Abstract
When cells are confronted with an insufficient supply of nutrients in their extracellular fluid, they may begin to cannibalize some of their internal proteins as well as whole organelles for reuse in the synthesis of new components. This process is termed autophagy and it involves the formation of a double-membrane structure within the cell, which encloses the material to be degraded into a vesicle called an autophagosome. The autophagosome subsequently fuses with a lysosome/vacuole whose hydrolytic enzymes degrade the sequestered organelle. Degradation of peroxisomes is a specific type of autophagy, which occurs in a selective manner and has been mostly studied in yeast. Recently, it was reported that a similar selective process of autophagy occurs in mammalian cells with proliferated peroxisomes. Here we discuss characteristics of the autophagy of peroxisomes in mammalian cells and present a comprehensive model of their likely mechanism of degradation on the basis of known and common elements from other systems.
Keywords: lysosome, pexophagy, protein transport, vacuole, yeast
1. Characteristic of peroxisome homeostasis
1.1. Morphology and function of peroxisomes
Peroxisomes are organelles found in nearly all eukaryotic cells. These organelles are usually elliptically shaped and have a diameter between 0.25 and 1 μm. In some types of tissues peroxisomes are tubularly shaped and connected to each other in a peroxisomal network, e.g. in rat hepatocytes (Yamamoto et al.,1987). They are an intracellular compartment involved in hydrogen peroxide metabolism and contain at least one hydrogen peroxide-producing enzyme along with catalase that is needed to eliminate this toxic compound.
In mammals, functional peroxisomes are required for a wide range of metabolic pathways, such as β- and α-oxidation of fatty acids, catabolism of amino acids, polyamines, and purines, as well as the synthesis of plasmalogens and cholesterol. A defect in peroxisomal function or formation causes several severe defects in humans. This is well demonstrated by human genetic disorders such as Zellweger syndrome, neonatal adrenoleukodystrophy, and infantile Refsum disease, which are characterized by mental retardation, severe neurologic, hepatic and renal abnormalities, and an early death (Small et al.,1988).
1.2. Peroxisome turnover
The size, number and enzyme content of peroxisomes can be very much influenced by the environment of the cell. For example peroxisomes are most abundant in tissues with active lipid metabolism, such as liver. There may be thousands of peroxisomes per cell in the mouse liver when animals are fed for a week with clofibrates, e.g. phthalate ester (DEHP). After withdrawal of the proliferators in 2 weeks, the number of peroxisomes dramatically decreases in rat liver cells (Reddy et al.,1974; Yokota1993). Apparently the massive proliferation of peroxisomes has necessitated the cell's maintenance of regulatory pathways to precisely control organelle activity and abundance. What are the mechanisms underlying this homeostatic process?
Previous reports on peroxisome turnover in insect fat bodies and rat liver describe a mechanism where the peroxisomal matrix enzymes are directly released to the cytoplasm in order to be decomposed (Locke et al.,1971; Yokota et al.,2001) . In contrast, other studies propose that the decay of peroxisomes is due to lysosomal degradation (Svoboda et al.,1967; Yokota2003). In the work by Svoboda and co-workers, the catalase that accounts for 40% of the total peroxisomal enzymatic activity pool, was used as a marker to monitor peroxisomes, and they show that its removal is dependent on lysosomal proteolysis. Similarly, in 2003 Yokota et al. report that during recovery from peroxisomal proliferator in the presence of the proteinase inhibitor leupeptin, peroxisomes are detected inside membranous structures, an observation that suggests autophagic degradation.
The latest studies of Iwata et al. (2006) show that removal of excess peroxisomes after DEHP withdrawal is inhibited in the liver of Atg7-deficient mice. Examination by western blot shows that the level of peroxisomal thiolase (PT) and peroxisomal bifunctional protein (BF) remain constant in the conditional atg7 mutant, whereas in the wild type situation the levels are reduced. Also accumulation of peroxisomes in the absence of Atg7 is revealed as numerous fluorescent dots with anti-PT antibodies; peroxisomes are removed under the same conditions in the wild type mouse. Conversion of the cytosolic form of LC3 (LC3-I) to the membrane-bound form (LC3-II) is commonly used as a tracer of autophagosome formation. This process is apparently impaired in the atg7 conditional mouse liver because predominantly the LC3-I accumulates. The LC3 modification assay result was confirmed by electron microscopy. When the protease inhibitor leupeptin was injected after 1 week of discontinuation of DEHP to wild type mice, the sequestered peroxisomes accumulate, and this mode of degradation morphologically resembles macroautophagy. However, under the same conditions in the atg7 mutant, sequestered peroxisomes are not detected, in agreement with the fact that Atg7 is essential for autophagosome formation in autophagy (Iwata et al.,2006). Conversely, another study using CHO cells indicated that peroxisome turnover occurs via microautophagy (Pan et al.,2005).
