Abstract
Paracellular junctions could play an important role in corneal endothelial fluid transport. In this study we explored the effects of different reagents on the tight junctional barrier by assessing the translayer specific electrical resistance (TER) across rabbit corneal endothelial preparations and cultured rabbit corneal endothelial cells (CRCEC) monolayers, the paracellular permeability (Papp) for fluorescein isothiocyanate (FITC) dextrans across CRCEC, and fluid transport across deepithelialized rabbit corneal endothelial preparations. Palmitoyl carnitine (PC), poly-L-lysine (PLL), adenosine triphosphate (ATP), and dibutyryl adenosine 3’:5’–cyclic monophosphate (dB-cAMP), were used to modulate corneal endothelial fluid transport and tight junctions (TJs). After seeding, the TER across CRCEC reached maximal values (29.2 ± 1.0 Ω·cm2) only after the 10th day. PC (0.1 mM) caused decreases both in TER (by 40%) and fluid transport (swelling rate: 18.5 ± 0.3 μm/h), and an increase in Papp. PLL resulted in increased TER rose and Papp but decreased fluid transport (swelling rate: 10 ± 0.3 μm/h). dB-cAMP (0.1 mM) and ATP (0.1 mM) decreased TER by 16% and 6%, increased Papp slightly, and stimulated fluid transport; the rates of de-swelling (in μm/h) were −5.4 ± 0.3 and −12.1 ± 0.4, respectively. PC might cause the junctions to open up unspecifically and thus increase passive leak. PLL is a known junctional charge modifier that may be adding steric hindrance to the tight junctions. The results with dB-cAMP and ATP are consistent with fluid transport via the paracellular route.
Keywords: paracellular pathway, paracellular permeability, translayer electrical resistance, electro-osmotic coupling, fluid transport
1. Introduction
The corneal endothelium lies on Descemet’s membrane and covers the posterior surface of the cornea. It forms a continuous monolayer of hexagonal-type cells linked by incomplete belts of tight junctions (TJs) (Hirsch et al., 1976). The junctions form a morphological and functional boundary between the apical and basolateral cell surface domains, and regulate transport along the paracellular route (Anderson et al., 1993; Anderson and Van Itallie, 1999; Balda and Matter, 2000). The transparency of the mammalian cornea is governed by the permeability and active transport characteristics of endothelial cells. Despite the incompleteness of its TJ belt, the endothelial layer functions as a barrier. On one hand, the layer allows access of nutrients to the stroma and epithelium presumably by diffusion across the junctions. On the other hand, it actively transports small solutes and fluid from the stromal side to the aqueous chamber to maintain corneal deturgescence and transparency (Maurice, 1972). Previous studies have demonstrated that the paracellular pathway between the endothelial cells presents the lowest electrical resistance to currents across this monolayer (Fischbarg, 1973) (Lim and Fischbarg, 1981). Compared to other transporting epithelia, this layer is quite leaky. It is permeable to molecules as large as horseradish peroxidase (Kaye et al., 1973), and it allows the passage of fluid driven by hydrostatic pressure differences between the anterior chamber and the stroma (Fischbarg et al., 1977).
The mechanism of fluid transport is still controverted(Fischbarg et al., 2006). We have suggested that electro-osmosis through the intercellular junctions is the primary process in a sequence of events that results in fluid transport across rabbit corneal endothelial preparation (Sanchez et al., 2002). In other words, the corneal endothelium removes water from the stroma through the paracellular route via electro-osmotic coupling taking place in tight junctions (TJs). The major determinant of paracellular permeability is the intercellular tight junction (Fanning et al., 1999), therefore, alterations in the properties of the tight junction by various factors will affect the ability of the corneal endothelium to transport fluid.
In this study, we altered junctional properties by employing a charge modifier (poly-L-lysine, (PLL) (McEwan et al., 1993)), had the junctional width manipulated (e.g., with palmitoyl carnitine (PC) (Knipp et al., 1997)), and investigated the effects of kinase activators and inhibitors known to have effects on either electrical potential difference or on corneal hydration (adenosine triphosphate (ATP), dibutyryl adenosine 3’:5’–cyclic monophosphate (dB-cAMP)). Assays of their effects include tests for changes in transendothelial specific electrical resistance (TER), diffusional permeability, and the transendothelial fluid transport using either rabbit corneal preparations or cultured cells grown on permeable supports. Our results are consistent with a paracellular route for fluid transport
2. Materials and Methods
2.1 Cultured rabbit corneal endothelial cells (CRCEC)
Rabbits were treated in accordance with the ARVO statement on the Use of Animals in Ophthalmic and Vision Research. In a typical session to harvest corneal endothelial cells, the cell layer and the underlying Descemet’s membrane were separated from six albino rabbit corneal stromas under sterile conditions using a jeweler’s forceps and viewing the procedure with a dissection microscope (Neufeld et al., 1986). The dissected materials were then treated with Dispase II (1 unit/ml) in Ca++, Mg++-free DPBS using a 37 C water shaker for 60 min. After incubation, cells were dissociated from clumps by repeated cycles of vigorous aspiration-ejection with a Pasteur pipette. They were next centrifuged for 5 min (@ 200 g). The cells thus pelleted were resuspended in DMEM containing 10% FBS, bFGF (2 ng/ml) and gentamycin (50 μg/ml), and the suspension placed in 25 cm2 culture flasks in a 5% CO2 incubator at 37 C. The medium was changed after 48 hours, when membrane fragments were discarded.
After the primary cultures of CRCEC thus obtained reached confluence, cells were sub-cultured (Kuang et al., 2004c) into 24.5 mm diameter, 0.4 μm pore size permeable membrane inserts (Transwell® Costar®; cells from one flask into six inserts). Each insert was placed into the well of a 6-well culture plate (Corning Transwell) containing 2 ml of culture medium. Cells were allowed to attach to the insert for one hour at room temperature, and the plates were then placed in a 5% CO2 incubator at 37 C. The culture medium (DMEM + 10% fetal bovine serum) was changed every 2–3 days. The media for experiments had no serum.
2.2. Measurement of specific transendothelial electrical resistance (TER) in cultured cell layers
A scheme of the inserts is shown in Fig. 1. TER for CRCEC was measured with an epithelial Voltohm-meter (EVOM, World Precision Instruments, Sarasota, FL), from the next day of subculture to 14 days. Measurements were done keeping the inserts inside the incubator, as it was found that readings depended crucially on temperature. Prior measurements of TER and TEPD for cultured bovine corneal endothelial cells we have reported (Narula et al., 1992) were done instead at room temperature. As a plateau for TER was reached only after 9 days, CRCEC inserts were used for the experiments after 14 days of culture. Monolayer resistance was obtained by subtracting the resistance determined for blank filters.
Fig. 1.
Chamber used for determinations of R and Papp in cultured cells. Cells grow on a Costar Transwell permeable support. Electrodes for R measurements are not shown.
