any growth-promoting effects of any of the c-Yes constructs within 24 to 72 hours of induction. If anything, overexpression of c-YesY537F, c-YesΔt6aa, and wtc-Yes could be slightly growth-inhibitory. These results were consistent with almost identical cell cycle profiles between investigated clones in induced (+Dox) and uninduced (-Dox) states (Figures 4 and W3). We did not observe any changes in cell viability following the induction of wtc-Yes or of the constitutively active mutants of c-Yes (Figure W4).
Figure 3.
Consequences of the induction of wtc-Yes, c-YesY537F, and c-YesΔt6aa on the growth of LS174T cells in vitro. (A) Quantification of adherent and detached cells in indicated LS174T Luc-wtYes, LS174T Luc-YesY537F, LS174T Luc-YesΔt6aa clone, LS174T Luc-only control clone, and parental wild-type LS174T cell line after 24 hours of growth in the presence or in the absence of Dox. Data are representative of three independent experiments performed in triplicate. (B) Four-day growth curves of LS174T Luc-wtYes clone 17, LS174T Luc-Yes537F clone 14, LS174T Luc-YesΔt6aa clone 23, and LS174T Luc-only control cells (clone B20) in the absence and in the presence of Dox. Data are representative of three independent experiments performed in triplicate.
Figure 4.
Consequences of inducible increases in c-Yes levels and activity on the cell cycle profile of LS174T cells. Histogram illustrates the percentage of cells in each cell cycle phase after 24 hours of growth in the presence and in the absence of Dox. Means and standard deviations are based on data from three independent experiments.
Inducible Increases in c-Yes Levels and Activity Do Not Promote the Growth of LS174T Tumor Xenografts
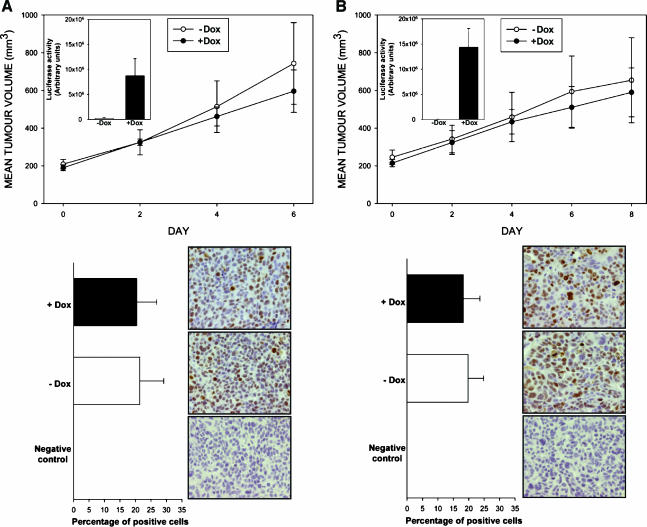
The experiments described so far demonstrated that increases in the levels and activity of c-Yes do not promote the proliferation of LS174T and HCT116 cells in vitro. To assess the consequences of Yes upregulation on cell growth in a more complex in vivo microenvironment, we grew LS174T clones inducing wtc-Yes and constitutively active c-YesY537F mutant (LS174T Luc-wtYes 17 and LS174T Luc-YesY537F 29) as xenografts in nude mice. As illustrated in Figure 5, neither induction of wtc-Yes nor induction of c-YesY537F resulted in any growth-promoting effects on LS174T tumor xenografts. Immunohistochemistry studies performed on tumor sections using antibody specific for the proliferation marker Ki67 also did not detect any significant differences in cell proliferation following wtc-Yes or cYesY537F induction (Figure 5).
Figure 5.
Consequences of the induction of wtc-Yes and c-YesY537F on the growth of LS174T cells in a xenograft model. LS174T Luc-wtYes 17 cells (A) and LS174T Luc-YesY537F 29 cells (B) were grown in vivo as described in the Materials and Methods section. There were at least five animals in each control group and Dox-treated group. Top panels show the growth curves for each clone in the presence and in the absence of Dox. Successful gene induction in Dox-treated animals was confirmed using luciferase reporter assay (histogram inserts in the top panels). The percentages of cells positive for the proliferation marker Ki67 scored following immunochistochemistry studies on xenograft sections and representative photographs of staining are shown in bottom panels. Ki67 staining was performed as described in the Materials and Methods section.
