Abstract
Glioblastoma multiformes (GBMs) express increased aquaporin (AQP) 1 compared to normal brain. AQPs may contribute to edema, cell motility, and shuttling of H2O and H+ from intracellular to extracellular space. We sought to gain insight into AQP1 function in GBM. In cultured 9L gliosarcoma cells, AQP1 expression was induced by dexamethasone, platelet-derived growth factor, NaCl, hypoxia, d-glucose (but not l-glucose), and fructose. Induction of AQP1 expression correlated with the level of glycolysis, maximized by increasing medium d-glucose or fructose and decreasing O2, and was quantified by measuring lactate dehydrogenase (LDH) activity and medium lactate concentration. Upregulation of the protease cathepsin B was also observed in 9L cells cultured under glycolytic conditions. Immunohistochemical staining of human GBM specimens revealed increased coincident expression of AQP1, LDH, and cathepsin B in glioma cells associated with blood vessels at the tumor periphery. GBMs are known to exhibit aerobic glycolysis. Increased glucose metabolism at the tumor periphery may provide a scenario by which upregulation of AQP1, LDH, and cathepsin B contributes to acidification of the extracellular milieu and to invasive potential of glioma cells in perivascular space. The specific upregulation and metabolic consequences of increased AQP1 in gliomas may provide a therapeutic target, both as a cell surface marker and as a functional intervention.
Keywords: Aquaporin, cathepsin B, glioma, glycolysis, invasion
Introduction
Gliomas are the most common primary brain tumors in adults. Glioblastoma multiformes (GBMs), the most malignant of the gliomas, are highly aggressive and invasive tumors. Among the phenotypic characteristics associated with malignant GBMs are rapid growth, high glucose consumption, intratumoral necrosis and hypoxia, abundant microvascular proliferation, blood-brain barrier breakdown and vasogenic brain edema, and perivascular infiltration of glioma cells [1–3]. Changes in the vasculature of GBMs have been the target of intense investigation because the extensive changes that occur in the vasculature associated with GBMs contribute to the progression, morbidity, and mortality of this disease [4,5]. Tumor-associated blood vessels display increased permeability and blood-brain barrier breakdown, resulting in brain tumor-associated edema. Proliferative changes in these vessels also contribute to a predisposition to intratumoral hemorrhage. In addition, vessels associated with GBMs express altered adhesion molecules, contributing to the invasive potential of glioma cells.
Aquaporins (AQPs) are a family of water-selective transmembrane transport channels that allow rapid movement of H2O across normally hydrophobic cell membranes according to osmotic gradients [6]. In normal brain, AQP1 and AQP4 are the most studied and have received attention as possible contributors to brain edema [7–9]. AQP4, mainly located in astrocytic endfeet around microvascular junctions, is thought to control water movement at the blood-brain barrier and to be involved in ischemia-induced cytotoxic brain edema [6,8–11]. Compared to normal mice, AQP4 knockouts exhibited reduced brain edema and neurologic improvement following ischemic brain injury [12,13]. AQP1, absent for the most part from normal brain, is expressed in choroid plexus epithelium and may be important in the formation of cerebrospinal fluid [8,14]. In brain tumors, AQP1 expression increases with the grade of malignancy [15–17]. In some cases, this expression is associated with tumor blood vessels, and this perivascular localization has fueled speculation that increased AQP1 contributes to vasogenic brain edema [18,19]. However, the role that AQP1 may play in these tumors is still speculative, and the mechanisms by which AQP1 is upregulated in this setting are unknown.
In addition to vascular changes associated with GBMs, these tumors also exhibit important metabolic changes compared to normal brain tissue. Glioma cells can engage in high rates of aerobic glycolysis, resulting in increased glucose consumption and production of lactic acid even under normoxia [20–25]. The increase in lactic acid production and subsequent acidification of extracellular space likely contribute to the invasive potential of cancer cells [26]. The ability of cells to transport excess H+ from intracellular to extracellular space may also require movement of H2O in the same direction [27,28], suggesting another potential function for AQP1 in brain tumors. Analysis of the AQP1 gene promoter revealed regions associated with increased growth (AP1 and Sp1) and an E-box element [29]. The presence of the E-box is particularly interesting in the study of gliomas because this carbohydrate-responsive element (ChoRE) provides a mechanism by which gene transcription is increased in response to increased glucose consumption and metabolism [30,31].
Increasingly, the importance of AQPs in several physiologic processes is becoming appreciated [32]. Our goal was to gain insight into the function of AQP1 in the brain tumor setting, with particular attention to the possibility that AQP1 levels may vary according to the metabolic state of the cell. We performed this by examining the regulation of AQP1 in the 9L gliosarcoma cell line and by examining the patterns of AQP1 expression in surgical specimens. The results suggest that AQP1 and other proteins are upregulated in response to increased glucose consumption and glycolysis in glioma cells, and that AQP1 may play a role in malignant gliomas that extends beyond the regulation of tumor-associated vasogenic brain edema.
Materials and Methods
Reagents
DMEM [both (+)-glucose and (-)-glucose], penicillin-streptomycin, and fetal calf serum were obtained from Gibco Invitrogen (Carlsbad, CA). Dexamethasone sodium phosphate, BSA, l-glucose, and β-d-(-)fructose were obtained from Sigma-Aldrich Co. (St. Louis, MO). d-Glucose was purchased from Mallinckrodt Chemical, Inc. (Phillipsburg, NJ). Recombinant rat platelet-derived growth factor (PDGFBB) was purchased from R&D Systems, Inc. (Minneapolis, MN). Rat brain, kidney, liver, and lung total RNA were obtained from BD Biosciences (Mountain View, CA).
Cell Culture
The C6 glioma cell line was purchased from the American Type Culture Collection (Manassas, VA). S635 rat glioma cells were generously provided by Dr. Darell Bigner (Duke University, Durham, NC) [33]. The development of the 9L rat gliosarcoma cell line has been described [34]. Cells were grown in DME supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 mg/ml streptomycin. All cell lines were incubated at 37°C in an atmosphere of 5% CO2. Cells were routinely grown in standard DME, as described above. In experiments examining the induction of AQP1 in 9L cells, cultures (at ∼ 75% confluency) were changed to serum-free medium for 24 hours before the addition of an inducer. In cultures exposed to varying concentrations of hexoses, cells were changed to glucose-free and serum-free medium for 24 hours before the addition of a hexose. Where indicated, cells were made hypoxic, as previously described [35]. Cells were harvested after a 24-hour exposure to the inducer.
Reverse Transcription-Polymerase Chain Reaction
Total RNA from cells or tissues was isolated using Qiagen's RNEasy kit (Valencia, CA). Three micrograms of total RNA was reverse-transcribed using the First-Strand cDNA Synthesis kit (Amersham Pharmacia Biotech, Piscataway, NJ). Three microliters of this reaction was used for polymerase chain reaction (PCR) using the Takara PCR kit according to the manufacturer's specifications (Takara, Otsu, Japan). Glyceraldehyde-3-phosphate-dehydrogenase control primers (Clontech, Mountain View, CA) were used as housekeeping genes. DNA was denatured for 1 minute at 95°C and amplified using 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute, with a final 10-minute extension at 72°C. PCR products were resolved on a 1.2% agarose gel containing ethidium bromide.