2.3. Paracellular permeability (Papp) (FITC-dextran Fluxes)
The permeability of the CREC monolayer was assessed by determining FITC-dextran fluxes across this layer. For these experiments, the solution utilized was a bicarbonate-HEPES medium (BH), containing (in mM): NaCl 113.9, NaHCO3 26.2, KCl 4.7, NaH2PO4 1.0, CaCl2 1.8, MgSO4 0.4, D-glucose 5.6, HEPES 10; pH was 7.4, and osmolarity 290 mOsm.
Experiments were conducted in a 37 C CO2 incubator. Inserts were washed three times with BH and allowed to stabilize in the incubator for 20 minutes. Subsequently, 2 ml of BH with or without reagents were added to the apical compartment, and 2 ml of solutions containing FITC-dextrans of 4, 20 or 40 kDa (1 mg/ml) or sodium-fluorescein (150 μg/ml) in BH were added to the basal compartment. Samples (50 μl) were removed from the apical and basal compartments at scheduled times (10, 20, 30, 40, 50, 60, 120 min), and placed into a cuvette containing 2.95 ml of BH. Measurements (photon counting) were performed using a PTI fluorometer (Kuang et al., 2004a) set for excitation wavelength= 492 nm and emission wavelength= 520 nm. The paracellular permeability coefficients (Papp) were calculated as described by Stutts (Stutts et al., 1981), namely:
volume= 2 cm3, A= 4.5 cm2.
2.5. Rabbit corneal endothelial preparation (RCEP) mounting
We used standard de-epithelialized rabbit corneal preparations (Kuang et al., 2001). Preparations were mounted in a chamber composed of two hemi-chambers as previously described (Lim and Fischbarg, 1981). Temperature was maintained at 36.5 C, and a 95% air-5% CO2 gas mixture was bubbled into the open funnels connected to the hemichambers to maintain oxygenation and physiological pH, since the solution used (BSG, see below) had HCO3- as the only buffer system. A hydrostatic pressure difference of 3 cm H2O applied to the aqueous side helped maintain the corneal curvature against a supporting hemispherical stainless steel net. All solution changes were made in the endothelial side.
2.6. Electrical determinations in RCEP
Methods were as described previously by us (Fischbarg and Lim, 1974; Lim and Fischbarg, 1981). The TEPD was determined with a Keithley model 616 electrometer connected to calomel electrodes and salt bridges; the TER was determined using an AC current (Fischbarg and Lim, 1974; Lim and Fischbarg, 1981). In this case, a 10 μA root mean square (r.m.s.), 120 Hz alternating current was applied via Ag/AgCl "current sending" electrodes connected to salt bridges at the far end of each hemi-chamber (Lim and Fischbarg, 1981). The resulting AC potential difference (V) was detected by Ag/AgCl electrodes rigidly mounted so that the exposed 1 mm wire tips rested at about 1 mm from the corneal surfaces. At 120 Hz, the reactance is negligible and impedance equals DC resistance. The impedance between electrodes (equal to the specific DC resistance, Rt) was determined with a lock-in amplifier (Princeton Applied Research Corp., model 129A). Area of the cornea was taken as 1.43 cm2.
To correct for the resistance of the solution between the electrodes, at the end of each experiment, one-half strength "Clorox" (sodium hypochlorite) solution was applied to the "aqueous side" hemi-chamber for 5 minutes to disrupt the endothelium. After washing off the hypochlorite solution with BSG, the specific resistance of stroma plus solution (Rs) was determined. The specific transendothelial resistance, TER, was then calculated as Rt - Rs. TER values obtained with the various compounds were determined 40 - 70 minutes after solution changes. Deviations are S.E.M.
2.6. Solutions for studies on RCPE
For this series, most experiments were performed using a balanced salt plus glucose solution (BSG) composed of (in mM) NaCl 112.9, NaHCO3 39.2, KHCO3 3.8, KH2PO4 1.0, MgSO4 0.78, CaCl2 1.7, D-glucose 6.9. 20 μM of Cytochalasin D (CytD) and dihydro-Cytochalasin B (diCytB) were dissolved in dimethylsulfoxide (DMSO, 0.1%). The two cyclic AMP Na salts, 8-Bromoadenosine 3':5'-cyclic monophosphate (Br-cAMP) and dB-cAMP were dissolved in BSG. CytD, diCytB and cAMP analogs were all obtained from Sigma Chemical Company (St. Louis, MO).
2.7. Measurements of corneal transendothelial water movements by automatic microscopy (Iserovich et al., 2004)
We used de-epithelialized rabbit corneal preparations (Kuang et al., 2001) mounted in a Dikstein-Maurice chamber (Dikstein and Maurice, 1972). A Zeiss universal microscope was modified to operate as a specular microscope with stationary slits for confocal optics, with both white and red diode laser illuminations. The corneal thickness was determined by moving the microscope stage using a micro-step motor under indexer and computer control. Light reflected from the different planes was collected by a photomultiplier and digitized; data went to a computer and were smoothed by low-pass filtering. Software to run the system was written in LABVIEW 5.1. An automatic microscope based on another algorithm has been previously developed and used successfully (Ruberti et al., 2000). Corneal preparations were kept at 36.5 C and superfused with BH solution at a rate of 1.4 ml/h by a syringe pump (Razed Scientific Instruments, Stamford, CT). Reagents were added when corneal thickness had stabilized after mounting (about 60 minutes).
3. Results
3.1. Transepithelial Electrical Resistance of CRCEC
For a given insert, a control TER was determined daily; TER values rose steadily (Fig. 2).However, although the layers seemed confluent by the 7th day under the microscope, the TER would not reach maximum value until the 9th day (28.2 ± 0.5 Ω·cm2). Therefore, all further experiments were conducted after two weeks of culture to ensure confluent CRCEC.
Figure 2.
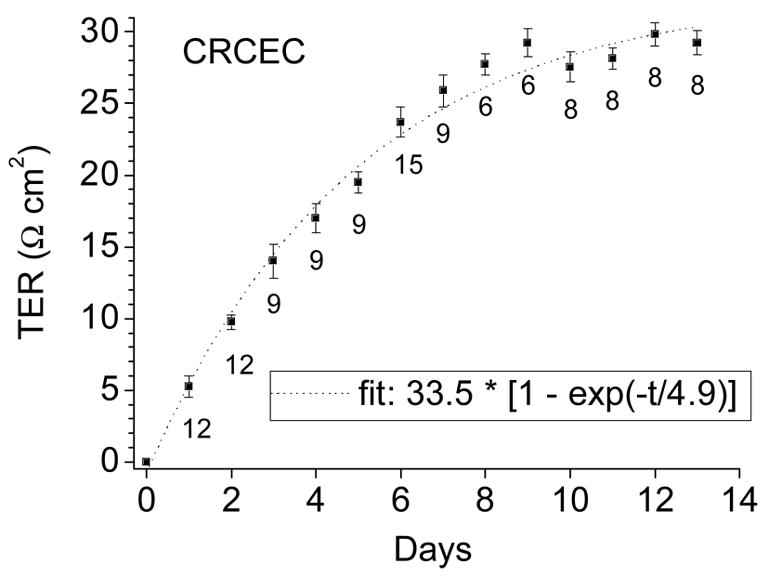
TER of CRCEC was determined at different stages of cell growth using an epithelial voltohmmeter. Resistance of blanks was subtracted. TER increased steadily to reach a plateau by the 10th day. Data represent average ± S.E.
