Abstract
In addition to their role in reverse cholesterol transport, high-density lipoproteins (HDL) exert several beneficial effects, including the prevention and correction of endothelial dysfunction. HDL promote endothelium proliferation and diminish endothelial apoptosis; they play a key role in vasorelaxation by increasing the release of nitric oxide and prostacyclin through the induction of the expression and the activity of endothelial nitric oxide synthase and the coupling of cyclooxygenase 2 and prostacyclin synthase. In addition, HDL affect coagulation, fibrynolisis, platelet adhesion, adhesion molecules, and protease expression, and they exert antioxidant activity. These effects are achieved at the gene expression level and are dependent on the activation of several intracellular signaling pathways, including PI3K/Akt, ERK1/2, PKC, and p38MAPK. The complexity of the signaling pathways modulated by HDL reflects the different effects of the components of this class of lipoproteins such as apolipoproteins or lipids on endothelial cell gene expression and the subsequent modulation of endothelial function observed. The in vivo relevance of these findings to endothelial recovery during physiological or pathological conditions remains to be addressed; nevertheless, the results of clinical studies with synthetic HDL, ApoA-I mimetics, and drugs that are becoming available that selectively affect HDL plasma levels and biological functions support the importance of the correction of endothelial function by HDL.
Keywords: HDL, endothelium, inflammation, molecular mechanisms, gene expression, intracellular kinases
Introduction
Numerous clinical and epidemiological studies have demonstrated the inverse association between high-density lipoprotein cholesterol (HDL-C) and the risk of coronary heart disease (CHD) events (Gordon and Rifkind 1989; Assmann et al 1996). Low HDL-C has been identified as the most frequent familial dyslipoproteinemia in patients with premature myocardial infarction (Genest et al 1992). In angiographic studies, markers of inflammation had a significant association with CHD, which was lost upon multivariate analysis taking HDL-C into account (Erren et al 1999). In several prospective studies, including the prospective ECAT angina pectoris study, both low serum levels of HDL cholesterol and high serum levels of C-reactive protein were independent risk factors of a second coronary event in patients with manifest CHD (Bolibar et al 2000; Ridker 2001). Of note, the results of the Veterans Affairs High-density Lipoprotein Intervention Trial study showed that raising HDL decreases the incidence of coronary artery disease events (Robins 2001).
The inverse correlation between HDL cholesterol levels and the risk of CHD is often explained by the ability of HDL to remove cholesterol from the periphery for delivery to the liver and excretion in the bile, the process termed reverse cholesterol transport (Silver et al 2000). The concept of reverse cholesterol transport provides the theoretical framework for understanding body cholesterol homeostasis. Distortion of reverse cholesterol transport may favor deposition of cholesterol in the arterial wall and thereby contribute to the development of arteriosclerosis. The concept of reverse cholesterol transport is supported by numerous studies in vitro and in vivo (for a review, see von Eckardstein et al 2001). Several evidences are accumulating to suggest that in addition to their role in reverse cholesterol transport HDL positively influence vascular functions including endothelial responses during atherogenesis (Calabresi 2003). The aim of this paper is to summarize the existing information on the role of HDL in preventing and correcting endothelial dysfunction and to present an overview on the molecular mechanisms responsible for these effects.
Endothelial dysfunction and cardiovascular disease
The endothelium is strategically located between the wall of blood vessels and the blood stream. It senses mechanical stimuli, such as pressure and shear stress, and hormonal stimuli, such as vasoactive substances. In response, it releases agents that regulate vasomotor function, trigger inflammatory processes, and affect hemostasis. Among the vasodilator substances produced by the endothelium are nitric oxide (NO), prostacyclin, various endothelium-derived hyperpolarizing factors, and C-type natriuretic peptide (CNP). Vasoconstrictors include endothelin-1 (ET-1), angiotensin II (Ang II), thromboxane A2 (TXA2), and reactive oxygen species (ROS). Inflammatory modulators include intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), E-selectin, and NF-κB. Modulation of hemostasis includes the release of plasminogen activator, tissue factor inhibitor, von Willebrand factor, NO, prostacyclin, TXA2, plasminogen-activator inhibitor-1 (PAI-1), and fibrinogen. The endothelium also contributes to mitogenesis, angiogenesis, vascular permeability, and fluid balance (Cines et al 1998).
Endothelial dysfunction was initially identified as impaired vasodilation to specific stimuli such as acetylcholine or bradykinin. A broader understanding of the term would include not only reduced vasodilation but also a proinflammatory and prothrombic state associated with dysfunction of the endothelium.
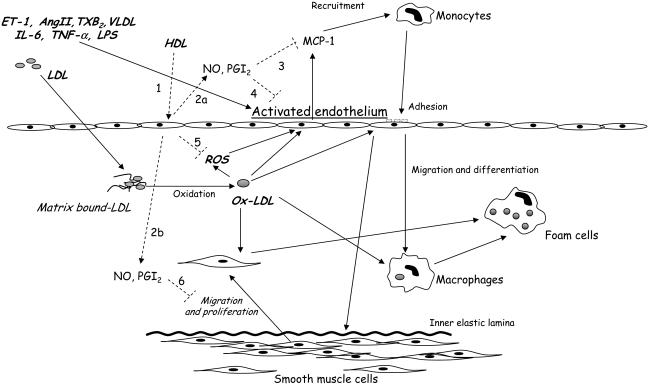
Endothelial dysfunction has been proposed to be an early event of pathophysiologic importance in the atherosclerotic process (Ross 1999; Libby 2002) and provides an important link between diseases such as hypertension, chronic renal failure, and diabetes and the high risk of cardiovascular events that patients with these conditions exhibit. Low NO bioavailability can up-regulate VCAM-1 in the endothelial cell layer via induction of NF-κB expression (Khan et al 1996). ROS, CD40 ligand, lectin-like oxidized LDL receptor-1 (LOX-1) and lipoproteins such as VLDL or oxidized-LDL (Ox-LDL) also up-regulate endothelial expression of adhesion molecules (Mach et al 1997; Libby 2002; Norata, Pirillo, et al 2003). The expression of VCAM-1, ICAM-1, and E-selectin plays a role in the initiation of the inflammatory process (Ross 1999). VCAM-1 binds monocytes and T lymphocytes, the first step of invasion of the vessel wall by inflammatory cells (Libby 2002). NO inhibits leukocyte adhesion (Kubes et al 1991). Reduction in NO results in induction of monocytechemoattractant protein-1 expression, which recruits mononuclear phagocytes (Zeiher et al 1995). Monocytes are then transformed to lipid-loaded foam cells. Ox-LDL, for example, are scavenged through LOX-1 (Yoshida et al 1998), which is highly expressed in blood vessels in hypertension, diabetes, and dyslipidemia (Mehta and Li 2002). Ox-LDL trigger a variety of actions: they reduce eNOS (endothelial nitric oxide synthase) expression (Mehta and Li 2002) and further stimulate adhesion molecule expression (Libby 2002), apoptosis (Norata et al 2002), and release of proinflammatory prostaglandins (Norata, Pirillo, et al 2004) (Figure 1).