Induction of c-Yes Results in Increased Motility of LS174T Cells
Considering that c-Src is known to promote cell motility (a process believed to be important for the metastatic spread of cancer cells) and that c-Yes has been reported to be elevated in colorectal cancer metastases to the liver, we analyzed the effects of inducible elevations in c-Yes levels and activity on the motility of LS174T cells in a commonly used wound-healing assay [25]. As illustrated in Figure 6, induction of wtc-Yes resulted in a clear increase in cell migration into the wound (representative images of migrating cells are shown in Figure W5). The effect was even more pronounced in the clones induced to express the constitutively active mutants of c-Yes. No increases in cell motility following Dox treatment were observed in control LS174T Luc-only cells (clone B20) and in the parental LS174T cell line.
Figure 6.
Effects of inducible increases in c-Yes levels and activity on the motility of LS174T cells characterized in a wound-healing assay. The assay was performed as described in the Materials and Methods section. Graphs show a quantitative analysis of the wound-healing process. They illustrate changes in “wound” width (presented as a percentage of the initial wound width) in time. Means and standard deviations are based on three independent experiments performed in quadruplicate.
Discussion
There is considerable evidence linking the aberrant signaling of nonreceptor tyrosine kinases from the Src family (SFK) to the development and progression of a variety of human tumors [2,3]. Consequently, SFK is believed to be an attractive target for cancer chemotherapy, and multiple SFK inhibitors have been developed [26]. Some of these inhibitors have recently entered phase I clinical trials as anticancer agents [17]. The success of Src-focused therapeutic strategies depends on a thorough understanding of SFK functions in relevant human cancer cell contexts. Unfortunately, the consequences of abnormal signaling from SFK and the degree of redundancy between particular members of the Src family are still poorly defined for the majority of human tumor types.
We have previously performed a detailed analysis of the consequences of increases in c-Src levels and activity on the growth of human colorectal cancer cells in vitro and in vivo [15]. In the present study, we characterized the effects of c-Yes upregulation on LS174T and HCT116 cells. Our results demonstrate that elevations in c-Yes levels and activity promote the motility of colorectal cancer cells but are unable to promote their proliferation. These data support the hypothesis that in more advanced stages of colorectal carcinogenesis, increases in the levels and activity of SFK may be involved in tumor spread and metastasis rather than in the direct promotion of tumor growth. Of course, we cannot exclude the possibility that elevations in c-Yes (or other members of the SFK) may trigger increased proliferation in some other colorectal cancer cell types, but in the context of our results, proliferation is unlikely to be a common response to SFK upregulation. This assumption finds strong support in a recent study by Serrels et al. [27], in which the SFK inhibitor dasatinib (BMS-354825), when used at concentrations sufficient to block the kinase activity of SFK, was shown to have no effect on the proliferation of 10 of 12 colorectal cancer cell lines tested. Consequently, it is tempting to speculate that in advanced colorectal carcinomas, SFK inhibitors may be more effective in suppressing other processes (e.g., invasion and metastasis) than in suppressing tumor growth. It is still possible, however, that SFK could promote cell proliferation at earlier phases of colorectal carcinogenesis (e.g., at dysplastic epithelium stage). This possibility is supported by observations that certain downstream effectors of SFK are able to promote the growth of colorectal cancer cells [28,29]. Undoubtedly, additional studies on the roles of SFK at diverse stages of colorectal carcinogenesis are necessary to optimize the use of SFK inhibitors in colorectal cancer therapy in the clinic.
