Abstract
The sensorimotor speech/voice deficits associated with Parkinson Disease have been well-documented in humans. They are largely resistant to pharmacological and surgical treatment, but respond to intensive speech treatment. The mechanisms underlying this phenomenon are not well understood and are difficult to systematically test in humans. Thus we turn to the rat as a model. The purpose of this study is to compare the ultrasonic vocalization (USV) of rats in three conditions: control, haloperidol-induced transient dopamine depletion, and unilateral 6-hydroxydopamine (6-OHDA) induced moderately-severe degeneration of dopamine neurons. It was hypothesized that both dopamine-altered conditions would lead to a change in the features of the USV acoustic signal. Results demonstrated that bandwidth decreased in the dopamine-altered rats. This is the first study to document a degradation of the acoustic signal of frequency-modulated 50-kHz calls as a result of interfering with dopamine synaptic transmission in rats. The data suggest that mild transient dopamine depletion with haloperidol or even unilateral degeneration of dopamine neurons is associated with changes in the USV acoustic signal. Thus, dopaminergic dysfunction appears to influence USV production. This study provides a foundation to examine the role of dopamine in sensorimotor processes underlying USV production and potentially to explore treatments for dopamine deficiency-related impaired vocal outcome.
Keywords: ultrasonic vocalization, Parkinson disease, sensorimotor control, dopamine, 6-OHDA, haloperidol, rat
1. Introduction
Much attention has been given to sensorimotor limb deficits that occur with Parkinson Disease (PD). However up to 89% of individuals with PD also have disordered speech and voice that significantly impacts their quality of life [20, 25, 28]. The most salient characteristics of speech/voice deficits in those with PD are: decreased vocal loudness, decreased frequency variability (monotone), breathiness, hoarse voice quality, and imprecise articulation [14, 15, 28]. These deficits in acoustic parameters can be thought of as a degradation of the acoustic signal. Voice disorders can occur as an early sign of PD suggesting that voice may be vulnerable to the primary disease pathology of dopamine depletion [23,42]. In contrast to the therapeutic effects of levodopa and deep brain stimulation on limb motor deficits, speech/voice disorders are often resistant to pharmacological and surgical intervention [27, 32, 41], but reliably show improvement with behavioral treatment [33–36]. The established animal models of PD provide an opportunity to investigate these unique manifestations of the disease process.
Rodent models provide a useful tool in studying sensorimotor deficits associated with PD [12, 18, 31, 38, 44], although to date there are no rodent models to examine qualitative changes in the USV acoustic signal. Rats produce several types of USVs that can be classified by frequency and complexity of the waveform. Generally, the peak energy of the calls falls into 2 main categories: 22-kHz or 50-kHz frequency ranges [8, 9]. These 22-kHz calls generally occur as an alarm. Compared to the 22-kHz calls, the 50-kHz calls are more complex. They are composed of either short constant frequency calls (constant calls) or with frequency modulated (FM) types [5, 10, 21, 50]. Recently, rodent (mice) vocalization has also been shown to display characteristics of song [26].
Type and complexity of the call will vary depending on the age, sex, social situation, affective state, and general situation of the rat. These calls carry semiotic value, so have symbolic reference (sign), and are capable of changing the behavior of the signal recipient [7]. Among many possible biological functions of the sign contained in the signal, the present study will be focused on those associated with reproductive/sexual and affiliation promoting function of the 50-kHz calls. Expression of 50-kHz vocalization is correlated with sexual motivation, as males vocalize more with increasing sexual experience and with receptive (estrous) females [4]. McGinnis and Vakulenko have found that when the males were allowed to ejaculate the number of vocalizations was significantly higher [30]. Their results suggest that environmental stimuli play an important role in enhancing or increasing the number of 50-kHz calls per recording session.
The mechanism of USV production in rats is poorly understood. Generally, respiratory, phonatory/laryngeal, and possibly articulatory subsystems of communication are utilized. Constant USVs are produced by a whistling mechanism by approximation and fixation of the vocal folds [49]. FM calls are possibly produced by a similar whistle mechanism and the modulation is thought to be produced by articulation at the level of the larynx. However, the respiratory and upstream (tongue) subsystems of the vocal tract are likely candidates for modulation as well. Understanding the mechanism of modulation will facilitate understanding the complexities of the communicative motor system and how it breaks down with neurological disease.