Figure 1.
Effects of pro-atherogenic factors on the vascular wall and biological actions of HDL (high-density lipoproteins) on the endothelium. ET-1 (endothelin-1), AngII (angiotensin II), TXB2 (tromboxane B2), VLDL (very low-density lipoprotein), IL-6 (interleukin 6), TNF-α(tumor necrosis factor alpha), LPS (lipopolysaccharides), NO (nitric oxide), PGI2 (prostacyclin), MCP-1 (monocyte chemoattractant protein-1), ROS (radical oxygen species), Ox-LDL (oxidized low-density lipoprotein) promote endothelial dysfunction by inducing adhesion molecule expression and chemotactic factor release. Once recruited, monocytes migrate and differentiate to macrophages in the vascular wall. Activated endothelium can also promote the migration and proliferation of smooth muscle cells. Both macrophages and smooth muscle cells scavenge Ox-LDL and become foam cells. HDL interact with endothelium (1) and induce NO and prostacyclin release (2a and 2b). HDL, directly and via NO and PGI2, can inhibit chemokine secretion (3) and adhesion molecule expression (4). In addition, HDL decrease oxygen radical production (5) and smooth muscle cell migration and proliferation (6). These mechanisms counteract the progression of atherosclerosis in the vascular wall.
As the atherosclerotic plaque progresses, growth factors secreted by macrophages in the plaque stimulate vascular smooth muscle cell growth and interstitial collagen synthesis (Libby 2002). The event that initiates the majority of myocardial infarctions is the rupture of the fibrous cap of the plaque, inducing thrombus formation. Decreased NO and oxidative excess may activate matrix metalloproteinases (MMP) (Eberhardt et al 2000; Uemura et al 2001), namely MMP-2 and MMP-9, which weaken the fibrous cap. Endothelial NOS has been indicated as the main enzyme that contributes to NO production in the arterial wall (Kawashima and Yokoyama 2004). Experimental studies in vitro have revealed that NO from eNOS constitutes an antiatherogenic molecule and that a deficiency of eNOS accelerates atherosclerotic lesion formation in eNOS knockout mice (Kawashima and Yokoyama 2004). Nevertheless, under conditions in which vascular tissue levels of tetrahydrobiopterin (BH4), a cofactor for NOS, are deficient or lacking, eNOS becomes dysfunctional and produces superoxide rather than NO. eNOS overexpression with hypercholesterolemia may promote atherogenesis via increased superoxide generation from dysfunctional eNOS (Ozaki et al 2002). Thus, eNOS may have 2 faces in the pathophysiology of atherosclerosis depending on tissue BH4 metabolisms.
Recent works indicate that vascular inflammatory responses can be limited by antiinflammatory counteregulatory mechanisms that maintain the integrity and homeostasis of the vascular wall (Uyemura et al 1996; Mallat et al 1999). These mechanisms include antiinflammatory signals such as HDL (Tedgui and Mallat 2001).
HDL effects on the endothelium
Several effects account for the endothelial protection by HDL, including: the control of cell proliferation; the inhibition of apoptosis; the modulation of the secretory functions; the regulation of coagulation, fibrinolysis, and platelet adhesion; and the inhibition of inflammatory processes (Table 1).
Table 1.
HDL effects on the endothelium
| HDL | Mimicked by ApoA-I | Mimicked by lysosphingolipids | Key references |
|---|---|---|---|
| Cell proliferation | |||
| ↑ proliferation | Yes | Yes | Darbon et al 1986 |
| ↓ apoptosis | Nofer et al 2000 | ||
| Vascular tone | |||
| ↑ NO | No | Yes | Yuhanna et al 2001 |
| Nofer et al 2004 | |||
| ↑ PGI2 | Yes | ? | Pomerantz et al 1985 |
| Norata, Pirillo, et al 2004 | |||
| ↓ endothelin 1 | ? | ? | Unoki et al 1999 |
| ↑ CNP | ? | ? | Sugiyama et al 1995 |
| Coagulation, fibrinolysis, platelet adhesion | |||
| ↓ factor X activation | Yes | ? | Carson 1981 |
| ↓ tissue factor | ? | ? | Kaneko et al 1994 |
| ↓ PAF | ? | ? | Sugatani et al 1996 |
| ↓ prothrombinase complex | Yes | ? | Epand et al 1994 |
| ↑ activated protein C and protein S | ? | ? | Griffin et al 1999 |
| Inflammation | |||
| ↓ VCAM-1 | No | Yes | Ashby et al 1998 |
| ↓ ICAM-1 | ? | ? | Barter et al 2004 |
| ↓ E-selectin | ? | Yes | Nofer et al 2003 |
| ↓ MMP-9 | ? | ? | Xu et al 1999 |
| ↓ ADAMT-s 1 | ? | ? | Norata, Bjork, et al 2004 |
| ↑ TGF-β2 | No | Yes | Norata et al 2005 |
Abbreviations are listed at the end of the paper.
HDL and cell proliferation
Rapid regeneration through the migration and proliferation of endothelial cells as well as diminished apoptosis have been described as key mechanisms in preventing endothelial damage by HDL. HDL stimulate the proliferation of bovine (Cohen et al 1982) and human endothelial cells (Darbon et al 1986), and enhance endothelial cell migration (Murugesan et al 1994). Initially, it was proposed that apolipoproteins A-I and C-I were responsible for the mitogenic effects (Tournier et al 1984; Darbon et al 1986); latest data suggest that lysosphingolipids, namely sphingosine 1-phosphate (S1P), sphingosylphosphorylcholine (SPC), and lysosulfatide (LSF), could also enhance endothelial cell proliferation (Nofer et al 2000; Kimura et al 2001).
