Abstract
Hemoglobin (Hb) released during hemolysis is a potent oxidant. Extracorpuscular Hb may enter the vessel wall and mediate low-density lipoprotein oxidation, thereby promoting the development and progression of atherosclerosis. Haptoglobin (Hp) is an antioxidant protein as a result of its ability to bind Hb and block Hb-induced oxidative damage. Hp also facilitates the removal of Hb from the extravascular compartment via the CD163 macrophage scavenger receptor. In man, there are two common alleles for Hp denoted 1 and 2, and correspondingly, three different possible genotypes: Hp1-1, Hp2-1, and Hp2-2. We have recently demonstrated in several longitudinal studies that Hp genotype is an independent risk factor for diabetic vascular complications. Specifically, we have shown that diabetic individuals with Hp 2-2 are more likely to develop nephropathy, retinopathy, and cardiovascular disease as compared with those with Hp2-1 or Hp1-1. Mechanistically, we have found significant Hp type differences in the antioxidant and CD163-mediated scavenging and activation functions of the different Hp protein types. Furthermore, we have demonstrated that these functions are modified in the diabetic state. In this review, we focus on the clinical studies associating the Hp polymorphism and diabetic vascular complications, and the molecular basis behind this interaction.
Keywords: diabetes, cardiovascular disease, haptoglobin polymorphism, hemoglobin, oxidative stress
Introduction
The overwhelming public health burden of diabetes mellitus (DM) is due in large part to the development of late vascular complications. The most prevalent of these complications is atherosclerotic cardiovascular disease (CVD). The percentage of total deaths from CVD in type 1 and type 2 diabetes has progressively increased so that more than 75% of all deaths among diabetics are directly attributable to CVD (Bierman 1992; Grundy et al 1999). Approximately 35% of diabetes type 1 patients die from CVD before the age of 55 (Krolewski et al 1987). Prospective epidemiologic studies of individuals at risk for CVD yield consistent temporal relationships between diabetes and both incident CVD and mortality (Resnick and Howard 2002). Diabetes increases the risk of CVD 2- to 5-fold compared with the general population (Kannal and McGee 1979; Stamler et al 1993; Howard et al 1999). The overall prevalence of CVD is over 55% in adults with diabetes as compared with 2%–4% of the general population. The rate of cardiovascular mortality is more than doubled in men and more than quadrupled in women with diabetes compared with nondiabetic individuals (Carroza et al 1993).
In addition to independent risk factors for CVD attributable to the diabetic state, such as insulin resistance, hyperinsulinemia, and hyperglycemia, several other known risk factors for CVD are more common in diabetics than the general population. These comorbid risk factors frequently associated with diabetes include hypertension, central obesity, dyslipidemia, family history of CVD, endothelial dysfunction, and coagulation abnormalities (Haydan and Reaven 2000; Sowers et al 2001). The development of diabetic vascular complications is related to age of onset of hyperglycemia, the duration of the disease, and the degree of glycemic control. Intensive therapy aiming to maintain blood glucose levels close to the normal range has been shown to effectively delay the onset and slow the progression of diabetic vascular complications (The Diabetes Control and Complications Trial Research Group 1993). However, the contribution of hyperglycemia and all conventionally measured risk factors together can account for no more than 25% of the excess CVD risk in diabetes (Pyorala et al 1987). Moreover, a significant percentage of diabetics have been shown to be resistant to the development of vascular complications in spite of poor glycemic control.
There exists a growing body of evidence that diabetic vascular disease develops only in those patients who are genetically susceptible (Chowdhury et al 1995, 1999; Parving et al 1996; Ruiz 1997; Marre 1999). Hyperglycemia appears to be a necessary but not sufficient condition for the development of diabetic vascular disease. Family studies have supported the hypothesis of a genetic predisposition for diabetic complications by demonstrating a familial clustering of diabetic nephropathy and CVD. Additionally, it has been demonstrated that there exist significant ethnic differences in the incidence of CVD in subjects with diabetes that could not be explained by differences in conventional cardiovascular risk factors between these ethnic groups (UK Prospective Diabetes Study Group 1998). Analysis of population groups, such as the Pima Indians, has shown markedly higher rates of diabetic retinopathy and nephropathy than would be expected based on the known risk factors (Imperatore et al 1998). Various candidate genes with known functional polymorphisms have been examined regarding their ability to demonstrate an association between CVD and diabetes, including the genes for paraxonase, angiotensin-converting enzyme, glutathione peroxidase, and superoxide dismutase (Ruiz et al 1995; Tarnow et al 2000). We have recently demonstrated in several prospective and cross-sectional population studies that a polymorphism in the haptoglobin (Hp) gene is an independent risk factor for diabetic vascular disease.
Haptoglobin polymorphism
Hp is a Hb-binding serum protein. It is an acute phase protein synthesized mainly in the liver and found at levels of 30–300 mg/dL in normal human serum (Katnik and Jadach 1996). Hp serum levels are increased up to 3- to 8-fold during the acute phase reaction and in response to injury (Dobryszycka 1997). The hepatic synthesis of Hp is induced by interleukin-6 (IL-6), IL-1 and tumor necrosis factor-α (TNF-α). Hp is also expressed in specific nonhepatic cells, including adipocytes and lung cells, and its levels are increased after inflammation similar to that observed in hepatocytes (Kalmovarin et al 1991). Moreover, Hp is highly expressed in arteries after sustained flow changes induced by shear stress and nitric oxide, which influence IL-6 expression. Arterial Hp is believed to play a role in cell migration and arterial restructuring (Smeets et al 2002).