Western Blot Analysis
Western blot analysis was performed on cultured 9L cells. Samples were homogenized in cell lysis buffer (5 mM DTT, 5 µg/ml aprotinin, 0.5 mM PMSF, and 5 µg/ml leupeptin in 10 mM Tris buffer) and centrifuged at 1000 rpm for 5 minutes at 4°C. Soluble cell extracts were prepared by ultrasonication followed by centrifugation at 15,000 rpm for 15 minutes. Protein concentration was determined by Bio-Rad protein assay (Bio-Rad, Hercules, CA). The extracts were subjected to SDS-PAGE under reducing conditions after boiling samples at 100°C for 5 minutes in a sample buffer + β-mercaptoethanol. Forty micrograms of each sample was loaded onto 12% Tris-glycine gels (Invitrogen) and run at 120 V for 1.5 hours at room temperature. Proteins were transferred to a nitrocellulose membrane (Invitrogen) for 3 hours at 4°C. The membrane was rinsed with PBS containing 0.02% Tween 20 (PBS-T) and then placed in a blocking solution (Zymed, San Francisco, CA) overnight at 4°C. The affinity-purified rabbit polyclonal anti-rat AQP1 antibody was diluted to 1 µg/ml and added to a blocking solution, then incubated with the membrane for 3 hours at room temperature. As a protein loading control, the membrane was also incubated with anti-µ-actin antibody. After washing thrice in PBS-T, the membrane was incubated in horseradish peroxidase-linked donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA; 1:20,000) in a blocking solution for an hour at room temperature. For µ-actin, anti-mouse IgG (Amersham Pharmacia, Piscataway, NJ) was used at a concentration of 0.05 µg/ml. The membrane was washed thrice in PBS-T, incubated with SuperSignal reagents (Pierce, Rockford, IL) for 5 minutes, and exposed to film (BioMax LIGHT; Eastman Kodak, Rochester, NY). For cathepsin B Western blot analysis, rabbit anti-rat cathepsin B antibody (2 µg/ml) was used as primary antibody.
Lactate Assay
Lactate assay was performed on cultured 9L cells. Conditioned medium was taken out of each well (six-well Cell Culture Cluster; Corning, Inc., Corning, NY) in which 9L cells (3.0 × 105) were cultured. Lactate reagent (Trinity Biotech, St. Louis, MO) was reconstituted with deionized water. One milliliter of 9L-conditioned medium was added into 1 ml of lactate reagent solution, incubated for 10 minutes, and read at 540 nm. Lactate concentration was determined according to the manufacturer's directions.
Lactate Dehydrogenase Assay
Lactate dehydrogenase (LDH) assay was performed on 9L cells plated in six-well dishes (3.0 × 105). Cells were lysed with cell lysis buffer with 0.1% Triton X-100, and soluble cell extracts were prepared by ultrasonication followed by centrifugation at 15,000 rpmfor 15 minutes. LDH assay kit (L-type LDH; Wako, Osaka, Japan) was used. Two microliters of cell lysate solution was added into 160 ml of coenzyme solution (10 mM Tris buffer, pH 9.5, containing 0.28 mM NADH) and incubated for 3 minutes at 37°C. Forty microliters of pyruvate solution (0.25 mM phosphate buffer, pH 7.0, containing 4.0 mM pyruvate acid) was added and incubated for 1 minute at 37°C, then read at 340 nm. LDH activity was calculated from the molar absorption coefficient of NADH. Protein concentration was determined by Bio-Rad protein assay.
Cathepsin B Enzyme Activity Assay
9L cells were plated at 2.5 × 105 cells/well in six-well plates (6 wells/condition) and incubated for 24 hours. The medium was changed to DME without glucose or serum. Hexose (d-glucose, l-glucose, and fructose) was added in doses of 0, 25, and 125 mM, and cells were incubated for an additional 24 hours. Control assays were carried out using 10 µM cathepsin B inhibitor. Cathepsin B activity assay kit (Bio Vision, Mountain View, CA) was used, and assays were performed according to the manufacturer's directions. Fluorescence was read using a Fluoroskan Ascent Fluorimeter (Labsystems, Franklin, MA) equipped with a 400-nm excitation filter and a 505-nm emission filter. Protein assays were performed using Bio-Rad protein assay.
Tissue Samples and Immunohistochemistry
Twenty-two human GBM specimens were obtained from the Surgical Neurology Branch at the National Institutes of Health in accordance with institutional guidelines. Specimens were frozen in isopentane (precooled on dry ice), coated with OCT compound, and frozen at -80°C until use.
For immunohistochemistry, tissues were sectioned at 8 µm thickness, and slides were warmed to 60°C for 15 minutes. Tissues were fixed in Histochoice (Amresco, Solon, OH) with 0.1% Triton X-100 (Research Products International Corp., Mount Prospect, IL) for 12 minutes. For detection of immunostaining by DAB method, endogenous peroxidase activity was quenched by immersion in 0.5% H2O2 in methanol for 20 minutes. Slides were incubated in a blocking reagent (PBS, pH 7.4, containing 2% BSA and 5% normal goat serum) for at least 3 hours before the application of primary antibody. Slides were incubated overnight in primary antibody in the blocking reagent at 4°C. On the following day, sections were incubated in biotinylated IgG secondary antibody for 1 hour at room temperature. Detection of antibody was performed using the Vectastain ABC reagent (1:50) and DAB according to the manufacturer's instructions (Vector Laboratories, Burlingame, CA). Sections were counterstained with Meyer's hematoxylin (Sigma-Aldrich Co.), dehydrated, cleared, and covered by microscopic cover glass using Permount (Fisher Scientific, Fair Lawn, NJ).
For double immunofluorescence staining, tumor tissue was fixed using Histochoice with 0.1% Triton X-100 for 12 minutes. Subsequently, the tissue was washed with PBS, blocked in 5% normal serum matching the secondary antibody host, and incubated with primary antibodies overnight at 4°C. Sections were incubated in a rhodamine-conjugated secondary antibody (Jackson ImmunoResearch) for 1 hour at 4°C. The second primary antibody was applied overnight at 4°C after incubation with normal serum matching the host of that secondary antibody. Sections were incubated in a fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Jackson ImmunoResearch). After washing in PBS, sections were mounted with Vectashield mounting medium (Vector Laboratories), and coverslips were applied.