Apart from the mitogenic activity, HDL prevent endothelial cell death. Suc et al (1997) showed that both HDL and apolipoprotein A-I diminish the induction of cell death by Ox-LDL. This activity was not related to the interaction between these two lipoproteins or to antioxidative properties of HDL. The inhibition of death of endothelial cells by HDL or apolipoprotein A-I (ApoA-I) was most evident after 24-h incubation. The antiapoptotic effect of HDL was abolished by protein synthesis inhibitors. Thus, it seems that weakening of the cytotoxic effect of Ox-LDL in the presence of HDL or ApoA-I is related to the expression of still unknown factors that prevent cell death. Also, triglyceride-rich lipoproteins have been shown to promote endothelial cell death (Norata, Pirillo, et al 2003), and HDL reduce endothelial necrosis induced by remnants of triglyceride-rich lipoproteins (Speidel et al 1990). In addition, Sugano et al (2000) found protective effects of HDL and ApoA-I against endothelial apoptosis induced by tumor necrosis factor alpha (TNF-α). HDL also prevent endothelial cell damage and necrosis resulting from the activation of the complement system. The C5a–C9 terminal complement complex was shown to be inversely correlated with HDL-C, but not total cholesterol, in dyslipidemic subjects (Pasqui et al 2000). Rosenfeld et al (1983) have shown that HDL inhibits complement-mediated cell lysis.
HDL and vascular tone
Several clinical studies have demonstrated a close association between plasma levels of HDL and endothelium-dependent flow-mediated dilation (O'Connell and Genest 2001). Recently, administration of reconstituted HDL was shown to restore abnormal endothelial function in hypercholesterolemic men (Spieker et al 2002). Several mechanisms underlying the effects of HDL on endothelial reactivity have been suggested, including synthesis of vasorelaxing prostanoids such as prostacyclin (PGI2) as well as activation of eNOS via SR-BI (Yuhanna et al 2001). Incubation of cultured endothelial cells with HDL activates eNOS in a process that involves the binding of ApoA-I to the scavenger receptor-BI (SR-BI) (Yuhanna et al 2001; Mineo et al 2003). However, eNOS is not activated by lipidfree ApoA-I. A recent study showed that lysosphingolipids present in HDL are responsible for these effects (Nofer et al 2004), while ApoA-I is fundamental for the binding to the membrane of endothelial cells.
PGI2 is a potent endothelium-derived vasodilator that binds IP receptors on vascular smooth muscle cells (SMC) and acts synergistically with NO to induce smooth muscle relaxation. PGI2 is synthesized from arachidonate derived from phospholipids of cellular membranes or from exogenous sources as phospholipids and cholesteryl esters present in circulating lipoproteins. The enzyme responsible for PGI2 production is cyclooxygenase (Cox), which exists as 2 different isoforms: a constitutive form (Cox-1) and an inducible form (Cox-2). Cox-2 has been implicated in several inflammatory processes including the induction of PGE2 (Norata, Pirillo, et al 2004) a proinflammatory prostaglandin that modulates MMPs production in the atherosclerotic plaque (Cipollone et al 2001).
Incubation of cultured endothelial cells with HDL causes a dose-dependent increase of PGI2 release, which is prevented by a Cox-2 inhibitor, suggesting a major role of this enzyme in HDL-mediated PGI2 synthesis; indeed, HDL have shown to induce Cox-2 expression (Norata, Callegari, et al 2004). Delipidated HDL apolipoproteins also enhance PGI2 production but to a lower extent than intact HDL (Pomerantz et al 1985). This suggests that different mechanisms could account for this effect, including the possibility that HDL can provide endothelial cells with arachidonate, which then acts as substrate for Cox-mediated PGI2 synthesis (Pomerantz et al 1985).
The role of Cox-2 in cardiovascular disease is of great interest, and several clinical trials have reported that selective Cox-2 inhibitors can increase the relative risk of myocardial infarction (Juni et al 2004; Furberg et al 2005), probably by decreasing prostacyclin production (McAdam et al 1999). Thus, it will be of interest to investigate whether HDLdependent Cox-2 induction and prostacyclin release can in part rescue from Cox-2 inhibitors side effects, and whether subjects with high HDL levels are less susceptible to the coronary side effects of Cox-2 inhibitors.
HDL also affect endothelin-1 (ET-1) synthesis (Hu et al 1994). ET-1 is a potent vasoconstrictor peptide that binds to specific G protein-coupled receptors on SMCs to reverse the response to NO. Previous studies have shown that subphysiological concentrations of native HDL increases ET-1 production in endothelial cells (Hu et al 1994). These findings are in contrast with those of a study where incubation of human endothelial cells cultured with HDL on a 2-chamber model system (a model reproducing the physiological state where ET-1 is released toward the underlying intimal smooth muscle in a polar fashion) inhibited the secretion of ET-1 on the opposite side of the culture on which they where applied (Unoki et al 1999), suggesting that HDL may indeed prevent the vasoconstrictor effects of ET-1.
Finally, HDL was shown to modulate the synthesis of CNP, which causes vasodilatation, inhibits proliferation of smooth muscle cells, and inhibits ET-1 secretion (Sugiyama et al 1995). Moreover, suppression of CNP synthesis in the presence of Ox-LDL is antagonized by HDL.