In man, there are two alleles for Hp, denoted 1 and 2, giving rise to three major phenotypes. Individuals homozygous for the 1 allele express the Hp1-1 phenotype at the protein level. Individuals homozygous for the 2 allele express the Hp2-2 phenotype, whereas heterozygotes express the Hp2-1 phenotype. The Hp gene locus on chromosome 16q22 consists of 5 exons encoding the 1 allele, or 7 exons encoding the 2 allele. The two alleles appear to have been generated from the 1 allele by an intragenic duplication of exons 3 and 4, presumably by a nonhomologous DNA crossing-over event (Maeda et al 1984). Hp is synthesized as a single chain which is cleaved to an amino-terminal α-chain and a carboxy-terminal β-chain, linked by disulfide bonds (Raugei et al 1983). The β-chain is identical in both Hp alleles, while the α-chains differ. The α-chain of the Hp1 allele binds one β-chain and one other α-chain, both by disulfide bonds. Therefore, subjects with the Hp1-1 genotype produce a single (αβ)2 homodimer with a molecular weight of 86 KDa. As the cysteine forming the intermolecular disulfide bond between the α-chains is duplicated in the 2 allele, human's homozygotes for the 2 allele (Hp2-2) have multimeric cyclic haptoglobin molecules with a molecular weight of 170–900 KDa. Individuals with the Hp2-1 genotype demonstrate a variety of linear homodimers and multimers with a molecular weight of 86–300 KDa. Each β-chain of Hp binds an αβ-dimer of Hb to form the Hp-Hb complex (Wejman et al 1984) (Figure 1).
Figure 1.
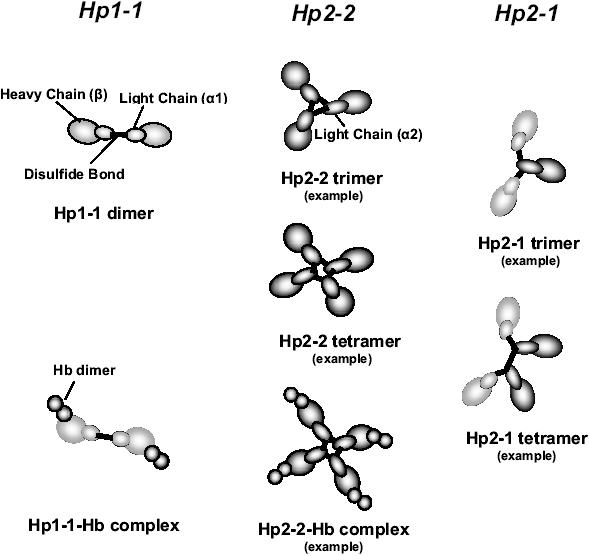
Subunit organization of Hp. The Hp1 monomer can bind just one other monomer to form a homodimer in Hp1-1 individuals or as one of the different possible complexes in Hp2-1 individuals. Hp2 monomer can bind two monomers to form a variety of cyclic multimers in Hp2-2 or a variety of linear complexes in Hp2-1 individuals. In the Hp-Hb complex, each β subunit of the Hp monomer binds an αβ-dimer of Hb.
Hp protein phenotypes are easily distinguished by nondenaturing gel electrophoresis of Hb-enriched serum. The Hp-Hb complex in the gel is identified by virtue of its peroxidase activity, and each Hp type gives rise to a signature banding pattern. The Hp type can also be determined from DNA by PCR (Koch et al 2002), and there exists complete correspondence between the Hp type assigned by the two methods. There exist marked geographic differences in the distribution of the Hp phenotype in different populations. The highest incidence of the Hp1 allele has been reported in Africa and South America (up to 92% in Mexican Lacandon), and the lowest in Southeast Asia (minimum 7% in Indian Irulas) (Langlois and Delanghe 1996). There are no differences in frequency of the two Hp alleles in diabetics and nondiabetics in any given population.
Haptoglobin function
The best known function of Hp is to bind free Hb released into the plasma to form a stable Hp-Hb complex. The two proteins form a complex with extremely high affinity (KD estimated to be greater than 10−15 M) (Hwang and Greer 1980; McCormick and Atassi 1990).
Intravascular hemolysis is a physiological phenomenon but may be increased in certain pathological conditions. Normally, senescent erythrocytes approaching the end of their 120-day lifespan are degraded in the spleen, liver, or bone marrow, but about 10% of red cell breakdown (about 650 mg per day or about 27 mg per hour) occurs within blood vessels (Garby and Noyes 1959). Free Hb in the circulation passes through the glomeruler filter resulting in renal damage. Furthermore, free Hb is involved in a variety of toxic side effects. Free Hb is considered as an extremely potent pro-oxidant agent and can catalyze various oxidative and peroxidative reactions, such as the Fenton reaction resulting in the generation of hydroxyl radical (Everse and Hsia 1997). Hp plays an essential role in capturing free Hb, thus preventing its deposition in the glomeruli and proximal tubule cells of the kidney. Moreover, Hp functions as an antioxidant through its ability to bind Hb and thereby preventing oxidative tissue damage mediated by free Hb. In vitro, Hp has been demonstrated to inhibit Hb-induced linolenic acid and low-density lipoprotein (LDL) oxidation (Gutteridge 1987; Miller et al 1997). Hp knockout mice are more prone to oxidative tissue damage (such as renal damage and endothelial dysfunction) and mortality following hemolysis, demonstrating the importance of Hp in the physiological defense against Hb toxicity (Lim et al 1998).
Most of the Hp-Hb complexes (90%) are taken up by hepatocytes through a poorly characterized transmembrane receptor. Recently, a specific high-affinity scavenger receptor for the Hp-Hb complex has been identified exclusively on monocytes and macrophages (Kristiansen et al 2001). This specific receptor-ligand interaction leads to uptake of Hp-Hb complexes from plasma as well as from injured or inflammated tissues (such as the vessel wall) within which free Hb may sieve. The specific binding of the Hp-Hb complex to CD163 leads to endocytosis and degradation of the complex inside the lysosome. Hb degradation leads to globin proteolysis and globin-derived heme conversion to iron and bilirubin (Gravensen et al 2002).