Antibodies and Immunohistochemistry Reagents
Vectastain ABC and DAB kits were purchased from Vector Laboratories. The sources of primary antibodies (the final concentration used for staining is listed in parentheses) are as follows: rabbit-anti-rat AQP1 (1 µg/ml) was purchased from Chemicon International, Inc. (Temecula, CA); mouse-anti-human factor VIII (2 mg/ml) and mouse-anti-human cathepsin B (0.5 µg/ml) were obtained from Serotec (Raleigh, NC); goat-anti-LDH (5 mg/ml) came from Abcam (Cambridge, MA); rabbit-anti-rat cathepsin B (2 mg/ml) was obtained from Upstate Biotechnology (Lake Placid, NY); mouse anti-β-actin (0.5 µg/ml) was purchased from Sigma-Aldrich Co.; rabbit IgG was obtained from Zymed; mouse IgG came from R&D Systems, Inc.; and goat IgG was obtained from Caltag Laboratories (Burlingame, CA). The secondary antibodies used were Rhodamine Red-X AffiniPure goat anti-rabbit IgG, Rhodamine Red-X AffiniPure rabbit anti-goat IgG, and horseradish peroxidase-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch). Biotinylated horse anti-mouse (rat-adsorbed) IgG and FITC avidin were obtained from Vector Laboratories.
Results
We examined the expression of AQPs using reverse transcription (RT) PCR, and total RNA was isolated from three rat brain tumor cell lines and normal brain (Table 1). AQP1 was strongly expressed in all three tumor cell lines; lesser amounts of AQP6 and AQP8 were also detected in all three lines. No other members of AQPs were detected. In normal brain, AQP4 was expressed predominantly, with lower levels of AQP1, AQP5, and AQP9.
Table 1.
Expression of AQPs in Rat Glioma Lines and Tissues By RT-PCR.
| AQP Family | ||||||||||
| Cell/Tissue | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 9L | - | ++ | - | - | - | - | + | - | + | - |
| S635 | - | +++ | - | - | - | - | + | - | + | - |
| C6 | - | ++ | - | - | - | - | + | - | + | - |
| Brain | - | + | - | - | ++ | + | - | - | - | + |
| Kidney | +++ | ++ | +++ | + | +++ | ++ | ||||
| Lung | + | |||||||||
| Liver | + | ++ | +++ | |||||||
| Primer | Sequence | Size (bp) | Reference |
| AQP-0 | F: acg gct caa gag tgt ttc tga | 189 | [63] |
| R: tcc cca cag tct ctt tct tca t | |||
| AQP-1 | F: ctg tgg tgg ctg agt tcc tg | 344 | [64] |
| R: att tcg gcc aag tga gtt ctc | |||
| AQP-2 | F: atg tgg gaa ctc aga tcc ata gcc ttc tcc | 816 | [64] |
| R: tca ggc ctt gct gcc gcg agg cag gct | |||
| AQP-3 | F: gag atg ctc cac atc cgc tac | 485 | [64] |
| R: cac aca ata agg gct gct gtg | |||
| AQP-4 | F: ctc tgc ttt gga ctc agc att g | 570 | [64] |
| R: ttc ctt tag gcg acg ttt gag | |||
| AQP-5 | F: gcc aca tca atc cag cca tt | 383 | [64] |
| R: aaa gat cgg gct ggg ttg at | |||
| AQP-6 | F: ctg ctt gta tgg tgt ccc tgg tgt | 262 | [65] |
| R: ggc ctt gga aaa cta act gga tgg | |||
| AQP-7 | F: atg gcc ggt tct gtg ctg | 810 | [66] |
| R: tct caa gaa ccc tgt ggt gg | |||
| AQP-8 | F: aag acc atg ctg cta att cc | 275 | [63] |
| R: tcc aca atg aca gag aaa cc | |||
| AQP-9 | F: atg cct tct gag aag gac gg | 888 | [67] |
| R: cta cat gat gac act gag ct | |||
| RT-PCR was performed as described in the Materials and Methods section. As positive controls, total RNA from rat kidney, lung, and liver were used. The size of PCR products for different AQP forms matched the predicted size. The relative intensity of protein bands is indicated as follows: (-) negative, (+) detectable, (++) strong, and (+++) very strong. In glioma cells lines, AQP1 was the predominant form, with lesser amounts of AQP6 and AQP8 also detectable. | |||
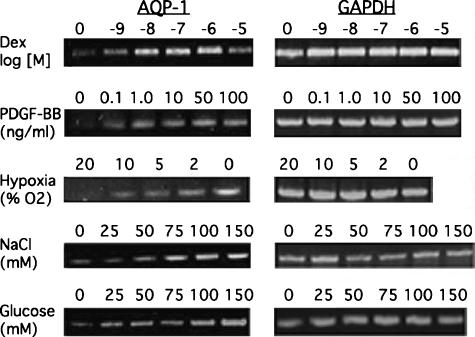
Numerous agents have been reported to regulate AQP1 expression in other cell types [36–42], but the extent to which these regulate AQP1 expression in glioma cells is unknown. Five potential regulators of AQP1 were tested in cultured 9L cells, and all were observed to increase AQP1 mRNA expression in a dose-dependent manner (Figure 1). Dexamethasone, previously reported to increase AQP1 in the lung and peritoneum when administered systemically [37,40], also increased expression in 9L cells. PDGF and NaCl also increased AQP1 expression, as has been reported in other cell types (see above). Of particular interest to the study of gliomas is the effect of hypoxia on AQP1 induction. Central necrosis and hypoxia are hallmarks of GBM, and induction of AQP1 has been observed under this condition. However, no hypoxia-responsive element has been identified in the AQP1 gene, suggesting that induction by hypoxia may occur by an indirect mechanism. In the presence of sufficient glucose, hypoxia also increases glycolysis, leading us to test the effect of glucose itself on the expression of AQP1. Under normoxic conditions, increasing glucose in the culture medium increased AQP1 expression, suggesting that the extent of glycolysis, rather than hypoxia per se, was the effector.
Figure 1.
Regulation of AQP1 expression in 9L cells. 9L gliosarcoma cells were cultured as described in the Materials and Methods section. Total RNA was harvested from cells exposed to the indicated condition for 24 hours. The concentration of the agent added to the culture medium is indicated above the appropriate lane. The amounts added equal the final concentration in the medium, except for NaCl. NaCl was added to the medium already containing a normal concentration of 110 mM. Standard DME was used, except for experiments examining the effect of glucose, in which case glucose was added to glucose-free medium to achieve the indicated final concentration. Levels of AQP1 transcripts were determined by RT-PCR using primers specific for rat AQP1. Glyceraldehyde-3-phosphate-dehydrogenase was used as a housekeeping gene. All of these conditions induced the expression of AQP1 in these cells. Experiments were duplicated in independent cultures. Dex, dexamethasone.