HDL, coagulation, fibrinolysis, and platelet adhesion
In contrast to atherogenic lipoproteins, like LDL and very low-density lipoprotein (VLDL), which stimulate both the secretion of tissue factor (TF) and the activation of extrinsic tenase, HDL per se does not stimulate the secretion of TF from endothelial cells or monocytes (Kaneko et al 1994). TF synthesis stimulated by VLDL is rather inhibited by HDL (Rosenson and Lowe 1998). Furthermore, HDL inhibit thrombin-induced human endothelial TF expression through inhibition of RhoA and activation of PI3K but not Akt/eNOS (Viswambharan et al 2004). In addition, HDL antagonize the activation of factor X induced by extrinsic tenase (Carson 1981). Besides HDL, ApoA-I also inhibits the activation of factor X. Recent studies have shown that the inhibitory effect of HDL may be related to the presence of tissue pathway factor inhibitor (TPFI) in this lipoprotein (Lesnik et al 1993). Both HDL and ApoA-I inhibit the calcium ionophoreinduced production of the pro-thrombinase complex on the surface of platelets (Epand et al 1994). HDL may affect the blood coagulation process via the regulation of activated protein C (APC), which is the crucial element regulating blood coagulation by the proteolytic inactivation of Va and VIIIa factors. The anticoagulatory effect of APC is enhanced by protein S. HDL augment APC-induced inactivation of Va and VIIIa factors (Griffin et al 1999). HDL also augment the activity of protein S to stimulate APC. This activity of HDL may arise from anticoagulants such as cardiolipin and phosphatidylethanolamine, which are present in these lipoproteins. It has been shown that not only coagulation but also fibrinolysis is regulated by HDL. Hypercholesterolemia and hypertriglyceridemia are associated with increased secretion of PAI-1 from endothelial cells (Stiko-Rahm et al 1990; Norata, Pirillo, et al 2003). By contrast, HDL cholesterol levels are negatively correlated with plasma levels of PAI-1 and tissue plasminogen activator (tPA) (Juhan-Vague et al 1996). This correlation may reflect the in vitro inhibitory effect of HDL on tPA and PAI secretion by the endothelium (Ren and Shen 2000). Of note, when HDL are oxidized, the resulting Ox-HDL have been reported to induce PAI-1 mRNA expression and protein release in endothelial cells via a p38MAPK-dependent pathway that promotes mRNA stabilization (Norata, Banfi, et al 2004).
In addition to coagulation and fibrinolysis, HDL also affect platelet adhesion. NO and prostacyclin have an antitrombotic effect besides being regulators of the vascular tone. HDL inhibit agonist-induced production of platelet-activating factor (PAF), a bioactive phospholipid that stimulates cell adhesion, platelet aggregation, and vascular permeability in endothelial cells (Sugatani et al 1996). Von Willebrand factor (vWF) is another protein expressed by endothelial cells that plays an essential role in platelet adhesion and aggregation; the circulating vWF levels are inversely correlated with plasma HDL (Conlan et al 1993), suggesting that HDL may inhibit vWF production. Therefore, by modulating the production/activity of a variety of endothelium-derived factors such as NO, PGI2, PAF, and vWF, HDL may affect both vascular tone and thrombogenicity.
HDL, angiogenesis, and cell migration
Two new aspects of the interaction between HDL and endothelial cells are stimulation of angiogenesis and cell motility and migration. An in vitro model of human coronary artery endothelial cell (HCEC) tube formation on a matrix gel has been used to explore the possible angiogenic effect of HDL (Miura et al 2003). The results have shown that HDL induce angiogenesis and have identified Ras as a key player in the angiogenic action of HDL (Miura et al 2003). The signaling pathway in HDL-induced tube formation is through pertussis toxin (PTX)-sensitive G-protein coupled receptors (GPCRs) and may be partly through receptors for S1P such as EDG-1 and EDG-3 (Miura et al 2003). This model assumes that lipoproteins induce angiogenic signals through PTX-sensitive GPCRs. HDL signaling through cholesteryl ester donation is an exciting possible route to the induction of angiogenesis. The scavenger receptor SR-BI mediates the selective cellular uptake of cholesterol from HDL. Consistent with this idea is the observation that HDL maintains the appropriate cellular localization of eNOS by replenishing endothelial cellular membranes with cholesteryl esters in an SR-BI–dependent fashion (Uittenbogaard et al 2000).
Endothelial genes implicated in angiogenesis and cell proliferation include matrix-degrading proteases. Proteases including MMPs have been shown to play a central role in endothelial dysfunction (Libby 2002). HDL inhibit MMP-9 expression induced by Ox-LDL in monocytes (Xu et al 1999) and the expression of ADAMTs-1 induced by lipopolysaccharide (LPS) on TNF-α in endothelial cells (Norata, Bjork, et al 2004).
ADAMTS-1, a disintegrin and metalloproteinase with thrombospondin motif, has been shown to inhibit endothelial cell proliferation by direct binding and sequestration of vascular endothelial growth factor (Luque et al 2003). It is thus possible that HDL can influence angionenic processes through proteases modulation (Norata, Pellegatta, et al 2003).
Although it is not clear whether HDL in its relation to angiogenesis induces the progression of atherosclerosis, the strong and specific angiogenic effect of HDL deserves attention in the development of therapeutic strategies to elevate plasma levels of these lipoproteins.
Control of the inflammatory response
As mentioned above, endothelial dysfunction promotes adhesion of leukocytes to endothelial cells sustaining the inflammatory processes during atherogenesis. Interaction of monocytes with endothelial cells is mediated by adhesion molecules located on the surface of these cells, which include VCAM-1, ICAM-1, and E-selectin. VCAM-1 and ICAM-1 mediate adhesion of mononuclear cells, including monocytes and lymphocytes. E-selectin enables tethering and rolling of monocytes and lymphocytes on the surface of endothelial cells. All three adhesion molecules are abundantly expressed in the atherosclerotic plaque (van der Wal et al 1992; Libby 2002).
Expression of VCAM-1 is induced by lysophosphatidylcholine present in Ox-LDL and by products of lipolysis (Kume et al 1992; Saxena et al 1992). Expression of VCAM-1, ICAM-1, and E-selectin is induced also by cytokines such as TNF-α, interleukin-1 (IL-1), and LPS (Libby 2002; Norata, Bjork, et al 2004). HDL inhibits the interaction of monocytes with endothelial cells and smooth muscle cells as well as the adhesion of monocytes to endothelial cells induced by Ox-LDL (Barter et al 2002). Recent studies have shown that cytokine-induced expression of VCAM-1, ICAM-1, and E-selectin is inhibited by HDL (Cockerill et al 1995; Ashby et al 1998; Nofer et al 2003; Barter et al 2004). The most pronounced inhibition of the expression of these proteins was observed at physiological HDL concentrations. Inhibition of VCAM-1 and E-selectin expression on endothelial cells is also induced by reconstituted HDL and depends strongly on the phospholipid composition of reconstituted particles as the inhibitory effects were not exerted by lipid-free apoproteins A-I and A-II (Ashby et al 1998; Calabresi et al 2003; Nofer et al 2003). Because the inhibitory effect of HDL is observed even after removal of these lipoproteins from the endothelial cell culture, it seems not to be related to the scavenging of free radicals by antioxidants contained in HDL.