CD163 is present on the surface of 15%–30% of freshly isolated monocytes and virtually all macrophages (Hogger et al 1998). The expression of CD163 is significantly increased in the late phase of the inflammatory process (Zwadlo et al 1987). CD163 expression is upregulated in response to antiinflammatory factors, such as glucocorticoids and IL-10 and in response to proinflammatory cytokines such as IL-6 and GM-CSF. Its expression is down-regulated by proinflammatory cytokines, such as lipopolysacharide, interferon-γ (INF-γ), and TNF-α (Buechler et al 2000).
Cross-linking of CD163 expressed on macrophages with monoclonal antibody has been shown to produce a protein tyrosine kinase-dependent signal that leads to secretion of proinflammatory cytokines such as IL1-β, IL-6, and GMCSF (Van Den Heuvel et al 1999). Moreover, CD163 receptor contributes to the adhesion of monocytes to activated endothelial cells (Wenzel et al 1996). On the other hand, Philippidis et al have shown that CD163 activation is linked to antiinflammatory effector pathways. They have demonstrated that Hp-Hb complex, bound to CD163, triggers IL-10 release and heme oxygenase-1 protein induction in human macrophages in vitro and in tissue macrophages ex vivo (Philippidis et al 2004).
There exists considerable in vitro and in vivo data supporting differences in the antioxidant, scavenging, and immunomodulatory properties between the different Hp types. Individuals with the Hp2-2 phenotype have been found to have significantly lower levels of serum vitamin C (Langlois et al 1997), higher serum iron, and higher circulating oxidized LDL (oxLDL)/LDL ratios compared with those with the Hp2-1 or the Hp1-1 type (Delanghe and Langlois et al 2002; Brouwers et al 2004). In our laboratory, we have demonstrated that there are functional differences in the antioxidant capacity of the different Hp types towards Hb-driven oxidation of linolenic acid and LDL (Melamed-Frank et al 2001). Regarding the Hp-Hb complex CD163 interaction, we and others have demonstrated several differences between Hp1-1-Hb and Hp2-2-Hb complexes. First, Hp2-2-Hb complexes exhibit a 10-fold higher affinity to CD163 compared with the Hp1-1-Hb complexes. Second, we have recently found that the rate of clearance of the Hp1-1-Hb by CD163 is markedly greater than that of Hp2-2-Hb. Third, we have shown that Hp2-2-Hb complex is superior to Hp1-1-Hb complex in activating the CD163 receptor and results in more inositol tri-phosphate (IP3) production and greater intracellular calcium mobilization (Asleh et al 2003). Finally, Philippidis et al (2004) have recently demonstrated that Hp1-1-Hb complexes result in a greater production of the antiinflammatory cytokine IL-10 as compared with Hp2-2-Hb complexes.
Clinical studies in humans associating Hp phenotype and diabetic vascular disease
Several reports from our laboratory have established a strong association between the Hp phenotype and diabetic vascular complications. Hp1-1 confers significant protection, Hp2-1 confers partial protection, and Hp2-2 can be regarded as a major risk factor for vascular complications in diabetics.
Nephropathy, retinopathy, and restenosis after angioplasty
Our initial studies with the Hp polymorphism and diabetic complications were from three cross-sectional studies done in Israel. We demonstrated that the Hp1-1 phenotype was associated with a marked decrease in the prevalence of diabetic nephropathy (DN), diabetic retinopathy (DR), and restenosis after percutaneous transluminal coronary angioplasty (PTCA).
Diabetic nephropathy
This study included 110 consecutive normotensive type 1 and type 2 diabetics. The difference in the incidence of DN between patients with the three Hp phenotypes was statistically significant for type 1 diabetic patients and for all diabetic patients combined. Of the 54 type 1 diabetics, 13 demonstrated DN. None (0/13) of the patients with Hp1-1 phenotype had DN, whereas 5 of 22 (23%) with Hp2-1 and 8 of 21 (38%) with Hp2-2 had evidence of DN (p < 0.04). For both types of diabetes combined, none (0/18) of the Hp1-1 patients showed any sign of nephropathy, as compared with 10 of 37 (27%) with Hp2-1 and 19 of 55 (34%) with Hp2-2 (p < 0.02).
There were 15 patients with macroalbuminuria in this study. The risk of developing macroalbuminuria was found to be significantly correlated with Hp phenotype for both types of diabetes combined. None (0/18) of the Hp1-1 patients had macroalbuminuria, whereas 3 of 37 (8%) patients with the Hp2-1 and 12 of 55 (22%) of the patients with Hp2-2 had macroalbuminuria (p < 0.03). This study demonstrated a graded-risk correlation between presence, as well as severity, of DN and Hp phenotype (Levy et al 2000; Nakhoul et al 2001).
Diabetic retinopathy
This study included 52 consecutive patients with type 1 diabetes, of which 25 had evidence of DR. There was significantly lower prevalence of DR in patients with the Hp1-1 compared with patients with the Hp2-1 and Hp2-2 phenotypes. Only 1 of 12 patients (8%) with Hp1-1 phenotype had DR, whereas 12 of 20 (60%) with Hp2-1 and 12 of 20 patients (60%) with Hp2-2 had evidence of DR (p < 0.002). This demonstrated that Hp1-1 diabetics are provided with increased protection against the development of DR compared with the other Hp phenotypes (Nakhoul et al 2000).
Restenosis after PTCA
Three studies investigating the relationship between the Hp type and restenosis have been reported (Roguin et al 2001, 2002, 2003). In two of these studies (Roguin et al 2001, 2002) we found a significantly reduced incidence of restenosis in Hp1-1 diabetic individuals, while the third study (Roguin et al 2003) failed to demonstrate any difference in the incidence of restenosis as a function of Hp type.