We acknowledge that rat and human communication differ, but to some degree they are analogous. Both systems utilize modifications of egressive airflow at the level of the larynx and through articulators upstream [51]. Further, human speech/vocalizations and rat USVs share semiotic/semantic nature of the signals. Thus, we believe that USV may be an adequate model to study phonatory/communication deficits in a sensorimotor context.
Speech and voice deficits occur early in PD, and during the hemi-Parkinson stage there is substantial, but subclinical dopamine depletion in the less depleted hemisphere so that limb sensorimotor symptom presentation is unilateral. It is unclear whether speech deficits in hemi-Parkinson disease require at least partial loss of dopamine terminals in both hemispheres. In an animal model, we began to address this issue by targeting dopamine neurons only in one hemisphere with the dopamine neurotoxin 6-OHDA and measuring ultrasonic vocalization. An additional dopamine depletion model was employed to test bilateral effects. Haloperidol was chosen based on its ability to block D1 and to a greater extent D2 dopamine receptors [13].
This is the first study to document dopamine deficiency-linked changes in acoustic parameters of the 50-kHz USV in rats. Although a high dose of a dopamine antagonist can reduce the absolute number of 50-kHz calls [11], other changes in frequency modulation of these calls have not been examined after doses that do not substantially reduce the number of 50-kHz USVs or after unilateral disruption of dopamine. In the present study we created a paradigm to examine the effects of unilateral 6-OHDA induced degeneration of dopamine neurons and low doses of the dopamine antagonist haloperidol on rat USV. It was hypothesized that the dopamine-altered conditions would cause a degradation of the acoustic signal.
2. Materials and Methods
2.1 Subjects
Male Long-Evans rats obtained from Charles River were used for all experiments. Ten animals were tested under both the control condition and with haloperidol, and 6-OHDA was infused unilaterally into the medial forebrain bundle of 3 additional rats. Animals were aged 6 months at the time of USV recording and housed in groups of two in standard polycarbonate cages with sawdust bedding on a reversed 12:12 hour light:dark cycle. All testing occurred during the dark period of the cycle. Food and water were available ad libitum. Rats were handled each day prior to the experiments for 7 consecutive days. Sexual encounters were used to elicit 50-kHz calls from the male rats. All rats were sexually experienced with estrous female rats to ensure immediate mounting and ejaculation took place prior to the experiment. Female rats were brought into estrous through i.p. injections of 10 μg of estradiol (Sigma, USA) and 500 μg of progesterone (Sigma, USA) at 48 hrs and 4 hrs prior to the behavioral testing, respectively. All experiments conducted were approved by the University of Texas Animal Care and Use Committee.
2.2 Dopaminergic Deficiency
Ten control rats were given i.p. injections of 0.1 mg/kg of haloperidol 1 hour before behavioral testing. The dose of haloperidol was based on dose-response analyses in our lab that indicated maintenance of normal locomotor function in an open field, mounting latency, absence of catalepsy, and number of calls in a given time period.
6-OHDA was infused into the medial forebrain bundle to cause partial degeneration of dopamine neurons on one side [22, 29, 44, 48, 52]. Prior to neurotoxin exposure, rats aged 10 weeks at the time of surgery were anesthetized with 90 mg/kg ketamine (i.p.) plus 10 mg/kg xylazine (i.p.). Upon anesthesia, a solution of 7 μg (free base weight) of 6-OHDA hydrobromide dissolved in 3 μl of artificial cerebrospinal fluid containing 0.05% (w/v) ascorbic acid was infused into the medial forebrain bundle (−3.3 AP; ±1.7 ML; −8.0 DV) at a rate of 0.3 μl/min for 10 min. 6-OHDA was infused into the hemisphere contralateral to the rat’s preferred limb, as determined from baseline behavioral data on forelimb-use. After surgery, rats recovered in a humidified incubator and upon waking were returned to their home cages.