3.2. Junctional modifiers on CRCEC
Several reagents which have been identified to disrupt the integrity of TJs were used in this study, namely: PC, which opens TJs by an unknown Ca++-independent mechanism; EGTA, an extracellular Ca2+ chelator; CytD, which binds to actin and inhibits actin polymerization; diCytB, which also binds to actin but less avidly than CytD.
Effects on TER are shown in Fig. 3A. In control time-course experiments, the TER of untreated CRCEC cells remained stable during 120 minutes. PC (1 mM) reduced TER to 54 ± 4% and 50 ± 2% at 1 and 2 hr. EGTA at 1 and 10 mM reduced TER to 41 ± 4% and 29 ± 4% at 1 hr and 37 ± 5% and 22 ± 3% at 2 hr. CytD and diCytB at 20 μM reduced TER to 52 ± 7% and 78 ± 7% at 1 hr and 50 ± 5% and 69 ± 6% at 2 hr, which seems in proportion to their actin binding affinities. As shown in Fig. 3A, there was a residual value of TER of about 20% after 10 mM EGTA challenge. The same compounds, predictably, increased the permeability of the layers (Fig. 3B).
Figure 3.
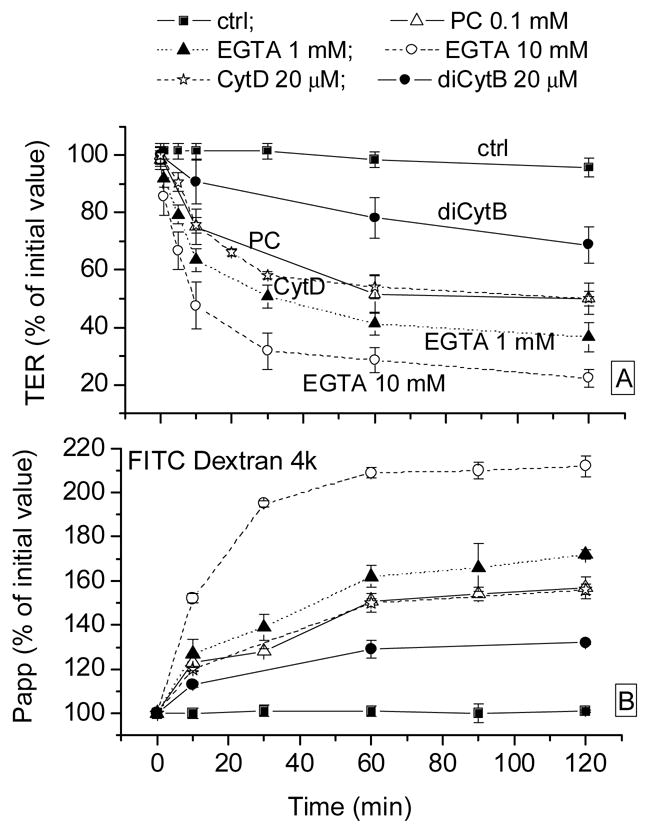
Effects of EGTA and PC on CRCEC TER (panel A) and Papp (FITC Dextran 4 kD, panel B). Papp: paracellular relative permeability to that at zero time (100 ×Pappt/Papp0) (FITC-4 kD dextran fluxes); TER: specific resistance relative to that at time zero (100 ×TERt/TER0). PC: palmitoyl carnitine; CytD: cytochalasin D; diCytB: dihycytochalasin B. Data represent average ± S.E.
3.3. Regulators/modulators on CRCEC
The effects on TER of another group of agents are shown in Fig. 4A. ATP had no significant effect, but dB-cAMP decreased TER to 84 ± 3% and 74 ± 6% at 1 hr and at 2 hr. The polycation PLL at 0.1 mg/ml caused a ~10% increase in TER; ouabain, the inhibitor of Na+-K+-ATPase at 1 mM decreased TER slightly (to 89 ± 5% at 30 minutes); the subsequent decrease in TER probably corresponds to unspecific deterioration.
Figure 4.
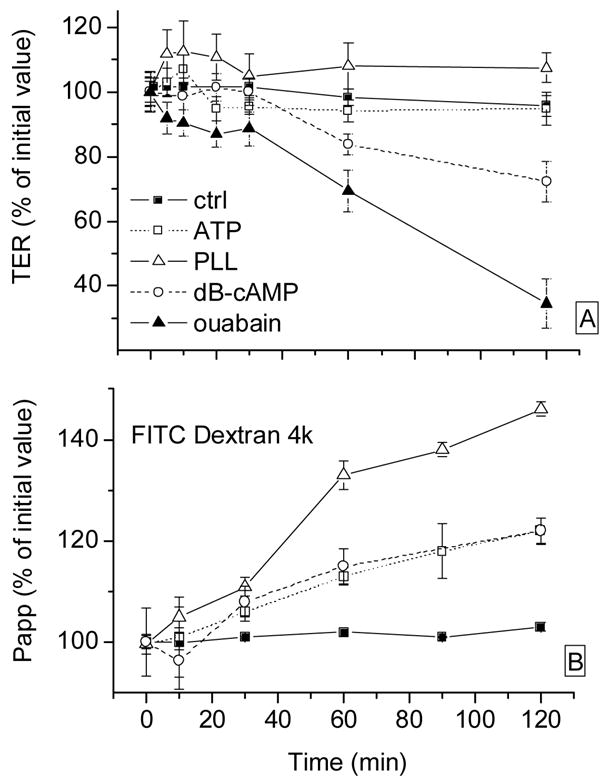
Effects of different reagents on CRCEC TER (panel A) and Papp (FITC Dextran 4 kD, panel B). Relative TER and Papp as in Fig. 3. PLL: poly-L-lysine. Data represent average ± S.E.
It is interesting to determine whether the permeability and resistance changes can be ascribed to a simple system of homogeneous pores that would merely change in diameter in response to the challenges. Towards this end, we have replotted some of the data in Figs. 3 and 4 expressing them as permeability times resistance, always as a function of time. These results appear in Fig. 5. As can be seen there, three patterns are discernible. (1) ATP and dB-cAMP do not deviate much from the control points, as if the increases in Papp and decreases in TER they generate could be mediated by similarly affected pores. (2) PLL results in an increase in Papp that outpaces the decrease in TER, and (3) conversely, for EGTA and PC the decrease in TER predominates over the increase in Papp.
Fig. 5.