Cardiovascular disease
We have established in four independent prospective longitudinal studies and 1 large cross-sectional population study that Hp genotype is an independent risk factor for CVD and that this relationship is specific for DM. Taken together these five studies suggest that Hp genotype may be useful in predicting which patients with diabetes will develop CVD.
Strong Heart Study
The goal of this study was to determine if the Hp phenotype was predictive of CVD in diabetes. The Strong Heart Study (SHS) was a population-based longitudinal study of CVD in American Indians. We analyzed the Hp phenotype in a matched case-control study of stored serum samples from the SHS. In multivariate analyses controlling for conventional CVD risk factors and DM characteristics, Hp phenotype was a highly statistically significant predictor of CVD in DM (Table 1). The odds ratio of having CVD in DM with the haptoglobin phenotype 2-2 was 5 times greater than that in DM with the Hp1-1 phenotype (p = 0.002). An intermediate risk of CVD was associated with the Hp2-1 phenotype. No significant association between Hp type and CVD risk was found in nondiabetic individuals (Levy et al 2002).
Table 1.
Relative risk of incident CVD according to DM status and Hp type in the Strong Heart Study adjusted for all CVD risk factors and DM characteristics
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| DM and Hp 2-1 (vs DM and Hp 1-1) | 1.63 | 0.74–3.63 | 0.228 |
| DM and Hp 2-2 (vs DM and Hp 1-1) | 4.96 | 1.85–13.33 | 0.002 |
| DM and Hp 2-2 (vs DM and Hp 2-1) | 3.04 | 1.30–7.09 | 0.010 |
| No DM, Hp 2-1 (vs no DM, Hp 1-1) | 1.46 | 0.44–4.87 | 0.542 |
| No DM, Hp 2-2 (vs no DM, Hp 1-1) | 2.73 | 0.81–9.26 | 0.107 |
| No DM, Hp 2-2 (vs no DM, Hp 2-1) | 1.88 | 0.73–4.80 | 0.190 |
Source: Data adapted from Levy et al (2002).
Abbreviations: CVD, cardiovascular disease; DM, diabetes mellitus; Hp, haptoglobin; OR, odds ratio; CI, confidence interval.
Munich Stent Study
The goal of this study was to determine if the Hp genotype was predictive of major adverse cardiac events after coronary artery stenting in individuals with diabetes. A consecutive series of 935 treated (oral agents and/or insulin) diabetic patients were followed for 1 year after stenting for major adverse cardiac events (MACE) defined as target vessel revascularization (TVR), myocardial infarction (MI), and death. In multivariate analysis controlling for all known determinants of outcome after stent placement we found that Hp genotype was a highly significant independent predictor of major adverse cardiac events in the 1-year period following stent placement in individuals with diabetes. We found significant differences in the risk of developing MI and in the need for TVR in the 1-year period following stent placement (Roguin et al 2003) (Table 2).
Table 2.
Incidence of major adverse cardiac events in the one year follow-up of 935 consecutive diabetic patients undergoing coronary artery stent placement
| Hp 1-1 | Hp 2-1 | Hp 2-2 | p | |
|---|---|---|---|---|
| n | 129 | 424 | 382 | |
| Death | 7 (5.4%) | 28 (6.6%) | 15 (3.9%) | 0.22 |
| Acute MI | 0 (0%) | 20 (4.7%) | 32 (8.4%) | <0.0001 |
| TVR | 21 (16.3%) | 82 (19.3%) | 91 (23.8%) | 0.040 |
| MACE | 27 (20.9%) | 112 (26.4%) | 120 (31.4%) | 0.015 |
Source: Data adapted from Roguin et al (2003).
Abbreviations: MI, myocardial infarction; TVR, target vessel revascularization; MACE, major adverse cardiac events.
Acute myocardial infarction
The goal of this study was to determine if the Hp phenotype was predictive of MI size and adverse cardiac events in the peri-infarct period in patients with DM. Hp phenotype was determined in a consecutive series of over 620 individuals presenting with acute MI to the coronary intensive care unit (224 with DM) of the Rambam Medical Center in Haifa, Israel. Major adverse cardiac events (death, reinfarction, and revascularization) were prospectively identified occurring within 30 days. MI size and the severity of left ventricular dysfunction were assessed by peak creatine kinase, ECHO, and Killup score. We found a significant interaction effect between Hp type and diabetes in predicting mortality and major adverse cardiac events at 30 days. Specifically, the mortality rate in DM Hp1-1 patients was significantly less than in DM patients with the Hp2 allele, but there was no difference between the Hp types in these outcomes in nondiabetic individuals. For the combined MACE end point the relative risk was 5 times greater in diabetic Hp2-2 individuals as compared with diabetic Hp1-1 individuals (p < 0.0001) (Table 3). There was no difference in mortality or in the combined endpoint according to Hp type in nondiabetic patients. Diabetic patients with Hp1-1 also had significantly smaller and less severe myocardial infarctions as compared with diabetic patients with Hp2-1 or Hp2-2 (Suleiman et al 2003).
Table 3.
Incidence of major adverse cardiac events at 30 days in 224 consecutive diabetic patients presenting with myocardial infarction
| Hp 1-1 | Hp 2-1 | Hp 2-2 | p-value | |
|---|---|---|---|---|
| n | 28 | 83 | 113 | |
| Death | 0 | 19 (23%) | 28 (25%) | 0.01 |
| Re-infarction | 0 | 2 (2%) | 6 (5%) | 0.31 |
| Revascularization | 3 (11%) | 23 (28%) | 35 (31%) | 0.09 |
| Composite | 3 (11%) | 42 (51%) | 62 (55%) | <0.0001 |
Source: Data adapted from Suleiman et al (2003).
