Several mammalian enzymes are capable of transferring electrons to molecular oxygen, sequentially forming the 1 electron-reduction product superoxide (O2•−) and the 2 electron-reduction product hydrogen peroxide (H2O2). These serve as progenitors for other reactive oxygen species (ROS), including peroxynitrite (ONOO−), hypochlorous acid, the hydroxyl radical, lipid peroxides, lipid peroxy- radicals, and lipid alkoxyl radicals. Another relevant group of molecules is the reactive nitrogen species, including NO, the nitrogen dioxide radical, and the nitrosonium cation. In the cardiovascular system, the most important enzymes that produce ROS are the Nox-based reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidases, xanthine oxidase, the mitochondrial electron transport system and, under certain circumstances, NO synthase. Conditions such as hypertension, atherosclerosis, hypercholesterolemia, diabetes, and insulin resistance increase either the activity or the expression of these enzymes, leading to elevated ROS production. ROS, in turn, contribute to these disorders. As examples, virtually every aspect of atherosclerotic lesion formation is augmented by oxidative events. Via several mechanisms involving vessels, the kidney, and the central nervous system, ROS augment hypertension. ROS have been implicated in causing insulin resistance and pancreatic damage leading to diabetes. Moreover, ROS, when produced in specific subcellular compartments in controlled amounts, can act as signaling molecules to regulate normal cellular functions. These reactions have been reviewed in depth elsewhere recently.1
Given the importance of ROS and reactive nitrogen species in physiology and pathophysiology, it has become essential that methods be adapted and standardized to quantify the levels of these molecules in cells and tissues. Numerous methods have been used for ROS detection, each with potential pitfalls and advantages. All of these techniques can yield errors, and it is advisable to use ≥2 methods to avoid a mistaken conclusion. In this review, we will discuss various methods used to detect and quantify ROS and consider their advantages and disadvantages.
General Comments
The major reason to measure ROS in biological systems is to determine whether they play a role in physiological or pathophysiological processes. To accomplish this, it is reasonable to adopt Koch's postulates. One should show that the offending species are present in the system of interest. Removal of the species (in the case of ROS, scavenging or knockout of the source) should remedy the condition, and introduction of the species should cause the phenomenon. There are several important caveats here. First, ROS can be highly localized such that assay of an entire cell or segment of tissue might not reflect a subcellular increase, although it usually does. Second, for the same reason, the addition of scavengers, particularly if not properly targeted, might not block the effect. For example, many enzymes produce O2•− intracellularly, and the addition of superoxide dismutase (SOD) outside the cell will have no effect. Third, one should be aware that most scavengers of O2•−, including SOD and most antioxidant vitamins, remove O2•− by producing H2O2. If H2O2 has a biological role, its effect might not be diminished and, in fact, could be increased by interventions that increase flux from O2•− to H2O2.
An ideal assay for ROS detection should be sufficiently sensitive to ensure that measurements are within the linear range of the assay and well above the limits of detection. Preferably, it should be specific for 1 ROS, at least in physiological/pathophysiological concentrations. Its signal should be substantially inhibited by a specific scavenger of the ROS in question. It should be robust, that is, applicable to a wide variety of experimental conditions and comparable between these applications. There are some things that should not be expected of assays for ROS. As discussed above, ROS production can be highly localized, and, therefore, most assays will not be able to detect ROS in all of the subcellular locations and extracellularly. Furthermore, the concentration of ROS at the site of production could be extremely high, and this almost certainly will not be reflected by measurements obtained in intact tissues, cells, or in the media in which tissues are incubated. Finally, it is very unlikely that any probe will react with all of the ROS produced, meaning that any assay cannot be viewed as completely quantitative.
Another general comment is that one should take great care in the preparation of buffers to minimize contamination with transition metals. Even the most fastidiously prepared buffers, made with the highest-grade chemicals, can contain trace amounts of transition metals, and great care should be made to minimize this when performing studies of isolated tissues, membranes, and enzyme systems. In our experience, if this is not done, fully one half of the O2•− formed in a study of isolated tissue homogenates can be attributed to transition metal contaminants. This artifact can be eliminated by treating buffers for several hours with chelex and by using diethylenetriamine-penta-acetic acid in near millimolar concentrations. Experiments with the buffer as a background control are also important to assure that the signals are arising from the tissue sample and not the buffer.
One should also be cautioned about the literature regarding assays for ROS, which is replete with in vitro experiments using very contrived experimental situations to disprove the validity of an assay for ROS.2-5 In any biological assay, it is possible to generate artifacts using extreme conditions that are not encountered in vivo. Future experiments should be designed to study these assays in the context of intact tissues and under proper physiological conditions, rather than in artificial “test tube” situations. Results from studies such as these should not be used to refute the use of these assays under properly controlled physiological circumstances.
Finally, there have been attempts to examine activities of various enzymes by adding their substrates to intact tissues. For example, NADPH added to intact vessels has been used as a measure of NADPH oxidase activity. These studies are hard to interpret, because it is unclear how extracellular NADPH enters the cell to be used as a substrate for the NADPH oxidase. There is no established mechanism for cellular uptake of NADPH, and the binding domain to the Nox proteins (the catalytic subunits of the NADPH oxidase) is intracellular. In fact, a recent article showed that NADPH exerts its effects by stimulating purinergic receptors.6 Thus, one is not studying NADPH oxidase activity using this approach but rather purinergic signaling. When subcellular enzyme fractions, such as membranes, are used, receptor signaling pathways are disrupted, and the added substrates are more likely to reach their respective enzyme binding sites to promote ROS production.
Cytochrome C Reduction
The Theory
Oxygen is a diradical, possessing 2 unpaired electrons. As mentioned above, when oxygen gains an additional electron, O2•− is formed (Figure 1A). Superoxide is generally considered an oxidant, because in most cases, it extracts an electron from another molecule, leading to formation of H2O2. For other molecules with higher electron potentials, O2•− actually donates its extra electron. This is the case for ferricytochrome c, which is reduced to ferrocytochrome c by receiving an electron from O2•−. When ferricytochrome c is reduced, its spectrophotometric absorbance is altered in a very specific fashion. Absorbance at 550 nm is increased, whereas absorption at 540 and 560 nm remain unaltered and can serve as isosbestic points (Figure 1B). Unfortunately, ferricytochrome c can be directly reduced by electrons donated from enzymes and other molecules, so this change in absorbance is not specific for O2•−. For this reason, the assay must be performed in the presence and absence of SOD and only the SOD-inhibitable signal used to calculate the amount of O2•− formed. In studies of the cardiovascular system, cytochrome c reduction has been used to measure O2•− produced by mouse aortic segments,7 human vessels,8 mesenteric arterioles,9 segments of the cardiac ventricles, and pieces of the left atrium.10
Figure 1.
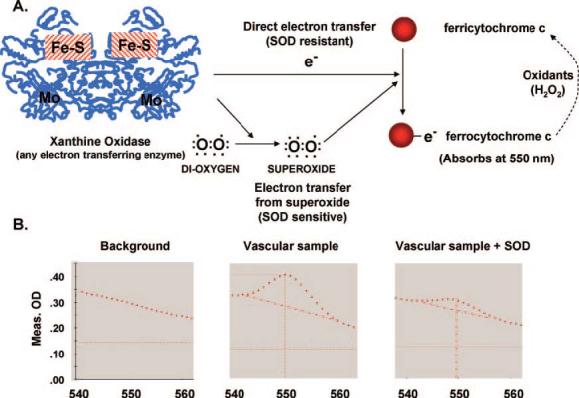
Detection of O2•− by reduction of ferricytochrome c. A, Various enzymes (shown as an example is xanthine oxidase) can generate electrons, which can directly reduce cytochrome c and also reduce oxygen leading to O2•− formation. Superoxide, in turn, can donate its electron to ferricytochrome c, leading to formation of ferrocytochrome c. SOD is used to differentiate between a direct reduction of ferricytochrome c vs reduction mediated by O2•−. B, Reduction of ferricytochrome c can be detected at 550 nm absorbance, using 540 and 560 nm as isosbestic points.