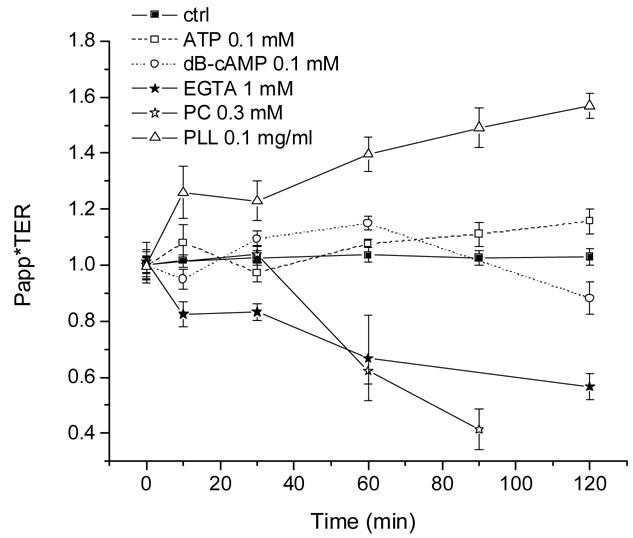
Some of the data from Figs. 3 and 4 recalculated as % fractional permeability times % fractional resistance.
3.4. CRCEC permeability
In prior work (unpublished results), Na2-fluorescein labeled medium was added to either the basolateral or the apical sides of inserts (while the contralateral side carried unlabeled medium). We found that the fluorescein unidirectional flux from the basolateral towards the apical side was some 10% larger than the opposite flux (ratio= 1.104 ± 0.034). Hence, a small net flux of fluorescein takes place in the direction of fluid transport (baso-lateral to apical). Since we are using the same marker here, we determined paracellular permeability only in that same direction.
3.5. Paracellular permeability of CRCEC and molecular weight of paracellular markers
We determined paracellular permeability (Papp) of CRCEC using fluorescein and several FITC dextrans. Papp values (cm/s) were: (a). sodium-fluorescein: 7.0 ± 0.5 x 10−4 ; (b). FITC-dextran 4 kDa: 4.1 ± 0.6 x 10−6; (c). FITC-dextran 20 kDa: 1.7 ± 0.1 x 10−6; (d). FITC-dextrans 40 kDa: 8.9 ± 0.5 x 10−7. These values are plotted in Fig. 6. As can be seen there, Papp was a smooth power function of the molecular weight, which is consistent with all labels traversing the same diffusional pathway. The 40 kDa dextran used can be calculated to have an effective diameter of about 46 Å. Given the elongated shape of dextrans, this value is consistent with priorly determined corneal endothelial junctional width values of 38 Å (from hydraulic conductance measurements, (Fischbarg et al., 1977)) and 43 Å obtained using probe molecules of different diameters (Kuang K. et al, unpublished data).
Figure 6.
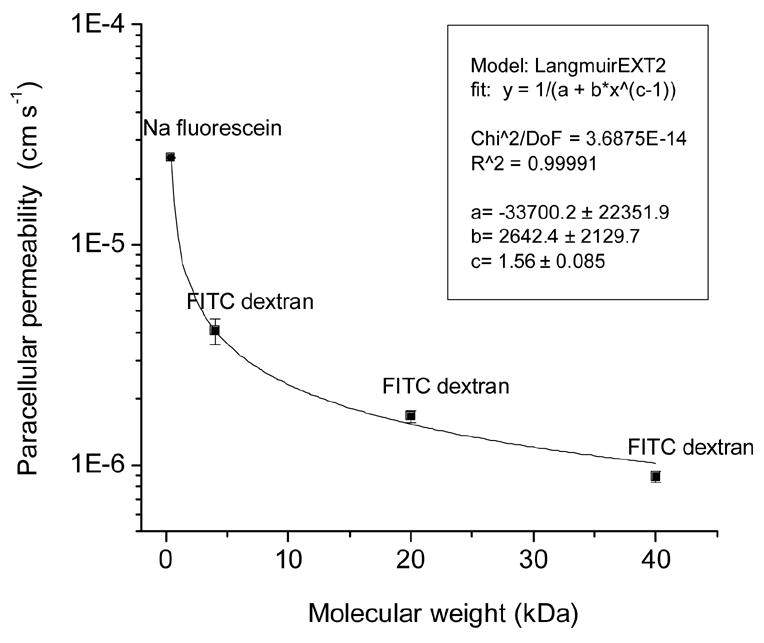
Paracellular permeability across CRCEC grown on inserts. Values from fluxes of fluorescein and several FITC dextrans.
3.6. Rabbit corneal preparations. Time course of endothelial specific resistance
In control experiments, BSG was exchanged with fresh BSG to ascertain the effects of such exchange on rabbit corneal preparations (Fig. 7). No significant effects were seen in resistance. In Fig. 7A, the relative change in resistance was calculated as the average of the three readings after an exchange over the prior reading; it was 1.02 ±0.03. Similarly, in the single exchange of Fig. 7B, the resistance increased by only 11%.
Figure 7.
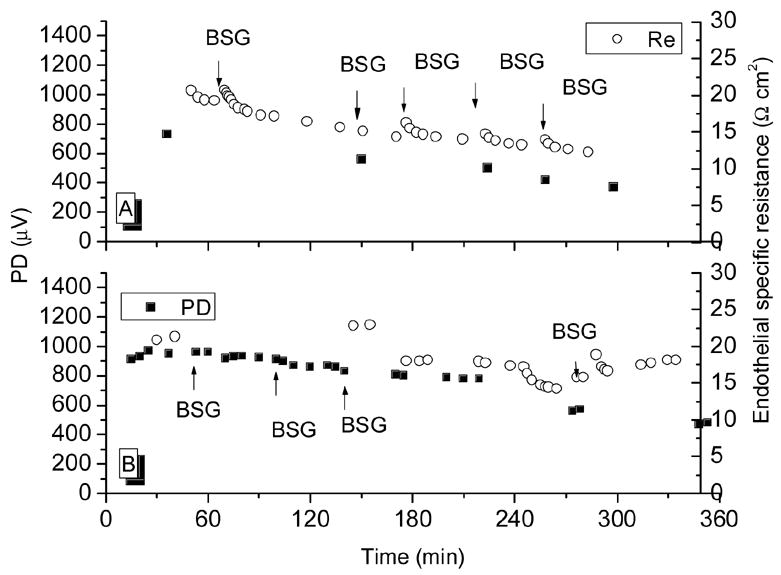
Time course of specific endothelial resistance and potential difference in a control experiment in which BSG was exchanged with fresh BSG to ascertain the effects of a solution exchange.