HOPE (Heart outcomes prevention evaluation)
The HOPE study evaluated the effects of daily administration of 400 IU of vitamin E and/or ramipril for 4.5 years on the development of MI, stroke, and cardiovascular death in people with CVD or diabetes plus 1 other risk factor (Yusuf et al 2000). In diabetic patients receiving double placebo (no ramipril or vitamin E), Hp phenotype was a significant predictor of the primary composite outcome (Hp1-1 20.8%, Hp2-1 16.4 %, Hp2-2 31.9%, p = 0.03). The effect of antioxidants on this risk will be discussed below.
Framingham Offspring Cohort
We sought to examine the relationship between Hp type, diabetes status, and coronary artery disease in a cross-sectional analysis of a large community-based cohort from the Framingham Heart Offspring Study (Kannal and McGee 1979). We determined the Hp phenotype in over 3200 participants of the study who attended the seventh examination of the study. This study therefore examined CVD prevalence (since this was a cross-sectional study) rather than incidence of CVD in individuals with and without diabetes segregated by Hp type. We demonstrated that there was a significant difference in the nature of association between Hp type and CVD in individuals with diabetes and those without diabetes (p = 0.01 for interaction term of Hp type and diabetes) (Levy et al 2004).
Molecular basis for the interaction between the Hp phenotype and diabetic CVD
The clinical studies reported above demonstrated that the polymorphism in the Hp gene is an independent risk factor for the development of CVD in individuals with diabetes. No relationship was found between the Hp type and the risk for CVD in the nondiabetic population. We have sought to understand the molecular mechanism whereby the Hp polymorphism confers different susceptibility to CVD and the reason this interaction is specific for the diabetic state.
A substantial body of evidence supports the hypothesis that oxidative modification of LDL or other oxidative events within the blood vessel wall, promotes the development and progression of atherosclerotic lesions leading to CVD. Among several molecular components that function as active catalyzers of LDL oxidation, free Hb is a potent oxidant of LDL by the Fenton reaction (Gutteridge 1987; Bamm et al 2003). As Hb concentration in the red cells is in the millimolar range, a minimal cell rupture suffices to achieve local micromolar concentrations of free Hb. At sites of endothelial injury, Hb may sieve into the subendothelial space catalyzing the oxidation of LDL. Fernandez and colleagues (2001) have shown that intravascular hemolysis increases oxidative stress and atherogenicity of diet-induced hypercholesterolemia in rabbits. In the diabetic condition, the erythrocyte membrane demonstrates structural and functional modifications, resulting in a reduced lifespan and an increase in its turnover (Venerando et al 2002). Furthermore, diabetes is associated with accelerated endothelial cell dysfunction and injury (Laight et al 1999), thus the amount of Hb penetrating into the blood vessel wall may be significantly increased. Hp, which is found in more than 400 molar excess compared with free Hb, can bind rapidly free intravascular Hb released in the circulation and prevent Hb-driven oxidation. However, this may not be true within the vessel wall where Hp is not normally found.
The antioxidant function of Hp appears to be inhibited in the diabetic state. We have recently demonstrated that the oxidation of LDL mediated by glycosylated Hb (GlyHb), a fraction that is markedly increased in diabetes, is not completely blocked by the binding of Hp. Therefore, unlike the Hp-Hb complex, the GlyHb-Hp complex is a potent potential oxidant of LDL within the vessel wall. It is critical in the diabetic state, where there is an increase in hemolysis, GlyHb concentration and endothelial injury, to capture and clear Hb-Hp complexes from the subendothelial space as rapidly as possible to minimize their oxidative potential and the atherogenic damage that they may cause. Indeed as discussed above we have shown that Hp1-1-Hb complexes are more rapidly scavenged as compared with the Hp2-2-Hb complexes. We have therefore proposed that the interaction between these processes results in significantly less oxidative damage within the vessel wall in diabetic individuals with Hp1-1 phenotype compared with those with Hp2-2 phenotype (Asleh et al 2003).
Several additional mechanisms have been identified that may be important in understanding the differential susceptibility to CVD in diabetic subjects with the different Hp types. First, the Hp polymers present in individuals with 1-1, 2-1, or 2-2 phenotypes differ dramatically in their shape and size. These differences confer differential sieving potential to enter the subendothelial space, where Hp is needed in order to neutralize the harmful oxidizing effect of free Hb that may be released at sites of vascular injury. Second, recent studies have highlighted the role of immune cells and inflammatory mediators in the initiation, growth, and rupture of atherosclerotic plaques (Greaves and Channon 2002). We have demonstrated marked differences between the two Hp types (Hp2-2 is more potent than Hp1-1) in two distinct markers of macrophage activation via CD163 receptor. We propose that these differences in cell activation may result in high production of inflammatory mediators implicated in atherosclerosis. Third, we found a profound and significant reduction in the percentage of monocytes expressing the surface CD163 in patients with diabetes, resulting in a decrease in the ability to scavenge the Hp-Hb complex in the diabetic state (Asleh et al 2003).
Recently, we have demonstrated a correlation between Hp phenotype and the amount of catalytically active redox active iron in the diabetic state. Iron has been hypothesized to be of significant importance in morbidity and mortality from atherosclerotic CVD, due to its strong oxidative activity by the Fenton reaction (Sullivan 1981; Fu et al 1998). It has been shown that iron induces cellular lipid peroxidation in macrophages, thus increasing cell-mediated LDL oxidation (Fuhrman et al 1994). Over 99% of the iron in the body is sequestered in specialized cells (over 75% is found in Hb inside the red cells), transport or storage proteins (transferring or ferritin), in which iron is not redox active and incapable of generating free radicals. It has been suggested that redox active iron, rather than total body iron status, is more likely to be associated with lipid peroxidation and clinically manifested atherosclerosis (Shah and Alam 2003). Redox active serum iron may exist in many forms, such as iron bound to albumin or citrate; iron bound to glycosylated proteins, which have an increased affinity for iron; or Hb-derived iron delocalized outside of the hydrophobic heme pocket as occurs when Hb is oxidized (Puppo and Halliwell 1988; Ferrali et al 1997).
