Abstract
The thymus is a lymphoid organ that selects T cells for release to the peripheral immune system. Unfortunately, thymopoiesis is highly susceptible to damage by physiologic stressors and can contribute to immune deficiencies that occur in a variety of clinical settings. No treatment is currently available to protect the thymus from stress-induced involution. Leptin-deficient (ob/ob) mice have severe thymic atrophy and this finding suggests that this hormone is required for normal thymopoiesis. In this study, the ability of leptin to promote thymopoiesis in wild-type C57BL/6 and BALB/c mice, as well as in leptin-deficient (ob/ob) and endotoxin-stressed (Escherichia coli LPS) mice, was determined. Leptin administration induced weight loss and stimulated thymopoiesis in ob/ob mice, but did not stimulate thymopoiesis in wild-type C57BL/6 nor BALB/c mice. In endotoxin-stressed mice, however, leptin prevented LPS-induced thymus weight loss and stimulated TCRα gene rearrangement. Coadministration of leptin with LPS blunted endotoxin-induced systemic corticosterone response and production of proinflammatory cytokines. Thus, leptin has a selective thymostimulatory role in settings of leptin deficiency and endotoxin administration, and may be useful for protecting the thymus from damage and augmenting T cell reconstitution in these clinical states.
A cute thymic atrophy results from stress in a variety of clinical settings, including bacterial infection (1), starvation (2), irradiation, or immunosuppressive therapy (3). Induced depletion of CD4+, CD8+ double positive (DP)3 thymocytes can exacerbate T cell deficiencies that occur in HIV/AIDS, bone marrow and stem cell transplantation, cancer chemotherapy, and bacterial sepsis (4–6). T cell reconstitution following bone marrow transplantation is frequently prolonged, and development of strategies to accelerate thymopoiesis is needed to decrease posttransplant infections (5). There is currently no treatment available to protect the thymus from induced involution and/or promote postnatal T cell recovery in these clinical settings.
Leptin is the 16-kDa product of the obese (ob) gene that is secreted by adipocytes and functions primarily as a satiety hormone. In addition to playing this critical role in regulating energy homeostasis, leptin has also been implicated in modulating innate and adaptive immunity similar to a cytokine (7). It has recently been appreciated that primary lymphoid tissues, such as the thymus, contain deposits of adipose tissue capable of producing leptin (8–10). The three-dimensional structure of leptin consisting of a four α-helix bundle motif is similar to that of the IL-6 family of cytokines (11), and the leptin receptor, ObR, has close homology with gp130, a class I cytokine receptor shared by all members of the IL-6 cytokine family for signal transduction (12). Leptin's functional receptor is expressed in the hypothalamus where it regulates the hypothalamus pituitary adrenal (HPA) axis and energy homeostasis, but has recently been reported to be expressed by all cell types involved in innate and adaptive immunity (7).
We have previously reported that the IL-6 family of cytokines, including leukemia inhibitory factor, oncostatin M, and IL-6, are critical mediators of thymic involution associated with aging and septic shock, and that thymic adipose tissue can produce these cytokines (8, 13). Adipose-derived leptin has been suggested to affect thymic homeostasis and, like other cytokines, leptin can modulate inflammatory cytokine production (7). Leptin deficiency in the obese (ob/ob) mouse (14) causes a syndrome of overeating, reduced energy output, hypothermia, hyperinsulinemia (15), hypercorticosteronemia (16), and reduced immune function (17, 18). Exogenous administration of leptin to these mice results in decreased food intake and weight loss (12, 16, 17). ob/ob mice also have decreased thymopoiesis compared with age-matched controls, with thymic atrophy that is reversed by exogenous leptin administration (18). Leptin has been postulated to provide a survival signal to developing CD4+, CD8+ DP thymocytes (2).
We hypothesized that administration of supraphysiologic doses of leptin would augment thymopoiesis in normal mice and/or prevent thymic atrophy in a systemic model of endotoxin-induced thymic involution. However, we found that leptin had little to no effect on thymopoiesis in normal nonobese mice, but did stimulate thymopoiesis in the setting of LPS-induced thymic atrophy and leptin deficiency. Thus, leptin is a thymopoietic hormone only in the setting of induced thymic atrophy.
Materials and Methods
Reagents
Recombinant mouse leptin was purchased from R&D Systems. The lyophilized protein was reconstituted at 1 mg/ml with 15 mM sterile HCl, 7.5 mM sterile NaOH, and sterile PBS (pH 7.4). The stock solution was diluted with PBS to 20 μg/100 μl.. Leptin-treated mice received 1 μg/g leptin by i.p. injection. Escherichia coli-derived LPS was purchased from Sigma-Aldrich (L-2880) and reconstituted at 1 mg/ml in PBS. LPS-challenged mice received 100 μg of LPS by i.p. injection.
Animals and treatments
Wild-type (WT) C57BL/6 and C57BL/6 ob/ob mice were purchased from The Jackson Laboratory. BALB/c mice were purchased from the National Cancer Institute-Charles River Laboratories. All mice were housed in a pathogen-free environment with 12-h light/dark cycles at 20–25°C in accordance with all Institutional Animal Care and Use Committee and American Association for the Accreditation of Laboratory Animal Care-approved animal protocols. In studies of chronic leptin administration, injections were given at 1 μg/g at 8:00 a.m. and 5:00 p.m. daily for 10 days. Mouse weights were recorded regularly just before the time of the morning injection. On the 11th day, 16 h following the final dose, blood was obtained from the retro-orbital venous plexus. Serum was isolated by centrifugation for 20 min at 2000 × g and transferred to a 96-well round-bottom culture plate and stored at −20°C until thawed for analysis of corticosterone, leptin, insulin, and glucagon levels. Mice were euthanized by CO2 administration for 10 min followed by cervical dislocation. Thymuses were removed and weighed. Organs were divided into two halves; one-half was placed in a 60-mm tissue culture dish containing 3 ml of RPMI 1640 (Invitrogen Life Technologies) with 5% FCS (tissue medium) and one-half was placed into a 1.8-ml cryotube and snap-frozen in an ethanol/dry ice bath.
In LPS-induced acute thymic involution studies, BALB/c mice were injected i.p. with 100 μg of LPS plus or minus leptin (day 0). Replicate groups of animals were bled at various time points to determine serum cytokine levels before euthanasia for tissue harvest (1 h to 28 days post-treatment). Mice were euthanized by CO2 administration for 10 min followed by cervical dislocation. Thymus tissues were removed and weighed. Organs were divided into two halves; one-half was placed in a 60-mm tissue culture dish containing 3 ml of RPMI 1640 (Invitrogen Life Technologies) with 5% FCS (tissue medium) and one-half was placed into a 1.8-ml cryotube and snap-frozen in an ethanol/dry ice bath.