A different aspect of our study is the issue of common and distinct functions of c-Yes and c-Src in colorectal cancer cells. It is interesting to notice that, despite relatively high expression levels of the constitutively active c-Yes mutants following induction in some of the generated HCT116 clones, we did not observe cell detachment, formation of large vesicular structures in the cytoplasm, nonapoptotic cell death, nor delay in the G2 phase of the cell cycle, which have been previously reported to be associated with high-level induction of the constitutively active c-Src mutant c-SrcY527F [15]. Although the reasons for these apparent differences in phenotype following high-level induction of c-SrcY527F and c-YesY537F remain unknown, it seems reasonable to assume that they reflect some intrinsic differences between c-Src and c-Yes signaling. It has been reported that c-Yes and c-Src are differentially regulated during the cell cycle progression of colorectal carcinoma cells [16]. Studies in fibroblasts indicate that c-Yes is unable to compensate for c-Src signaling in several processes that require dynamic regulation of the actin cytoskeleton and that this can be attributed to the diverse structure of SH4-Unique-SH3-SH2 domains of these proteins [30]. Differences in c-Src and c-Yes signaling with respect to a variety of intracellular processes have also been described in several other cell types (reviewed in Summy et al. [11]). In the context of these published reports and in the light of our observations, potential differences between c-Src and c-Yes signaling in colorectal cancer cells deserve further attention.
c-Src signaling is well known to promote cell motility in multiple cellular backgrounds, including colorectal cancer cells [31]. Our data provide evidence that in a colorectal cancer cell background, c-Yes activation is also associated with increases in cell motility. Although the contribution of these c-Yes-induced increases in cell motility to cancer invasion and metastasis requires further evaluation, it is tempting to speculate that inhibitors of SFK may prove useful as antimetastatic agents.
Supplementary Material
Acknowledgements
We would like to thank Jeff Barry and Mike Hughes for their technical assistance with flow cytometry. Caron Abbey and Garry Ashton are acknowledged for their technical assistance with preparing xenograft sections. We would also like to thank Steve Bagley for his technical assistance with microscopy and Deema Hussein for her help with cell cycle analysis.
Footnotes
This article refers to supplementary material, which is designated by “W” (i.e., Figure W1) and is available online at www.bcdecker.com.
This study was supported by a Cancer Research UK program grant (C147) to C. Dive.
References
- 1.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 2.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 3.Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114–130. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 4.Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartwright CA, Meisler AI, Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci USA. 1990;87:558–562. doi: 10.1073/pnas.87.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao W, Irby R, Coppola D, Fu L, Wloch M, Turner J, Yu H, Garcia R, Jove R, Yeatman TJ. Activation of c-Src by receptor tyrosine kinases in human colon cancer cells with high metastatic potential. Oncogene. 1997;15:3083–3090. doi: 10.1038/sj.onc.1201496. [DOI] [PubMed] [Google Scholar]
- 7.Iravani S, Mao W, Fu L, Karl R, Yeatman T, Jove R, Coppola D. Elevated c-Src protein expression is an early event in colonic neoplasia. Lab Invest. 1998;78:365–371. [PubMed] [Google Scholar]
- 8.Park J, Meisler AI, Cartwright CA. c-Yes tyrosine kinase activity in human colon carcinoma. Oncogene. 1993;8:2627–2635. [PubMed] [Google Scholar]
- 9.Muro S, Takemasa I, Oba S, Matoba R, Ueno N, Maruyama C, Yamashita R, Sekimoto M, Yamamoto H, Nakamori S, et al. Identification of expressed genes linked to malignancy of human colorectal carcinoma by parametric clustering of quantitative expression data. Genome Biol. 2003;4:R21. doi: 10.1186/gb-2003-4-3-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han NM, Curley SA, Gallick GE. Differential activation of pp60(c-src) and pp62(c-yes) in human colorectal carcinoma liver metastases. Clin Cancer Res. 1996;2:1397–1404. [PubMed] [Google Scholar]