We have demonstrated Hp type dependent differences in the amount of serum redox active iron activity in the diabetic condition both in vitro and in vivo. First, we have shown a dramatic increase in the amount of chelatable redox active iron associated with Hp2-2-Hb as compared with Hp1-1-Hb complexes in vitro. Second, we have demonstrated marked diabetes dependent differences in the amount of redox active iron present in the plasma of mice genetically modified to express the Hp2 allele as compared with mice expressing the Hp1 allele (wild type). Finally, we have shown that redox active iron is increased in the plasma of humans with the Hp2-2 phenotype. These findings provide further clues as to why diabetic individuals carrying the Hp2-2 phenotype have an increased incidence of diabetic vascular disease.
Future directions
An increase in oxidative stress has been proposed to be of fundamental importance in the development of diabetic microvascular and macrovascular complications (Nishikawa et al 2000). We have proposed a new pathway for generation of this oxidative stress involving Hp type dependent differences in Hb-induced damage in the vessel wall in diabetic patients. Based on this model, we suggest that antioxidant therapy may be more beneficial in diabetic patients with the Hp2-2 phenotype as compared with those with the Hp1-1 phenotype. Several recent reports failed to demonstrate any benefit from antioxidant supplementation with vitamin E, alone or in combination with other antioxidant vitamins, in reducing the incidence of major adverse cardiovascular events in people with or without diabetes (Yusuf et al 2000; MRC/BHF Heart Protection Study 2002). The Heart Outcomes Prevention Study (HOPE) trial was one such study that did not demonstrate any cardiovascular benefit of daily administration of 400 IU of vitamin E for 4.5 years in either the overall study population and in study participants with diabetes. We were able to determine the relative risk reduction associated with vitamin E therapy according to Hp type in patients with and without diabetes in all of the Canadian participants of the HOPE study (3176 participants). We found that there was no benefit of vitamin E supplementation detected in either the entire group or in the diabetic subset. However, in diabetic participants with Hp2-2, vitamin E supplementation was associated with a statistically significant reduction in cardiovascular death (relative risk 0.45, 95% confidence interval (CI) 0.23–0.90) and non-fatal myocardial infarction (relative risk 0.57, 95% CI 0.33–0.97). While clearly these findings need to be confirmed prospectively, they may suggest that antioxidant therapy with vitamin E may reduce cardiovascular morbidity and mortality in diabetic patients with Hp2-2. Moreover, we have demonstrated that redox active iron is also increased in Hp2-2 diabetic individuals, contributing to the increased oxidative stress in this subgroup. As this redox active iron is chelatable, these findings suggest that iron chelators may be beneficial in reducing CVD in diabetic population with Hp2-2 phenotype. If antioxidant supplementation, such as vitamin E or iron chelators or a combination of both, is validated prospectively, Hp phenotyping may become a useful tool to identify diabetic individuals for whom antioxidant therapy may be effective in reducing the incidence and severity of CVD.
Acknowledgments
This work was supported by grants from the Rappaport Family Medical Research Fund, Kennedy Leigh Charitable Trust, Binational Science Foundation, Israel Science Foundation, D Cure Diabetes Care in Israel, and the Russell Berrie Foundation (APL).
References
- Asleh R, Marsh S, Shilkrut M, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92:1193–200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- Bamm VV, Tsemakhovich VA, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin-hemichrome. Int J Biochem Cell Biol. 2003;35:349–58. doi: 10.1016/s1357-2725(02)00255-8. [DOI] [PubMed] [Google Scholar]
- Bierman EL. George Lyman Duff Memorial Lecture. Atherogenesis in diabetes. Arterioscler Thromb. 1992;12:647–56. doi: 10.1161/01.atv.12.6.647. [DOI] [PubMed] [Google Scholar]
- Brouwers A, Langlois M, Delanghe J, et al. Oxidized low-density lipoprotein, iron stores, and haptoglobin polymorphism. Atherosclerosis. 2004;176:189–95. doi: 10.1016/j.atherosclerosis.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Buechler C, Ritter M, Orso E, et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- Carrozza JP, Kuntz RE, Fishman RF, et al. Restenosis after arterial injury caused by coronary stenting in patients with diabetes mellitus. Ann Intern Med. 1993;118:344–9. doi: 10.7326/0003-4819-118-5-199303010-00004. [DOI] [PubMed] [Google Scholar]
- Chowdhury TA, Dyer PH, Kumar S, et al. Genetic determinants of diabetic nephropathy. Clin Sci. 1999;96:221–30. [PubMed] [Google Scholar]
- Chowdhury TA, Kumar S, Barnett AH, et al. Nephropathy in type 1 diabetes: the role of genetic factors. Diabet Med. 1995;12:1059–67. doi: 10.1111/j.1464-5491.1995.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Delanghe JR, Langlois MR. Haptoglobin polymorphism and body iron stores. Clin Chem Lab Med. 2002;40:212–16. doi: 10.1515/CCLM.2002.035. [DOI] [PubMed] [Google Scholar]
- Dobryszycka W. Biological functions of haptoglobin – new pieces to an old puzzle. Eur J Clin Chem Clin Biochem. 1997;35:647–54. [PubMed] [Google Scholar]
- Everse J, Hsia N. The toxicities of native and modified hemoglobins. Free Radic Biol Med. 1997;22:1075–99. doi: 10.1016/s0891-5849(96)00499-6. [DOI] [PubMed] [Google Scholar]