1.3. Selectivity in the autophagy of peroxisomes
Interestingly, only proliferated peroxisomes are specifically removed for degradation. The study by Yokota et al. shows that during recovery from proliferator in the conditions when autophagic proteolysis is inhibited, only low density peroxisomes, that correspond to those induced during drug treatment, are trapped inside the lysosomes. Also, another study shows that the kinetics of degradation of peroxisomal PT and BF differ from those of non-peroxisomal proteins (Iwata et al.,2006). Therefore, autophagic peroxisome turnover in mammalian cells might proceed in a selective manner. But despite multiple attempts to study peroxisomal turnover in mammals, apart from some morphological events, not much is known yet about the molecular basis of this process.
2. Molecular mechanism of peroxisome autophagy
Quite a bit of progress has been made in studies of yeast peroxisome autophagy in recent years. Yeast cell adjust their peroxisome level in response to variation in nutritional sources. For instance when cells are grown in conditions (e.g., methanol, alkanes, urate, primary amines or oleate) requiring peroxisomal function, peroxisomes are massively proliferated. After a shift back to glucose the peroxisomes are degraded via autophagy. This process of peroxisome degradation in yeast is called pexophagy. Pexophagy has been reported to operate in a selective way and two possible modes of pexophagy have been described. Macroautophagy (termed as macropexophagy) involves sequential and selective degradation of individual organelles via sequestration and subsequent fusion of the sequestered compartment with the vacuole (Fig. 1). In microautophagy (micropexophagy), a cluster of peroxisomes is directly internalized by the vacuole without prior sequestration. Both pathways result in the degradation of peroxisomes within the lysosome by proteolysis.
Figure 1.

Selective degradation of peroxisomes via macropexophagy and micropexophagy. Morphological events. Macropexophagy starts at a single peroxisome. This process involves targeting of peroxisome for initial membrane nucleation, sequestration–formation of autophagosomes–and hydrolytic degradation inside the vacuole/lysosome. In contrast, during micropexophagy a cluster of peroxisomes without prior sequestration is directly engulfed by vacuolar protrusions. These peroxisomes are released and degraded in the vacuolar lumen similar to the last stages of macropexophagy. P, peroxisome.
2.1. Atg proteins involved in pexophagy
Many of the proteins involved in pexophagy have been identified. Genetic analyses in various yeast species has resulted in the cloning of over 25 genes involved in autophagy-related pathways. The genes that encode these proteins were initially indicated as APG, AUT, CVT, GSA, PAG, PAZ, PDD and PDG, but they were recently renamed ATG (AuTophaGy related genes) (Klionsky et al.,2003).
Most Atg proteins are clearly involved in the formation of sequestering vesicles during macroautophagy of peroxisomes, whereas some of them are essential for fusion of the vesicles with the vacuole/lysosome and actual degradation inside the lysosome. Much less is known about the exact role of Atg proteins in micropexophagy. It has been proposed that some of these proteins are required specifically for membrane formation during the microautophagic process. The resulting membranous structure is called the micropexophagic apparatus (Mukaiyama et al.,2004) and it is necessary for closing up vacuolar protrusions around the peroxisomes in order to complete the sequestration process.
2.2. Initiation of pexophagy
How are nutritional signals sensed to initiate pexophagy? In yeast, for example, depletion of nitrogen inactivates the Tor protein kinase, which operates as an inhibitor of autophagy; thus, the inhibition of Tor kinase induces autophagy. It is not clear what regulatory mechanism is upstream of Tor. However, in mammalian cells mTOR is regulated by a class I phosphatidylinositol (PtdIns) 3-kinase complex. In methylotrophic yeast pexophagy is activated when yeast are moved from growth on peroxisome-requiring conditions to glucose or ethanol. Nothing is known so far regarding the signaling of this pathway.