All rats were tested for forelimb use asymmetry on post-surgery days 7, 14 and 55 to confirm moderately severe unilateral dopaminergic terminal loss [39]. Rats were placed in an upright translucent acrylic cylinder, measuring 30 cm high and 20 cm in diameter, to encourage rearing and vertical exploration with the forepaws. Forelimb-use asymmetry was calculated as ipsilateral limb wall contacts, plus 1/2 the number of “both” contacts, divided by the total number of contacts (limited to 20 per session). Higher scores (>50%) indicate greater behavioral deficits [40]. The average cylinder score for the 6-OHDA treated animals was 84%, with individual scores ranging from 61–97%. In addition, at 4 weeks post-surgery, animals were treated with the dopamine receptor agonist apomorphine (0.5 mg/kg, s.c.) and the net number of contralateral quarter turns made during a 5 min period (20–25 min post-apomorphine injection) was recorded. Apomorphine administration induces dose-dependent contralateral rotation linked to degeneration of dopamine terminals and compensatory upregulation of dopamine receptors [24, 47]. The mean number of net contralateral quarter turns was 84. Taken together with the limb-use asymmetry data, this suggests that the unilateral degeneration of dopamine neurons was moderately-severe [40].
2.3 Ultrasound recording
A limitation in most USV studies has been the inability to isolate male rat calls in the presence of females. Thus, for this experiment a new recording environment was designed. An ultrasonic microphone with high directional properties for recording 50-kHz USVs (CM16, Avisoft, Germany) with a flat frequency response of up to 150-kHz and a working frequency response range of 10–180-kHz, was attached to a panel. The panel was then placed in the top center of a 10 × 10 × 12 cm sound-isolated Plexiglas chamber to divide the space into upper and lower compartments. The subject male rat was placed in the bottom area with the microphone. During the control and experimental recordings, the high frequency gain was kept at the same level. The female estrous rat was placed on top of the divider. Four 3 mm holes were drilled into the panel to allow odor to pass to the male chamber. The diameter of the holes was smaller than the 50-kHz USV wavelength to sufficiently attenuate female USVs. Additionally, an inlet and exhaust fan were installed to create positive pressure within the chamber to enable the female’s odor to pass down to the lower chamber.
2.4 Experimental Protocol
Each male rat was placed in its home cage with an estrous female. Sexual behavior of darting and time to mount were observed. Time to mount is an important parameter to monitor to ensure that the dopamine-altered rats are behaving similarly to the control rats in regard to interest and motivation. After 2 successful mounts without ejaculation, the male rat was placed in the recording chamber for 5 min with home cage bedding. This process was repeated for three consecutive days prior to vocalization recording experiments.
On recording day, after 2 mounting attempts with the female estrous rat in its home cage, the subject male rat was placed inside the recording chamber with home cage bedding for a 2 min habituation period. The female rat was then placed in the superior portion of the chamber. USVs were recorded for 5 min. The vocalization recording protocol was completed for 4 consecutive days in the control condition, and 1 day in the haloperidol condition. The 6-OHDA treated rats underwent the same protocol as rats in the control condition.
2.5 Data analysis and statistics
Video recordings with a Panasonic PV-DV800 Infrared Camcorder were made with each session to ensure that behavior and distance from the microphone were similar among all rats. Only those calls that occurred when the rat was upright facing the microphone and mouth-to-microphone distance was within 6–8 cm were included in the analyses. Thus, only 6 of the 10 rats injected with haloperidol were included in the analyses.
USV recordings were collected on a computer and transferred to an external hard drive for storage and analysis. Analog recordings were digitized through a D/A card (National Instruments, USA) at 200-kHz sampling rate with 16 bit resolution. Recorded USVs were analyzed with Saslab Pro (Avisoft, Germany). Sonograms were generated under a 512 FFT-length and 75% overlap frame setup. A 120 s duration of vocalization recording was inspected after bypassing the initial 30 s transient phase. Individual calls were then separated into single WAV files for further parametric analysis. Time to mount and absolute number of calls per recording session were analyzed.