Cell isolation and flow cytometry
Thymus tissue was teased to a single-cell suspension through a 70-μm cell strainer (BD Labware) in tissue medium. Thymocytes were centrifuged at 1500 rpm for 5 min and resuspended in 3 ml of tissue medium for cell counts and immunofluorescent staining. Cell counts were performed in triplicate on a Coulter Z1 Dual Threshold Cell Counter (Coulter) and the mean was recorded. Total thymus cell counts were extrapolated based on the percentage weight of the teased portion of thymus relative to the whole thymus weight. Direct immunofluorescence staining was performed with anti-mouse directly conjugated mAbs: anti-CD3 FITC (BD Biosciences), anti-CD4 PE (BD Biosciences), and anti-CD8 CyChrome (BD Biosciences). Cell suspensions were added to PBS Wash (1× PBS + 1% BSA + 0.1% sodium azide) and diluted Ab, incubated for 45 min at 4°C, washed, and resuspended in PBS Wash containing 0.4% (w/v) paraformaldehyde. Immunophenotype data were acquired on a FACSVantage SE (BD Biosciences), and data analyzed with CellQuest software (Duke University Human Vaccine Institute Flow Facility, Durham, NC).
Quantitative real-time PCR for mouse signal joint TCR δ excision circles (sjTREC)
Total genomic DNA from thymus tissue was extracted using TRIzol Reagent (Invitrogen Life Technologies) per manufacturer's protocol. DNA was quantified by spectrophotometry (260 nm). Numbers of signal joint murine TREC (mTREC) were quantified as previously described (19). Briefly, an excess of forward and reverse DNA primers for the mTREC sequence and DNA probe conjugated to a fluorescent dye was added to genomic thymus DNA. Real-time PCR using BioRad iCycler IQ allowed detection and quantification of mTREC numbers per 1 μg of DNA in each sample. Numbers of mTREC were normalized to reflect levels per milligram of thymus tissue.
Corticosterone immunoassay
Serum corticosterone levels were determined using a corticosterone ELISA kit purchased from R&D Systems.
Bead-based ELISAs
Serum glucagon, insulin, and leptin levels were analyzed using the LincoPlex mouse endocrine immunoassay panel according to the manufacturer's protocol (Linco Research). Serum was incubated with a mixture of anti-insulin, anti-leptin, and anti-glucagon Abs immobilized on Luminex spheres followed by a mixture of biotinylated detection Abs and then streptavidin-PE. Serum cytokine levels were determined by multiplex bead-based assays using BioPlex mouse cytokine/chemokine kits according to the manufacturer's protocol (Bio-Rad). All bead assay samples were quantified on the BioPlex protein array reader (Bio-Rad) in the Duke University Human Vaccine Institute Immune Reconstitution Core Facility (Durham, NC).
Statistics
A two-tailed Student t test was used to compare the means between data sets. Differences were considered significantly different with p ≤ 0.05.
Results
Leptin administration induced thymopoiesis in ob/ob mice, but not in young WT C57BL/6 mice
The impact of daily leptin administration on thymopoiesis in leptin-deficient ob/ob (C57BL/6 background) vs WT C57BL/6 mice was determined by treating mice for 10 days twice daily with leptin (1 μg/g; i.p.) or saline as a control. Leptin administration in this regimen resulted in significant weight loss in both ob/ob and WT C57BL/6 mice during treatment (Fig. 1). On day 11, 16 h following the final treatment, there was a mean weight change from baseline of −22% (p < 0.0001) in leptin-treated ob/ob mice, compared with +17% in saline control mice (Fig. 1A). In WT mice, there was a mean weight change from baseline of −8% on day 11 (p < 0.001) with leptin treatment, compared with +1% in saline control mice (Fig. 1B).
FIGURE 1.
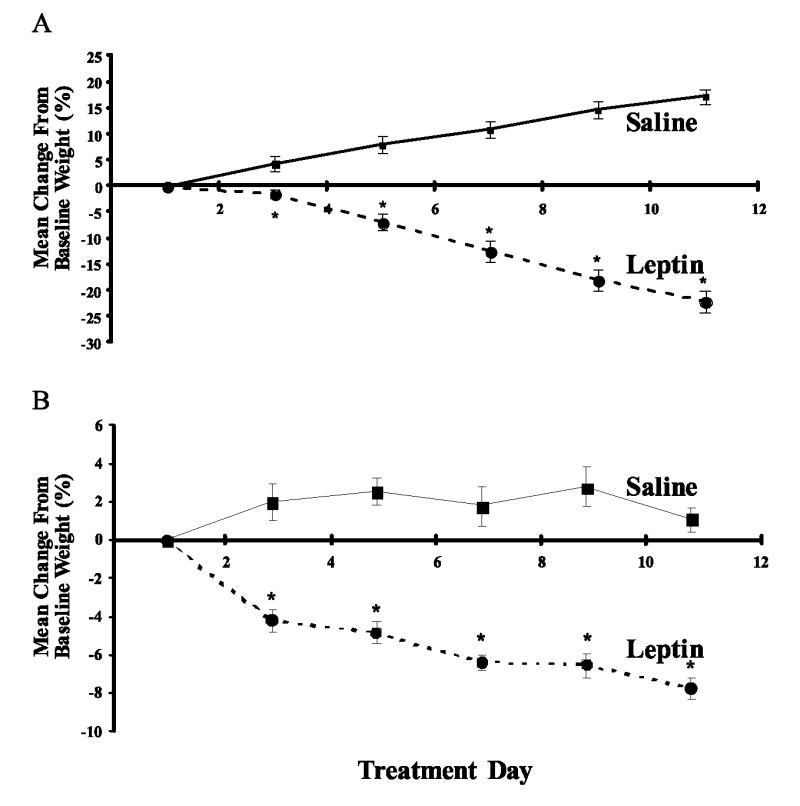
Effect of leptin on body weight in ob/ob (A) and WT C57BL/6 (B) mice over time. Animals were given i.p. injections of leptin (1 μg/g body weight) or saline twice daily for 10 days. Change in body weight data are expressed as mean of percentage change in body weight (grams) from baseline weight recorded before treatment on day 1 ± SEM. ob/ob saline, n = 3; ob/ob leptin, n = 4; C57BL/6 saline, n = 7; C57BL/6 leptin, n = 9. *, p ≤ 0.05 compared with saline-treated animals.
Next, thymus weight, absolute thymocyte number, and thymocyte phenotype in ob/ob and WT mice were determined 11 days after leptin administration. In WT mice, leptin treatment significantly lowered thymus weights (p = 0.019) (Fig. 2A) while absolute thymocyte numbers were not reduced compared with saline controls (Fig. 2B). Thymuses from untreated ob/ob mice were surrounded with dense adipose tissue, resulting in higher thymus weights in this group compared with leptin-treated mice. However, as previously reported by others (18), mean absolute thymocyte numbers were significantly elevated in leptin-treated ob/ob mice (p = 0.048) compared with saline controls (Fig. 2B).
FIGURE 2.