The Assay
For measurement of O2•− from a segment of vessel or other tissues, it is necessary to divide the tissue equally. For mouse aortas, two 3-mm rings are placed in a buffer composed of (mmol/L): NaCl, 145; KCl, 4.86; NaH2PO4, 5.7; CaCl2, 0.54; MgS04, 1.22; glucose, 5.5; deferoxamine mesylate, 0.1; and 50 μmol/L of acetylated ferricytochrome c. Two other 3-mm segments of aorta are added to a similar buffer containing manganese superoxide dismutase (100 U/mL). The tissues are incubated in a 96-well plate to minimize the volume and, thus, maximize the concentration of O2•− released from tissues exposed to ferricytochrome c. Acetylation of cytochrome c improves quantification and specificity for O2•− because it strongly inhibits direct enzymatic reduction, improves stability of the reduced cytochrome c, and has minimal effects on reaction with O2•−.11-13 To further ensure that any reduced ferricytochrome c is not reoxidized, catalase (125 U/mL) is added to the reaction, which removes any H2O2 formed. The tissue pieces are incubated in these reaction mixtures for 30 minutes at 37°C. The tissue is then removed, and the buffer absorbances measured at 540, 550, and 560 nm using a 96-well plate reader. We have found the BioTek Powerwave spectrophotometer to be very useful for these assays, although similar instruments are probably also effective. Figure 1B shows examples of scans encompassing these wavelengths for a background sample, a tissue sample alone, and a tissue sample in the presence of SOD.
To calculate the amount of O2•− produced, the increase in optical density (ΔOD) at 550 nm over baseline is calculated by averaging the absorbances at 540 and 560 nm and subtracting this value from the reading at 550 nm. The amount of O2•− formed is calculated from the formula:
where ΔODs with and without SOD represent the shifts in absorbance at 550 nm above the average of OD at 540 and 560 nm. The extinction coefficient for reduced cytochrome c is 21.1 mmol/L−1 cm−1; thus, the value for O2•− is in millimoles per liter.
Strengths
Reduction of cytochrome c is considered by many the “gold standard” for detection of O2•−. In the case of professional phagocytes, which produce huge amounts of O2•−, or for isolated enzymes like xanthine oxidase, it is a very powerful and useful method. It allows quantification of O2•− without addition of a standard, because the extinction coefficient of reduced cytochrome c is known. When corrected for the small volume, and considering that one is measuring changes in OD of microunits, it is clear that one can obtain reasonably accurate estimates of O2•− in the picomole range, given the caveats mentioned below. The assay can be performed with equipment available in most laboratories.
Weaknesses
Although cytochrome c reduction is a good method for quantifying O2•− released in large amounts during the respiratory burst of neutrophils or by isolated enzymes, for other tissues, like vessels, vascular smooth muscle cells, endothelial cells, and pieces of cardiac tissue, the level of O2•− produced is much lower. This means that one is working at the lower limit of the range of detection for O2•− production using this assay. Tiny errors in the measurement of absorbance, weighing of tissue, pipetting, and so forth, are amplified substantially in the final calculation. For this reason, it is often better to quantify the results per length of vessel used (ie, per 2-mm segments) or volume of tissue rather than to normalize to weight. Another drawback is the assumption that the 2 bits of tissue used in the assay (with and without SOD) are identical. This is never true. For blood vessels, flow profiles have large effects on ROS production, and even small differences in the location of the vessel can affect the mechanical forces to which segment of tissue are exposed. Thus, to normalize a piece of aorta from the mid thorax to one slightly nearer the diaphragm might lead to spurious results and to substantial variability in measurements. This does not entirely preclude the use of cytochrome c for these purposes, but means that caution must be used and that higher numbers of experiments are often necessary to yield useful results. We have also found that the assay is not robust, that is, that it cannot be used under a variety of different conditions. For example, when one adds various enzyme inhibitors, like oxypurinol, apocynin, and NG-nitro-L-arginine methyl ester, this may inhibit the direct enzymatic reduction of cytochrome c, and the shifts in baseline absorbances can be large. Although appropriate blanks should correct for this, we have found that these baseline shifts make comparisons between various conditions difficult. Finally, cytochrome c reduction only detects extracellular O2•−. Intracellular sources of O2•− are likely underestimated by this approach.
The Verdict
Cytochrome c reduction is a time-honored approach for measuring O2•−. It is difficult, but not impossible, to apply to vascular and myocardial tissues because one is working at the lower limit of the assay's sensitivity. The results are more variable than other methods, making it necessary to use a large “n.” Given these caveats, it is almost always accepted by reviewers without question.
Chemiluminescence-Based Techniques
The Theory
On exposure to O2•−, chemiluminescent probes release a photon, which, in turn, can be detected by a scintillation counter or a luminometer. Because most of these compounds are cell permeable, the O2•− measured reflects extracellular, as well as intracellular, O2•− production. The most commonly used chemiluminescence technique for measurement of O2•− is lucigenin-enhanced chemiluminescence. For this reason, this section will focus on lucigenin with a brief discussion of other chemiluminescence methods.
The reactions (1 through 3) involved in lucigenin-amplified chemiluminescence (LC) are as follows: (1) reaction 1: O2•−+LC2+→LC•++O2; (2) reaction 2: LC•++O2•−→LCO2; and (3) reaction 3: LCO2→2N-methylacridone+hν. Thus, O2•− reduces lucigenin (LC2+) to its cation radical (LC•+), which, in turn, reacts with a second O2•− to form the energy-rich dioxetane molecule (LCO2), which then emits a photon.
The Assay
Two 3-mm segments of vessels or similar-sized pieces of other tissues are dissected free of adjacent tissues in a Krebs/HEPES buffer. For the assay, a Krebs/HEPES buffer containing 5 μmol/lucigenin of is prepared. We have found that the results are most consistent if this buffer is “dark adapted.” The buffer is placed in either a luminometer or scintillation counter set to the out-of-coincidence mode and incubated until background counts have stabilized. Tissue segments are then added to the reaction vial, and, after 15 minutes of equilibration, counts are obtained every minute for the next 5 minutes and averaged. Counts can be expressed as chemiluminescence units, can be normalized to surface area of the tissue or to segments of tissue if great care is taken to prepare segments of identical size, or can be normalized to wet or dry weight.
Strengths of Lucigenin-Enhanced Chemiluminescence
This method has been widely used, and an enormous amount of new information has been gained from its use. It is reasonably specific for O2•−, and one does not need to routinely prepare a second sample with SOD to prove that the signal is derived from O2•−. If one chooses to use an O2•− scavenger, it should be kept in mind that lucigenin penetrates cells, and, therefore, unmodified SOD, which is not cell permeable, will not reduce the lucigenin signal completely (usually ≤50%). Generally the lucigenin-enhanced chemiluminescence signal is well within the linear range of pathological samples, although normal tissues produce low levels of O2•−, which are generally only slightly above background. The assay is also inexpensive and can be performed using equipment that is commonly available.