Average peak frequency was measured to ensure that all animals were calling within the 50-kHz range. Bandwidth was analyzed as a dependent variable using SasLabPro (Avisoft, Germany). Bandwidth was defined as the maximum frequency (Hz) minus the minimum frequency in each call.
A mixed ANOVA was used to analyze the following dependent variables: time to mount, number of calls, and bandwidth. The within-subjects factor was control vs. haloperidol and the between-subjects factors were control vs. 6-OHDA and 6-OHDA vs. haloperidol.
3. Results
3.1 Mounting time and number of calls
Time to mount was not significantly different among any of the conditions (F [2, 18] = 1.526, p = .247), although there was considerable variability in both dopamine deficiency conditions. The haloperidol condition yielded a mean latency of 18.5 ± 12.9 s, the 6-OHDA condition 13.7 ± 11.2 s, and the control condition 5.4 ± 1.9 s. It should be noted that one rat’s time to mount in the haloperidol condition was 120 s and this elevated the group mean.
There was a significant main effect for the number of calls per session for all groups (F [2, 18] = 4.926, p = .002). Follow-up tests revealed that the 6-OHDA animals (X̄ = 335 ± 47) were not significantly different from the control animals (X̄ =263 ± 159, p = .647), although the 6-OHDA group did on average produce more calls per session. The haloperidol treated animals (X̄ = 88 ± 79) produced significantly fewer calls per session when compared with the control condition (p = .045) or with the 6-OHDA animals (p = .038).
3.2. Average Peak Frequency
Average peak frequency obtained from spectrum analysis for the control, 6-OHDA, and haloperidol treated rats was 57.83 ± 7.3 kHz, 53.81 ± 3.84 kHz, 52.97 ± 4.83 kHz, respectively, indicating that all calls analyzed were 50-kHz calls.
3.3. Bandwidth
Traditionally, all types of calls with a peak frequency above 30 kHZ have been grouped together as 50-kHz FM calls. However, when averaging acoustic parameters such as frequency (in kHz), bandwidth, and duration, these variables tend to ‘wash-out.’ For example, a call with a slowly rising increase in fundamental frequency is likely produced by a ‘whistle’ mechanism with stationary positioning of the articulators with little modulation. A call with a ‘trill’ likely involves oscillation somewhere in the production mechanism. If one averages the bandwidth of both types of calls, the complex nature of a trill call will be lost in that function. For the purposes of this paper, only dependent variables for the trill type of calls, defined as rapid fluctuations in frequency in both positive and negative directions without the presence of harmonics, are being reported (Figure 1). However, all other types of high frequency calls showed marked degradations in the 6-OHDA and haloperidol treated rats.
Fig. 1.
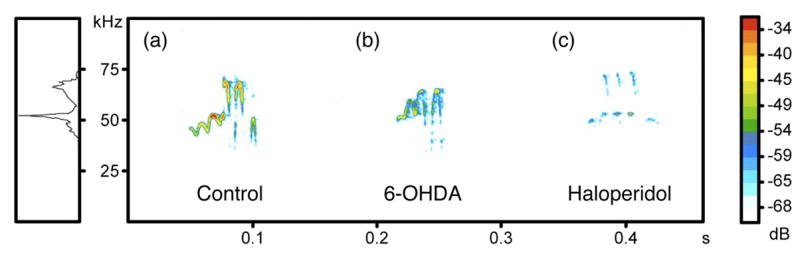
Sonogram of “trill” type ultrasonic vocalization (USV) for control, unilateral 6-OHDA and bilateral Haloperidol treated male subject rats in middle box. Ordinate represents the frequency change, abscissa represents time. Colors indicate relative level of signal intensity in the call. Right box color indicates different relative intensity (dB). Left box represents overall energy level of these 3 calls distributed among the frequency axis with a peak located at 50 kHz.
There was a significant main effect for bandwidth (Figure 2) among all groups (F [2, 18] = 9.970, p = .002). The mean bandwidth was reduced for the 6-OHDA animals (X̄ = 5200 ± 3050 Hz, p = .005) and after haloperidol administration (X̄ = 13500 ± 1550 Hz, p = .008), as compared with the control animals (X̄ = 23600 ± 5360 Hz). The difference in bandwidth between the 6-OHDA and haloperidol conditions was not statistically significant (p = .837). As evident in the representative sonograms in Figure 2, the trill structure of the dopamine-altered rats’ acoustic signal appeared to be markedly diminished.