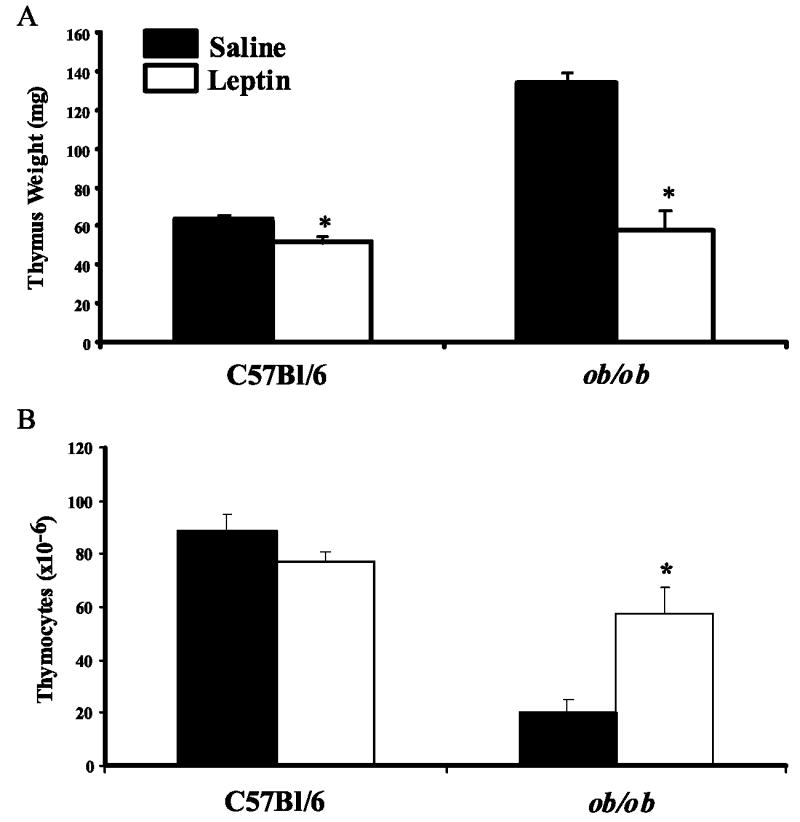
Effect of chronic leptin treatment on thymopoiesis. C57BL/6 and ob/ob (C57BL/6 background) mice were given i.p. injections of leptin (1 μg/g body weight) or saline twice daily for 10 days. Thymus tissues was dissected from euthanized animals on day 11. Mean thymus weight (A) and absolute number of thymocytes (B) ± SEM are shown for both mouse strains treated with either saline or leptin. ob/ob saline, n = 3; ob/ob leptin, n = 4; C57BL/6 saline, n = 7; C57BL/6 leptin, n = 9. *, p ≤ 0.05 compared with saline-treated animals.
Data from flow cytometric analyses of thymocyte subset frequencies in leptin-treated ob/ob mice vs controls demonstrated a significant increase in the percentage and absolute number of DP thymocytes (p ≤ 0.05) with a trend toward decreased frequencies of double-negative (DN), CD4+ single-positive (SP), and CD8+ SP thymocytes compared with saline-treated control ob/ob mice (Table I). Leptin-treated ob/ob mice also demonstrated a significant increase in the DP-DN thymocyte ratio compared with saline-treated control mice. Taken together, these data suggested that leptin treatment enhanced development of DP thymocytes from DN thymocytes in ob/ob mice.
Table I.
Frequency and absolute number of thymocyte subsets in wild-type C57BL/6 and ob/ob-deficient mice after chronic treatment with leptin
| C57BL/6 | ob/ob | |||
|---|---|---|---|---|
| Saline (n = 7) | Leptin (n = 9) | Saline (n = 2) | Leptin (n = 4) | |
| Frequency of T cell subset (%) | ||||
| CD4+CD8+ DP | 90 ± 0.4 | 88 ± 1 | 63 ± 5 | 84 ± 3a |
| CD4− CD8− DN | 2 ± 0.1 | 3 ± 0.2 | 25 ± 5 | 7 ± 2a |
| DP/DN ratio | 50 ± 3 | 38 ± 4a | 3 ± 1 | 21 ± 7a |
| CD4+ SP | 6 ± 0.3 | 8 ± 0.4 | 9 ± 0.4 | 7 ± 0.3 |
| CD8+ SP | 2 ± 0.1 | 2 ± 0.2 | 3 ± 0.03 | 2 ± 0.2 |
| Absolute number of T cell subset (×10−6) | ||||
| CD4+ CD8+ DP | 77 ± 17 | 64 ± 12 | 11 ± 5 | 43 ± 17a |
| CD4− CD8− DN | 2 ± 0.4 | 2 ± 0.3 | 4 ± 0.1 | 3 ± 1 |
| CD4+ SP | 5 ± 1 | 5 ± 1 | 2 ± 0.6 | 3 ± 0.9 |
| CD8+ SP | 2 ± 0.4 | 2 ± 0.3 | 1 ± 0.2 | 1 ± 0.2 |
Value of p < 0.05 vs saline control.
To quantify thymopoiesis at the level of TCRα gene rearrangement, the absolute number of mTREC was determined on genomic DNA extracted from thymus tissue obtained at harvest from ob/ob mice (19). The mean ± SEM number of molecules of mTREC per milligram of thymus tissue from leptin-treated ob/ob mice (230,138 ± 35,494; n = 4) was 18-fold greater than the number of mTREC from saline-treated ob/ob mice (12,767 ± 259; n = 4). Thus, mTREC analysis demonstrated that leptin treatment of ob/ob mice stimulated TCRα gene rearrangement, and therefore significantly increased thymopoiesis (saline vs leptin treatment; p = 0.013).
Leptin treatment of WT mice induced a slight decrease in the DP-DN thymocyte ratio (p = 0.021), but had no significant affect on absolute numbers of thymocyte subsets compared with saline administration (Table I, data not shown). mTREC analysis of thymic genomic DNA indicated no significant difference in thymopoiesis in leptin-treated (119,043 ± 30,999; n = 8) vs saline-treated WT mice (202,789 ± 48,552; n = 6; p = NS). While the results obtained in ob/ob mice of leptin induction of thymopoiesis validated and extended previous findings (2), these data demonstrated that leptin did not stimulate thymopoiesis in WT mice.
Leptin administration did not induce thymopoiesis in BALB/c WT mice
To determine whether the lack of effect of leptin on thymopoiesis in normal mice was strain specific, the impact of leptin treatment on thymopoiesis was determined in a second commonly used inbred mouse strain (BALB/c). The BALB/c mouse strain was selected for study because it is acutely sensitive to LPS-induced thymic involution, and is an excellent model for identifying agents that modulate thymopoiesis/thymic atrophy during stress (8, 13). BALB/c mice were injected twice daily with leptin (1 μg/g) for 10 days, i.e., the same regimen that was thymopoietic for ob/ob mice. As observed in ob/ob and WT C57BL/6 mice, leptin treatment induced satiety and resulted in significant total body weight loss (data not shown). Before sacrifice on day 11, leptin-treated BALB/c mice demonstrated a mean weight loss of −10% (p < 0.001) compared with −2% in control BALB/c mice.