2.3. Origin of the sequestering membranes
In order to be degraded via macroautophagy, peroxisomes first have to be isolated by the membranous layers that form an autophagosome. For this to occur, a lot of membranes–or the structural components of membrane, phospholipids–have to be transported to the place where this process is initiated. So far, pre-existing membranes that are involved in autophagosome formation have not been found. Instead mitochondria are often seen in close association with sequestering membranes during the initial stages of pexophagy in yeast (Dunn et al.,2005). The mitochondria may play a role as a source of phospholipids in the formation of sequestering membranes. The autophagy factor Atg9 is a transmembrane protein essential for sequestering membrane formation, and is shown to localize to the mitochondria in yeast (Reggiori et al.,2005). The S. cerevisiae Atg9 cycles between mitochondria, cytosol and autophagosomes in the membrane/lipid bound form. In this way phospholipids or whole vesicles are transported to the autophagosome formation site. In another yeast species, P. pastoris, Atg9 is proposed to localize to peripheral membranous structures in close proximity to the mitochondria and the endoplasmic reticulum (ER), and it moves to the sequestering membranes when pexophagy is induced (Chang et al.,2005). A key protein implicated in autophagosome formation is Atg8, which can occur in two states, soluble and membrane associated. Its phospholipid association (Atg8 is conjugated to phosphatidylethonolamine; (Ichimura et al.,2000)), cleavage and subsequent rebinding is proposed to play a role in the arrangement of sequestering membranes (Kirisako et al.,2000). In yeast, Atg8 and Atg9 may be key elements bringing membranes in close vicinity to the vacuolar pre-autophagosomal structure/phagophore assembly site (PAS), where autophagosomes are nucleated. In higher eukaryotes, structures reminiscent of the PAS have been seen but the mammalian counterparts of Atg9 localize to the ER. EM studies suggest that the ER is an origin of the autophagosomes in mammalian cells (Mizushima et al.,2001). In yeast, mutations impairing the integrity of the ER or Golgi block bulk and selective autophagy (Reggiori et al.,2004). Therefore, the ER (and ER derived structures) and mitochondria are the best candidates as possible sources of lipids or lipid bilayers for building and maintenance of autophagic membranes.
2.4. Mechanism of peroxisome targeting for degradation
The peroxisomal membrane protein Pex14 in yeast, as well as its counterpart in higher eukaryotes, is a key component of the peroxisomal import machinery and may be the initial docking site for the targeting of sequestering membranes. For initiation of pexophagy, peroxisomes require a specific tag on the organelle, which serves as a target for the sequestering membrane. In methylotrophic yeast, the peroxisomal membrane-bound peroxins Pex14 and Pex3 play a role in initiation of macropexophagy (Bellu et al.,2002; Bellu et al.,2002; Veenhuis et al.,2003). Pex14 is involved in the import of matrix proteins into peroxisomes. Together with other peroxins (Pex13, Pex17) it forms a docking complex at the peroxisomal surface for the soluble protein receptor Pex5. In PEX14 null mutants of H. polymorpha, the defect in the matrix protein import machinery, and hence peroxisome formation, can be largely suppressed by overproduction of Pex5. However, upon induction of macropexophagy in these cells, these organelles are protected from degradation. Moreover, detailed electron microscopy analysis revealed that sequestration of peroxisomes is also not observed in these cells. Based on these observations, it was concluded that Pex14 is essential for macropexophagy and is involved in an early step prior to initiation of sequestration (Bellu et al.,2001).
Pex3 also plays a role in the formation and maintenance of peroxisomal membranes (Baerends et al.,2000). At the onset of macropexophagy, Pex3 is rapidly removed from the peroxisome membranes (Bellu et al.,2002). Strains that fail to remove Pex3 are blocked in macropexophagy, indicating that not the presence but rather the removal of Pex3 is essential for macropexophagy.