Weaknesses and Alternate Chemiluminescence Probes
Despite the above strengths, the validity of lucigenin-enhanced chemiluminescence has been questioned on the grounds that O2•− production might be artificially overestimated because of a phenomenon known as redox cycling, in which the lucigenin radical can react with oxygen to generate O2•−.2
Reaction 4 is as follows: LC•++O2→O2•−+LC2+. This reaction is catalyzed by flavin containing enzymes such as endothelial NO synthase, xanthine oxidase, and cytochrome P450 monooxygenases, and, as shown in reaction 4, leads to oxygen consumption. Although there is no question that reaction 4 occurs in biological tissues, the main issue is how much it contributes to the total signal one detects in an assay of tissue. When 5 μmol/L of lucigenin is used, the amount of artifact is probably insignificant.14 As discussed above, many of the experiments demonstrating lucigenin redox cycling have been performed in artificial situations and do not reflect the manner in which this assay is used under normal circumstances.
Other chemiluminescent probes have been used to detect ROS of various kinds. One of the oldest is luminol (5-amino-2,3-dihydro-1,4-phthalazinedione).15 This compound is oxidized by a variety of ROS, including H2O2, hydroxyl radical, hypochlorous acid, and peroxynitrite. The assay is essentially identical to that described above for lucigenin. One can add specific scavengers to assess the contribution of various ROS, that is, catalase (≈100 U/mL) for H2O2 and urate (250 μmol/L) for peroxynitrite. In the past several years L-012, a modified form of luminol, has been proposed to be the specific probe for O2•−. However, this molecule may also detect ONOO− and probably other ROS.16 Its light emission is reported to be greater than lucigenin, but beyond this, it may not provide any clear cut advantage to lucigenin. L-012 has been reported not to undergo redox cycling, but examination of its structure, as well as the structure of luminol, suggests that they have potential to do so.
Beginning in the 1980s, a series of fluorescent compounds were developed from coelenterazine (2-[4-hydroxybenzyl]-6-[4-hydroxyphenyl]-8-benzyl-3,7 dihydroimidazo[1,2-a]pyrazin-3-1).17 Coelenterazine is the molecule responsible for the fluorescence of various bioluminescent marine organisms in the genus cypradina and is the light-emitting component of the fluorescent protein aeqourin. Coelenterazine does not undergo redox cycling and was found to be useful as a probe for the detection of O2•−.4 Modifications of the cypridina luciferin led to production of a series of new compounds, including the cypridina luciferin analog (CLA) and the methylated-modified CLA (MCLA). Of these, MCLA seems to have the highest signal:background ratio and emits >100-fold more light for the same stimulus as coelenterazine. CLA and MCLA have been used to measure O2•− production by vessels,18 cultured endothelial cells,19 vascular smooth muscle cells,20 and other tissues.21 One report indicated that MCLA could also emit light in response to the hydroxyl radical in bicarbonate buffers,22 and high levels of other ROS have been shown to elicit MCLA chemiluminescence.23
The Verdict
Chemiluminescence-based techniques, and, in particular, lucigenin-enhanced chemiluminescence have provided enormous insight into how various diseases like atherosclerosis, hypertension, diabetes, and heart failure affect O2•− production in various tissues. Virtually every result with lucigenin has been confirmed using other methodologies. Despite this, these assays remain controversial, and reviewers often criticize articles using these techniques. Concerns persist about how important redox cycling is, what ROS are being measured, and whether the signals might be nonspecific. In the case of lucigenin, when low concentrations are used with intact tissues, redox cycling probably contributes to the entire signal only minimally. The cypradina luciferin-based molecules CLA and MCLA are promising probes for O2•−, but their use has been limited and not validated under a variety of conditions. Given these concerns, it is highly recommended that a second assay, preferably not based on chemiluminescence, be used to confirm the results.
Electron Spin Resonance
The Theory
Electron spin resonance (or electron paramagnetic resonance; ESR) is based on absorption of microwave radiation stimulated by an electromagnetic field in molecules such as free radicals and transition metal ions with unpaired electrons. The typical ESR spectrometer consists of a microwave generator, a resonator cavity flanked by a pair of electrical magnets (Figure 2A). Microwaves are delivered from a generator to the resonator cavity, and when the spectrometer is tuned, all of the energy delivered to the resonator is absorbed so that no microwave energy is reflected back from the resonator cavity. When a magnetic field is applied to the sample, the paramagnetic unpaired electrons act like tiny compasses that orient either in a direction parallel or antiparallel to the magnetic field. This creates 2 distinct energy levels for the unpaired electrons such that the absorption of microwave energy causes a transition from the lower state to the higher state (Figure 2B). This transition occurs when the magnetic field (Bo) causes a difference between the 2 states of electron energy equal to the microwave energy (hv=g×μB×B), where “hv” is the microwave energy, “g” is the factor constant equal to 2.002 for most organic samples, and “μB” is the Bohr magneton constant.24 In summary, ESR detects the absorption of microwave energy, which occurs on transition of unpaired electrons in an applied magnetic field. The amplitude of the ESR signal is proportional to the number of the unpaired electrons present in the sample (Figure 2B), allowing quantification of free radicals.
Figure 2.
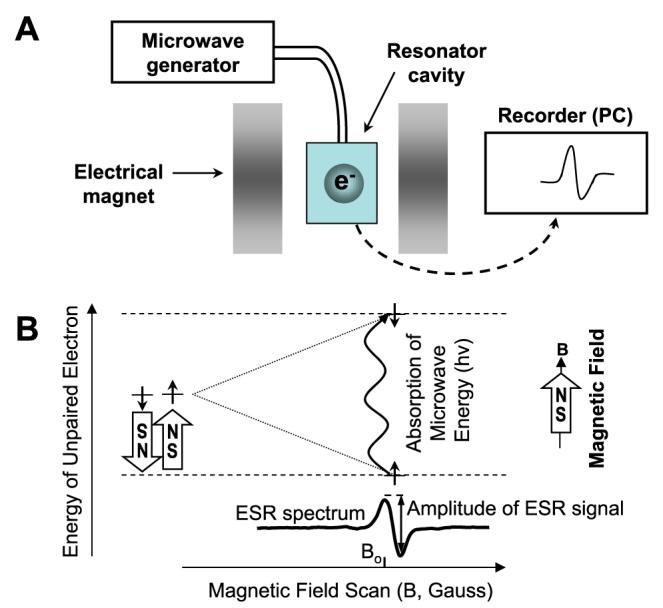
The typical ESR spectrometer (A) consisting of a microwave generator, a resonator, and magnets. ESR detects the absorption of microwave energy on the transition of unpaired electrons in an applied magnetic field (B). Because the electron has a magnetic moment, it acts like a compass when it placed in a magnetic field (B). It will have a state of lowest energy when the moment of the electron is aligned with the magnetic field (↑) and a state of highest energy when it aligned against the magnetic field (↓). The energy of the unpaired electron, therefore, is a function of the magnetic field: E=±0.5g μBB, where “g” is the g-factor constant, “μB” is the Bohr magneton constant, and “B” is the magnetic field.