Leptin-induced weight loss in BALB/c mice was further studied by determining serum concentrations of leptin, insulin, glucagon, and corticosterone (Table II). The results showed that serum leptin levels were undetectable 16 h following the 10th day of daily treatment. Insulin levels were significantly reduced on day 11 (p = 0.05) and there was a trend, though not statistically significant, of increased serum glucagon in leptin-treated mice compared with saline controls. In contrast, there were no differences in serum corticosterone concentrations between leptin-treated and saline-control mice. Although leptin-treated BALB/c mice were not environmentally or pharmacologically stressed, they demonstrated metabolic signs of starvation/fasting (weight loss, decreased insulin, and a trend toward increased glucagon).
Table II.
Serum concentrations of leptin, insulin, glucagon, and corticosterone in wild-type BALB/c mice after chronic treatment with leptin
| Saline (n = 5) | Leptin (n = 5) | |
|---|---|---|
| Leptin (pM) | 98 ± 45 | 0.0 ± 0.0 |
| Insulin (pM) | 188 ± 23 | 89 ± 17a |
| Glucagon (pM) | 8 ± 0.5 | 18 ± 7 |
| Corticosterone (ng/ml) | 170 ± 38 | 140 ± 33 |
Value of p < 0.05 compared to saline-treated mice.
The absence of serum leptin after daily injection suggests that the chronic daily exposure to supraphysiologic leptin suppressed the endogenous production of leptin in these mice. This may be due to profound weight loss or a negative feedback loop in the leptin-production pathway. To verify that our exogenous i.p. treatment with leptin was indeed inducing elevated serum levels of leptin, we treated groups of mice (n = 5) with saline or leptin and monitored their serum leptin levels for 24 h (Fig. 3). Following a single exogenous injection of leptin, we observed a 5-fold increase over endogenous levels (saline control) in serum leptin concentration within 30 min. Serum leptin rapidly returned to baseline endogenous levels by 4 h post-leptin injection.
FIGURE 3.
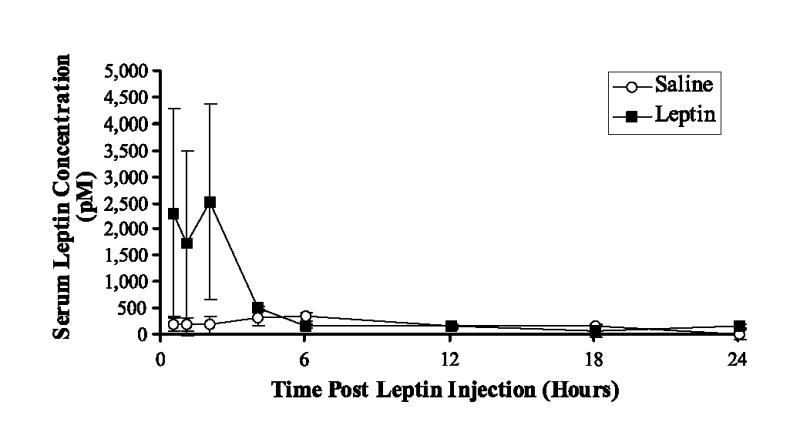
Effect of exogenous leptin on endogenous serum leptin concentrations in BALB/c mice over time. Animals were given a single i.p. injection of leptin (1 μg/g body weight) or saline. Serum leptin levels were determined 0.5, 1, 2, 4, 6, 12, 18, and 24 h after injection (mean picomoles ± SEM); n = 5 mice/time point/group.
Ten days of daily leptin administration to BALB/c mice did not alter thymus size. Thymus weights (35 ± 2 vs 41 ± 3 mg) and absolute thymocyte numbers (44 × 106± 8 × 106 vs 41 × 106± 5 × 106 cells/thymus) were similar in leptin-treated compared with saline-treated BALB/c mice (n = 9 for leptin, n = 7 for saline). All thymocyte subpopulations in the treatment group remained at levels equivalent to saline controls as did the DP-DN thymocyte ratio (Table III). No significant difference in number of mTREC per milligram of thymus tissue was observed between leptin-treated BALB/c mice (1,545,239 ± 322,604; n = 7) and saline-treated BALB/c mice (1,432,916 ± 380,791; n = 7). Thus, these findings demonstrated that leptin did not induce thymopoiesis in normal young BALB/c mice.
Table III.
Frequency (%) of thymocyte subsets in BALB/c mice after chronic treatment with leptin
| Lymphocyte Subset | Saline (n = 7) | Leptin (n = 9) |
|---|---|---|
| Thymocytes | ||
| CD4+CD8+ DP | 86 ± 1 | 85 ± 1 |
| CD4−CD8− DN | 2.6 ± 0.2 | 3.1 ± 0.3 |
| DP-DN ratio | 34 ± 2 | 30 ± 3 |
| CD4+CD8− SP | 9 ± 0.4 | 10 ± 1 |
| CD4−CD8+ SP | 2 ± 0.1 | 2 ± 0.2 |
Leptin had a thymopoietic effect in the LPS-induced acute thymic atrophy model
Bacterial LPS induces severe acute thymic atrophy that is mediated in part by cytokines and corticosteroids (1, 13). To study the effect of leptin in this model system, we first determined the kinetics of LPS-induced thymic atrophy. We found thymus weight and cellularity decreased for 7 days after a single LPS treatment on day 0 (100 μg; i.p.). Following LPS induction of thymic atrophy over 7 days, a rapid increase in thymopoiesis occurred over the next 10 days. By 14 days after LPS administration, thymus weights and cellularity rebounded and surpassed those of saline controls (Fig. 4A). This pattern was mirrored by parallel changes in indicators of thymopoiesis, i.e., CD4/CD8 DP T cell numbers and mTREC numbers per milligram of thymus tissue (Fig. 4, B and C).
FIGURE 4.