It is suggested (Hazra et al.,2002) that Pex3 connects two known peroxisome membrane bound protein complexes, namely the receptor docking site (Pex13, Pex14 and Pex17) and a ring finger protein complex (Pex2, Pex10 and Pex12) to form a large supercomplex (Fig. 2). Also, Pex8 may play a role in the formation of these supercomplexes (Agne et al.,2003). Data from Hazra et al. (2002) indicate that Pex3 connects these two sub-complexes. Therefore, removal of Pex3 at the onset of macropexophagy will disrupt the complex and hence may lead to a block of further matrix protein import in the peroxisomes that are destined for degradation (Fig. 3). Also, dissociation of the supercomplex may result in exposure of the N-terminal domain of Pex14, which has been shown to be essential for macropexophagy (Bellu et al.,2001). This domain may be recognized by a yet unknown protein (perhaps Atg11) to tag the organelle for sequestration by the autophagosome.
Figure 2.
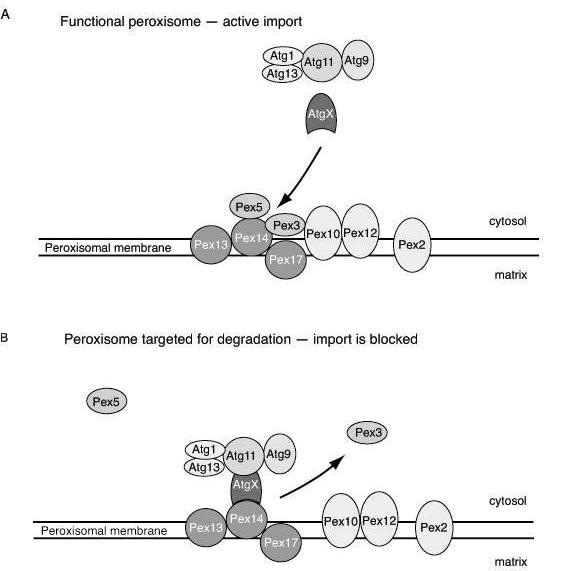
Targeting of peroxisome for degradation. Model of molecular mechanism underlying initiation of pexophagy. A. By docking to the peroxisomal membrane and subsequent translocation, Pex5 shuttles between the cytosol and matrix, taking newly synthesized peroxisomal proteins into the lumen. The peroxisomal membrane protein Pex3 bridges the docking peroxisomal complex (Pex13, Pex14 and Pex17) and the putative translocation site (Pex12, Pex10 and Pex2). In this way peroxisome matrix proteins are imported and the peroxisome is functional. At this stage Pex3 presumably shields Pex14.
B. When peroxisomes are no longer functional, the docking and translocation complexes are apart. Pex3 is removed, thus exposing Pex14 for targeting by a hypothetical receptor (AtgX). Via AtgX and Atg11 the peroxisome is connected to the other Atg components, allowing autophagic sequestration to occur.
Figure 3.

Molecular mechanism of autophagosome formation. A single import-incompetent peroxisome is targeted for degradation. Peroxisomal membrane complex modifications from import–competent to incompetent allow docking of sequestering membranes to the peroxisome. This process occurs through multiple sequential steps carried out by Atg proteins. The Atg1-Atg13 and PtdIns 3-kinase complexes are involved in forming a scaffold that allows for the initiation of autophagosome formation. Atg8 and Atg9 are involved in the building of membranes by bringing lipids by recruitment and subsequent release. Atg9 cycles to the mitochondria, which possibly is the donor of lipids. Atg8 is involved in the recruitment of the Atg12–Atg5-Atg16 complex, which forms a coat that might be necessary for membrane curvature. Prior to completion of the autophagosome most of the Atg proteins dissociate from the membrane.
2.5. Molecular model of pexophagy: steps and protein complexes
Binding of Atg11 to Atg19 marks one of the first steps of cargo recognition in one of the selective type of autophagy, the cytoplasm to vacuole targeting (Cvt) pathway, in yeast. The receptor protein Atg19 binds to the propeptide of the Ape1 precursor. Interaction of cargo-bound Atg19 with Atg11 is required for its PAS localization (Scott et al.,2001; Shintani et al.,2002). In pexophagy, the cargo selection mechanism may be organized in a similar way, as Atg11 is required for pexophagy in yeast; however, S. cerevisiae atg19 mutants are not blocked in peroxisome degradation. Hence, another protein is likely to be involved in recognition of peroxisomes by autophagosomes in yeast.