If an unpaired electron existed in the sample by itself, the spectrum obtained would be quite boring, consisting of only 1 peak. Fortunately, electrons are always accompanied by nuclei, which have magnetic moments that produce a local magnetic field at the unpaired electron. This tiny local magnetic field subtracts from or adds to the exogenous magnetic field applied by the spectrometer, depending on the alignment of the magnetic moment of the nucleus, phenomena referred to as hyperfine interactions.25 In the case where the nucleus adds to the electron's magnetic field, it takes a lower amount of external magnetic energy to cause absorption of microwaves and yield a signal. In contrast, when the magnetic moment of nearby nuclei subtracts from the electron's magnetic field, a higher exogenous magnetic field is needed to cause electron transition. Hyperfine interactions result in several peaks being detected by the spectrometer as the magnetic field is increased and provide information about the identity and number of atoms that make up a molecule, as well as their distances from the unpaired electron.
Unprocessed ESR absorption signals are very broad and unresolved. Because of this, ESR spectroscopy uses a technique known as phase-sensitive detection to enhance sensitivity of the spectrometer. The magnetic field is modulated sinusoidally at the modulation frequency, and, in case of the ESR signal, microwaves reflected from the cavity are also amplitude modulated at the same frequency. This transforms the ESR signal into a sine wave with the amplitude proportional to the slope of the signal, that is, a first derivative of the original absorption curve. The amplitude of the sine wave is proportional to the amount of the free radical.
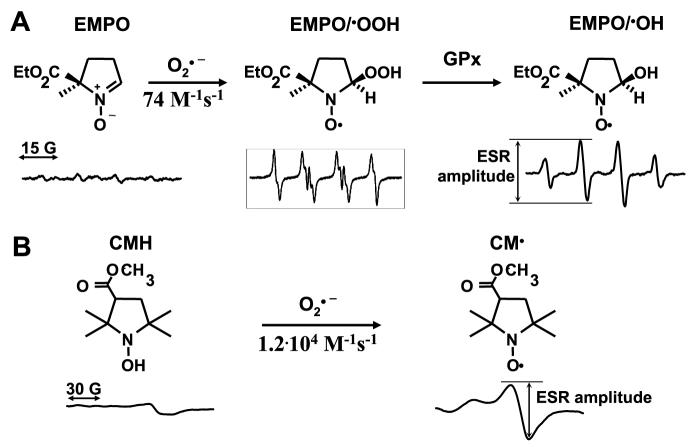
Most of the biologically relevant radicals are very short lived and, therefore, impossible to detect in biological samples. For this reason, compounds have been used that form stable adducts with radicals.26 The molecules, termed “spin traps,” include nitrone compounds such as N-t-butyl-α-phenylnitrone, α-(4-pyridyl 1-oxide)-N-tert-butylnitrone, 5, 5-dimethyl-1-pyrroline-N-oxide, 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide, and 2-ethoxycarbonyl-2-methyl-3,4-dihydro-2H-pyrrole-1-oxide.27 The reactions of spin traps with radicals results in adducts that yield specific spectra when subjected to ESR spectroscopy. Some of these (N-t-butyl-α-phenylnitrone and α-[4-pyridyl 1-oxide]-N-tertbutylnitrone) work well for trapping carbon-centered radicals, such as lipid radicals,28 whereas others (5,5-dimethyl-1-pyrroline-N-oxide and 5-[diethoxyphosphorylor-5-methyl-1-pyrroline-N-oxide) are often used for trapping O2•− and •OH.29 Spin traps can be used to both identify and quantify the original radical formed. Theoretically, spin trapping with nitrones should be an ideal way to detect radicals in biological tissues. Unfortunately, this turns out not to be the case. First, nitrone radical adducts are unstable. For example, when 5,5-dimethyl-1-pyrroline-N-oxide and 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide react with O2•−, they initially form adducts that yield hyperfine splits typical of the O2•− adduct. Within seconds to minutes, however, intracellular glutathione peroxidase rapidly converts these to alcohols, which are identical to OH-radical adducts.30,31 Thus, one cannot discern formation of O2•− from •OH without using specific scavengers. This negates one of the advantages of the nitrone spin traps. Second, spin traps have low reactivity with O2•− (74 mol/L−1 s−1) compared with other O2•− probes, such as cytochrome c (3×106 mol/L−1 s−1).32 More importantly, nitrone radical adducts are very susceptible to bioreduction when exposed to cells or tissues, and their 1-electron reduction by flavins, thiols, or ascorbate converts them to ESR silent species (Figure 3A). Because of this, it is difficult to accurately quantify radicals formed in intact tissues, cells, or homogenates using the currently available nitrone spin traps. Recently, a caged nitrone spin trap has been developed and reported to specifically detect O2•− in biological samples and to be resistant to degradation by reductants.33 Additional studies are needed to determine whether this will be useful in biological samples.
Figure 3.
Detection of superoxide by ESR. A, Spin trapping of O2•− by spin trap EMPO produces superoxide radical adduct EMPO/OOH, which is decomposed by glutathione peroxidase (GPx) into OH-adduct and reduced by flavin enzymes and ascorbate into ESR silent hydroxylamine. B, The reaction of O2•− with spin probe CMH produces stable nitroxide radical, which can be conveniently detected in frozen samples.
Because of these problems using nitrone spin traps, investigators have turned to other molecules as probes for detection of ROS in tissues. One such class of compounds, the cyclic hydroxylamines, has proven extremely effective for use in tissues and cultured cells.34 These molecules are not spin traps, in that they do not “trap” radicals, but they are oxidized to form stable radicals with a half-life of several hours, which can readily be detected by ESR. Cyclic hydroxylamines, such as 1-hydroxy-3-carboxy-2,2,5,-tetramethyl-pyrrolidine hydrochloride (CPH), can provide quantitative measurements of O2•− radicals with high sensitivity.35 The half life of 3-carboxy-proxyl radical has been shown to vary from 330 minutes in smooth muscle cells to 87 minutes in the presence of ascorbate.36 CPH was used for in vivo O2•− detection,34 whereas 3-carboxy-proxyl radical clearance was mainly dependent on renal excretion and was not depend on reaction with ascorbate.37,38 CPH reacts rapidly with O2•− (3.2×103 mol/L−1 s−1) and ONOO− (Figure 3B), and the resultant 3-carboxy-proxyl radical is very stable, undergoing minimal bioreduction.36 Because these compounds can be oxidized by several ROS, it is necessary to perform paired experiments where SOD, an ONOO− scavenger, or other scavengers are added to define the ROS causing the reaction. We have found that SOD lowers the signal by >70% in cultured cells or isolated vessels.39 Another cell permeable cyclic hydroxylamine, 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH), has been used for detection of intracellular O2•− in cultured cells and tissue samples (Figure 3B).35,39 Recently, we have validated 1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yltrimethylammonium and found this to be extremely useful for detecting extracellular O2•− produced by intact tissues.9 This probe seems particularly resistant to auto-oxidation.