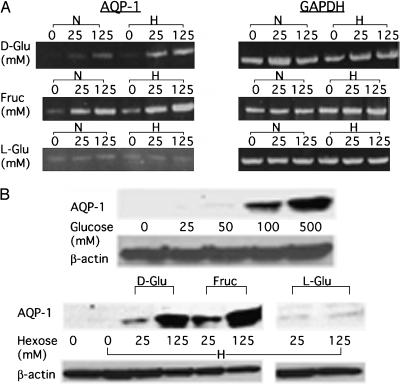
If the effect of glucose on AQP1 expression is related to the extent of glycolysis rather than to a change in osmolarity [36], then the effect should be dependent on the capacity of the cell to metabolize hexose. In fact, both glucose and fructose induced AQP1, whereas nonmetabolized l-glucose did not (Figure 2A). In addition, reducing the oxygen content of the culture medium in the presence of ample glucose or fructose (conditions that force an increased level of glycolysis) resulted in additional AQP1 induction. By contrast, L-glucose, which is not metabolized, did not result in an increase in AQP1 mRNA regardless of oxygen status. The lack of induction by L-glucose alone suggests that changes in osmolarity are not responsible for AQP1 upregulation by hexoses in this setting. The levels of AQP1 protein were also increased under glycolytic culture conditions (Figure 2B).
Figure 2.
Effect of glycolytic conditions on AQP1 induction. (A) The effect of different hexoses on AQP1 expression as determined by RT-PCR. 9L cells were cultured for 24 hours at the indicated final concentration of hexose (no hexose, 25 mM, or 125 mM), and total RNA was isolated: d-glucose (d-Glu), fructose (Fruc), or l-glucose (l-Glu). During this 24 hours of hexose exposure, cells were maintained under either normoxic (N) or hypoxic (H) conditions, as described in the Materials and Methods section. The greatest increase in AQP1 transcripts was observed with the combination of metabolized sugar and hypoxia. (B) The effect of different hexoses on AQP1 expression, as determined by Western blot analysis. 9L cells were cultured as described above, and cells were harvested for Western blot analysis as described in the Materials and Methods section. AQP1 protein levels increased in the presence of increasing glucose under normoxic conditions (top panel). Consistent with the PCR results above, hypoxia alone did not markedly increase AQP1, but in the presence of readily metabolized hexoses (d-glucose and fructose), protein levels were dramatically increased. Nonmetabolized l-glucose had no effect. Protein loading was assessed by probing for --actin. Experiments were duplicated in independent cultures.
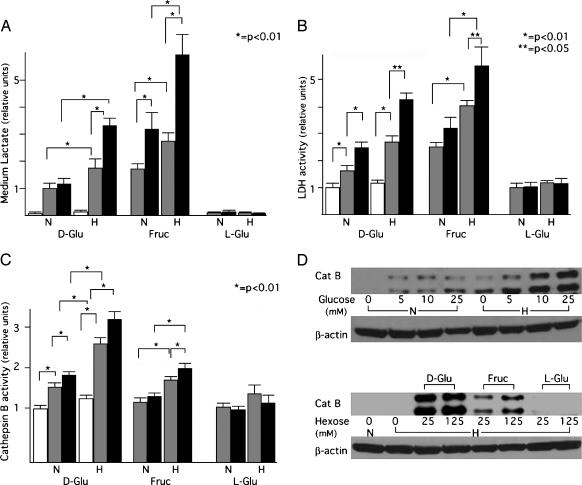
The increased AQP1 expression observed in the presence of metabolized hexoses combined with hypoxia suggested that the extent of glucose consumption/glycolysis was driving the expression of AQP1. Therefore, we examined the levels of medium lactate, a measure of the extent of glycolysis, in 9L cells cultured under these conditions (Figure 3A). The extent of glycolysis correlated well with induction of AQP1, suggesting specific upregulation of AQP1 under glycolytic conditions. Such upregulation has been reported for genes that have an E-box/ChoRE in their promoter [30,31]. Because the AQP1 promoter contains this element, we examined the regulation of two other genes containing the same responsive element LDH [20,23], which catalyzes the final step of glycolysis, and cathepsin B [43,44], a proteolytic enzyme involved in glioma invasion. Under the same conditions described in Figure 3A, LDH activity (Figure 3B), and cathepsin B activity and protein level (Figure 3, C and D) were also increased. The similar upregulation of AQP1, LDH, and cathepsin B suggests that increased glycolysis alters the expression of these genes in a coordinated fashion.
Figure 3.
Effect of glycolytic culture conditions on medium lactate, cellular LDH, and cathepsin B. (A–C) X-axes are the same for the three graphs and indicate the conditions under which 9L cells were cultured for 24 hours before harvest. Hexose (d-glucose, fructose, or l-glucose) was present at a concentration of 0 (open bars), 25 mM (gray bars), or 125 mM (black bars), and was maintained under either normoxic (N) or hypoxic (H) conditions. Medium lactate (A), LDH (B), and cathepsin B (C) activities were determined as described in the Materials and Methods section. All three parameters increased in the presence of metabolized hexoses (d-glucose and fructose). Data are presented as mean ± standard deviation. (A and B) n = 8; (C) n = 6. Statistical significance (*P < .01; **P < .05) was determined using Student's t test. The data sets being compared are indicated by brackets above the bars. (D) Analysis of cathepsin B (Cat B) protein by Western blot analysis. Culture conditions are as described in the Materials and Methods section and are indicated above and below the appropriate lanes. The intensity of protein bands is similar to the results obtained from measuring cathepsin B activity. These experiments were performed twice with different samples.
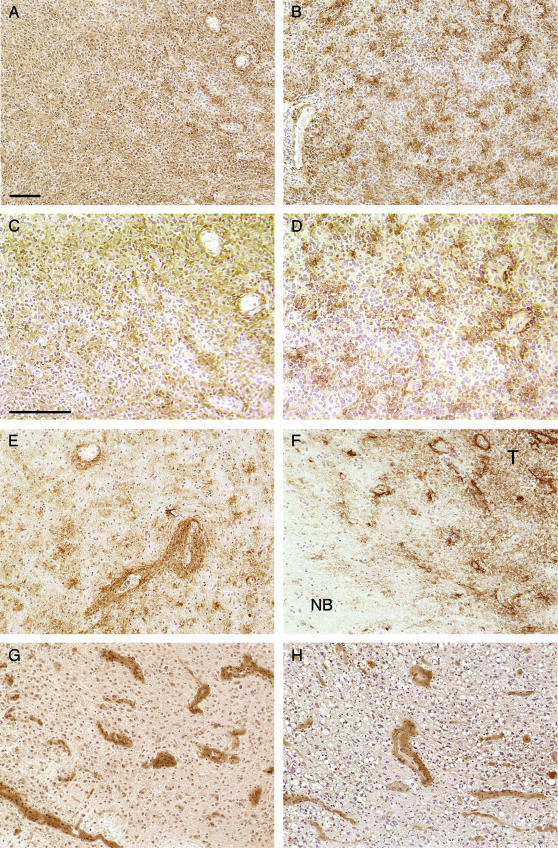
To relate these results to the clinical setting, we examined the expression of these three proteins immunohistochemically in human GBM surgical specimens. We evaluated AQP1 expression in 22 cases of GBM by immunohistochemistry. All of the GBM specimens stained positively for AQP1. Two general patterns of staining were observed (Figure 4). In some samples, there was an overall increase in AQP1 staining throughout the tumor, with no particular association to a specific structure (Figure 4, A and C). Also observed was a more clustered pattern (Figure 4, B and D) related to perivascular distribution. This is more clearly evident around larger microvessels (Figure 4E) and is most notable at the tumor periphery (Figure 4F). Areas of normal brain adjacent to the tumor did not stain (Figure 4F). We observed no increase in the perinecrotic staining of AQP1 (not shown). Immunohistochemical detection of LDH and cathepsin B (Figure 4, G and H) in GBM specimens also revealed a perivascular staining pattern for these enzymes.