The PAS most likely carries out some type of recruitment process to organize the Atg proteins involved in the initial steps of autophagy. The exact biochemical composition of the PAS is not known yet. This structure contains at least one protein that has typical features of integral membrane proteins, Atg9, and it might harbor phospholipids because it contains a functional PtdIns 3-kinase complex; however, there is no direct evidence for this.
Generally three major sets of proteins comprise the PAS; the Atg1-Atg13 and PtdIns 3-kinase complexes, and the Atg12–Atg5-Atg16 proteins. Initially, these proteins are probably independently targeted to the PAS. The PtdIns 3-kinase complex phosphorylates phosphatidylinositol (PI) that allows binding of Atg18, and together with Atg1 and Atg9 they recruit Atg2. The latter plays a role in the interaction of Atg18 with Atg9. Next, Atg18 and Atg2 together with Atg1-Atg13 facilitate release of Atg9 from the PAS. This step is important for the completion of the autophagosomes.
Atg12 conjugates to Atg5 by a ubiquitination-like reaction. Initially Atg7 activate Atg12, which is subsequently transferred to Atg10. Atg10 serves as a functional equivalent of E2 enzymes, although it is not a homologue. Atg5 associates with Atg16, a small coiled-coil protein, whose subsequent oligomerization serves as a link in the formation of the Apg12–Apg5-Apg16 multimeric protein complex. The oligomerization step has been shown to be necessary for proper Atg12–Atg5 function (Kuma et al.,2002). Immediately before or after completion of the autophagosome, the Atg12–Atg5 conjugate dissociates from the autophagosomal membrane (Mizushima et al.,2001).
Atg9 is important for Atg12–Atg5 conjugate formation and these events facilitate Atg8 conjugation to PE and recruitment to the PAS. The membrane-recruited form of Atg8 is lipidated. The lipidation process involves specific posttranslational modifications of the protein via a process that shares features with the ubiquitin conjugation mechanism (Kim et al.,2001; Kirisako et al.,1999). Lipidation requires cleavage of the arginine residue located at the extreme C terminus, which is removed by the cysteine protease Atg4. The exposed C-terminal glycine residue is subsequently covalently linked to PE. This process is catalyzed by Atg7 (a protein that is homologous to the E1-ubiquitin activating enzyme) and Atg3 (functions similar to E2-ubiquitin conjugating enzymes) (Ichimura et al.,2000) and leads to the recruitment of Atg8–PE to the PAS. During autophagy, Atg8 is initially localized on both the outer and inner membrane of the autophagosome. After completion of the autophagosome, Atg8 molecules that are associated with the inner autophagosomal membrane surface are, together with the autophagosomal contents, degraded in the vacuole. The remaining fraction of Atg8 that is associated with the outer autophagosomal membrane is recycled to the PAS after a second cleavage by Atg4 to remove it from PE.
2.6. Involvement of the cytoskeleton in pexophagy
Studies on mammalian cells offer some information concerning the effects of a series of inhibitors on cytoskeleton function in autophagy. For example, in rat normal kidney epithelial cells the cytochalasins, microfilament depolymerizing agents, interfere with formation of the autophagosomes (Aplin et al.,1992), whereas microtubule inhibitors block fusion of the autophagosome with the lysosome in both rat hepatocytes and normal rat kidney epithelial cells (Aplin et al.,1992; Seglen et al.,1996). However, microtubules in yeast are not essential for autophagy (Kirisako et al.,1999; Reggiori et al.,2005). In contrast, the actin cytoskeleton is required for two types of selective autophagy including pexophagy (Reggiori et al.,2005). This work shows that the cycling of Atg9, which is needed for autophagosomes formation, is dependent on functional Factin. However, it still remains completely unclear exactly how actin plays a role in the autophagy of peroxisomes.
3. Concluding remarks and perspectives
How organelles are degraded in mammalian cells is not well characterized but autophagy is the only mechanism with the capacity to sequester entire membrane compartments. Recent reports indicate that peroxisome autophagy is a selective process. Interestingly, the peroxisome appears to be the only organelle for which there is clear evidence of selective autophagy in mammalian cells. At present there is a rough idea about the mechanistic basis of peroxisome turnover in mammals, based primarily on morphological studies, but little information about the molecular mechanism. Atg7 is the first molecular factor discovered in mammalian pexophagy. In yeast pexophagy this protein works in a complex that resembles the ubiquitin conjugation machinery. Presumably other components of this complex are also involved in mammalian pexophagy. One of them, Atg8, has at least three mammalian homologues and all of them have been reported to be essential for autophagy.