The Assay
To minimize hydroxylamine auto-oxidation, the spin probe stock solutions must be prepared in argon-bubbled ice-cold 0.9% NaCl in the presence of the chelating agent diethylenetriamine-penta-acetic acid (0.1 mmol/L) or a combination of 25 μmol/L of deferoxamine and 2 μmol/L of diethyldithiocarbamate. Three 2-mm mouse aortic rings or similar-sized pieces of other tissues are incubated for 60 minutes at 37°C in 1 mL of Krebs/HEPES buffer containing 2 μmol/L of diethyldithiocarbamate, 50 μmol/L of deferoxamine, and 0.5 mmol/L of CMH. The segments are then frozen in liquid nitrogen, and ESR spectra are recorded using the following ESR settings: field sweep, 80 G; microwave frequency, 9.39 GHz; microwave power, 2 mW; modulation amplitude, 5 G; conversion time, 327.68 ms; time constant, 5242.88 ms; 512 points resolution; and receiver gain, 1×104. The amplitude of the signal is measured, and the concentration of CM-radical is determined by calibration with standard concentrations of the nitroxide TEMPOL. The portion of the signal because of O2•− is determined by preincubation of duplicate samples with polyethylene glycol–SOD (100 U/mL) for 3 to 4 hours in Krebs/HEPES buffer. After the ESR measurement, rings are transferred into Eppendorf tubes, dried for 48 hours at 37°C, and their dry weight is determined. The formation of 3-methoxycarbonyl-proxyl (CM)-nitroxide is normalized to the dry weight of aorta rings. A similar approach can be used to measure extracellular O2•−, using the cell-impermeable 1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium spin probe.9 In this case, aortas are incubated for 60 minutes at 37°C in 210 μL of Krebs/HEPES buffer containing 0.5 mmol/L of 1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium in the presence or absence of Cu, ZnSOD (100 U/mL). The buffer without the vessel is then aspirated into a tuberculin syringe, snap frozen in liquid nitrogen, and scanned as described for CMH.
We have also found cyclic hydroxylamines to be very useful in detecting O2•− released from intact cells. For example, cultured cells can be harvested and placed in buffer containing either CPH or CMH, which is then transferred to an ESR capillary tube and placed in the ESR resonator at room temperature. The rate of rise of the low field peak of the spectrum can be used to calculate the rate of ROS formation. In the case of CPH, a similar assay can be performed in the presence of SOD. When the cell-permeable CMH is used, polyethylene-glycolated SOD (100 U/mL) can be added 4 hours before cells are studied to determine the contribution of O2•− to the signal.40 We have used this approach to detect O2•− production for cultured endothelial cells, neutrophils, and vascular smooth muscle cells.
ESR can be used to monitor the activity of various enzymes in cell or tissue homogenates. In these cases, either whole homogenates or membrane fractions can be added to the buffer in the presence of a spin probe like CPH,10,41 and the enzyme activity stimulated by adding the specific substrate, for example, xanthine in the case of xanthine oxidase or NADPH in the case of the NADPH oxidase. Again, the rate of rise of the low field peak of the ESR spectra is monitored. The rate of O2•− production can be determined at room temperature by time scan after the amplitude low field peak of the ESR spectra, or when frozen samples are used, the height of the spectra obtained from a field scan can be used.
Strengths
Electron spin resonance is an excellent approach for the detection of radicals. When studying isolated enzymes or chemical reactions, the nitrone spin traps are very useful for determining the precise product formed or for detecting reaction intermediates. For studies of intact tissues, cells, or homogenates, the cyclic hydroxylamines offer a distinct advantage over nitrone spin traps for measurement of O2•− and other radicals, because they yield very stable products and strong ESR signals. These methods are also quantitative. By selecting the proper spin probe, one can restrict measurements to intracellular or extracellular compartments. The values obtained when studying cultured cells, intact vessels, or other segments of tissue are well within the sensitivity of ESR when cyclic hydroxylamines are used, which can reach 1 nmol/L.
Weaknesses
As discussed above, the nitrone spin traps, often considered ideal for detection of various radicals, cannot be used with intact tissues or cells, because they are converted to spin inactive products by bioreductants. The cyclic hydroxylamines are, therefore, preferable; however, the product formed on reaction with different radicals yields the same nitroxide regardless of the radical or oxidant trapped,35 and the resultant ESR spectrum is, therefore, identical for several different species. To overcome this limitation, it is necessary to perform additional studies using various antioxidants and inhibitors specific for the ROS in question. For example, O2•− radicals can be determined as SOD-inhibitable 3-carboxyproxyl radical–nitroxide formation, and ONOO− can be measured as urate-inhibited 3-carboxy-proxyl radical–nitroxide formation.42 New spin probes are constantly being developed to improve their stability and selectivity.
A major weakness of ESR is that the spectrometers are very expensive, often costing several hundred thousand dollars. They also occupy a large amount of space, generally requiring a 600-ft2 laboratory room for proper operation. This is prohibitive for many investigators. Tabletop spectrometers are available, which are much less expensive and less space consuming. These are not as sensitive or versatile as the larger floor models. Because of the strength of the signals generated by the cyclic hydroxylamines, it has been possible to use these tabletop models for studies of vessels and cells.
A final weakness of ESR is that it is almost essential to have extensive training to operate the spectrometers correctly. Unlike other methods for ROS detection, one cannot simply read manuals or papers to understand all of the nuances of this technique or the equipment involved.
The Verdict
ESR is an elegant method for the detection of radicals. For studies of vessels and other tissues, the best currently available approach is to use cyclic hydroxylamines as probes. Newly developed nitrone spin traps that are resistant to bioreduction may provide a superior approach, because they could yield specific spectra for various radicals. The major detractions of ESR are its expense and the need for a trained spectroscopist to oversee use of this equipment.
Detection of Intracellular O2•− With Dihydroethidium and High-Performance Liquid Chromatography
The Theory
Initially, it was thought that dihydroethidium (DHE) reacted with O2•− to form ethidium, which would, in turn, intercalate with nDNA. Tissues were visualized using fluorescence microscopy, and the intensity of the signal was interpreted as reflecting the level of O2•− present. Recently, it has been found that this is not precisely correct. The reaction of dihydroethidium with O2•− yields a very specific product, 2-hydroxyethidium (Figure 4A), with a molecular weight 16 U greater than DHE.43,44 The rate constant of the reaction of DHE with O2•− is estimated as high as 2.6×106 mol/L−1 s−1.43 In cells and tissues, ethidium can also be formed from DHE, but this is not a specific O2•− product and seems to reflect the redox status of the cell (Figure 4B through 4E).45 2-Hydroxyethidium can be readily separated from its parent DHE and ethidium by performing high-performance liquid chromatography (HPLC), and the 2-hydroxyethidium peak can then be used to estimate the intracellular production of O2•−.45
Figure 4.
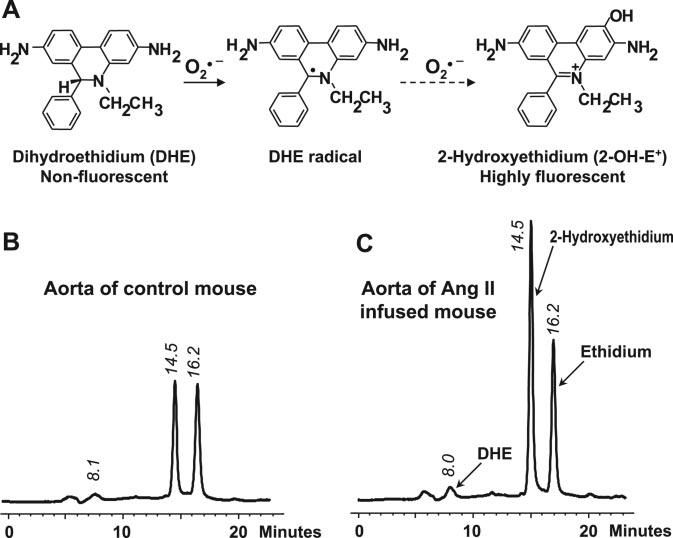
Detection of O2•− by DHE and HPLC. A, The reaction of O2•− with DHE produces 2-hydroxyethidium (2-HE+). B, HPLC tracing of untreated control mouse aortic tissue incubated with 50 μmol/L of DHE for 30 minutes. C, HPLC tracing of angiotensin II–infused mouse (0.7 mg/kg per day for 14 days) aortic tissue incubated with 50 μmol/L of DHE for 30 minutes. Angiotensin II significantly increases production of the O2•−-specific product 2-HE+.