Figure 4.
Immunohistochemical analysis of AQP1, LDH, and cathepsin B in human glioblastoma. Sections were stained with the indicated primary antibody and appropriate secondary as described in the Materials and Methods section and detected by DAB method. Staining for AQP1 (A–F) revealed specific patterns of expression. In some areas of the tumor, AQP1 staining appeared fairly homogenous within the tumor section (A). AQP1 staining also revealed a more clustered appearance (B). (C and D) A higher magnification of (A) and (B), respectively, demonstrates a clustered appearance often associated with microvessels. Intense perivascular staining around larger vessels is also observed (E). Sections containing the interface between normal brain (NB) and tumor (T) demonstrate the absence of AQP in the normal brain and upregulation in the tumor periphery, particularly around vessels (F). Staining for LDH (G) and cathepsin B (H) also shows intense vascular-associated patterns of expression. Negative controls were performed using nonimmune IgG from the same species as the primary antibody, and staining was not observed. Sections were counterstained with hematoxylin. Bar = 100 µm. (A), (B), (E)–(H) are of the same magnification. (C) and (D) are of the same magnification.
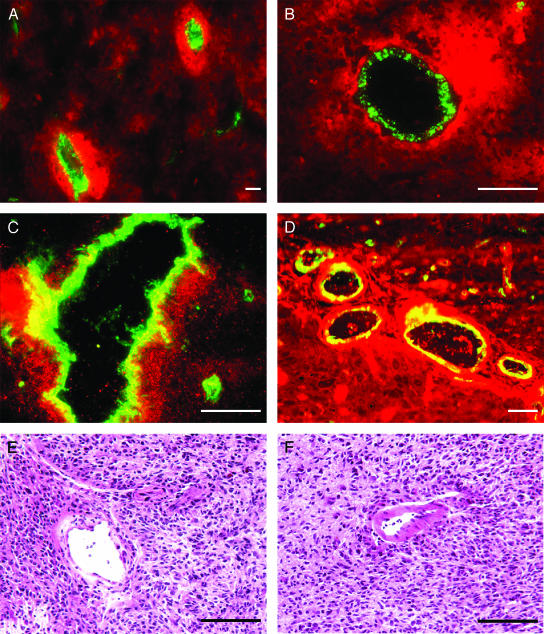
To further assess the cellular localization of these proteins, double immunofluorescent staining with factor VIII was performed, and similar staining patterns were confirmed. Double staining for AQP1 and factor VIII indicates that AQP1 expression is associated with perivascular glioma cells and not with endothelial cells (Figure 5, A and B). Double staining with LDH and factor VIII also demonstrates perivascular upregulation of LDH in glioma cells, but some LDH is also present in endothelial cells (Figure 5C). Staining for cathepsin B also reveals a perivascular pattern of expression in glioma cells, but cathepsin B is also clearly present in endothelial cells associated with the tumor (Figure 5D).
Figure 5.
Localization of AQP1, LDH, cathepsin B, and factor VIII in human glioblastoma. Double immunofluorescent staining was performed. Images were overlaid using Adobe Photoshop. In all cases, factor VIII appears green (FITC). (A and B) The distribution of AQP1 (red) and factor VIII demonstrates AQP1 expression in perivascular tumor cells, but not in endothelial cells. (C) LDH (red) and factor VIII also demonstrate LDH staining in perivascular glioma cells. Some endothelial cells also stain for LDH, as indicated in yellow. (D) Cathepsin B (red) and factor VIII staining displays strong perivascular staining for cathepsin B in glioma cells. In this case, cathepsin B staining was also observed in endothelial cells, as indicated in yellow. (E and F) Hematoxylin-eosin sections of glioblastoma specimens demonstrate the presence of tumor cells surrounding vessels. Bar = 100 µm.
Discussion
The results of this study indicate that AQP1 is upregulated by increased glucose consumption and glycolysis in glioma cells, both in vitro and in vivo. The induction of AQP1 by glycolysis most likely occurs through the E-box/ChoRE transcriptional element in the AQP1 gene promoter [30,31]. This increase in gene transcription results from a buildup of glycolytic intermediates. Particularly striking is the intense perivascular staining pattern of AQP1 in the tumor periphery of GBM surgical specimens. The coordinated upregulation of LDH in these specimens indicates that increased glycolysis also occurs in this perivascular area. Although glycolysis is often associated with hypoxic conditions, gliomas are well known for exhibiting high rates of aerobic glycolysis, resulting in increased lactic acid production even in the presence of normal levels of oxygen [21,22,25]. For cells engaging in aerobic glycolysis, the highest rate of glycolysis may occur where the highest levels of substrate are available [45]. The well-perfused perivascular space at the periphery of the tumor would have the greatest access to circulating nutrients and potentially to the highest level of glycolysis. Although hypoxic areas in tumors would switch metabolism to anaerobic glycolysis, nutrient depletion in that same area might prevent a buildup of glycolytic intermediates.
In addition to AQP1, upregulation of LDH and cathepsin B can also be explained by the E-box/ChoRE transcriptional element present in their promoters [20,23,43,44]. Although coordinate upregulation of these three proteins supports the hypothesis that glycolysis is an inducer of these genes in gliomas, it is not proof of it. Myc, often upregulated in tumor cells, can directly stimulate the transcription of E-box- containing genes [20]. The perivascular staining pattern also suggests that increased levels of extravasated serum and growth factors might stimulate the transcription of these genes through other promoters such as AP1 and Sp1 [23, 29,44]. Although the interplay of factors contributing to the coincident induction of AQP1, LDH, and cathepsin B in GBMs is not fully understood, the observation is notable.