Fig. 2.
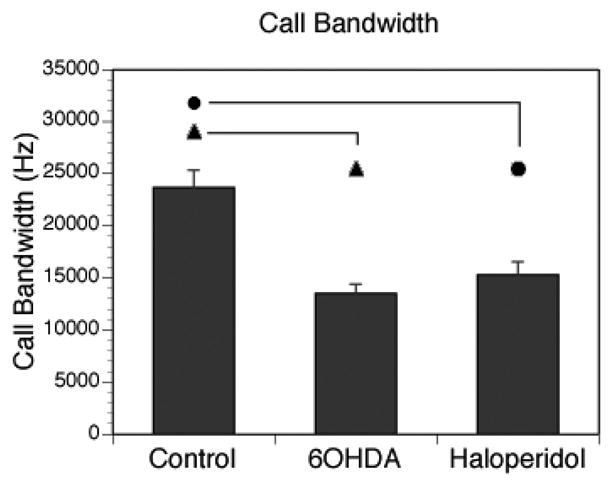
Mean call bandwidth calculated from max-min frequency of “trill” type USV for 3 different groups. Control group showed wider bandwith in USV compared to 60HDA, Haloperidol treated animals. Vertical bars represents the S.E.M. Symbols represents statistic significance between groups. p < 0.01.
4. Discussion
To have some insight into the postulated role of dopamine in human speech/voice, we analyzed changes in rat USV associated with unilateral infusion of the dopamine neurotoxin 6-OHDA and low doses of the dopamine antagonist haloperidol. Sexually experienced male rats were paired with estrous female rats and their USVs were recorded in a sound-treated chamber that isolated male rat calls. USVs were analyzed in terms of acoustic parameters for the most common type of call (‘trill’) for the control, 6-OHDA, and haloperidol conditions. Results indicated that bandwidth of the sonograms were significantly reduced in both dopamine-altered conditions. The data do not appear to reflect a decreased interest in the female, as all rats mounted the female and the time to mount was not significantly different between control and dopamine-altered conditions.
This is the first study to document a qualitative change in the acoustic signal of frequency-modulated 50-kHz calls as a result of interfering with dopamine synaptic transmission in rats. These findings may be useful since speech/voice deficits are prevalent in individuals with PD and the neural mechanisms underlying speech/voice disorders in PD are unclear [1, 2, 17, 20, 45]. Bradykinesia and hypokinesia associated with PD are the most frequently implicated causes of the speech-system disorders [20, 37, 45] and are likely linked with dopamine depletion. However, non-dopaminergic events, such as the depletion of non-dopaminergic neurons, cannot be ruled out [6]. Speech/voice impairment in PD presumably emerges as a result of faulty sensorimotor processing, which could lead to a reduced gain in the motor command for selecting and reinforcing movement amplitude [3, 16, 46]. These subtle changes in amplitude gain commands may interfere with shifting sets (high/low) and degrade the ability of the system to produce complex output (e.g. frequency modulation).
Dopamine has also been implicated in other speech/voice disorders involving the nigrostriatal system including speech changes associated with major depressive illness [19]. However, what complicates this clinical picture is the resistance of the speech motor system’s therapeutic response to drugs such as levodopa that target increasing dopamine levels. Again, non-dopaminergic neurons begin to degenerate early in Parkinson’s disease [6].