The Assay
Three 2-mm mouse aortic rings or similar segments of tissue are incubated for 30 minutes at 37°C in 1 mL of Krebs/HEPES buffer containing 50 μmol/L of DHE at 37°C. Sources of intracellular O2•− can be analyzed by preincubation of the tissue with the xanthine oxidase inhibitor oxypurinol, the NADPH oxidase inhibitor apocynin, or the endothelial NO synthase inhibitor NG-nitro-l-arginine methyl ester. The tissue is then homogenized in 300 μL of methanol, and 50 μL of homogenate are used for protein determination. The remainder of the sample is passed through a 0.22-μm syringe filter and then used for HPLC analysis. DHE, 2-hydroxyethidium, and ethidium are separated using a C-18 reverse-phase column (Nucleosil 250 to 4.5 mm) and a mobile phase containing 0.1% trifluoroacetic acid and an acetonitrile gradient (from 37% to 47% over 23 minutes) at a flow rate of 0.5 mL/min (Figure 4). Ethidium and 2-hydroxyethidium are detected with a fluorescence detector using an emission wavelength of 580 nm and an excitation of 480 nm. UV absorption at 355 nM is used for detection of DHE. The 2-hydroxyethidium peak reflects the amount of O2•− formed in the tissue during the incubation and is expressed per milligram of protein. Superoxide appears to be the major source of the basal level of 2-hydroxyethidium product formed in untreated control-type tissue (Figure 4). There is often, however, a small background signal from the DHE stock.45 We use a Beckman Gold HPLC with a Jasco fluorescence detector, but other similar devices can be used.
It is important to mention a potential advantage of using DHE fluorescence imaging for detection of localization of O2•− generation in a tissue preparation such as a vascular segment. This provides a spatial distribution of O2•− production and can provide a qualitative evidence of increased O2•−.46
Strengths
Unlike ESR, this assay can be performed with equipment commonly available in research departments. Aside from familiarity with HPLC, specific training is not required. The product 2-hydroxyethidium specifically reflects the reaction of O2•− with DHE and is easily quantified using this approach. When vessels are used, the values of 2-hydroxyethidium obtained are well within the linear range of the assay and above the detection limit. This assay provides results that have been correlated with other methods, such as the cytochrome c assay; however, it provides advantages over the cytochrome c assay, because it can be used to detect intracellular O2•−. Moreover, samples can be stored after incubation and the HPLC assays performed at later time. We have found that samples stored at −20°C are stable for several months. This can also allow frozen samples to be sent to another laboratory for analysis if needed.
Weaknesses
DHE is a light-sensitive dye. For this reason, all of the procedures should be performed in dim light. DHE can react with oxygen in solution. We, therefore, recommend that DHE be prepared as a stock solution in argon-purged buffers using dark tubes.
The Verdict
The detection of 2-hydroxyethidium formation from DHE is a simple and accurate method for estimating intracellular O2•−. Given that 2-hydroxyethidium is not formed by other common oxidants, this assay is as close to being a “gold standard” for detecting O2•− in intact tissues or cells as anything currently available.
Detection of Intracellular H2O2 With DCF-DA
The Theory
One of the most commonly used probes for the detection of intracellular ROS formation is 2′7′-dichlorofluorescein diacetate (DCFH-DA).47-49 DCFH-DA is cell permeable, and, after uptake, it is cleaved by intracellular esterases to 2′,7′-dichlorofluorescin (DCFH), trapped within the cells, and oxidized to the fluorescent molecule 2′,7′-dichlorofluorescein (DCF) by a variety of ROS (Figure 5A).50 DCFH-DA is considered a general indicator of ROS, reacting with H2O2, ONOO−, lipid hydroperoxides, and, to a lesser extent, O2•−. Because H2O2 is a secondary product of O2•−, DCF fluorescence has been used to implicate O2•− production.
Figure 5.
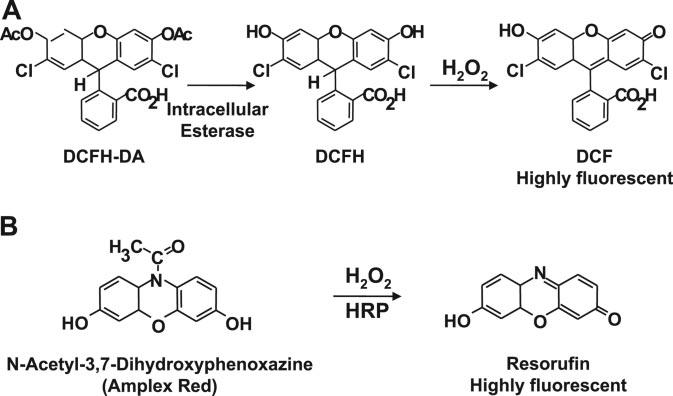
Detection of hydrogen peroxide with DCFH-DA and N-acetyl-3,7-dihydroxyphenoxazine (Amplex Red). A, DCFH-DA is cleaved by intracellular esterases to DCFH and oxidized by ROS to the highly fluorescent molecule DCF. B, Nonfluorescent Amplex Red is oxidized by the horseradish peroxidase and H2O2 to highly fluorescent resorufin.
The Assay
For in situ localization of H2O2, cultured cells or frozen sections of arteries are incubated with DCFH-DA (5 μmol/L; Molecular Probes)51 for 30 minutes at 37°C.52 H2O2 detection is confirmed by simultaneously treating replicate sections or dishes with polyethylene glycol-catalase (350 U/mL). Sections or dishes incubated with vehicle can serve as negative controls. All of the images are acquired at identical settings. For cultured cells, DCFH-DA fluorescence can also be measured using fluorescence-activated cell sorter analysis.53
Strengths
As discussed above, once cleaved, DCFH is cell impermeable and, therefore, has been used to detect intracellular ROS. Another strength is the ability to localize cellular production of ROS within the tissue, and the sensitivity to detect ROS within a single cell. Analysis of intracellular H2O2 with DCFH-DA by flow cytometry or imaging has also been used to measure intracellular ROS. Recently, Molecular Probes has developed 5,6-chloromethyl-2,7-dichlorodihydrofluorescein diacetate,54 which is reported to be retained in living cells better than DCFH-DA.
Weaknesses
Despite its advantages, the DCFH-DA technique is often criticized. Photoreduction of DCF results in artificial production of a semiquinone radical that in turn can reduce oxygen to O2•−.55 In addition, the oxidation of DCFH to the DCF can be self-catalyzed by peroxidases.56 Third, redox reactions with DCF and DCFH can lead to formation of the DCF-free radical anion57 which not only generates O2•−, but also reacts with antioxidants such as ascorbate and thiols.58 Dismutation of this O2•− to H2O2 can result in self-amplification of DCF fluorescence. Finally, oxidation of DCFH by H2O2 is a very indirect reaction and requires peroxidase activity, transition metals, and heme enzymes.59 Therefore, conditions that alter cellular peroxidase levels could affect DCF fluorescence independent of ROS levels.
The Verdict
Although DCF-DA and its related compounds have been used extensively, there is substantial evidence to suggest that these molecules can generate ROS and, thus, artifacts. Great care should be taken in interpreting results obtained using this probe, and the use of alternate probes is encouraged.