Autophagy appears to be a highly conserved process from unicellular to multicelullar eukaryotes.
Although many autophagic factors identified in yeast are also found in mammals, Atg proteins specific for selective autophagy in yeast do not have homologues in higher eukaryotes. How the substrate specificity is determined and the molecular mechanism of selectivity is still unclear. Thus, there is still much to be understood about the mechanism of pexophagy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agne B, Meindl NM, Niederhoff K, Einwachter H, Rehling P, Sickmann A, Meyer HE, Girzalsky W, Kunau WH. Pex8p: An intraperioxisomal organizer of the peroxisomal import machinery. Mol. Cell. 2003;11:635–646. doi: 10.1016/s1097-2765(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Aplin A, Jasionowski T, Tuttle DL, Lenk SE, Dunn WA. Cytoskeletal elements are required for the formation and maturation of autophagic vacuoles. J. Cell. Physiol. 1992;152:458–466. doi: 10.1002/jcp.1041520304. [DOI] [PubMed] [Google Scholar]
- Baerends RJS, Faber KN, Kram AM, Kiel JAKW, van der Klei I, Veenhuis M. A stretch of positively charged amino acids at the N terminus of Hansenula polymorpha Pex3p is involved in incorporation of the protein into the peroxisomal membrane. J Biol Chem. 2000;275:9986–9995. doi: 10.1074/jbc.275.14.9986. [DOI] [PubMed] [Google Scholar]
- Bellu AR, Komori M, van der Klei I, Kiel JAKW, Veenhuis M. Peroxisome biogenesis and selective degradation converge at Pex14p. J Biol Chem. 2001;276:44570–44574. doi: 10.1074/jbc.M107599200. [DOI] [PubMed] [Google Scholar]
- Bellu AR, Salomons FA, Kiel JAKW, Veenhuis M, van der Klei I. Removal of Pex3p is an important initial stage in selective peroxisome degradation in Hansenula polymorpha. J Biol Chem. 2002;277:42875–42880. doi: 10.1074/jbc.M205437200. [DOI] [PubMed] [Google Scholar]
- Chang T, Schroder LA, Thomson JM, Klocman AS, Tomasini A, Stromhaug PE, Dunn WA. PpATG9 encodes a novel membrane protein that traffics to vacuolar membranes, which sequester peroxisomes during pexophagy in Pichia pastoris. Mol. Biol. Cell. 2005;16:4941–4953. doi: 10.1091/mbc.E05-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WA, Gregg JM, Kiel JA, van der Klei IJ, Oku M, Sakai Y, Sibirny A, Stasyk OV, Veenhuis M. Pexophagy: The Selective Autophagy of Peroxisomes. Autophagy. 2005;1:75–83. doi: 10.4161/auto.1.2.1737. [DOI] [PubMed] [Google Scholar]
- Hazra PP, Suriapranata I, Snyder WB, Subramani S. Peroxisome remnants in pex3Δ cells and the requirement of Pex3p for interactions between the peroxisomal docking and translocation subcomplexes. Traffic. 2002;3:560–574. doi: 10.1034/j.1600-0854.2002.30806.x. [DOI] [PubMed] [Google Scholar]
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- Iwata J, Ezaki J, Komatsu M, Yokota S, Ueno T, Tanida I, Chiba T, Tanaka K, Kominami E. Excess peroxisomes are degraded by autophagic machinery in mammals. J. Biol. Chem. 2006;281:4035–4041. doi: 10.1074/jbc.M512283200. [DOI] [PubMed] [Google Scholar]
- Kim J, Huang W-P, Klionsky DJ. Membrane recruitment of Aut7p in the autophagy and cytoplasm to vacuole targeting pathways requires Aut1p, Aut2p, and the autophagy conjugation complex. J Cell Biol. 2001;152:51–64. doi: 10.1083/jcb.152.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999;147:435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, Ohsumi Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 2000;151:263–275. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Mizushima N, Ishihara N, Ohsumi Y. Formation of the approximately 350-kDa Apg12-Apg5·Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem. 2002;277:18619–18625. doi: 10.1074/jbc.M111889200. [DOI] [PubMed] [Google Scholar]