HDL has also been shown to affect cytokines, chemokine, and chemokine receptor expression; using a cDNA microarray approach, it has been shown that under basal conditions, transforming growth factor-β2 (TGF-β2) is the only gene within a panel of 96 genes involved in inflammation that is significantly induced upon incubation with HDL in endothelial cells (Norata et al 2005). The effect of HDL is specific for TGF-β2, as neither TGF-β1 nor TGF-β3 expression is modulated by HDL. TGF-β possesses antiinflammatory properties and stabilizes the plaque (Grainger 2004), thus suggesting a novel target for the antiatherosclerotic effect of HDL. Also, IL-18 receptor and MIP1β expression appear to be modulated by HDL (Norata et al 2005), but the relevance of this finding needs further investigation.
Signaling pathways modulated by HDL
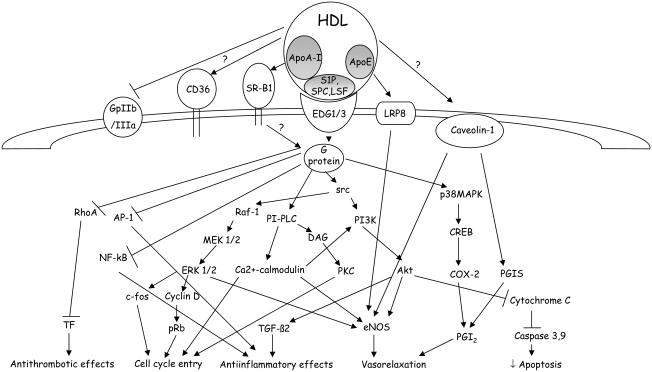
Several intracellular signaling pathways account for the effects of HDL on the endothelium (see Figure 2 and Table 2). Both ApoA-I and the lysosphingolipids SPC, LSF, and sphingosine 1-phosphate present in the HDL are responsible for intracellular signaling activation. ApoA-I binding to the scavenger receptor-BI (SR-B1) is required for HDL activation of eNOS (Yuhanna et al 2001); however, eNOS is not activated by lipid-free ApoA-I. This discrepancy was elegantly solved by Nofer et al (2004), which showed that the interaction of ApoA-I with SR-B1 is required for localizing the HDL particles at the membrane level where lysosphingolipids interact with specific receptors as EDG1/3 (also called S1P3) activating several intracellular signaling cascades. Moreover, deficiency of the lysophospholipid receptor S1P3 (also known as LPB3 and EDG3) abolishes the vasodilatory effects of SPC, S1P, and LSF and reduces the effect of HDL by approximately 60%. In endothelial cells from S1P3-deficient mice, Akt phosphorylation, and HDL-dependent calcium increase is severely reduced. In vivo, intra-arterial administration of HDL or lysophospholipids lowered mean arterial blood pressure in rats (Nofer et al 2004). A second mechanism has been proposed by Mineo et al (2003), which suggests that HDL via interaction with SR-B1 activate a tyrosine kinase most likely belonging to the src family kinase that phosphorylate both Akt and MAPK. The PI3K/Akt pathway plays a central role in the modulation of HDL-induced eNOS expression and activity (Mineo et al 2003; Nofer et al 2004). The activation of the PI3K/Akt pathway by HDL also maintains mitochondrial integrity by inhibiting cytochrome C release, caspase 3 and 9 activation, and apoptosis (Nofer, Levkau, et al 2001). PI3K/Akt activation by HDL has also been involved in the modulation of the expression of TGF-β2 (Norata et al 2005), a cytokine with antiinflammatory properties (Mallat and Tedgui 2002). HDL have been shown to activate also the protein kinase network Raf-1, MEK1/2, and ERK1/2 (Nofer, Junker, et al 2001; Norata, Callegari, et al 2004) that is responsible for the cell cycle entry and for eNOS activation (Nofer, Junker, et al 2001; Mineo et al 2003). ERK1/2 activation leads to the expression of cyclin D1 and c-fos, resulting in the phosphorylation of the retinoblastoma protein (pRb), which initiates the progression of the cell cycle (Nofer, Junker, et al 2001).
Figure 2.
Intracellular signaling pathways activated by high-density lipoprotein (HDL). HDL interact with several membrane proteins, including scavenger receptor-B1 (ApoA-I), EDG1/3 G-coupled receptor (lysosphingolipids S1P, SPC, and LSF) and other putative receptors LRP8 (ApoE), GpIIa/IIIb, CD36 or membrane proteins, caveolin-1, thus resulting in the phopshorylation of several kinases, MAPK, PI3K/Akt, PCK, p38MAPK, and the modulation of transcription factor activity. (Abbreviations are listed at the end of the paper.)
Table 2.
The HDL-induced intracellular signaling
| Signaling type | Resulting effect | Key references |
|---|---|---|
| Membrane interaction | ||
| Interaction with SR-B1 | Akt, eNOS phosphorylation | Yuhanna et al 2001 |
| Interaction with EDG1/3 | Akt, eNOS phosphorylation | Nofer et al 2004 |
| Interaction with LRP8 | eNOS phosphorylation | Riddell 1999 |
| Interaction with GpIIa/IIIb | Possible antagonism | Nofer 1998 |
| Interaction with caveolae | eNOS phosphorylation | Uittenbogaard et al 2000 |
| PGI-synthase shuttling | Norata, Callegari, et al 2004 | |
| Kinase phosphorylation | ||
| MEK1/2-ERK1/2 | eNOS phopshorylation | Mineo et al 2003 |
| Cell cycle entry | Nofer, Junker, et al 2001 | |
| PI3K/Akt | eNOS phopshorylation | Nofer et al 2004 |
| ↓ apoptosis/caspases | Nofer, Levkau, et al 2001 | |
| ↑ TGF-β2 | Norata et al 2005 | |
| PI-PLC/PKC | Cell cycle entry | Darbon et al 1986 |
| P38MAPK | Cox-2 synthesis | Norata, Callegari, et al 2004 |
| RhoA | ↓ phopshorylation / ↓TF | Viswambharan et al 2004 |
| Transcription factors | ||
| NF-κB | ↓ activity | Xia et al 1999; Park et al 2003 |
| AP-1 | ↓ activity | Park et al 2003 |
| CREB | ↑ activity-Cox-2 expression | Norata, Callegari, et al 2004 |
Abbreviations are listed at the end of the paper.