Detection of Extracellular H2O2 With Amplex Red
The Theory
The Amplex Red assay, developed by Molecular Probes, involves measurement of H2O2 by the horseradish peroxidase–catalyzed oxidation of the colorless and nonfluorescent molecule N-acetyl-3, 7-dihydroxyphenoxazine (Amplex Red) to resorufin, which, when excited at 530 nm, strongly emits light at 590 nm (Figure 5B).60
The Assay
Three aortic segments (2-mm rings) are placed in the well of a 96-well plate and incubated with Amplex Red (10 μmol/L) and horseradish peroxidase (0.2 U/mL) for 60 minutes at 37°C in Krebs Ringer's phosphate glucose buffer (145 mmol/L of NaCl, 5.7 mmol/L of sodium phosphate, 4.86 mmol/L of KCl, 0.54 mmol/L of CaCl2, 1.22 mmol/L of MgSO4, and 5.5 mmol/L of glucose) protected from light. The tissue is removed from the buffer, and the buffer's fluorescence is detected at 590 nm using an excitation 530 nm. Background fluorescence, determined using a reaction without tissue, is subtracted from each value. H2O2 release, calculated using H2O2 standards or standard solutions of resorufin, is expressed as picomoles per milligram of dry tissue.61 In some experiments, SOD (40 U/mL) or catalase (200 U/mL) can be added to the tissue samples.
Strengths
The Amplex Red assay is highly specific and sensitive, with a limit of detection of ≈5 pmol of H2O2. We have found that the signal is consistently abolished by exogenous catalase. The stoichiometry of Amplex Red and H2O2 is 1:1, and, therefore, the assay results are linear over the range of values encountered in tissues and cells. Resorufin is a very stable product that allows detection of H2O2 both in oxidative and reductive conditions. The assay is quite simple and can be performed using standard, readily available equipment. Because of its commercial availability, it does not require special reagents.
Weaknesses
The Amplex Red dye is somewhat unstable. At high concentrations (50 μmol/L) it can be auto-oxidized and produce O2•− and H2O2. Low concentrations of Amplex Red (10 μmol/L) minimize this problem. Amplex Red detects H2O2 released from tissues and does not provide a measure of intracellular H2O2. H2O2 is diffusible, however, and reaches equilibrium with the tissue's surrounding buffer, so that values measured in the buffer should provide an index of what was originally produced by the tissue.
The Verdict
The Amplex Red assay is useful for the measurement of H2O2 released by cells and tissues and can be applied to cell-free systems.
Perspectives
The precise measurement of ROS in vascular cells and tissues represents a challenge because of their low levels and transient lifetimes. The choice of an assay for a particular application will depend on the tissue to be studied, the ROS of interest, and the amount generated. Given the limitations of each assay, and the availability of alternative methodology, we recommend the concomitant use of ≥2 ROS assays to ensure accuracy and specificity of measurements. As outlined in this review, many techniques have been developed, refined, and applied successfully. Continued efforts should be made to refine these techniques and increase their specificity, permitting us to expand our understanding of the role of ROS in cardiovascular physiology and pathophysiology.
Footnotes
Sources of Funding
This work was supported by National Institutes of Health grants HL39006, HL38206, HL58863, and HL59248; National Institutes of Health program project grants HL58000 and HL075209; Department of Veterans Affairs Merit Grant; and American Heart Association grant SDG 0430201N.
Disclosures
None.
References
- 1.Harrison DG, Dikalov S. Oxidative events in cell and vascular biology. In: Re R, DiPette D, Schiffrin E, Sowers J, editors. Molecular Mechanisms in Hypertension. 1st ed. Taylor & Francis Medical Books; Abingdon, United Kingdom: 2006. pp. 297–320. [Google Scholar]
- 2.Liochev SI, Fridovich I. Lucigenin (bis-N-methylacridinium) as a mediator of superoxide anion production. Arch Biochem Biophys. 1997;337:115–120. doi: 10.1006/abbi.1997.9766. [DOI] [PubMed] [Google Scholar]
- 3.Rost M, Karge E, Klinger W. What do we measure with luminol-, lucigenin- and penicillin-amplified chemiluminescence? 1. Investigations with hydrogen peroxide and sodium hypochlorite. J Biolumin Chemilumin. 1998;13:355–363. doi: 10.1002/(SICI)1099-1271(199811/12)13:6<355::AID-BIO502>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Tarpey MM, White CR, Suarez E, Richardson G, Radi R, Freeman BA. Chemiluminescent detection of oxidants in vascular tissue. Lucigenin but not coelenterazine enhances superoxide formation. Circ Res. 1999;84:1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- 5.Votyakova TV, Reynolds IJ. Detection of hydrogen peroxide with Amplex Red: interference by NADH and reduced glutathione auto-oxidation. Arch Biochem Biophys. 2004;431:138–144. doi: 10.1016/j.abb.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Judkins CP, Sobey CG, Dang TT, Miller AA, Dusting GJ, Drummond GR. NADPH-induced contractions of mouse aorta do not involve NADPH oxidase: a role for P2X receptors. J Pharmacol Exp Ther. 2006;317:644–650. doi: 10.1124/jpet.105.096610. [DOI] [PubMed] [Google Scholar]
- 7.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 9.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 10.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 11.Azzi A, Montecucco C, Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun. 1975;65:597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- 12.Green TR, Pratt KL. A reassessment of the product specificity of the NADPH:O2 oxidoreductase of human neutrophils. Biochem Biophys Res Commun. 1987;142:213–220. doi: 10.1016/0006-291x(87)90473-6. [DOI] [PubMed] [Google Scholar]
- 13.Nasrallah VN, Jr, Shirley PS, Myrvik Q, Waite M. The use of acetylated cytochrome c in detecting superoxide anion production in rabbit alveolar macrophages. J Immunol. 1983;131:2104–2106. [PubMed] [Google Scholar]
- 14.Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- 15.Faulkner K, Fridovich I. Luminol and lucigenin as detectors for O2−. Free Radic Biol Med. 1993;15:447–451. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- 16.Daiber A, Oelze M, August M, Wendt M, Sydow K, Wieboldt H, Kleschyov AL, Munzel T. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radic Res. 2004;38:259–269. doi: 10.1080/10715760410001659773. [DOI] [PubMed] [Google Scholar]
- 17.Nakano M, Sugioka K, Ushijima Y, Goto T. Chemiluminescence probe with Cypridina luciferin analog, 2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one, for estimating the ability of human granulocytes to generate O2. Anal Biochem. 1986;159:363–369. doi: 10.1016/0003-2697(86)90354-4. [DOI] [PubMed] [Google Scholar]
- 18.Warnholtz A, Nickenig G, Schulz E, Macharzina R, Brasen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Bohm M, Meinertz T, Munzel T. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 19.Kimura C, Oike M, Ito Y. Hypoxia-induced alterations in Ca(2+) mobilization in brain microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H2310–H2318. doi: 10.1152/ajpheart.2000.279.5.H2310. [DOI] [PubMed] [Google Scholar]
- 20.Kimura C, Cheng W, Hisadome K, Wang YP, Koyama T, Karashima Y, Oike M, Ito Y. Superoxide anion impairs contractility in cultured aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002;283:H382–H390. doi: 10.1152/ajpheart.00574.2001. [DOI] [PubMed] [Google Scholar]
- 21.Kuwahara K, Oizumi N, Fujisawa S, Tanito M, Ohira A. Carteolol hydrochloride protects human corneal epithelial cells from UVB-induced damage in vitro. Cornea. 2005;24:213–220. doi: 10.1097/01.ico.0000141232.41343.9d. [DOI] [PubMed] [Google Scholar]
- 22.Oosthuizen MM, Greyling D. Hydroxyl radical generation: the effect of bicarbonate, dioxygen and buffer concentration on pH-dependent chemiluminescence. Redox Rep. 2001;6:105–116. doi: 10.1179/135100001101536111. [DOI] [PubMed] [Google Scholar]
- 23.Kambayashi Y, Ogino K. Reestimation of Cypridina luciferin analogs (MCLA) as a chemiluminescence probe to detect active oxygen species–cautionary note for use of MCLA. J Toxicol Sci. 2003;28:139–148. doi: 10.2131/jts.28.139. [DOI] [PubMed] [Google Scholar]
- 24.Bruker_Biospin What is EPR? 2006 Available at: http://www.brukerbiospin.com/brukerepr/whatisepr continuouswave.html. Accessed January 9, 2007.