- Fernandez AZ, Lopez F, Tablante A, et al. Intravascular hemolysis increases atherogenicity of diet-induced hypercholesterolemia in rabbits in spite of heme oxygenase-1 gene and protein induction. Atherosclerosis. 2001;158:103–11. doi: 10.1016/s0021-9150(01)00422-1. [DOI] [PubMed] [Google Scholar]
- Ferrali M, Signorini C, Caciotti B, et al. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997;416:123–9. doi: 10.1016/s0014-5793(97)01182-4. [DOI] [PubMed] [Google Scholar]
- Fu S, Davies MJ, Stocker R, et al. Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem J. 1998;333:519–25. doi: 10.1042/bj3330519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman B, Oiknine J, Aviram M. Iron induces lipid peroxidation in cultured macrophages, increases their ability to oxidatively modify LDL, and affects their secretory properties. Atherosclerosis. 1994;111:65–78. doi: 10.1016/0021-9150(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Garby L, Noyes WD. Study on hemoglobin metabolism II. Pathways of hemoglobin iron metabolism in normal men. J Clin Invest. 1959;38:1484–6. doi: 10.1172/JCI103926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graversen JH, Madsen M, Moestrup SK. CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol. 2002;34:309–14. doi: 10.1016/s1357-2725(01)00144-3. [DOI] [PubMed] [Google Scholar]
- Greaves DR, Channon KM. Inflammation and immune responses in atherosclerosis. Trends Immunol. 2002;11:535–41. doi: 10.1016/s1471-4906(02)02331-1. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 2002;100:1134–46. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM. The antioxidant activity of haptoglobin towards haemoglobin-stimulated lipid peroxidation. Biochim Biophys Acta. 1987;917:219–23. doi: 10.1016/0005-2760(87)90125-1. [DOI] [PubMed] [Google Scholar]
- Hayden JM, Reaven PD. Cardiovascular disease in diabetes mellitus type 2: a potential role for novel cardiovascular risk factors. Curr Opin Lipidol. 2000;11:519–28. doi: 10.1097/00041433-200010000-00010. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- Hogger P, Dreier J, Droste A, et al. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoidinducible member of the scavenger receptor cysteine-rich family (CD163) J Immunol. 1998;161:1883–90. [PubMed] [Google Scholar]
- Howard BV, Lee ET, Cowan LD, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389–95. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- Hwang PK, Greer J. Interaction between hemoglobin subunits in the hemoglobin-haptoglobin complex. J Biol Chem. 1980;255:3038–41. [PubMed] [Google Scholar]
- Imperatore G, Hanson RL, Pettitt DJ, et al. Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes Group. Diabetes. 1998;47:821–30. doi: 10.2337/diabetes.47.5.821. [DOI] [PubMed] [Google Scholar]
- Kalmovarin N, Friedrichs WE, O'Brien HV, et al. Extrahepatic expression of plasma protein genes during inflammation. Inflammation. 1991;15:369–79. doi: 10.1007/BF00917353. [DOI] [PubMed] [Google Scholar]
- Kannal WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120–6. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- Katnik I, Jadach J. Haptoglobin concentration in serum and other body fluids measured by comparison of its reactivity with hemoglobin and concanavalin A. Arch Immunol Ther Exp (Warsz) 1996;44:45–50. [PubMed] [Google Scholar]
- Koch W, Latz W, Eichinger M, et al. Genotyping of the common haptoglobin Hp 1/2 polymorphism based on PCR. Clin Chem. 2002;48:1377–82. [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the hemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Krolewski AS, Warram JH, Rand LI, et al. Epidemiologic approach to the etiology of type I diabetes mellitus and its complications. N Engl J Med. 1987;317:1390–8. doi: 10.1056/NEJM198711263172206. [DOI] [PubMed] [Google Scholar]
- Laight DW, Carrier MJ, Anggard EE. Endothelial cell dysfunction and the pathogenesis of diabetic macroangiopathy. Diabetes Metab Res Rev. 1999;15:271–82. doi: 10.1002/(sici)1520-7560(199907/08)15:4<274::aid-dmrr46>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42:1589–600. [PubMed] [Google Scholar]
- Langlois MR, Delanghe JR, De Buyzere ML, et al. Effect of haptoglobin on the metabolism of vitamin C. Am J Clin Nutr. 1997;66:606–10. doi: 10.1093/ajcn/66.3.606. [DOI] [PubMed] [Google Scholar]
- Langlois MR, Martin ME, Boelaert JR, et al. The haptoglobin 2-2 phenotype affects serum markers of iron status in healthy males. Clin Chem. 2000;46:1619–25. [PubMed] [Google Scholar]
- Levy AP, Hochber I, Jablonski K, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: the strong heart study. J Am Coll Card. 2002;40:1984–90. doi: 10.1016/s0735-1097(02)02534-2. [DOI] [PubMed] [Google Scholar]
- Levy AP, Larson MG, Corey D, et al. Haptoglobin phenotype and prevalent coronary heart disease in the Framingham offspring cohort. Atherosclerosis. 2004;172:361–5. doi: 10.1016/j.atherosclerosis.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Levy AP, Roguin A, Hochberg I, et al. Haptoglobin phenotype and vascular complications in patients with diabetes. N Engl J Med. 2000;343:969–70. doi: 10.1056/NEJM200009283431313. [DOI] [PubMed] [Google Scholar]
- Lim SK, Kim H, Bin AA, et al. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 1998;92:1870–7. [PubMed] [Google Scholar]
- Maeda N, Yang F, Barnett DR, et al. Duplication within the haptoglobin Hp2 gene. Nature. 1984;309:131–5. doi: 10.1038/309131a0. [DOI] [PubMed] [Google Scholar]
- Marre M. Genetics and the prediction of complications in type 1 diabetes. Diabetes Care. 1999;22:53–8. [PubMed] [Google Scholar]