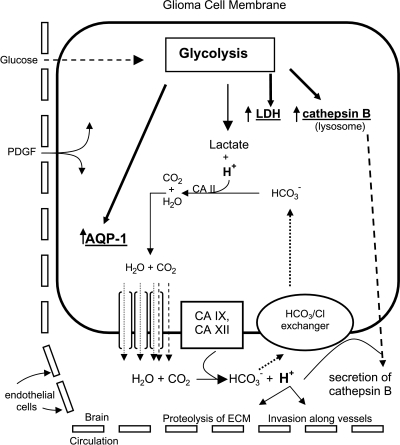
What might be the importance (in brain tumors) of an increase in glycolysis (indicated by increased LDH) and upregulation of AQP1 in this perivascular space at the periphery of brain tumors? Increased AQP1 might contribute to maintaining the viability of these highly metabolic glioma cells. Glycolysis results in intracellular lactic acidosis, but the intracellular pH of tumors remains slightly alkaline [46]. Low intracellular pH is detrimental, so cells respond by shuttling H+ from the intracellular to the extracellular compartment. However, this movement of H+ probably also requires movement of H2O in the same direction (Figure 6). Along with carbonic anhydrases (CAs), which are known to be expressed by glioma cells [47], AQPs may be required to relieve intracellular lactic acidosis and subsequent cellular swelling that might otherwise occur even under normoxia. CA II catalyzes the reaction of H+ and HCO-3, producing H2O and CO2 whose products probably must move to the extracellular compartment to prevent acidosis and cytotoxic edema [27,28]. Although clearly speculative, an increase in AQP1 may be important to maintaining the viability of the tumor cell itself and may contribute to the acidification of the extracellular compartment (Figure 6).
Figure 6.
Speculation on the possible relationship of glycolysis, AQP1, and cathepsin B. Glioma cells take up glucose from the circulation as source of energy and preferentially engage the glycolytic pathway to produce lactic acid. The increase in glycolytic intermediates may result in upregulation of AQP1, LDH, and cathepsin B through the E-box/ChoRE. Production of lactic acid from glycolysis results in intracellular acidosis and excess H+. As described [27,28], intracellular CA II catalyzes the formation of H2O and CO2 from H+ and bicarbonate. The excess H2O generated from this reaction leaves tumor cells through increased AQP1 in the tumor cell membrane and prevents cytotoxic edema. CO2 may or may not also leave the cells through AQP1 [62]. Membrane-bound extracellular CA IX and XII may regenerate H+ from extracellular H2O and CO2, thereby “shuttling” H+ from the inside to the outside of the cell and decreasing extracellular acidic pH. Maintaining an acidic environment in the perivascular space would encourage secretion of cathepsin B and would contribute to the ability of glioma cells to invade the brain along the perivascular space. Upregulation of AQP1 and stimulation of glucose uptake might also occur due to the presence of extravasated serum growth factors such as PDGF.
Furthermore, the capacity to maintain an acidic environment has ramifications for the invasive potential of GBMs. Glioma cells have long been observed to migrate throughout the brain, often following a perivascular path [48]. The upregulation of cathepsin B in the perivascular area has previously been reported, and its expression in tumor cells along blood vessels, as well as in tumor endothelial cells, correlates well with the aggressiveness and invasive potential of GBMs [49–52]. An acidic environment increases cathepsin B release from the cell [53]. If AQP1 contributes to the glycolysis-dependent acidification of the extracellular environment, then the coordinate upregulation of AQP1 and cathepsin B in the perivascular area at the tumor periphery would provide an optimal environment for the invasion of glioma cells [26]. In fact, the contribution of AQP1 to invasive potential has been described in other experimental systems [54,55]. In addition, the glycolysis-dependent increase in lysosomal cathepsin B, together with the possible increase in lysosomal membrane permeabilization occurring in metabolically stressed cells, might contribute to tumor cell death if those same cells experienced an intracellular release of cathepsin B [56]. However, the relief of intracellular acidosis by AQP1 induction, together with lysosomal membrane stabilization by Hsp70 expressed in glioblastomas, might be another route for promoting the survival and subsequent invasion of perivascular glioma cells subjected to glycolytic stress [57,58].
Although our study focused on the effects of glycolytic conditions on AQP1 expression, the possibility that glucocorticoids might increase the expression of AQP1 has also been raised. This is of interest in the study of brain tumors because of the widespread clinical use of dexamethasone for the management of brain tumor-associated vasogenic edema. A glucocorticoid response element has been noted in the mouse AQP1 gene, and administration of systemic steroid to rats increases AQP1 expression in the lung [37,39]. A possible interest in studying AQP1 in the context of rodent models of brain tumor-associated edema led us to examine the regulation of AQP1 in the rat 9L gliosarcoma line [35]. Although dexamethasone increased AQP1 mRNA in 9L cells (this study), we did not observe AQP1 upregulation by dexamethasone in human glioma cells, although upregulation under glycolytic conditions was observed (Y.H., preliminary results). In fact, a glucocorticoid response element has not been reported in the human AQP1 gene [29]. This suggests that there may be a species difference with regard to AQP1 induction by steroids, and the extent to which upregulation of AQP1 by a direct effect of dexamethasone occurs in human GBMs is unclear.
In summary, the regulatory and expression patterns of AQP1 observed in this study suggest that the microenvironment and metabolic state of glioma cells are major determinants of the level of AQP1 expression. Increased AQP1, along with CAs, may provide a mechanism for relieving the intracellular acidosis and edema that would otherwise occur in highly glycolytic cells. The resulting acidification of extracellular space and coordinate upregulation of proteases, such as cathepsin B, particularly along vessels, may provide optimal conditions for glioma cell migration and invasion. In the treatment of brain tumors, the primary tumor focus, including the area of central necrosis, is usually removed surgically. However, invasion along blood vessels has already occurred, and it is these initially undetectable invasive cells that eventually appear as clinically significant recurrence [59]. Interestingly, AQP4 is also upregulated in GBM [60,61] but exhibits increased staining more diffusely at the center of the tumor, compared to the intensely perivascular staining observed for AQP1 in this study. The rather selective upregulation of AQP1 in gliomas may provide a therapeutic target both as a cell surface marker and for functional intervention. Inhibition of AQP1 expression (by siRNA, for example) or AQP1 function (with a blocking antibody or a small inhibitory molecule) may result in increased intracellular acidosis and cytotoxicity—and hence reduced invasive potential—of perivascular glioma cells.
Acknowledgements
The authors thank Alexander Vortmeyer and Hiroaki Okamoto of the National Institute of Neurological Disorders and Stroke for glioblastoma diagnosis and technical assistance. We thank Kazuyasu Chihara of the National Institute of Arthritis and Musculoskeletal and Skin Disease for assistance with cathepsin B activity assay, Kengo Furuichi of the National Institute of Allergy and Infectious Disease for assistance with immunofluorescent staining, and Tsuyoshi Nishioku of the National Institute of Mental Health for assistance with LDH activity assay.