As can be seen in Fig. 7, TER and TEPD decayed slowly over time by about 5% per hour. Since the agents utilized in this study caused typical reductions of >= 10 Ω cm2, solution exchanges and spontaneous resistance decay played only a minute role in all the results below. In freshly mounted preparations, the TER was typically 21 Ω cm2 (see Fig. 7). Similarly, the addition of the 0.1% DMSO amount required to solubilize the cytochalasins did not affect TER (Fig. 8). In five experiments, the BSG in the endothelial (aqueous) side hemi-chamber was replaced with Ca2+ and Mg2+-free-BSG containing 2 mM EDTA. Fig. 9 shows three of these experiments. After the exchange, TER began to decline; some 20 minutes afterwards, it had gone from control levels of (in Ω cm2) 18.5 ± 0.6 down to 3.6 ± 0.8 (see Fig. 9, top panel). When this solution was replaced with BSG, TER recovered to 12.9 ± 0.8 (75% of original) after 25 minutes. The TEPD declined from 673 ± 89 μV to 112 ± 50 μV in the Ca++ and Mg++-free plus EDTA solution and recovered to 393 ± 20 μV (58% of original) after BSG restoration.
Figure 8.
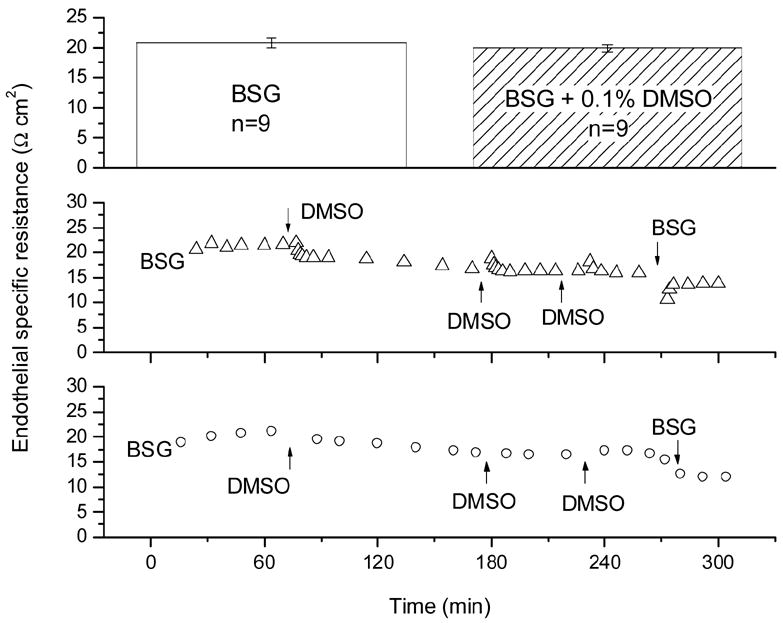
Time course of endothelial resistance control experiments in which BSG was exchanged with fresh BSG containing the 0.1% DMSO required to solubilize cytochalasins. Top: TER in BSG and in BSG + DMSO; Bottom: two typical time course experiments.
Figure 9.
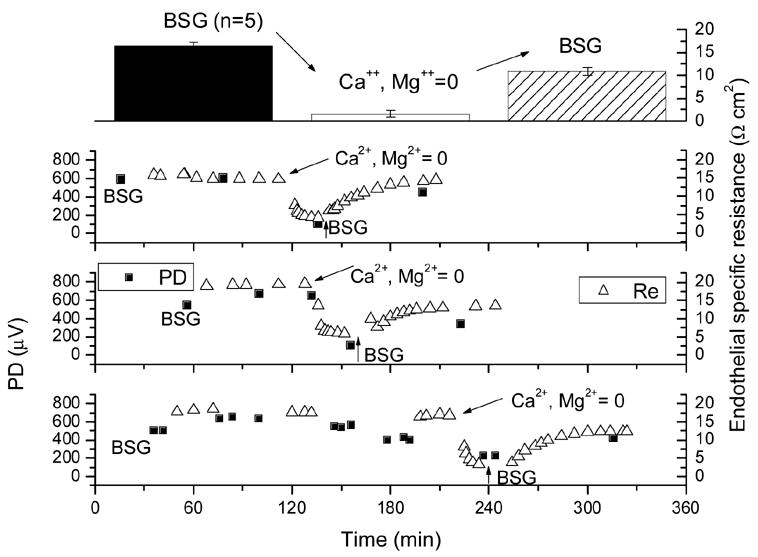
Time course of transendothelial specific resistance and potential difference in experiments in which BSG in the endothelial (aqueous) side hemi-chamber was replaced with a solution devoid of Ca2+ and Mg2+ and containing 2 mM EDTA.
In four experiments, the corneal endothelium was challenged with 20 μM of CytD for 60-90 minutes. TER declined from (in Ω cm2) 18.7 ± 0.7 to 8.8 ± 0.6, and afterwards recovered by less than 1 when the solution was replaced with BSG (Fig. 10). The TEPD declined from 590 ± 50 μV to 465 ± 5 μV with no recovery upon BSG washing (not shown). Another actin binder, diCytB was also tested in the fresh preparation. As mentioned above, this molecule binds actin less avidly than CytD but its exact affinity is not well known. A 20 μM concentration was tested in six experiments using the same protocol as above. diCytB caused only a minor decline of TER, from 20.0 ± 0.5 to 17.5 ± 0.6 Ω cm2 (Fig. 10). The effects of its inhibition were not reversible either.
Figure 10.
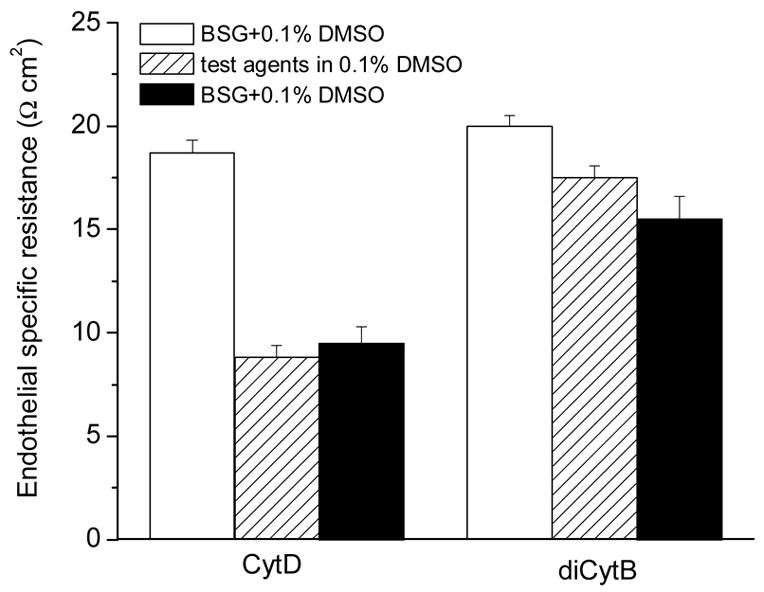
Effects of actin filament inhibitors (20 g/ml in DMSO) analogs on transendothelial specific resistance. Data represent average ± S.E.
3.7. Measurements of corneal transendothelial water movements
Figure 11 allows a comparison of our results on the time course of fluid transport across rabbit corneal endothelium (A) and of the TER across the CRCEC layer (B). Three patterns of effects are apparent. ATP and dB-cAMP (both 0.1 mM) stimulated fluid transport (A), and decreased resistance (B). PLL resulted in decreased fluid transport and increased resistance. PC drastically decreased both fluid transport and resistance.