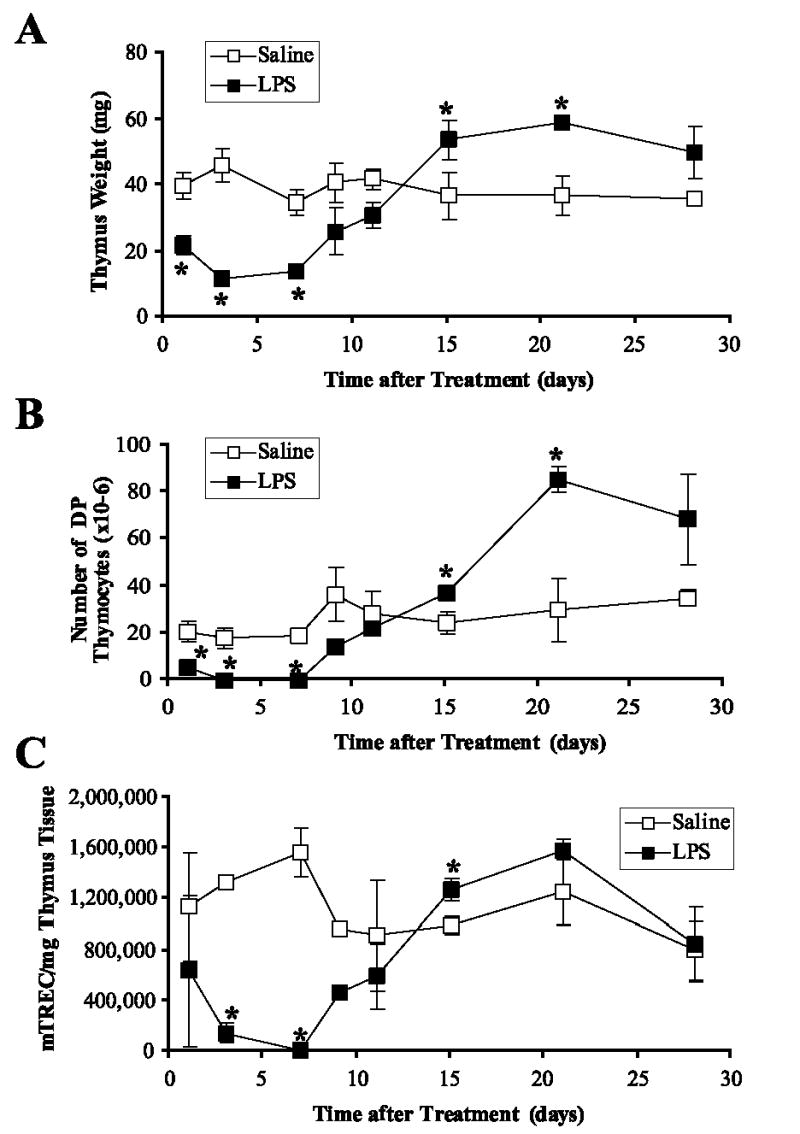
A single administration of E. coli LPS induced acute thymic atrophy with subsequent recovery. Groups of female BALB/c mice were treated with saline or LPS (100 μg i.p.) on day 0, and three mice per group were euthanized on days 1, 3, 7, 11, 15, 21, and 28 to monitor thymopoiesis. Mean thymus weight (A), absolute number of CD4/CD8 DP thymocytes (B), and molecules of mTREC per milligram of thymus tissue (C) ± SEM were determined at each harvest time. *, p ≤ 0.05 compared with saline-treated controls.
To investigate the therapeutic effects of leptin in mice subjected to LPS-induced acute thymic atrophy, groups of BALB/c mice were given a single i.p. injection of saline or leptin 1 μg/g with or without 100 μg of LPS, and mice were sacrificed after 1, 7, or 14 days. One day after LPS challenge, we found that a single dose of leptin administered with LPS resulted in a significant increase in thymus weight compare with LPS-treated animals that received only saline (Fig. 5) (p = 0.036), thus suggesting leptin inhibited LPS-induced acute thymic atrophy. One week after LPS-induced acute thymic atrophy, mice given the single dose of leptin vs saline control had significantly larger thymuses (p = 0.002) (Fig. 5) and a trend toward increased thymocyte numbers (31 ± 5 × 106 vs 21 ± 3 × 106 cells/thymus, p = 0.14; data not shown). By 2 wk after LPS treatment, the thymus had recovered to normal size and the leptin thymopoietic effect was no longer observed (Fig. 5). Similar to what we observed in Fig. 3 and Table III with chronic leptin administration to BALB/c mice, a single dose of leptin had no effect on thymus weights (p = 0.48) (Fig. 5) or thymocyte numbers (161 ± 4 × 106 vs 166 ± 3 × 106 cells/thymus, p = 0.73) in non-LPS-treated BALB/c mice (Fig. 5).
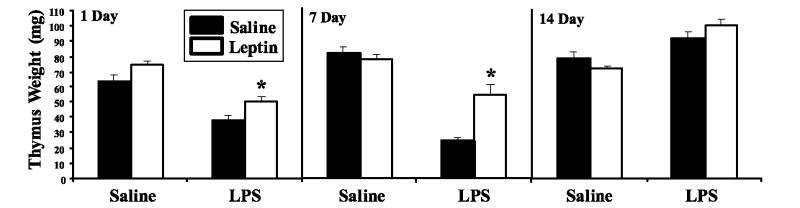
FIGURE 5.
Leptin prevents LPS-induced acute thymic atrophy in BALB/c mice. Female BALB/c mice were simultaneously administered (i.p.) either saline or leptin (1 μg/g body weight) and challenged with either saline or E. coli LPS (100 μg/mouse). Ten mice per group were euthanized on days 1, 7, and 14 to monitor the effect of leptin on LPS-induced acute thymic atrophy. Mean thymus weight ± SEM was determined at each harvest time as an indicator of thymic atrophy. *, p ≤ 0.05 compared with saline-treated controls.
The most striking evidence that leptin augmented thymopoiesis after LPS-induced thymic atrophy was seen in the number of molecules of mTREC per milligram of thymus tissue in LPS-treated mice. mTREC per milligram of thymus tissue dropped significantly 1 day after LPS challenge alone (saline control 561,166 ± 135,366 vs LPS-treated 147,120 ± 55,882; p < 0.05, n = 10/group). Mice that received a simultaneous injection of leptin with LPS, had an equivalent number of mTREC per milligram of thymus tissue compared with saline control or leptin-only treated animals (saline control 561,166 ± 135,366 vs saline/leptin 557,803 ± 102,518 vs LPS/leptin 537,751 ± 130,768; p = NS, n = 10/group). Taken together, these data definitively demonstrated that leptin augmented thymopoiesis in the setting of LPS-induced thymic atrophy.
Leptin reduced peak serum corticosterone levels
Systemic and intrathymic glucocorticoids are critical mediators of stress-induced thymic atrophy (13). To determine whether leptin modulated LPS-induced elevations in glucocorticoids, serum corticosterone levels were quantified during the first 24 h after administration of LPS with or without a single dose of leptin. Mean serum corticosterone concentrations were significantly elevated in all mice challenged with LPS compared with saline- or leptin-only controls with a peak response at 4 h. However, coadministration of leptin significantly blunted this peak corticosterone response (Fig. 6; p ≤ 0.004). These data suggest that inhibition of serum corticosterone may be part of the mechanism by which leptin protects the thymus from sepsis (LPS)-induced thymic atrophy.
FIGURE 6.