Abbreviations
- AQP1
aquaporin-1
- CA
carbonic anhydrase
- ChoRE
carbohydrate-responsive element
- GBM
glioblastoma multiforme
- LDH
lactate dehydrogenase
- PBS-T
PBS with 0.02% Tween 20
Footnotes
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
References
- 1.Behin A, Hoang-Xuan K, Carpentier AF, Delattre J-Y. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 2.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 3.Padma MV, Said S, Jacobs M, Hwang DR, Dunigan K, Satter M, Christian B, Ruppert J, Bernstein T, Kraus G, et al. Prediction of pathology and survival by FDG PET in gliomas. J Neuro-Oncol. 2003;64:227–237. doi: 10.1023/a:1025665820001. [DOI] [PubMed] [Google Scholar]
- 4.Demeule M, Regina A, Annabi B, Bertrand Y, Bojanowski MW, Beliveau R. Brain endothelial cells as pharmacological targets in brain tumors. Mol Neurobiol. 2004;30:157–183. doi: 10.1385/MN:30:2:157. [DOI] [PubMed] [Google Scholar]
- 5.Stiver SI. Angiogenesis and its role in the behavior of astrocytic brain tumors. Front Biosci. 2004;9:3105–3123. doi: 10.2741/1463. [DOI] [PubMed] [Google Scholar]
- 6.King LS, Yasui M, Agre P. Aquaporins in health and disease. Mol Med Today. 2000;6:60–65. doi: 10.1016/s1357-4310(99)01636-6. [DOI] [PubMed] [Google Scholar]
- 7.Badaut J, Brunet JF, Grollimund L, Hamou MF, Magistretti PJ, Villemure JG, Regli L. Aquaporin 1 and aquaporin 4 expression in human brain after subarachnoid hemorrhage and in peritumoral tissue. Acta Neurochir Suppl. 2003;86:495–498. doi: 10.1007/978-3-7091-0651-8_101. [DOI] [PubMed] [Google Scholar]
- 8.Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos MC, Saadoun S, Binder DK, Manley GT, Krishna S, Verkman AS. Molecular mechanisms of brain tumor edema. Neuroscience. 2004;129:1011–1020. doi: 10.1016/j.neuroscience.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 2004;129:999–1010. doi: 10.1016/j.neuroscience.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulos MC, Krishna S, Verkman AS. Aquaporin water channels and brain edema. Mt Sinai J Med. 2002;69:242–248. [PubMed] [Google Scholar]
- 12.Manley GT, Binder DK, Papadopoulos MC, Verkman AS. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience. 2004;129:983–991. doi: 10.1016/j.neuroscience.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 13.Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758:1085–1093. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Mobasheri A, Marples D. Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am J Physiol Cell Physiol. 2004;286:C529–C537. doi: 10.1152/ajpcell.00408.2003. [DOI] [PubMed] [Google Scholar]
- 15.Boon K, Edwards JB, Eberhart CG, Riggins GJ. Identification of astrocytoma associated genes including cell surface markers. BMC Cancer. 2004;4:39–46. doi: 10.1186/1471-2407-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshio K, Binder DK, Liang Y, Bollen A, Feuerstein B, Berger MS, Manley GT. Expression of the aquaporin-1 water channel in human glial tumors. Neurosurgery. 2005;56:375–381. doi: 10.1227/01.neu.0000148904.57841.6b. [DOI] [PubMed] [Google Scholar]
- 17.Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87:621–623. doi: 10.1038/sj.bjc.6600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo M, Jain RK, Witwer B, Brown D. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc Res. 1999;58:89–98. doi: 10.1006/mvre.1999.2158. [DOI] [PubMed] [Google Scholar]
- 19.Venero JL, Machado A, Cano J. Importance of aquaporins in the physiopathology of brain edema. Curr Pharm Des. 2004;10:2153–2161. doi: 10.2174/1381612043384150. [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Zeller KI, Wang Y, Jegga AG, Aronow BJ, O'Donnell KA, Dang CV. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangiardi JR, Yodice P. Metabolism of the malignant astrocytoma. Neurosurgery. 1990;26:1–19. doi: 10.1097/00006123-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Rajendran JG, Mankoff DA, O'Sullivan F, Peterson LM, Schwartz DL, Conrad EU, Spence AM, Muzi M, Farwell DG, Krohn KA. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245–2252. doi: 10.1158/1078-0432.ccr-0688-3. [DOI] [PubMed] [Google Scholar]
- 23.Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warburg O. The metabolism of the carcinoma cell. In: Warburg O, editor. The Metabolism of Tumours: Investigations from the Kaiser Wilhem Institute for Biology. New York: Richard R. Smith; 1931. pp. 129–169. [Google Scholar]
- 25.Ziegler A, von Kienlin M, Décorps M, Rémy C. High glycolytic activity in rat glioma demonstrated in vivo by correlation peak 1H magnetic resonance imaging. Cancer Res. 2001;61:5595–5600. [PubMed] [Google Scholar]
- 26.Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potter CP, Harris AL. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer. 2003;89:2–7. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umenishi F, Verkman AS. Isolation of the human aquaporin-1 promoter and functional characterization in human erythroleukemia cell lines. Genomics. 1998;47:341–349. doi: 10.1006/geno.1997.5125. [DOI] [PubMed] [Google Scholar]
- 30.Dentin R, Denechaud P-D, Benhamed F, Girard J, Postic C. Hepatic gene regulation by glucose and polyunsaturated fatty acids: a role for ChREBP. J Nutr. 2006;136:1145–1149. doi: 10.1093/jn/136.5.1145. [DOI] [PubMed] [Google Scholar]
- 31.Foufelle F, Girard J, Ferre P. Glucose regulation of gene expression. Curr Opin Clin Nutr Metab Care. 1998;1:323–328. doi: 10.1097/00075197-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 33.Lee YS, Bigner SH, Eng LF, Molnar P, Kuruvilla A, Groothuis DR, Bigner DD. A glial fibrillary acidic protein-expressing and tumorigenic cell line derived from an avian sarcoma virus-induced rat astrocytoma. J Neuropathol Exp Neurol. 1986;45:704–720. doi: 10.1097/00005072-198611000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Barker M, Hoshino T, Gurcay O, Wilson CB, Nielsen SL, Downie R, Eliason J. Development of an animal brain tumor model and its response to therapy with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1973;33:976–986. [PubMed] [Google Scholar]
- 35.Heiss JD, Papavassiliou E, Merrill MJ, Nieman L, Knightly JJ, Walbridge S, Edwards NA, Oldfield EH. Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor. J Clin Invest. 1996;98:1400–1408. doi: 10.1172/JCI118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenq W, Cooper DR, Bittle P, Ramirez G. Aquaporin-1 expression in proximal tubule epithelial cells of human kidney is regulated by hyperosmolarity and contrast agents. Biochem Biophys Res Commun. 1999;256:240–248. doi: 10.1006/bbrc.1999.0306. [DOI] [PubMed] [Google Scholar]