- 25.Weil JA, Bolton J, Wertz J. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications. John Wiley and Sons; New York, NY: 1994. [Google Scholar]
- 26.Janzen EG. Spin trapping. Methods Enzymol. 1984;105:188–198. doi: 10.1016/s0076-6879(84)05025-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Joseph J, Vasquez-Vivar J, Karoui H, Nsanzumuhire C, Martasek P, Tordo P, Kalyanaraman B. Detection of superoxide anion using an isotopically labeled nitrone spin trap: potential biological applications. FEBS Lett. 2000;473:58–62. doi: 10.1016/s0014-5793(00)01498-8. [DOI] [PubMed] [Google Scholar]
- 28.Dikalova AE, Kadiiska MB, Mason RP. An in vivo ESR spin-trapping study: free radical generation in rats from formate intoxication–role of the Fenton reaction. Proc Natl Acad Sci U S A. 2001;98:13549–13553. doi: 10.1073/pnas.251091098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dikalov S, Jiang J, Mason RP. Characterization of the high-resolution ESR spectra of superoxide radical adducts of 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide (DEPMPO) and 5,5-dimethyl-1-pyrroline N-oxide (DMPO). Analysis of conformational exchange. Free Radic Res. 2005;39:825–836. doi: 10.1080/10715760500155688. [DOI] [PubMed] [Google Scholar]
- 30.Alegria AE, Samuni A, Mitchell JB, Riesz P, Russo A. Free radicals induced by adriamycin-sensitive and adriamycin-resistant cells: a spin-trapping study. Biochemistry. 1989;28:8653–8658. doi: 10.1021/bi00447a056. [DOI] [PubMed] [Google Scholar]
- 31.Zwicker K, Dikalov S, Matuschka S, Mainka L, Hofmann M, Khramtsov V, Zimmer G. Oxygen radical generation and enzymatic properties of mitochondria in hypoxia/reoxygenation. Arzneimittelforschung. 1998;48:629–636. [PubMed] [Google Scholar]
- 32.Koppenol WH, van Buuren KJ, Butler J, Braams R. The kinetics of the reduction of cytochrome c by the superoxide anion radical. Biochim Biophys Acta. 1976;449:157–168. doi: 10.1016/0005-2728(76)90130-4. [DOI] [PubMed] [Google Scholar]
- 33.Bardelang D, Rockenbauer A, Karoui H, Finet JP, Biskupska I, Banaszak K, Tordo P. Inclusion complexes of EMPO derivatives with 2,6-di-Omethyl-beta-cyclodextrin: synthesis, NMR and EPR investigations for enhanced superoxide detection. Org Biomol Chem. 2006;4:2874–2882. doi: 10.1039/b606062e. [DOI] [PubMed] [Google Scholar]
- 34.Dikalov S, Fink B, Skatchkov M, Bassenge E. Comparison of glyceryl trinitrate-induced with pentaerythrityl tetranitrate-induced in vivo formation of superoxide radicals: effect of vitamin C. Free Radic Biol Med. 1999;27:170–176. doi: 10.1016/s0891-5849(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 35.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 36.Dikalov S, Skatchkov M, Fink B, Bassenge E. Quantification of superoxide radicals and peroxynitrite in vascular cells using oxidation of sterically hindered hydroxylamines and electron spin resonance. Nitric Oxide. 1997;1:423–431. doi: 10.1006/niox.1997.0139. [DOI] [PubMed] [Google Scholar]
- 37.Seimenis I, Foster MA, Lurie DJ, Hutchison JM, Whiting PH, Payne S. The excretion mechanism of the spin label proxyl carboxylic acid (PCA) from the rat monitored by X-band ESR and PEDRI. Magn Reson Med. 1997;37:552–558. doi: 10.1002/mrm.1910370413. [DOI] [PubMed] [Google Scholar]
- 38.Vianello F, Momo F, Scarpa M, Rigo A. Kinetics of nitroxide spin label removal in biological systems: an in vitro and in vivo ESR study. Magn Reson Imaging. 1995;13:219–226. doi: 10.1016/0730-725x(94)00121-i. [DOI] [PubMed] [Google Scholar]
- 39.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 40.Beckman JS, Minor RL, Jr, White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
- 41.Hanna IR, Hilenski LL, Dikalova A, Taniyama Y, Dikalov S, Lyle A, Quinn MT, Lassegue B, Griendling KK. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic Biol Med. 2004;37:1542–1549. doi: 10.1016/j.freeradbiomed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354. doi: 10.1016/j.bcp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 44.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 46.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 48.Kooy NW, Royall JA, Ischiropoulos H. Oxidation of 2′,7′-dichlorofluorescin by peroxynitrite. Free Radic Res. 1997;27:245–254. doi: 10.3109/10715769709065763. [DOI] [PubMed] [Google Scholar]
- 49.Zulueta JJ, Sawhney R, Yu FS, Cote CC, Hassoun PM. Intracellular generation of reactive oxygen species in endothelial cells exposed to anoxia-reoxygenation. Am J Physiol. 1997;272:L897–L902. doi: 10.1152/ajplung.1997.272.5.L897. [DOI] [PubMed] [Google Scholar]
- 50.Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- 51.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109:520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 53.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 54.Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN, Britigan BE. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. J Med Virol. 2005;76:489–497. doi: 10.1002/jmv.20388. [DOI] [PubMed] [Google Scholar]
- 55.Marchesi E, Rota C, Fann YC, Chignell CF, Mason RP. Photoreduction of the fluorescent dye 2′-7′-dichlorofluorescein: a spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic Biol Med. 1999;26:148–161. doi: 10.1016/s0891-5849(98)00174-9. [DOI] [PubMed] [Google Scholar]
- 56.Rota C, Chignell CF, Mason RP. Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radic Biol Med. 1999;27:873–881. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 57.Wrona M, Wardman P. Properties of the radical intermediate obtained on oxidation of 2′,7′-dichlorodihydrofluorescein, a probe for oxidative stress. Free Radic Biol Med. 2006;41:657–667. doi: 10.1016/j.freeradbiomed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Rota C, Fann YC, Mason RP. Phenoxyl free radical formation during the oxidation of the fluorescent dye 2′,7′-dichlorofluorescein by horseradish peroxidase. Possible consequences for oxidative stress measurements. J Biol Chem. 1999;274:28161–28168. doi: 10.1074/jbc.274.40.28161. [DOI] [PubMed] [Google Scholar]
- 59.Kim YM, Lim JM, Kim BC, Han S. Cu, Zn-superoxide dismutase is an intracellular catalyst for the H(2)O(2)-dependent oxidation of dichlorodihydrofluorescein. Mol Cells. 2006;21:161–165. [PubMed] [Google Scholar]
- 60.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 61.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;288:H37–H42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]