The activation of other signaling pathways has been implicated in HDL-dependent endothelial cell proliferation. Initially, it was proposed that HDL-induced proliferation occurs through a protein kinase C-mediated pathway, and HDL apolipoproteins were required for this effect (Darbon et al 1986). More recent data suggest that the mitogenic effect of HDL is mediated by a rise in intracellular pH and calcium (Tamagaki et al 1996), initiated by phospholipase C activation (Honda et al 1999), and that the lipid fraction of HDL is responsible for the rise of intracellular calcium. These observations suggest that activation of two different signaling pathways by HDL apolipoproteins and lipids may ultimately enhance proliferation of endothelial cells. Furthermore, the rise in calcium and subsequent activation of calcium/calmodulin kinase has been implicated in the activation of the PI3K/Akt pathway leading to increased eNOS expression and activity (Nofer et al 2004). HDL also induce the phosphorylation of p38MAPK, which is implicated in the activation of CREB and subsequent HDL dependent Cox-2 induction and prostacyclin release in endothelial cells (Norata, Callegari, et al 2004).
Of interest, studies have shown that HDL can modulate the activity of proteins present in plasma membrane microdomains, known as caveolae, including eNOS and PGI synthase (PGI-S) (Frank et al 2003). These effects are achieved both through the maintenance of cholesterol content in caveolae for eNOS (Uittenbogaard et al 2000; Everson and Smart 2001) and the phosphorylation of caveolin1 (the main protein of the caveolae) and subsequent PGI-S shuttling and Cox-2 coupling in the perinuclear area of endothelial cells (Norata, Callegari, et al 2004).
Despite the activation of all these intracellular signaling pathways, the molecular mechanisms by which HDL exert their antiinflammatory effects are poorly understood. As the activation of the nuclear factor kappa B (NF-κB) is responsible for TNF-α–dependent adhesion molecule expression, it has been proposed that it may act by inhibiting NF-κB activation. HDL block the TNF-α–induced nuclear translocation and DNA binding of NF-κB by interrupting a sphingosine kinase signaling pathway upstream of NF-κB activation (Xia et al 1999) and inhibiting NF-κB and AP-1 translocation and transactivation (Park et al 2003). Some of the inflammatory effects of HDL may also result from the induction of TGF-β2 expression (Norata et al 2005).
Conclusion
Endothelial dysfunction plays a key role during atherogenesis, and HDL have been shown to protect the endothelium through the modulation of the expression of several genes leading to increased cell proliferation, diminished apoptosis, increased vasorelaxation, and decreased inflammation. Apolipoproteins, lipids, and enzymes associated with HDL are implicated in these effects in vitro. Injection of reconstituted HDL (Badimon et al 1990) or ApoA-I mimetics in animal models (Chiesa et al 2002; Chiesa and Sirtori 2002) and humans (Nissen et al 2003) provide protection against atherosclerosis; however, the specific effects on the endothelium have not yet been determined.
The effects of HDL on endothelial cells depend on the activation of several intracellular pathways. The PI3K/Akt pathway has been implicated in many of the effects of HDL on the endothelium; furthermore, mice overexpressing ApoA-I present an increased Akt phosphorylation in the arterial wall (Norata et al 2005) and mice lacking one of the lysosphingolipid receptors show a reduced Akt phosphorylation in the arterial wall (Nofer et al 2004).
Other signaling pathways, including ERK1/2, calcium/calmodulin kinase, PKC, and p38MAPK, are activated by HDL and are involved in the effects of HDL on the endothelium.
A body of evidence thus suggests that in addition to their role in reverse cholesterol transport, HDL positively affect endothelial function in vitro; further studies are needed to address the pleiotropic effects of HDL in vivo and the molecular mechanisms involved.
Abbreviations
- ApoA-I
apolipoprotein A-I
- CNP
C-type natriuretic peptide
- Cox-2
cyclooxygenase-2
- CREB
cyclic AMP-response element binding protein
- DAG
diacylglycerol
- eNOS
endothelial nitric oxide synthase
- ERK1/2
extracellular signal-regulated kinases
- HDL
high-density lipoprotein
- ICAM
intercellular adhesion molecule-1
- LRP8
low-density lipoprotein receptor-related protein 8
- LSF
lysosulfatide
- MMP-9
matrix metalloproteinases-9
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- p38MAPK
p38–mitogen-activated protein kinase
- PAF
platelet-activating factor
- PGI2
prostacyclin2
- PGIS
prostacyclin synthase
- PI3K
phosphoinositide 3-kinase
- PI-PLC
phosphatidylinositol-specific phospholipase
- PKC
protein kinase C
- pRb
retinoblastoma protein
- S1P
sphingosine-1-phosphate
- SR-BI
scavenger receptor-BI
- SPC
sphingosyl-phosphorylcholine
- TGF-β2
transforming growth factor-β2
- TF
tissue factor
- VCAM-1
vascular adhesion molecule-1
References
- Ashby DT, Rye KA, Clay MA, et al. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:1450–5. doi: 10.1161/01.atv.18.9.1450. [DOI] [PubMed] [Google Scholar]
- Assmann G, Schulte H, von Eckardstein A, et al. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;(124 Suppl):S11–20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]
- Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterolfed rabbit. J Clin Invest. 1990;85:1234–41. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barter PJ, Baker PW, Rye KA. Effect of high-density lipoproteins on the expression of adhesion molecules in endothelial cells. Curr Opin Lipidol. 2002;13:285–8. doi: 10.1097/00041433-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- Bolibar I, von Eckardstein A, Assmann G, et al. Short-term prognostic value of lipid measurements in patients with angina pectoris. The ECAT Angina Pectoris Study Group: European Concerted Action on Thrombosis and Disabilities. Thromb Haemost. 2000;84:955–60. [PubMed] [Google Scholar]
- Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol. 2003;23:1724–31. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- Carson SD. Plasma high density lipoproteins inhibit the activation of coagulation factor X by factor VIIa and tissue factor. FEBS Lett. 1981;132:37–40. doi: 10.1016/0014-5793(81)80422-x. [DOI] [PubMed] [Google Scholar]