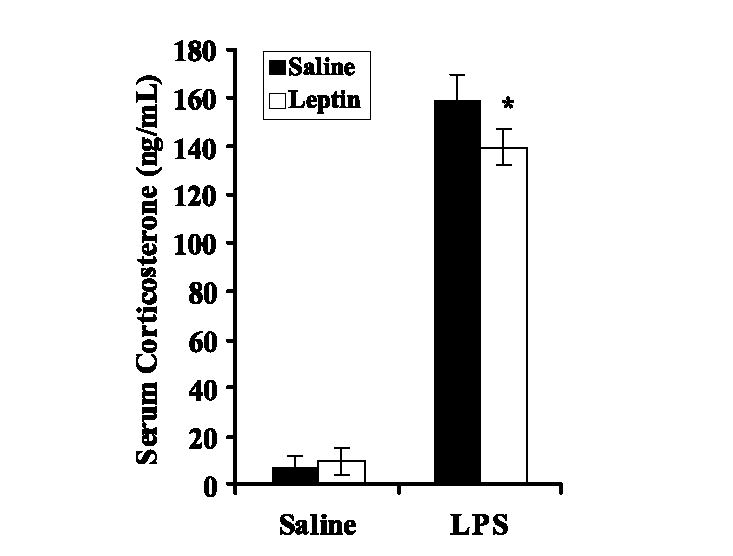
Effect of leptin on serum corticosterone levels induced by E. coli LPS administration. Female BALB/c mice were simultaneously administered (i.p.) either saline or leptin (1 μg/g body weight) and challenged with either saline or E. coli LPS (100 μg/mouse). Serum was obtained from five animals per group 4 h after above treatment. Serum corticosterone concentration was determined by ELISA and the data are reported as mean ± SEM. *, p ≤ 0.05 compared with LPS-treated non-leptin controls.
Leptin modulates inflammatory cytokine responses
In addition to elevated glucocorticoid responses, LPS challenge is also known to initiate a systemic cytokine cascade that contributes to many aspects of LPS-induced shock and subsequent thymic damage. To further elucidate mechanistic pathways by which leptin is thymostimulatory in the setting of LPS-induced thymic involution, serum levels of inflammatory cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, G-CSF, GM-CSF, KC, MCP-1, MIP-1α, MIP-1β, TNF-α, RANTES, and IFN-γ) were quantified at sequential time points (0, 0.5, 1, 2, 4, 6, 12, 18, 24 h) during the first 24 h after administration of LPS with or without a single dose of leptin.
LPS treatment vs saline or leptin treatment alone or 1-h time point induced significant peak inductions in serum levels (picograms per milliliter) of all measured cytokines at either 2, 4, or 6 h and then resolved to basal levels within 12–24 h (Table IV and Fig. 7, p < 0.05 vs saline, data not shown). Leptin alone had no effect on basal cytokine levels (data not shown). However, leptin coadministered at the time of LPS challenge had one of two effects on the LPS-induced cytokine response profile: 1) no change in relation to LPS-only treated mice (IL-3, IL-9, IL-10, IL-12p40, IL-13, G-CSF, KC, MCP-1, MIP-1α, MIP-1β, TNF-α, and RANTES) or 2) a significant blunting of the peak or postpeak cytokine level and a more rapid resolve to basal levels (Table IV). Shown in Fig. 7 are kinetic plots of serum IL-1β and IL-12 p70 induction during the first 12 h after LPS or LPS + leptin treatment showing the significant blunting of the peak or postpeak serum cytokine levels.
Table IV.
Six-hour serum cytokine levels post LPS treatment ± a single dose of leptin
| Cytokine | Salinea | Leptina | LPSa | LPS +Leptina | Percent Change LPS vs LPS + Leptin (%) | p Value |
|---|---|---|---|---|---|---|
| IL-1α | 63 ± 26 | 48 ± 27 | 505 ± 59 | 395 ± 113 | −22 | 0.0147 |
| IL-1β | 29 ± 6 | 20 ± 7 | 458 ± 107 | 322 ± 68 | −30 | 0.0032 |
| IL-2 | 4 ± 0.3 | 2 ± 2 | 32 ± 6 | 22 ± 4 | −31 | 0.0003 |
| IL-4 | 0.2 ± 0.1 | 0.1 ± 0.1 | 4 ± 0.4 | 2 ± 0.4 | −39 | 0.0000 |
| IL-5 | 9 ± 3 | 19 ± 15 | 55 ± 25 | 29 ± 10 | −47 | 0.0001 |
| IL-6 | 8 ± 7 | 0.4 ± 0.9 | 3658 ± 841 | 1505 ± 283 | −59 | 0.0000 |
| IL-12 p70 | 43 ± 28 | 31 ± 37 | 653 ± 175 | 320 ± 83 | −51 | 0.0011 |
| IL-17 | 7 ± 2 | 9 ± 9 | 53 ± 17 | 37 ± 13 | −30 | 0.0001 |
| GM-CSF | 6 ± 6 | 5 ± 2 | 97 ± 10 | 83 ± 9 | −14 | 0.0042 |
| IFN-γ | 33 ± 30 | 10 ± 11 | 186.7 ± 38 | 159 ± 19 | −15 | 0.0702 |
Mean ± SD cytokine level in serum (picograms per milliliter), n = 10 mice/group.
FIGURE 7.
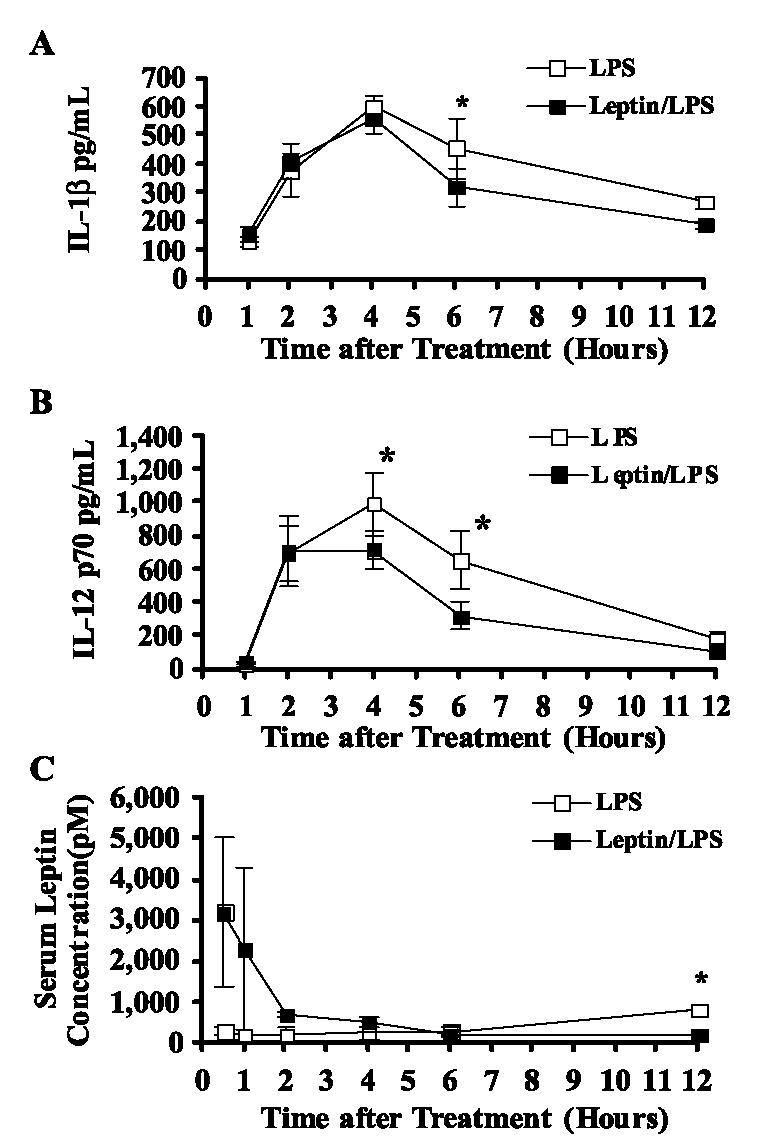
Effect of leptin on serum IL-1β (A), IL-12 p70 (B), and leptin (C) levels induced by E. coli LPS administration. Female BALB/c mice were simultaneously administered (i.p.) either saline or leptin (1 μg/g body weight) and challenged with either saline or E. coli LPS (100 μg/ mouse). Serum was obtained from 10 animals per group 1–12 h after above treatment. Serum cytokine (picograms per milliliter) and hormone (picomole) levels were determined by Luminex bead-based array and the data are reported as mean ± SD. *, p ≤ 0.05 compared with LPS-treated controls.