- 11.Summy JM, Sudol M, Eck MJ, Monteiro AN, Gatesman A, Flynn DC. Specificity in signaling by c-Yes. Front Biosci. 2003;8:s185–s205. doi: 10.2741/1011. [DOI] [PubMed] [Google Scholar]
- 12.Clump DA, Qazi IH, Sudol M, Flynn DC. c-Yes response to growth factor activation. Growth Factors. 2005;23:263–272. doi: 10.1080/08977190500199360. [DOI] [PubMed] [Google Scholar]
- 13.Irby R, Mao W, Coppola D, Jove R, Gamero A, Cuthbertson D, Fujita DJ, Yeatman TJ. Overexpression of normal c-Src in poorly metastatic human colon cancer cells enhances primary tumor growth but not metastatic potential. Cell Growth Differ. 1997;8:1287–1295. [PubMed] [Google Scholar]
- 14.Jones RJ, Avizienyte E, Wyke AW, Owens DW, Brunton VG, Frame MC. Elevated c-Src is linked to altered cell-matrix adhesion rather than proliferation in KM12C human colorectal cancer cells. Br J Cancer. 2002;87:1128–1135. doi: 10.1038/sj.bjc.6600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welman A, Cawthorne C, Ponce-Perez L, Barraclough J, Danson S, Murray S, Cummings J, Allen TD, Dive C. Increases in c-Src expression level and activity do not promote the growth of human colorectal carcinoma cells in vitro and in vivo. Neoplasia. 2006;8:905–916. doi: 10.1593/neo.06475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J, Cartwright CA. Src activity increases and Yes activity decreases during mitosis of human colon carcinoma cells. Mol Cell Biol. 1995;15:2374–2382. doi: 10.1128/mcb.15.5.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–1401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 18.Welman A, Cawthorne C, Barraclough J, Smith N, Griffiths GJ, Cowen RL, Williams JC, Stratford IJ, Dive C. Construction and characterization of multiple human colon cancer cell lines for inducibly regulated gene expression. J Cell Biochem. 2005;94:1148–1162. doi: 10.1002/jcb.20342. [DOI] [PubMed] [Google Scholar]
- 19.Welman A, Barraclough J, Dive C. Generation of cells expressing improved doxycycline-regulated reverse transcriptional transactivator rtTA2S-M2. Nat Protoc. 2006;1:803–811. doi: 10.1038/nprot.2006.117. [DOI] [PubMed] [Google Scholar]
- 20.Cawthorne C, Swindell R, Stratford IJ, Dive C, Welman A. Comparison of doxycycline delivery methods for Tet-inducible gene expression in a subcutaneous xenograft model. J Biomol Tech. 2007;18:120–123. [PMC free article] [PubMed] [Google Scholar]
- 21.Veillette A, Foss FM, Sausville EA, Bolen JB, Rosen N. Expression of the Ick tyrosine kinase gene in human colon carcinoma and other non-lymphoid human tumor cell lines. Oncogene Res. 1987;1:357–374. [PubMed] [Google Scholar]
- 22.Hirsch CL, Smith-Windsor EL, Bonham K. Src family kinase members have a common response to histone deacetylase inhibitors in human colon cancer cells. Int J Cancer. 2006;118:547–554. doi: 10.1002/ijc.21383. [DOI] [PubMed] [Google Scholar]
- 23.Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 24.Kmiecik TE, Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987;49:65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- 25.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez RH, Kantarjian HM, Cortes JE. The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer. 2006;107:1918–1929. doi: 10.1002/cncr.22215. [DOI] [PubMed] [Google Scholar]
- 27.Serrels A, Macpherson IR, Evans TR, Lee FY, Clark EA, Sansom OJ, Ashton GH, Frame MC, Brunton VG. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5:3014–3022. doi: 10.1158/1535-7163.MCT-06-0382. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Zhang Q, Zhang Y, Wang S, Ding Y. Lentivirus-mediated silencing of Tiam1 gene influences multiple functions of human colorectal cancer cell line. Neoplasia. 2006;8:917–924. doi: 10.1593/neo.06364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summy JM, Qian Y, Jiang BH, Guappone-Koay A, Gatesman A, Shi X, Flynn DC. The SH4-Uni Rue-SH3-SH2 domains dictate specificity in signaling that differentiate c-Yes from c-Src. J Cell Sci. 2003;116:2585–2598. doi: 10.1242/jcs.00466. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto M, Takamura M, Ino Y, Miura A, Genda T, Hirohashi S. Involvement of c-Src in carcinoma cell motility and metastasis. Jpn J Cancer Res. 2001;92:941–946. doi: 10.1111/j.1349-7006.2001.tb01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.