- McCormick DJ, Atassi MZ. Hemoglobin binding with haptoglobin: delineation of the haptoglobin binding site on the alpha-chain of human hemoglobin. J Protein Chem. 1990;9:735–42. doi: 10.1007/BF01024768. [DOI] [PubMed] [Google Scholar]
- Melamed-Frank M, Lache O, Enav BI, et al. Structure/function analysis of the anti-oxidant properties of haptoglobin. Blood. 2001;98:3693–8. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- Miller YI, Altamentova SM, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin stems from a heme-initiated globin radical: antioxidant role of haptoglobin. Biochemistry. 1997;36:12189–98. doi: 10.1021/bi970258a. [DOI] [PubMed] [Google Scholar]
- Nakhoul FM, Marsh S, Hochberg I, et al. Haptoglobin genotype as a risk factor for diabetic retinopathy. JAMA. 2000;284:1244–5. doi: 10.1001/jama.284.10.1244-a. [DOI] [PubMed] [Google Scholar]
- Nakhoul FM, Zoabi R, Kanter Y, et al. Haptoglobin phenotype and diabetic nephropathy. Diabetologia. 2001;44:602–4. doi: 10.1007/s001250051666. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Parving HH, Tarnow L, Rossing P. Genetics of diabetic nephropathy. J Am Soc Nephrol. 1996;7:2509–17. doi: 10.1681/ASN.V7122509. [DOI] [PubMed] [Google Scholar]
- Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–26. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- Puppo A, Halliwell B. Formation of hydroxyl radicals from hydrogen peroxides in the presences of iron. Is hemoglobin a biological Fenton reagent? Biochem J. 1988;249:185–90. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyorala K, Laasko M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- Raugei G, Bensi G, Colantuoni V, et al. Sequence of human haptoglobin cDNA: evidence that the alpha and beta subunits are coded by the same mRNA. Nucleic Acids Res. 1983;11:5811–19. doi: 10.1093/nar/11.17.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HE, Howard BV. Diabetes and cardiovascular disease. Annu Rev Med. 2002;53:245–67. doi: 10.1146/annurev.med.53.082901.103904. [DOI] [PubMed] [Google Scholar]
- Roguin A, Hochberg I, Nikolsky E, et al. Haptoglobin phenotype as a predictor of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 2001;87:330–2. doi: 10.1016/s0002-9149(00)01368-0. [DOI] [PubMed] [Google Scholar]
- Roguin A, Koch W, Kastrati A, et al. Haptoglobin genotype is predictive of major adverse cardiac events in the one year period after PTCA in individuals with diabetes. Diabetes Care. 2003;26:2628–31. doi: 10.2337/diacare.26.9.2628. [DOI] [PubMed] [Google Scholar]
- Roguin A, Ribichini F, Ferrero V, et al. Haptoglobin phenotype and the risk of restenosis after coronary artery stent implantation. Am J Cardiol. 2002;89:806–10. doi: 10.1016/s0002-9149(02)02189-6. [DOI] [PubMed] [Google Scholar]
- Ruiz J. Diabetes mellitus and the late complications: influence of the genetic factors. Diabetes Metab. 1997;23:57–63. [PubMed] [Google Scholar]
- Ruiz J, Blanche H, James RW, et al. Gln-Arg192 polymorphism of paraoxonase and coronary heart disease in type 2 diabetes. Lancet. 1995;346:869–72. doi: 10.1016/s0140-6736(95)92709-3. [DOI] [PubMed] [Google Scholar]
- Shah SV, Alam MG. Role of iron in atherosclerosis. Am J Kid Dis. 2003;41:80–3. doi: 10.1053/ajkd.2003.50091. [DOI] [PubMed] [Google Scholar]
- Smeets MB, Pasterkamp G, Lim SK, et al. Nitric oxide synthesis is involved in arterial haptoglobin expression after sustained flow changes. FEBS Lett. 2002;529:221–4. doi: 10.1016/s0014-5793(02)03343-4. [DOI] [PubMed] [Google Scholar]
- Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–9. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- Suleiman M, Kapeliovich MR, Roguin A, et al. Haptoglobin phenotype and mortality in the 30 day period following acute myocardial infarction. Diabetes Care. 2003;26:2699–700. doi: 10.2337/diacare.26.9.2699. [DOI] [PubMed] [Google Scholar]
- Sullivan JL. Iron and sex differences in heart disease risk. Lancet. 1981;1:1293–4. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- Tarnow L, Rossing P, Nielsen FS, et al. Cardiovascular morbidity and early mortality cluster in parents of type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2000;23:30–3. doi: 10.2337/diacare.23.1.30. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group Ethnicity and cardiovascular disease. The incidence of myocardial infarction in white, South Asian and Afro-Carribean patients with type 2 diabetes. Diab Care. 1998;21:1271–7. doi: 10.2337/diacare.21.8.1271. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MM, Tensen CP, Van As JH, et al. Regulation of CD 163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol. 1999;66:858–66. doi: 10.1002/jlb.66.5.858. [DOI] [PubMed] [Google Scholar]
- Venerando B, Fiorilli A, Croci G, et al. Acidic and neutral sialidase in the erythrocyte membrane of type 2 diabetic patients. Blood. 2002;99:1064–70. doi: 10.1182/blood.v99.3.1064. [DOI] [PubMed] [Google Scholar]
- Wejman JC, Hovsepian D, Wall JS, et al. Structure and assembly of haptoglobin polymers by electron microscopy. J Mol Biol. 1984;174:343–68. doi: 10.1016/0022-2836(84)90342-5. [DOI] [PubMed] [Google Scholar]
- Wenzel I, Roth J, Sorg C. Identification of a novel surface molecule, RM3/1, that contributes to the adhesion of glucocorticoid-induced human monocytes to endothelial cells. Eur J Immunol. 1996;26:2758–63. doi: 10.1002/eji.1830261131. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Zwadlo G, Voegeli R, Osthoff KS, et al. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55:295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]