Previous research has shown that bilateral 6-OHDA-induced degeneration of dopamine neurons can diminish the number of calls [11]. Others have shown that haloperidol injected into the nucleus accumbens caused a significant increase in the number of 50-kHz calls, although this was related to injection site [43]. Additionally, data showed that haloperidol did not block the animal’s ability to produce 50-kHz calls, but may have reversed glutamatergic effect to the level of spontaneously emitted calls [50]. However, examining only the number of calls that occur in a situation may overlook important data concerning the degree of signal degradation of the calling. Although the number of calls per session was diminished in some of the animals following haloperidol, all were within the range of number of calls observed in previous habituation periods. The data do not appear to reflect simply a decrease in the salience of the female stimulus. The time to mount an estrous female was not statistically different between control and dopamine-altered conditions, although the mounting times for the control condition were more reliable and less variable. Sensorimotor feedback impairment, incentive salience, and fatigue influences cannot be ruled out as contributing factors, but seem insufficient to account for the reduced bandwidth and diminished trill structure of the acoustic signal while maintaining the absolute number of calls. It would be interesting to provide reinforcement to promote complex calling to determine whether numbers of calls and/or their complexity can be enhanced, and whether or not such rehabilitation “therapy” is most optimal when conducted under the influence of levodopa.
Although bandwidth was significantly reduced, one limitation to this work is low number of 6-OHDA treated rats. In our current paradigm, the rats moved freely in the test chamber. This varied the distance from the mouth to microphone, which in turn limited our ability to analyze sound pressure level adequately (although the sonograms hint that sound pressure was substantially reduced). It should be noted that only calls that occurred when the rats were upright facing the microphone and mouth-to-microphone distance was within 6–8 cm were included in the analysis. Thus, our results do not reflect acoustic changes as a function of distance from the microphone. Moreover, female calls could be excluded completely. The degradation in the acoustic signal therefore appears to be related to real changes in bandwidth. Future studies will include a method to stabilize mouth to microphone distance and include data on acoustic sound pressure level.
Based on our findings from rats treated unilaterally with 6-OHDA and with low-dose haloperidol, alteration of dopamine synaptic transmission appears to contribute to the degradation of the acoustic signal in rat 50-kHz USVs. Examining qualitative acoustic changes in rat USV may be useful for systematically investigating potential pharmacological, surgical, and behavioral treatments in dopamine-altered rats.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle Ciucci, Institute for Neuroscience, Department of Communication Sciences and Disorders, University of Texas, Austin, TX 78712 USA.
Teh-Sheng Ma, Institute for Neuroscience, University of Texas, Austin, TX 78712 USA.
Cynthia Fox, National Center for Voice and Speech, Denver, CO, Department of Neurology, University of Arizona, Tucson, AZ 85750 USA.
Jacqueline Kane, Department of Psychology, University of Texas, Austin, TX 78712 USA.
Lorraine Ramig, Department of Speech, Language, Hearing Sciences, University of Colorado-Boulder, Boulder, CO 80303 USA, National Center for Voice and Speech, Denver, Colorado 80204 USA.
Timothy Schallert, Department of Psychology, University of Texas, Austin, TX 78712 USA.
References