- Chiesa G, Monteggia E, Marchesi M, et al. Recombinant apolipoprotein A-I(Milano) infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ Res. 2002;90:974–80. doi: 10.1161/01.res.0000018422.31717.ee. [DOI] [PubMed] [Google Scholar]
- Chiesa G, Sirtori CR. Use of recombinant apolipoproteins in vascular diseases: the case of apoA-I. Curr Opin Investig Drugs. 2002;3:420–6. [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61. [PubMed] [Google Scholar]
- Cipollone F, Prontera C, Pini B, et al. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation. 2001;104:921–7. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]
- Cockerill GW, Rye KA, Gamble JR, et al. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–94. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- Cohen DC, Massoglia SL, Gospodarowicz D. Correlation between two effects of high density lipoproteins on vascular endothelial cells. The induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and the support of cellular proliferation. J Biol Chem. 1982;257:9429–37. [PubMed] [Google Scholar]
- Conlan MG, Folsom AR, Finch A, et al. Associations of factor VIII and von Willebrand factor with age, race, sex, and risk factors for atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study. Thromb Haemost. 1993;70:380–5. [PubMed] [Google Scholar]
- Darbon JM, Tournier JF, Tauber JP, et al. Possible role of protein phosphorylation in the mitogenic effect of high density lipoproteins on cultured vascular endothelial cells. J Biol Chem. 1986;261:8002–8. [PubMed] [Google Scholar]
- Eberhardt W, Beeg T, Beck KF, et al. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 2000;57:59–69. doi: 10.1046/j.1523-1755.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- Epand RM, Stafford A, Leon B, et al. HDL and apolipoprotein A-I protect erythrocytes against the generation of procoagulant activity. Arterioscler Thromb. 1994;14:1775–83. doi: 10.1161/01.atv.14.11.1775. [DOI] [PubMed] [Google Scholar]
- Erren M, Reinecke H, Junker R, et al. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol. 1999;19:2355–63. doi: 10.1161/01.atv.19.10.2355. [DOI] [PubMed] [Google Scholar]
- Everson WV, Smart EJ. Influence of caveolin, cholesterol, and lipoproteins on nitric oxide synthase: implications for vascular disease. Trends Cardiovasc Med. 2001;11:246–50. doi: 10.1016/s1050-1738(01)00119-0. [DOI] [PubMed] [Google Scholar]
- Frank PG, Woodman SE, Park DS, et al. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–8. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- Furberg CD, Psaty BM, FitzGerald GA. Parecoxib, valdecoxib, and cardiovascular risk. Circulation. 2005;111:249. doi: 10.1161/01.CIR.0000155081.76164.17. [DOI] [PubMed] [Google Scholar]
- Genest JJ, Jr, Martin-Munley SS, McNamara JR, et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85:2025–33. doi: 10.1161/01.cir.85.6.2025. [DOI] [PubMed] [Google Scholar]
- Gordon DJ, Rifkind BM. High-density lipoprotein – the clinical implications of recent studies. N Engl J Med. 1989;321:1311–16. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- Grainger DJ. Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol. 2004;24:399–404. doi: 10.1161/01.ATV.0000114567.76772.33. [DOI] [PubMed] [Google Scholar]
- Griffin JH, Kojima K, Banka CL, et al. High-density lipoprotein enhancement of anticoagulant activities of plasma protein S and activated protein C. J Clin Invest. 1999;103:219–27. doi: 10.1172/JCI5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda HM, Wakamatsu BK, Goldhaber JI, et al. High-density lipoprotein increases intracellular calcium levels by releasing calcium from internal stores in human endothelial cells. Atherosclerosis. 1999;143:299–306. doi: 10.1016/s0021-9150(98)00302-5. [DOI] [PubMed] [Google Scholar]
- Hu RM, Chuang MY, Prins B, et al. High density lipoproteins stimulate the production and secretion of endothelin-1 from cultured bovine aortic endothelial cells. J Clin Invest. 1994;93:1056–62. doi: 10.1172/JCI117055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhan-Vague I, Pyke SD, Alessi MC, et al. Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. ECAT Study Group. European Concerted Action on Thrombosis and Disabilities. Circulation. 1996;94:2057–63. doi: 10.1161/01.cir.94.9.2057. [DOI] [PubMed] [Google Scholar]
- Juni P, Nartey L, Reichenbach S, et al. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364:2021–9. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Wada H, Wakita Y, et al. Enhanced tissue factor activity and plasminogen activator inhibitor-1 antigen in human umbilical vein endothelial cells incubated with lipoproteins. Blood Coagul Fibrinolysis. 1994;5:385–92. [PubMed] [Google Scholar]
- Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- Khan BV, Harrison DG, Olbrych MT, et al. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci U S A. 1996;93:9114–19. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Sato K, Kuwabara A, et al. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–5. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–44. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnik P, Vonica A, Guerin M, et al. Anticoagulant activity of tissue factor pathway inhibitor in human plasma is preferentially associated with dense subspecies of LDL and HDL and with Lp(a) Arterioscler Thromb. 1993;13:1066–75. doi: 10.1161/01.atv.13.7.1066. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem. 2003;278:23656–65. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- Mach F, Schonbeck U, Sukhova GK, et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A. 1997;94:1931–6. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Tedgui A. The role of transforming growth factor beta in atherosclerosis: novel insights and future perspectives. Curr Opin Lipidol. 2002;13:523–9. doi: 10.1097/00041433-200210000-00008. [DOI] [PubMed] [Google Scholar]
- McAdam BF, Catella-Lawson F, Mardini IA, et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999;96:272–7. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta JL, Li D. Identification, regulation and function of a novel lectin-like oxidized low-density lipoprotein receptor. J Am Coll Cardiol. 2002;39:1429–35. doi: 10.1016/s0735-1097(02)01803-x. [DOI] [PubMed] [Google Scholar]