Figure 11.
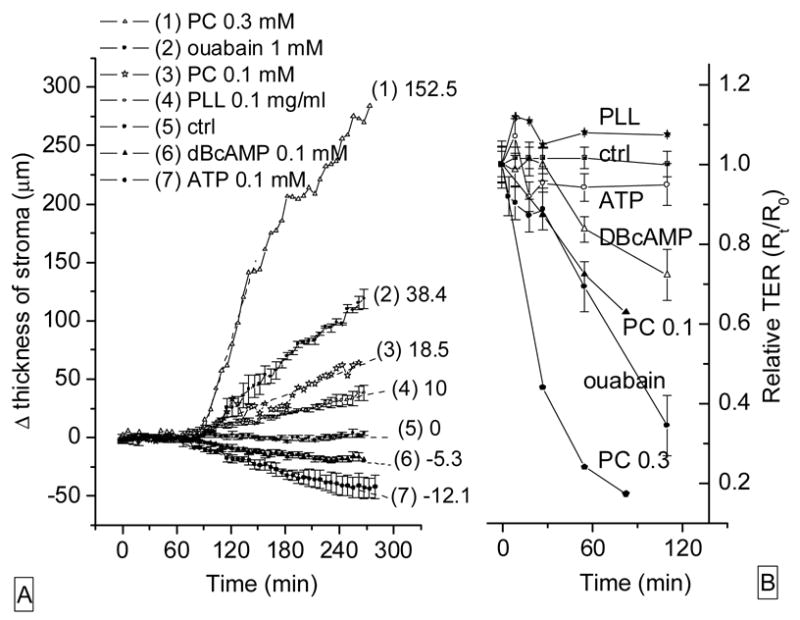
Effects of agents on stromal thickness and relative electrical resistance. (A): rabbit corneal endothelial preparations. Numbers to the right identify the curves and represent rates (μm/h) of swelling of corneal stroma for 60 minutes after drug application. (B): TER across CRCEC layers on permeable supports challenged with the same compounds used in the left (A) panel. Data represent average ± S.E.
4. Discussion
In corneal endothelium (and similarly in other leaky epithelia), paracellular resistance is only ~2% of transcellular resistance (Lim and Fischbarg, 1981). In addition, the junctional resistance can be calculated to be ~0.90 of the paracellular resistance (Zhu et al., 1996). Hence, we take TER as an indicator of junctional function.
In CRCEC layers growing on permeable inserts the TER increases as junctions develop, requiring two weeks to reach a plateau (28.2 ± 0.5 Ω·cm2). Both the time required and the values reached are consistent with a previous report by Geroski and Hadley (Geroski and Hadley, 1992), who found values increased for 14 days to 33-34 Ω cm2. Cultured endothelial cells form a suitable barrier, since their specific resistance is somewhat larger than that seen in rabbit corneal endothelial preparations, typically 21 ohms cm2 (Lim and Fischbarg, 1981). Hence CRCEC (just like cultured bovine corneal endothelial cells) appear a suitable system on which to study paracellular flows, as done here.
Of the compounds studied, EGTA is known to disrupt junctions by its Ca2+ chelating effect (Franchi and Camatini, 1985), and PC is also known to affect junctions by a yet unknown mechanism (Duizer et al., 1998). As expected, both EGTA and PC disrupted the integrity of the TJs (Fig. 3): they caused rapid decreases in TER (Fig. 3A) and increases in Papp (Fig. 3B). 0.1 mM PC resulted in a 50% decrease in TER (Fig. 3A). This is in line with its effect of nearly doubling the equivalent pore radius in other studies of ours (Kuang et al., 2004b). In addition, PC (both at 0.1 mM and 0.3 mM) caused sizable stromal swelling (Fig. 11, left). In both cases, TER fell appreciably (Fig. 11B). The effects on both TER and Papp can be best explained by an unspecific increase in the passive leak from the aqueous humor to the stroma.
Junctions were also modified by more standard means. In rabbit corneal endothelial preparations, changing to (Ca2+ and Mg2+)-free plus 2 mM EDTA solution caused both TER and TEPD to decrease by about 80%. Junctional disruption was partially reversible, as TER and TEPD returned to 75% and 58% of their original values respectively upon reintroduction of those ions (Fig. 9).
Cytoskeletal disruption subsequent to actin inhibition also affected TJs. Cytochalasin D (CytD), which inhibits actin polymerization, and a weaker actin inhibitor, dihydro cytochalasin B (dicytB), lowered TER (Fig. 3A; to 50% and 70%) and increased Paap (Fig. 3B; to 50% and 30%) in CRCEC correspondingly. Similar effects on TER by CytD and dicytB were also found in rabbit corneal preparations (Fig. 10). These results are consistent with work on small intestine in which CytD caused redistribution of labeled actin leading to lowered resistance and increased paracellular permeability (Madara and Pappenheimer, 1987).
Interestingly, ouabain resulted in a decrease in resistance (Fig. 4). The decrease was small (~10%) during the initial 0.5 hr. This might account for prior reports of lack of immediate effect of ouabain on transendothelial resistance in rabbit corneal endothelial preparations (Fischbarg, 1973) (Riley et al., 1998). However, at later times, the resistance fell drastically (Fig. 4). In this connection, an effect of ouabain in inducing endocytosis of plasmalemmal Na/K ATPase has been described (Liu et al., 2004). Furthermore, by that mechanism, ouabain leads to loss of cell-cell- and cell-substrate-attaching molecules (Contreras et al., 2004), which might account for the resistance decrease observed. The polycation PLL increased TER slightly (~10%) in CRCEC (Fig. 4A), which is consistent with it nullifying part of the negative fixed charges in the endothelial TJs (Lim et al., 1983; Rubashkin et al., 2005) and hence preventing migration of positive counterions. Addition of PLL changed the direction of electro-osmotic fluid movement in rabbit corneal endothelium (Sanchez et al., 2002), which is consistent with it changing the sign of junctional charges. However, given the TER increase, why the resulting positive fixed charges seemingly do not result in increased migration of negative counterions is not immediately apparent. Perhaps the PLL molecules could add to steric hindrance for the migration of ions in the restricted space of the TJs. In keeping with this interpretation, PLL inhibited fluid transport moderately in rabbit corneal preparations (Fig. 11A). However, surprisingly, PLL also substantially increased Papp (Fig. 4B). This may mean that the new paracellular pathways resulting from PLL addition cannot be accessed by anions by reasons that are unexplained at present.