- 1.Ackermann H, Konczak J, Hertrich I. The temporal control of repetitive articulatory movements in Parkinson’s disease. Brain Lang. 1997;56:312–319. doi: 10.1006/brln.1997.1851. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann H, Ziegler W. Articulatory deficits in parkinsonian dysarthria: an acoustic analysis. J Neurol Neurosurg Psychiatry. 1991;54:1093–1098. doi: 10.1136/jnnp.54.12.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berardelli A, Rothwell JC, Thompson PD, Hallet M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124:2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- 4.Bialy M, Rydz M, Kaczmarek L. Procontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci. 2000;114:983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard RJ, Agullana R, McGee L, Weiss S, Blanchard DC. Sex differences in the incidence and sonographic characteristics of antipredator ultrasonic cries in the laboratory rat (Rattus norvegicus) J Comp Psychol. 1992;106:270–277. doi: 10.1037/0735-7036.106.3.270. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 7.Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- 8.Brudzynski SM, Bihari F, Ociepa D, Fu XW. Analysis of 22 kHz ultrasonic vocalization in laboratory rats: long and short calls. Physiol Behav. 1993;54:215–221. doi: 10.1016/0031-9384(93)90102-l. [DOI] [PubMed] [Google Scholar]
- 9.Brudzynski SM, Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiol Behav. 1992;52:655–660. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- 10.Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J Comp Psychol. 2002;116:73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- 11.Burgdorf J, Wood PL, Kroes R, Moskal JR, Panksepp J. The role of the mesolimbic dopamine system in reward related 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.03.010. in press. [DOI] [PubMed] [Google Scholar]
- 12.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- 13.Cohen BM, Lipinski JF. In vivo potencies of antipsychotic drugs in blocking alpha 1 noradrenergic and dopamine D2 receptors: implications for drug mechanisms of action. Life Sci. 1986;39:2571–2580. doi: 10.1016/0024-3205(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 14.Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res. 1969;12:246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- 15.Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in the dysarthrias. J Speech Hear Res. 1969;12:462–496. doi: 10.1044/jshr.1203.462. [DOI] [PubMed] [Google Scholar]
- 16.Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. Eur J Neurosci. 2004;19:2871–2880. doi: 10.1111/j.0953-816X.2004.03395.x. [DOI] [PubMed] [Google Scholar]
- 17.Estenne M, Hubert M, De Troyer A. Respiratory-muscle involvement in Parkinson’s disease. N Engl J Med. 1984;311(23):1516–1517. doi: 10.1056/NEJM198412063112314. [DOI] [PubMed] [Google Scholar]
- 18.Fleming SM, Delville Y, Schallert T. An intermittent, controlled-rate, slow progressive degeneration model of Parkinson’s disease: antiparkinson effects of Sinemet and protective effects of methylphenidate. Behav Brain Res. 2005;156:201–213. doi: 10.1016/j.bbr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Flint AJ, Black SE, Campbell-Taylor I, Gailey GF, Levinton C. Abnormal speech articulation, psychomotor retardation, and subcortical dysfunction in major depression. J Psychiatr Res. 1993;27:309–319. doi: 10.1016/0022-3956(93)90041-y. [DOI] [PubMed] [Google Scholar]
- 20.Fox CM, Morrison CE, Ramig LO, Sapir S. Current perspectives on the Lee Silverman Voice Treatment (LSVT) for individuals with idiopathic Parkinson disease. Amer J of Speech-Language Pathology. 2002;11:111–123. [Google Scholar]
- 21.Fu XW, Brudzynski SM. High-frequency ultrasonic vocalization induced by intracerebral glutamate in rats. Pharmacol Biochem Behav. 1994;49:835–841. doi: 10.1016/0091-3057(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 22.Fulceri F, Biagioni F, Lenzi P, Falleni A, Gesi M, Ruggieri S, Fornai F. Nigrostriatal damage with 6-OHDA: validation of routinely applied procedures. Ann N Y Acad Sci. 2006;1074:344–348. doi: 10.1196/annals.1369.032. [DOI] [PubMed] [Google Scholar]
- 23.Harel B, Cannizzaro M, Snyder PJ. Variability in fundamental frequency during speech in prodromal and incipient Parkinson’s disease: a longitudinal case study. Brain Cogn. 2004;56:24–29. doi: 10.1016/j.bandc.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Herrera-Marschitz M, Casas M, Ungerstedt U. Caffeine produces contralateral rotation in rats with unilateral dopamine denervation: comparisons with apomorphine-induced responses. Psychopharmacology. 1988;94:38–45. doi: 10.1007/BF00735878. [DOI] [PubMed] [Google Scholar]
- 25.Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behavioral Neurology. 1998;11:131–137. [PubMed] [Google Scholar]
- 26.Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 28.Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J Speech Hear Disord. 1978;43:47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- 29.Marshall JF. Somatosensory inattention after dopamine-depleting intracerebral 6-OHDA injections: spontaneous recovery and pharmacological control. Brain Res. 1979;177:311–324. doi: 10.1016/0006-8993(79)90782-0. [DOI] [PubMed] [Google Scholar]