To determine the magnitude and kinetics of exogenous leptin treatment that mediated these cytokine changes, we quantified the serum level of leptin (picomoles) in LPS- and leptin/LPS-treated mice (Fig. 7C). As has been reported in the literature, LPS alone induced a mild yet significant rise in serum leptin at 12 h postinjection. Exogenous administration of leptin to LPS-treated mice induced a significant rise in serum leptin at 30 min postinjection, similar to leptin treatment alone (Fig. 3). However, the circulating leptin level in leptin/LPS-treated mice rapidly resolved to baseline at 2 h and remained at baseline through 12 h postinjection. Together these data suggest that leptin plays a critical and rapid role in modulating the systemic cytokine response induced by LPS challenge and subsequent LPS-induced damage to the thymus.
Discussion
This study demonstrated that leptin administration augments thymopoiesis in the setting of leptin deficiency and LPS-induced shock, but not in normal mice. Previous findings that leptin treatment of ob/ob mice promoted weight loss while increasing thymus size were validated by the results of this study, and were extended with the demonstration that leptin stimulated an increase in mTREC per milligram of thymus tissue in ob/ob mice. Administration of leptin to ob/ob mice may stimulate production of hemopoietic stem cells, accelerate thymocyte maturation, protect thymocytes from apoptosis, or effect thymic stromal cells that drive thymopoiesis. Alone or together, these signals may result in activating thymopoiesis in the chronically atrophic ob/ob thymus, as evidenced by leptin-induced increases in DP thymocyte frequency, TCRα gene rearrangement and DP-DN thymocyte ratio. While the absolute number of DP thymocytes is increased with leptin treatment, this does not directly indicate that leptin induces maturation of DP from DN thymocytes. It is also possible that leptin rescues thymic atrophy by preventing cell death and/or promoting cell survival in the DP thymocyte compartment.
It is important to note that the presentation of mTREC data as number of copies per milligram of thymic tissue may be overestimated in these studies. Leptin has a potent effect on thymic weight, thus, the ratio of thymocyte genomic DNA to thymic tissue DNA would increase when thymic tissue is reduced by leptin treatment. Furthermore, if leptin promotes DP thymocyte survival and reduces thymic weight, then there would be an additive effect on the calculated number of mTREC.
It has been demonstrated that fasting or malnutrition can induce acute thymic atrophy in normal mice which can be prevented with leptin treatment (2, 20). In the present study, both normal C57BL/6 and BALB/c mice showed significant weight loss with leptin treatment which may have been sufficient to result in fasting-induced thymic atrophy. If this were the case, then the exogenous leptin treatment in WT animals may have modestly stimulated thymopoiesis and counterbalanced the thymosuppressive effect of satiety caused by the supraphysiologic dose of leptin, thus resulting in observed stable thymopoiesis. Future studies in which leptin-induced satiety is inhibited are needed to expand understanding of leptin's impact on thymopoiesis. For example, gold thioglucose-mediated destruction of the ventromedial hypothalamus would permit observation of leptin-mediated effects on the thymus in the presence of obesity and in the absence of leptin deficiency (21). Obesity models in which leptin is increased such as carboxypeptidase E mutant mice (Cpefat) or high fat diet-induced obesity may also be of value to future studies.
The protein hormone leptin has been shown to have emerging effects on the immune system and has been associated with the impact of endotoxin (LPS) challenge in mice, rats, and hamsters (22–24). Leptin is the 16-kDa product from the ob gene, and is secreted by adipose tissue. Leptin-deficient ob/ob mice are severely obese, generally weighing twice that of their lean littermates (2). They also exhibit suppressed cell-mediated immunity, chronic thymic atrophy, and decreased numbers of total thymocytes and splenocytes (2). The HPA axis system is chronically stimulated in ob/ob mice, which results in hypercorticosteronemia (25). Leptin administration has been shown to reverse hypercorticosteronemia and suppress the HPA axis (25). Others have shown that starvation-induced leptin deficiency dramatically decreases survival after LPS challenge (26). It has also been demonstrated that leptin-deficient ob/ob mice have increased susceptibility to endotoxic shock measured by an increase in LPS-induced mortality (27).
The thymus is a central component of the immune system responsible for production and education of new T cells throughout life (5). The state of thymopoiesis is a barometer of the stress level of the body and is acutely sensitive to atrophy/involution from stress in a variety of clinical settings, including 1) bacterial infection, 2) starvation, 3) irradiation or immunosuppressive therapy. Currently, there are no treatments available to protect the thymus from induced involution and/or promote postnatal T cell recovery in these clinical settings. Our studies demonstrated that leptin administration at the time of LPS challenge resulted in significant protection of the thymus from involution.
Given that corticosteroids and inflammatory cytokines have been mechanistically associated with endotoxin-induced severe acute thymic atrophy (1, 13), the impact of leptin on these pathways was determined. Corticosteroids induce apoptosis of immature thymocytes and play a critical role in acute thymic involution (28). LPS activates the HPA axis to elevate systemic corticosteroids (26, 29). Leptin in turn has been shown to have a negative feedback effect on the HPA axis by inhibiting the corticotropin-releasing hormone protein (25). Studies reported herein demonstrate that leptin blunts peak (4 h) LPS-induced corticosterone and suggested an active role for leptin in suppressing systemic corticosteroids. This inhibition of corticosteroid response significantly alleviates the negative impact of LPS on the thymus. However, the reduction effect was not dramatic enough to fully explain the magnitude of thymostimulatory/protective effect of leptin in the LPS-induced thymic atrophy model. Thus suggesting other leptin-driven mechanisms, such as inflammatory cytokines, must be functioning alongside the suppression of the corticosterone response for thymus protection.