- Locke M, Mcmahon JT. Origin and fate of microbodies in fat body of an insect. J. Cell Biol. 1971;48:61. doi: 10.1083/jcb.48.1.61. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 2001;152:657–667. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaiyama H, Baba M, Osumi M, Aoyagi S, Kato N, Ohsumi Y, Sakai Y. Modification of a ubiquitin-like protein Paz2 conducted micropexophagy through formation of a novel membrane structure. Mol Biol Cell. 2004;15:58–70. doi: 10.1091/mbc.E03-05-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Pan X, Wang N, Ghazizadeh M, Yeldandi A. Characterization of the degradation of recombinant rat urate oxidase in tetracycline controlled gene expression cells. Journal of Electron Microscopy. 2005;54:385–392. doi: 10.1093/jmicro/dfi048. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Azarnoff DL, Svoboda DJ, Prasad JD. Nafenopin-induced hepatic microbody (peroxisome) proliferation and catalase synthesis in rats and mice - absence of sex difference in response. J. Cell Biol. 1974;61:344–358. doi: 10.1083/jcb.61.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Wang C-W, Nair U, Shintani T, Abeliovich H, Klionsky DJ. Early stages of the secretory pathway, but not endosomes, are required for Cvt vesicle and autophagosome assembly in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:2189–2204. doi: 10.1091/mbc.E03-07-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Monastyrska L, Shintani T, Klionsky DJ. The actin cytoskeleton is required for selective types of autophagy, but not nonspecific autophagy, in the yeast Saccharomyces cerevisiae. Molecular Biology of the Cell. 2005;16:5843–5856. doi: 10.1091/mbc.E05-07-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Shintani T, Chong H, Nair U, Klionsky DJ. Atg9 Cycles Between Mitochondria and the Pre-Autophagosomal Structure in Yeasts. Autophagy. 2005;1:101–109. doi: 10.4161/auto.1.2.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SV, Guan J, Hutchins MU, Kim J, Klionsky DJ. Cvt19 is a receptor for the cytoplasm-to-vacuole targeting pathway. Mol Cell. 2001;7:1131–1141. doi: 10.1016/s1097-2765(01)00263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen PO, Berg TO, Blankson H, Fengsrug M, Holen I, Stromhaug PE. Structural aspects of autophagy. Adv Exp Med Biol. 1996;389:103–111. doi: 10.1007/978-1-4613-0335-0_12. [DOI] [PubMed] [Google Scholar]
- Shintani T, Huang W-P, Stromhaug PE, Klionsky DJ. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell. 2002;3:825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GM, Santos MJ, Imanaka T, Poulos A, Danks DM, Moser HW, Lazarow PB. Peroxisomal integral membrane-proteins in livers of patients with Zellweger syndrome, infantile refsums disease and X-linked adrenoleukodystrophy. J. Inherit. Metab. Dis. 1988;11:358–371. doi: 10.1007/BF01800425. [DOI] [PubMed] [Google Scholar]
- Svoboda D, Azarnoff D. Peroxisomes (microbodies) in experimentally altered cells. Am. J. Pathol. 1967;50:A53. doi: 10.1083/jcb.35.1.127. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhuis M, Kiel JAKW, van der Klei I. Peroxisome assembly in yeast. Microsc Res Tech. 2003;61:139–150. doi: 10.1002/jemt.10323. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Fahimi HD. 3-dimensional reconstruction of a peroxisomal reticulum in regenerating rat-liver - evidence of interconnections between heterogeneous segments. J. Cell Biol. 1987;105:713–722. doi: 10.1083/jcb.105.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S. Formation of autophagosomes during degradation of excess peroxisomes induced by administration of dioctyl phthalate. Eur. J Cell Biol. 1993;61:67–80. [PubMed] [Google Scholar]
- Yokota S. Degradation of normal and proliferated peroxisomes in rat hepatocytes: Regulation of peroxisomes quantity in cells. Micr. Res. Tech. 2003;61:151–160. doi: 10.1002/jemt.10324. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oda T, Fahimi HD. The role of 15-lipoxygenase in disruption of the peroxisomal membrane and in programmed degradation of peroxisomes in normal rat liver. J. Histochem Cytochem. 2001;49:613–621. doi: 10.1177/002215540104900508. [DOI] [PubMed] [Google Scholar]