- 30.McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiology & Behavior. 2003;80:81–88. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- 31.Meredith GE, Kang UJ. Behavioral models of Parkinson’s disease in rodents: a new look at an old problem. Mov Disord. 2006;21:1595–1606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- 32.Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzou P. Treatments for dysarthria in Parkinson’s disease. Lancet Neurology. 2004;3:547–556. doi: 10.1016/S1474-4422(04)00854-3. [DOI] [PubMed] [Google Scholar]
- 33.Ramig LO, Countryman S, O’Brien C, Hoehn M, Thompson L. Intensive speech treatment for patients with Parkinson’s disease: short-and long-term comparison of two techniques. Neurology. 1996;47:1496–1504. doi: 10.1212/wnl.47.6.1496. [DOI] [PubMed] [Google Scholar]
- 34.Ramig LO, Countryman S, Thompson LL, Horii Y. Comparison of two forms of intensive speech treatment for Parkinson disease. J Speech Hear Res. 1995;38:1232–1251. doi: 10.1044/jshr.3806.1232. [DOI] [PubMed] [Google Scholar]
- 35.Ramig LO, Sapir S, Countryman S, Pawlas AA, O’Brien C, Hoehn M, Thompson LL. Intensive voice treatment (LSVT) for patients with Parkinson’s disease: a 2 year follow up. J Neurol Neurosurg Psychiatry. 2001;71:493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramig LO, Sapir S, Fox C, Countryman S. Changes in vocal loudness following intensive voice treatment (LSVT®) in individuals with Parkinson’s disease: a comparison with untreated patients and normal age-matched controls. Mov Disord. 2001;16:79–83. doi: 10.1002/1531-8257(200101)16:1<79::aid-mds1013>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 37.Sapir S, Ramig LO, Fox C. The Lee Silverman Voice Treatment [LSVT®] for Voice, Speech, and Other Orofacial Disorders in People with Parkinson’s Disease. Future Neurology. 2006;1:563–570. [Google Scholar]
- 38.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 39.Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich DF, Dean RL III, Sanberg PR, editors. Central Nervous System Diseases: Innovative models of CNS diseases from molecule to therapy. Totowa, NJ: Humana Press; 2000. pp. 131–151. [Google Scholar]
- 40.Schallert T, Woodlee MT. Orienting and placing. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat. New York: Oxford University Press; 2005. pp. 129–140. [Google Scholar]
- 41.Schulz GM. The effects of speech therapy and pharmacological treatments on voice and speech in Parkinson s disease: a review of the literature. Curr Med Chem. 2002;9:1359–1366. doi: 10.2174/0929867023369808. [DOI] [PubMed] [Google Scholar]
- 42.Stewart C, Winfield L, Hunt A, Bressman SB, Fahn S, Blitzer A, Brin MF. Speech dysfunction in early Parkinson’s disease. MovDisord. 1995;10:562–565. doi: 10.1002/mds.870100506. [DOI] [PubMed] [Google Scholar]
- 43.Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trail M, Fox C, Ramig LO, Sapir S, Howard J, Lai EC. Speech treatment for Parkinson’s disease. NeuroRehabilitation. 2005;20:205–221. [PubMed] [Google Scholar]
- 46.Turner RS, Grafton ST, McIntosh AR, DeLong MR, Hoffman JM. The functional anatomy of parkinsonian bradykinesia. Neuroimage. 2003;19:163–179. doi: 10.1016/s1053-8119(03)00059-4. [DOI] [PubMed] [Google Scholar]
- 47.Ungerstedt U. Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol Scand Suppl. 1971;367:49–68. doi: 10.1111/j.1365-201x.1971.tb10999.x. [DOI] [PubMed] [Google Scholar]
- 48.Ungerstedt U, Arbuthnott G. Quantitative recording of rotational behavior in rats after 6-0HDA lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 49.Weisz DJ, Yang BY, Fung K, Amirali A. The mechanism of ultrasonic vocalization in rat. Soc Neurosci. 2001;Abstract 27:#88.19. [Google Scholar]
- 50.Wintink AJ, Brudzynski SM. The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacol Biochem Behav. 2001;70:317–323. doi: 10.1016/s0091-3057(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 51.Zemlin WR. Speech and Hearing Science Anatomy and Physiology. Needham Heights: Allyn & Bacon; 1998. pp. 144–196. [Google Scholar]
- 52.Zigmond JM, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensatory responses to partial loss of dopaminergic neurons: studies with 6-hydroxydopamine. In: Schneider JS, Gutpa M, editors. Current Concepts in Parkinson’s Disease Research. Hogrefe & Huber Publishers; Toronto: pp. 99–140. [Google Scholar]