Many cytokines have been implicated in the induction of leptin, and conversely, leptin has been reported to have many downstream effects on cytokine networks (27). LPS is known to specifically induce IL-1, TNF-α, IL-6, as well as other inflammatory cytokines (30). Both IL-1 and TNF-α have been shown to independently induce arise in endogenous leptin levels (31). Specifically, IL-1β has been indicated as a mediator of leptin induction, since the stimulation of leptin by LPS and other cytokines is inhibited in IL-1β knockout mice (31). Leptin has also been reported to up-regulate the IL-1R antagonist, which functions of a negative feedback signal in the hypothalamus to regulate fever (32). In addition, several proinflammatory cytokines have been shown to be regulated by leptin, including IL-12 (32, 33), IL-6 (32, 33), IFN-γ (26, 32), and TNF-α (32, 33). Leptin has been generally defined in the immune system to promote phagocytosis, promote the development of the Th 1 subset, and stimulate hemopoiesis (32).
Leptin production is enhanced in response to LPS, suggesting an important regulatory role for leptin in inflammation and LPS-induced shock (22, 34). In our study, we demonstrated that supraphysiologic leptin levels decrease the LPS-induced cytokine response and resulted in augmentation of thymopoiesis. Data presented in Table IV demonstrate that leptin significantly inhibits specific LPS-induced cytokines by 15–59%. We speculate that the single dose of leptin at the time of LPS challenge acutely interferes with the LPS-induced cytokine cascade and suppresses the overall impact of sepsis on the thymus gland. These findings suggest that leptin acts in concert with multiple proinflammatory factors during stress to regulate thymopoiesis. Theses data are compatible with the hypothesis that leptin could be used to therapeutically protect the thymus from acute stress-induced atrophy.
Finally, it is important to note that leptin only induced thymopoiesis in the setting of LPS- and leptin deficiency-induced thymic atrophy and not in normal (WT) mice. These data suggest that LPS treatment and leptin deficiency possibly both simulate expression of a factor required for leptin's ability to stimulate thymopoiesis that is normally absent in untreated WT mice. In this regard, it will be of great interest to carry out expression array analysis of genes selectively induced in LPS-treated and leptin-deficient animals. These studies would determine genes induced in these settings that might be the factor(s) required for leptin's thymopoietic effect.
In summary, these studies demonstrated that leptin has a selective thymopoietic effect in leptin-deficient (ob/ob) mice and in LPS-treated mice, but not in normal mice. It is clear that leptin is needed for proper immune function and inflammatory response since leptin deficient (ob/ob) and leptin receptor deficient (db/db) mice have reduced immune responses (27, 35). Therefore, leptin plays a pleiotropic role in cytokine modulation and is an important link between the neuroendocrine and immunologic systems. Leptin administration may be an important therapeutic strategy to enhance T cell reconstitution in the human clinical settings of stress and leptin depletion.
Acknowledgments
We acknowledge the expert technical assistance of Maria E. Stauffer, Richard M. Scearce, Patrice M. McDermott, and the secretarial assistance of Kim R. McClammy. Flow cytometry and multiplex ELISAs were performed in the Duke Center for Translational Research (AI-51445) Flow Cytometry and Proteomics Core Laboratories, respectively.
Abbreviations
- DP
double positive
- HPA
hypothalamus pituitary adrenal
- TREC
TCR δ excision circle
- sjTREC
signal joint TREC
- mTREC
murine TREC
- DN
double negative
- SP
single positive
Footnotes
This work was supported by a predoctoral scholarship from the Eugene A. Stead Scholarship Committee (to R.W.H.) and by National Institutes of Health HL-67314 (to G.D.S.).
Disclosures:
The authors have no financial conflict of interest.
References
- 1.Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]
- 2.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller-Hermelink HK, Sale GE, Borisch B, Storb R. Pathology of the thymus after allogeneic bone marrow transplantation in man: a histologic immunohistochemical study of 36 patients. Am J Pathol. 1987;129:242–256. [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes BF. The human thymic microenvironment. Adv Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
- 5.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Ann Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 6.Haynes BF, Scearce RM, Lobach DF, Hensley LL. Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J Exp Med. 1984;159:1149–1168. doi: 10.1084/jem.159.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matarese G. Leptin and the immune system: how nutritional status influences the immune response. Eur Cytokine Netw. 2000;11:7–14. [PubMed] [Google Scholar]
- 8.Sempowski GD, Hale LP, Sundy JS, Massey JM, Koup RA, Douek DC, Patel DD, Haynes BF. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol. 2000;164:2180–2187. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- 9.Pond CM. Adipose tissue: quartermaster to the lymph node garrisons. Biologist. 2000;47:147–150. [PubMed] [Google Scholar]
- 10.Pond CM. Adipose tissue, the anatomists' Cinderella, goes to the ball at last, and meets some influential partners. Postgrad Med J. 2000;76:671–673. doi: 10.1136/pmj.76.901.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 12.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 13.Sempowski GD, Rhein ME, Scearce RM, Haynes BF. Leukemia inhibitory factor is a mediator of Escherichia coli lipopolysaccharide-induced acute thymic atrophy. Eur J Immunol. 2002;32:3066–3070. doi: 10.1002/1521-4141(200211)32:11<3066::AID-IMMU3066>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 15.Bray GA, York DA. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979;59:719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- 16.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 17.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 18.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 19.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 20.Fujita Y, Murakami M, Ogawa Y, Masuzaki H, Tanaka M, Ozaki S, Nakao K, Mimori T. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol. 2002;128:21–26. doi: 10.1046/j.1365-2249.2002.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Laey P, Dent C, Terry AC, Quinn EH. Food and water intake in gold thioglucose-induced obese Charles River mice. Arch Int Pharmacodyn Ther. 1975;213:145–162. [PubMed] [Google Scholar]
- 22.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci USA. 1999;96:7047–7052. doi: 10.1073/pnas.96.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1β mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 25.Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138:3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 26.Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000;156:1781–1787. doi: 10.1016/S0002-9440(10)65049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–R142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 28.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous nuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 29.Madiehe AM, Mitchell TD, Harris RB. Hyperleptinemia and reduced TNF-α secretion cause resistance of db/db mice to endotoxin. Am J Physiol. 2003;284:R763–R770. doi: 10.1152/ajpregu.00610.2002. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Poole B, Mitra A, Falk S, Fantuzzi G, Lucia S, Schrier R. Role of leptin deficiency in early acute renal failure during endotoxemia in ob/ob mice. J Am Soc Nephrol. 2004;15:645–649. doi: 10.1097/01.asn.0000113551.14276.0b. [DOI] [PubMed] [Google Scholar]
- 31.Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, 3rd, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juge-Aubry CE, Meier CA. Immunomodulatory actions of leptin. Mol Cell Endocrinol. 2002;194:1–7. doi: 10.1016/s0303-7207(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 33.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 34.Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–R142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 35.Chandra RK. Cell-mediated immunity in nutritional imbalance. Fed Proc. 1980;39:3088–3092. [PubMed] [Google Scholar]