- 37.King LS, Nielsen S, Agre P. Aquaporin-1 water channel protein in lung: ontogeny, steroid-induced expression, and distribution in rat. J Clin Invest. 1996;97:2183–2191. doi: 10.1172/JCI118659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai KN, Li FK, Lan HY, Tang S, Tsang AW, Chan DT, Leung JC. Expression of aquaporin-1 in human peritoneal mesothelial cells and its upregulation by glucose in vitro. J Am Soc Nephrol. 2001;12:1036–1045. doi: 10.1681/ASN.V1251036. [DOI] [PubMed] [Google Scholar]
- 39.Moon C, King LS, Agre P. Aqp1 expression in erythroleukemia cells: genetic regulation of glucocorticoid and chemical induction. Am J Physiol. 1997;273:C1562–C1570. doi: 10.1152/ajpcell.1997.273.5.C1562. [DOI] [PubMed] [Google Scholar]
- 40.Stoenoiu MS, Ni J, Verkaeren C, Debaix H, Jonas J-C, Lameire N, Verbavatz J-M, Devuyst O. Corticosteroids induce expression of aquaporin-1 and increase transcellular water transport in rat peritoneum. J Am Soc Nephrol. 2003;14:555–565. doi: 10.1097/01.asn.0000053420.37216.9e. [DOI] [PubMed] [Google Scholar]
- 41.Umenishi F, Schrier RW. Hypertonicity-induced aquaporin-1 (AQP1) expression is mediated by the activation of MAPK pathways and hypertonicity-responsive element in the AQP1 gene. J Biol Chem. 2003;278:15765–15770. doi: 10.1074/jbc.M209980200. [DOI] [PubMed] [Google Scholar]
- 42.Umenishi F, Schrier RW. Identification and characterization of a novel hypertonicity-responsive element in the human aquaporin-1 gene. Biochem Biophys Res Commun. 2002;292:771–775. doi: 10.1006/bbrc.2002.6709. [DOI] [PubMed] [Google Scholar]
- 43.Tournu C, Obled A, Roux M-P, Deval C, Ferrara M, Béchet DM. Glucose controls cathepsin expression in Ras-transformed fibroblasts. Arch Biochem Biophys. 1998;360:15–24. doi: 10.1006/abbi.1998.0916. [DOI] [PubMed] [Google Scholar]
- 44.Yan S, Jane DT, Dufresne MJ, Sloane BF. Transcription of cathepsin B in glioma cells: regulation by an E-box adjacent to the transcription initiation site. Biol Chem. 2003;384:1421–1427. doi: 10.1515/BC.2003.157. [DOI] [PubMed] [Google Scholar]
- 45.Schornack PA, Gillies RJ. Contributions of cell metabolism and H+ diffusion to the acidic pH of tumors. Neoplasia. 2003;5:135–145. doi: 10.1016/s1476-5586(03)80005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cadoux-Hudson TA, Blackledge MJ, Rajagopalan B, Taylor DJ, Radda GK. Human primary brain tumour metabolism in vivo: a phosphorus magnetic resonance spectroscopy study. Br J Cancer. 1989;60:430–436. doi: 10.1038/bjc.1989.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proescholdt MA, Mayer C, Kubitza M, Schubert T, Liao S-Y, Stanbridge EJ, Ivanov S, Oldfield EH, Brawanski A, Merrill MJ. Expression of hypoxia-inducible carbonic anhydrases in brain tumors. Neuro-Oncology. 2005;7:465–475. doi: 10.1215/S1152851705000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scherer HJ. The forms of growth in gliomas and their practical significance. Brain. 1940;63:1–35. [Google Scholar]
- 49.Mikkelsen T, Yan PS, Ho KL, Sameni M, Sloane BF, Rosenblum ML. Immunolocalization of cathepsin B in human glioma: implications for tumor invasion and angiogenesis. J Neurosurg. 1995;83:285–290. doi: 10.3171/jns.1995.83.2.0285. [DOI] [PubMed] [Google Scholar]
- 50.Rempel SA, Rosenblum ML, Mikkelsen T, Yan PS, Ellis KD, Golembieski WA, Sameni M, Rozhin J, Ziegler G, Sloane BF. Cathepsin B expression and localization in glioma progression and invasion. Cancer Res. 1994;54:6027–6031. [PubMed] [Google Scholar]
- 51.Sivaparvathi M, Sawaya R, Wang SW, Rayford A, Yamamoto M, Liotta LA, Nicolson GL, Rao JS. Overexpression and localization of cathepsin B during the progression of human gliomas. Clin Exp Metastasis. 1995;13:49–56. doi: 10.1007/BF00144018. [DOI] [PubMed] [Google Scholar]
- 52.Strojnik T, Kos J, Zidanik B, Golouh R, Lah T. Cathepsin B immunohistochemical staining in tumor and endothelial cells is a new prognostic factor for survival in patients with brain tumors. Clin Cancer Res. 1999;5:559–567. [PubMed] [Google Scholar]
- 53.Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994;54:6517–6525. [PubMed] [Google Scholar]
- 54.Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1228–1236. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- 55.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 56.Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- 57.Hermisson M, Strik H, Rieger J, Dichgans J, Meyermann R, Weller M. Expression and functional activity of heat shock proteins in human gliobastoma multiforme. Neurology. 2000;54:1357–1365. doi: 10.1212/wnl.54.6.1357. [DOI] [PubMed] [Google Scholar]
- 58.Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer-Hansen M, Weber E, Multhoff G, Rohde M, Jaattela M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425–435. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burger PC, Heinz ER, Shibata T, Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg. 1988;68:698–704. doi: 10.3171/jns.1988.68.5.0698. [DOI] [PubMed] [Google Scholar]
- 60.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warth A, Simon P, Capper D, Goeppert B, Tabatabai G, Herzog H, Dietz K, Stubenvoll F, Ajaaj R, Becker R, et al. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood-brain barrier disturbance but not with patient survival. J Neurosci Res. 2007;85:1336–1346. doi: 10.1002/jnr.21224. [DOI] [PubMed] [Google Scholar]
- 62.Hub JS. Does CO2 permeate through aquaporin-1? Biophys J. 2006;91:842–848. doi: 10.1529/biophysj.106.081406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huebert RC, Splinter PL, Garcia F, Marinelli RA, LaRusso NF. Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J Biol Chem. 2002;277:22710–22717. doi: 10.1074/jbc.M202394200. [DOI] [PubMed] [Google Scholar]
- 64.Parvin MN, Tsumura K, Akamatsu T, Kanamori N, Hosoi K. Expression and localization of AQP5 in the stomach and duodenum of the rat. Biochim Biophys Acta. 2002;1542:116–124. doi: 10.1016/s0167-4889(01)00172-0. [DOI] [PubMed] [Google Scholar]
- 65.Capurro C, Rivarola V, Kierbel A, Escoubet B, Farman N, Blot-Chabaud M, Parisi M. Vasopressin regulates water flow in a rat cortical collecting duct cell line not containing known aquaporins. J Membr Biol. 2001;179:63–70. doi: 10.1007/s002320010037. [DOI] [PubMed] [Google Scholar]
- 66.Calamita G, Mazzone A, Bizzoca A, Svelto M. Possible involvement of aquaporin-7 and -8 in rat testis development and spermatogenesis. Biochem Biophys Res Commun. 2001;288:619–625. doi: 10.1006/bbrc.2001.5810. [DOI] [PubMed] [Google Scholar]
- 67.Nicchia GP, Frigeri A, Nico B, Ribatti D, Svelto M. Tissue distribution and membrane localization of aquaporin-9 water channel: evidence for sex-linked differences in liver. J Histochem Cytochem. 2001;49:1547–1556. doi: 10.1177/002215540104901208. [DOI] [PubMed] [Google Scholar]