With this background of relatively unspecific results, by contrast the results with 0.1 mM ATP and 0.1 mM dB-cAMP appear as the most important and revealing in this study. These two reagents resulted in: (1) moderately decreased TER (Fig. 4A, Fig. 10B); (2) moderately increased Papp (Fig. 4B) in CRCEC; and (3) sizable stimulation of fluid transport across rabbit corneal endothelium (Fig. 11A). Our data is consistent with prior findings of decreased TER and increased inulin permeability by 0.1 mM dBcAMP in CBCEC (Le Varlet et al., 1995), and stimulation of fluid transport by 1 mM dBcAMP (Riley et al., 1998). The effects of ATP described here appear to be a novelty. Relevant prior findings for ATP on corneal endothelium are that it leads to Ca2+ mobilization (Srinivas et al., 1998), enhances apical HCO3- permeability (Zhang et al., 2002), and opposes loss of barrier integrity (Satpathy et al., 2005). In this last study, ATP resulted in increased permeability to horseradish peroxidase across cultured bovine corneal endothelial layers. Similarly, we report here an ATP-induced permeability increase for FITC-dextran 4kD (Fig. 4).
Other results in the paper by Riley et al. cited above (Riley et al., 1998) merit special discussion. Working with rabbit corneal preparations, they reported: a) carboxyfluorescein (CF) permeability decrease in response to db-cAMP, which is opposite to what we describe here; and b) CF permeability decrease after challenge with 30 μM H-8, a potent inhibitor for cyclic nucleotide-dependent protein kinases. These two effects are in contradiction since both an activator as well as an inhibitor of PKA appear to result in the same effect.
Consideration of the mechanism for the dBcAMP and ATP effects is informative. The stimulation of fluid transport (Fig. 11A) was deduced from thickness changes recorded in deepithelialized stroma originally in the steady state. Under those conditions, before the challenges, the stromal thickness was constant, indicating that the rate of fluid pumping was equal to the rate of stromal swelling via the leak. With both compounds the leak increased, since TER decreased (Fig. 4A) and Papp increased (Fig. 4B). From these results alone one would expect the stromal thickness to increase, as the leak would predominate against the pump. Yet the opposite was observed: stromal thickness went down as fluid transport increased. The explanation we favor at present is that both the pump and the leak follow paracellular pathways, and that dBcAMP and ATP increased paracellular electrical conductance allowing increased paracellular fluid transport. This explanation is consistent with evidence we have reported for an electro-osmotic origin for fluid transport (Sanchez et al., 2002), as a decreased TER would allow a more intense local electrical current and hence increased fluid movement. Of course, from the current evidence we cannot rule out a hypothetical increased transcellular fluid transfer that would overcompensate for the increased leak. Some evidence militates against this, as, cAMP analogs did not affect corneal TEPD significantly (not shown). Yet, to ascertain this more definitively would require additional work.
Concerning which structures in the junction account for the changes described in Papp and TER, the results presented in Fig. 5 cannot be explained by a junction endowed with simple homogeneous pores through which both solutes and ions flow. The results with PLL, EGTA and PC indicate that solutes and ions travel by different routes, with PLL increasing permeability while resistance is steady, and EGTA and PC which would require at least two different systems of pores. Junctions with pores of two different sizes have of course been described for kidney proximal tubule (Guo et al., 2003); the corneal endothelium appears to conform to those characteristics as well.
In summary, the role of TJs in regulating both the paracellular leak and fluid transport is only poorly understood. Hence, the present results may contribute to lay a foundation for future analysis. The present conclusions are in line with the model we have proposed for paracellular, electro-osmotically driven fluid transport in this preparation (Diecke et al., 2006; Fischbarg and Diecke, 2005; Fischbarg et al., 2006).
Acknowledgments
Supported by NIH grant EY06178, and by Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JM, Balda MS, Fanning AS. The structure and regulation of tight junctions. Curr Opin Cell Biol. 1993;5:772–778. doi: 10.1016/0955-0674(93)90024-k. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM. Tight junctions: closing in on the seal. Curr Biol. 1999;9:R922–924. doi: 10.1016/s0960-9822(00)80105-0. [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K. Transmembrane proteins of tight junctions. Semin Cell Dev Biol. 2000;11:281–289. doi: 10.1006/scdb.2000.0177. [DOI] [PubMed] [Google Scholar]
- Contreras RG, Flores-Maldonado C, Lazaro A, Shoshani L, Flores-Benitez D, Larre I, Cereijido M. Ouabain binding to Na+,K+-ATPase relaxes cell attachment and sends a specific signal (NACos) to the nucleus. J Membr Biol. 2004;198:147–158. doi: 10.1007/s00232-004-0670-2. [DOI] [PubMed] [Google Scholar]
- Diecke FP, Ma L, Iserovich P, Fischbarg J. Corneal endothelium transports fluid in the absence of net solute transport. 2006. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikstein S, Maurice DM. The metabolic basis of the fluid pump in the cornea. J Physiol (Lond) 1972;221:29–41. doi: 10.1113/jphysiol.1972.sp009736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duizer E, van der Wulp C, Versantvoort CH, Groten JP. Absorption enhancement, structural changes in tight junctions and cytotoxicity caused by palmitoyl carnitine in Caco-2 and IEC-18 cells. J Pharmacol Exp Ther. 1998;287:395–402. [PubMed] [Google Scholar]
- Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Fischbarg J. Active and passive properties of the rabbit corneal endothelium. Exp Eye Res. 1973;15:615–638. doi: 10.1016/0014-4835(73)90071-7. [DOI] [PubMed] [Google Scholar]
- Fischbarg J, Diecke FP. A Mathematical Model of Electrolyte and Fluid Transport Across Corneal Endothelium: The 9th World Multi-Conference on Ssytemics; Cybernetics and Informatics. 2005. pp. 115–121. [DOI] [PubMed] [Google Scholar]
- Fischbarg J, Diecke FP, Iserovich P, Rubashkin A. The role of the tight junction in paracellular fluid transport across corneal endothelium. Electro-osmosis as a driving force. J Membr Biol. 2006;212:117–130. doi: 10.1007/s00232-005-0850-8. [DOI] [PubMed] [Google Scholar]
- Fischbarg J, Lim JJ. Role of cations, anions and carbonic anhydrase in fluid transport across rabbit corneal endothelium. J Physiol (Lond) 1974;241:647–675. doi: 10.1113/jphysiol.1974.sp010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J, Warshavsky CR, Lim JJ. Pathways for hydraulically and osmotically-induced water flows across epithelia. Nature. 1977;266:71–74. doi: 10.1038/266071a0. [DOI] [PubMed] [Google Scholar]
- Franchi E, Camatini M. Evidence that a Ca2+ chelator and a calmodulin blocker interfere with the structure of inter-Sertoli junctions. Tissue Cell. 1985;17:13–25. doi: 10.1016/0040-8166(85)90012-6. [DOI] [PubMed] [Google Scholar]
- Geroski DH, Hadley A. Characterization of corneal endothelium cell cultured on microporous membrane filters. Curr Eye Res. 1992;11:61–72. doi: 10.3109/02713689209069168. [DOI] [PubMed] [Google Scholar]
- Guo P, Weinstein AM, Weinbaum S. A dual-pathway ultrastructural model for the tight junction of rat proximal tubule epithelium. J Physiol Renal Physiol. 2003;285:F241–F257. doi: 10.1152/ajprenal.00331.2002. [DOI] [PubMed] [Google Scholar]
- Hirsch M, Renard G, Faure JP, Pouliquen Y. Formation of intercellular spaces and junctions in regenerating rabbit corneal endothelium. Exp Eye Res. 1976;23:385–397. doi: 10.1016/0014-4835(76)90166-4. [DOI] [PubMed] [Google Scholar]
- Iserovich P, Rosensweig A, Sanchez JM, Fischbarg J. Computerized technology applied to measurements of corneal transendothelial water movements. ARVO Abstract. 2004:424. [Google Scholar]
- Kaye GI, Hoefle FB, Donn A. Studies on the cornea. VIII Reversibility of the effects of in vitro perfusion of the rabbit corneal endothelium with calcium-free medium. Invest Ophthalmol. 1973;12:98–113. [PubMed] [Google Scholar]
- Knipp GT, Ho NF, Barsuhn CL, Borchardt RT. Paracellular diffusion in Caco-2 cell monolayers: effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J Pharm Sci. 1997;86:1105–1110. doi: 10.1021/js9700309. [DOI] [PubMed] [Google Scholar]
- Kuang K, Li Y, Wen Q, Wang Z, Li J, Yang Y, Iserovich P, Reinach PS, Sparrow J, Diecke FP, et al. Corneal endothelial NKCC: molecular identification, location, and contribution to fluid transport. Am J Physiol Cell Physiol. 2001;280:C491–C499. doi: 10.1152/ajpcell.2001.280.3.C491. [DOI] [PubMed] [Google Scholar]
- Kuang K, Li Y, Yiming M, Sanchez JM, Iserovich P, Cragoe EJ, Diecke FP, Fischbarg J. Intracellular [Na+], Na+ pathways, and fluid transport in cultured bovine corneal endothelial cells. Exp Eye Res. 2004a;79:93–103. doi: 10.1016/j.exer.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Kuang K, Ma L, Sanchez JM, Fischbarg J. Claudin Expression And Paracellular Permeability In Cultured Rabbit Corneal Endothelial Cells (rce) Invest Ophthalmol Vis Sci. 2004b;45 E-Abstract 1090. [Google Scholar]
- Kuang K, Yiming M, Wen Q, Li Y, Ma L, Iserovich P, Verkman AS, Fischbarg J. Fluid Transport across Cultured Layers of Corneal Endothelium from Aquaporin-1 Null Mice. Exp Eye Res. 2004c;78:791–798. doi: 10.1016/j.exer.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Le Varlet B, Ducroc R, Dagonet FB, Pouliquen Y, Vandewalle A, Hirsch M. Dibutyryl cyclic adenosine monophosphate and forskolin alter the paracellular pathway in cultured corneal endothelial cells. Invest Ophthalmol Vis Sci. 1995;36:2503–2513. [PubMed] [Google Scholar]
- Lim JJ, Fischbarg J. Electrical properties of rabbit corneal endothelium as determined from impedance measurements. Biophys J. 1981;36:677–695. doi: 10.1016/S0006-3495(81)84758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JJ, Liebovitch LS, Fischbarg J. Ionic selectivity of the paracellular shunt path across rabbit corneal endothelium. J Membr Biol. 1983;73:95–102. doi: 10.1007/BF01870344. [DOI] [PubMed] [Google Scholar]
- Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, Shapiro JI. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66:227–241. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Maurice DM. The location of the fluid pump in the cornea. J Physiol (Lond) 1972;221:43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan GT, Jepson MA, Hirst BH, Simmons NL. Polycation-induced enhancement of epithelial paracellular permeability is independent of tight junctional characteristics. Biochim Biophys Acta. 1993;1148:51–60. doi: 10.1016/0005-2736(93)90159-w. [DOI] [PubMed] [Google Scholar]
- Narula PM, Xu M, Kuang K, Akiyama R, Fischbarg J. Fluid transport across cultured bovine corneal endothelial cell monolayers. Am J Physiol. 1992;262:C98–C103. doi: 10.1152/ajpcell.1992.262.1.C98. [DOI] [PubMed] [Google Scholar]
- Neufeld AH, Jumblatt MM, Matkin ED, Raymond GM. Maintenance of corneal endothelial cell shape by prostaglandin E2: effects of EGF and indomethacin. Invest Ophthalmol Vis Sci. 1986;27:1437–1442. [PubMed] [Google Scholar]
- Riley MV, Winkler BS, Starnes CA, Peters MI, Dang L. Regulation of corneal endothelial barrier function by adenosine, cyclic AMP, and protein kinases. Invest Ophthalmol Vis Sci. 1998;39:2076–2084. [PubMed] [Google Scholar]
- Rubashkin A, Iserovich P, Hernandez J, Fischbarg J. Epithelial fluid transport: protruding macromolecules and space charges can bring about electro-osmotic coupling at the tight junctions. J Membr Biol. 2005;208:251–263. doi: 10.1007/s00232-005-0831-y. [DOI] [PubMed] [Google Scholar]
- Ruberti JW, Klyce SD, Smolek MK, Karon MD. Anomalous acute inflammatory response in rabbit corneal stroma. Invest Ophthalmol Vis Sci. 2000;41:2523–2530. [PubMed] [Google Scholar]
- Sanchez JM, Li Y, Rubashkin A, Iserovich P, Wen Q, Ruberti JW, Smith RW, Rittenband D, Kuang K, Diecke FPJ, et al. Evidence for a Central Role for Electro-Osmosis in Fluid Transport by Corneal Endothelium. J Membr Biol. 2002;187:37–50. doi: 10.1007/s00232-001-0151-9. [DOI] [PubMed] [Google Scholar]
- Satpathy M, Gallagher P, Jin Y, Srinivas SP. Extracellular ATP opposes thrombin-induced myosin light chain phosphorylation and loss of barrier integrity in corneal endothelial cells. Exp Eye Res. 2005;81:183–192. doi: 10.1016/j.exer.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Srinivas SP, Yeh JC, Ong A, Bonanno JA. Ca2+ mobilization in bovine corneal endothelial cells by P2 purinergic receptors. Curr Eye Res. 1998;17:994–1004. doi: 10.1076/ceyr.17.10.994.5242. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Boucher RC, Bromberg PA, Gatzy JT. Effects of ammonium and nitrate salts on lon transport across the excised canine trachea. Toxicol Appl Pharmacol. 1981;60:91–105. doi: 10.1016/0041-008x(81)90139-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xie Q, Sun XC, Bonanno JA. Enhancement of HCO(3)(−) permeability across the apical membrane of bovine corneal endothelium by multiple signaling pathways. Invest Ophthalmol Vis Sci. 2002;43:1146–1153. [PubMed] [Google Scholar]
- Zhu Z, Kuang K, Kang F, Li J, Fischbarg J. Platelet activating factor inhibits fluid transport by corneal endothelium. Invest Ophthalmol Vis Sci. 1996;37:1899–1906. [PubMed] [Google Scholar]