- Mineo C, Yuhanna IS, Quon MJ, et al. High density lipoprotein induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem. 2003;278:9142–9. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- Miura S, Fujino M, Matsuo Y, et al. High density lipoprotein-induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:802–8. doi: 10.1161/01.ATV.0000066134.79956.58. [DOI] [PubMed] [Google Scholar]
- Murugesan G, Sa G, Fox PL. High-density lipoprotein stimulates endothelial cell movement by a mechanism distinct from basic fibroblast growth factor. Circ Res. 1994;74:1149–56. doi: 10.1161/01.res.74.6.1149. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Fobker M, Hobbel G, et al. Activation of phosphatidylinositol-specific phospholipase C by HDL-associated lysosphingolipid. Involvement in mitogenesis but not in cholesterol efflux. Biochemistry. 2000;39:15199–207. doi: 10.1021/bi001162a. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Geigenmuller S, Gopfert C, et al. High density lipoprotein-associated lysosphingolipids reduce E-selectin expression in human endothelial cells. Biochem Biophys Res Commun. 2003;310:98–103. doi: 10.1016/j.bbrc.2003.08.126. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Junker R, Pulawski E, et al. High density lipoproteins induce cell cycle entry in vascular smooth muscle cells via mitogen activated protein kinase-dependent pathway. Thromb Haemost. 2001;85:730–5. [PubMed] [Google Scholar]
- Nofer JR, Levkau B, Wolinska I, et al. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem. 2001;276:34480–5. doi: 10.1074/jbc.M103782200. [DOI] [PubMed] [Google Scholar]
- Nofer JR, van der Giet M, Tolle M, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–81. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofer JR, Walter M, Kehrel B, et al. HDL3-mediated inhibition of thrombin-induced platelet aggregation and fibrinogen binding occurs via decreased production of phosphoinositide-derived second messengers 1,2-diacylglycerol and inositol 1,4,5-tris-phosphate. Arterioscler Thromb Vasc Biol. 1998;18:861–9. doi: 10.1161/01.atv.18.6.861. [DOI] [PubMed] [Google Scholar]
- Norata GD, Banfi C, Pirillo A, et al. Oxidised-HDL induces the expression of PAI-1 in human endothelial cells. Role of p38MAPK activation and mRNA stabilization. Br J Haematol. 2004;127:97–104. doi: 10.1111/j.1365-2141.2004.05163.x. [DOI] [PubMed] [Google Scholar]
- Norata GD, Bjork H, Hamsten A, et al. High-density lipoprotein subfraction 3 decreases ADAMTS-1 expression induced by lipopolysaccharide and tumor necrosis factor-alpha in human endothelial cells. Matrix Biol. 2004;22:557–60. doi: 10.1016/j.matbio.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Norata GD, Callegari E, Inoue H, et al. HDL3 induces cyclooxygenase-2 expression and prostacyclin release in human endothelial cells via a p38 MAPK/CRE-dependent pathway: effects on COX-2/PGI-synthase coupling. Arterioscler Thromb Vasc Biol. 2004;24:871–7. doi: 10.1161/01.ATV.zhq0504.1403. [DOI] [PubMed] [Google Scholar]
- Norata GD, Callegari E, Marchesi M, et al. High-density lipoproteins induce transforming growth factor beta 2 expression in human endothelial cells. Circulation. 2005 doi: 10.1161/CIRCULATIONAHA.104.472886. In press. [DOI] [PubMed] [Google Scholar]
- Norata GD, Pellegatta F, Hamsten A, et al. Effects of HDL3 on the expression of matrix-degrading proteases in human endothelial cells. Int J Mol Med. 2003;12:73–8. [PubMed] [Google Scholar]
- Norata GD, Pirillo A, Callegari E, et al. Gene expression and intracellular pathways involved in endothelial dysfunction induced by VLDL and oxidised VLDL. Cardiovasc Res. 2003;59:169–80. doi: 10.1016/s0008-6363(03)00335-3. [DOI] [PubMed] [Google Scholar]
- Norata GD, Pirillo A, Pellegatta F, et al. Native LDL and oxidized LDL modulate cyclooxygenase-2 expression in HUVECs through a p38-MAPK, NF-kappaB, CRE dependent pathway and affect PGE2 synthesis. Int J Mol Med. 2004;14:353–9. doi: 10.3892/ijmm.14.3.353. [DOI] [PubMed] [Google Scholar]
- Norata GD, Tonti L, Roma P, et al. Apoptosis and proliferation of endothelial cells in early atherosclerotic lesions: possible role of oxidised LDL. Nutr Metab Cardiovasc Dis. 2002;12:297–305. [PubMed] [Google Scholar]
- O'Connell BJ, Genest J., Jr High-density lipoproteins and endothelial function. Circulation. 2001;104:1978–83. doi: 10.1161/hc3901.096667. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Kawashima S, Yamashita T, et al. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest. 2002;110:331–40. doi: 10.1172/JCI15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Park JH, Kang JS, et al. Involvement of transcription factors in plasma HDL protection against TNF-alpha-induced vascular cell adhesion molecule-1 expression. Int J Biochem Cell Biol. 2003;35:168–82. doi: 10.1016/s1357-2725(02)00173-5. [DOI] [PubMed] [Google Scholar]
- Pasqui AL, Bova G, Puccetti L, et al. Complement activation in hypercholesterolemia. Nutr Metab Cardiovasc Dis. 2000;10:137–42. [PubMed] [Google Scholar]
- Pomerantz KB, Fleisher LN, Tall AR, et al. Enrichment of endothelial cell arachidonate by lipid transfer from high density lipoproteins: relationship to prostaglandin I2 synthesis. J Lipid Res. 1985;26:1269–76. [PubMed] [Google Scholar]
- Ren S, Shen GX. Impact of antioxidants and HDL on glycated LDL-induced generation of fibrinolytic regulators from vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1688–93. doi: 10.1161/01.atv.20.6.1688. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Vinogradov DV, Stannard AK, et al. Identification and characterization of LRP8 (apoER2) in human blood platelets. J Lipid Res. 1999;40:1925–30. [PubMed] [Google Scholar]
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–18. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- Robins SJ. Targeting low high-density lipoprotein cholesterol for therapy: lessons from the Veterans Affairs High-density Lipoprotein Intervention Trial. Am J Cardiol. 2001;88:19N–23N. doi: 10.1016/s0002-9149(01)02148-8. [DOI] [PubMed] [Google Scholar]
- Rosenfeld SI, Packman CH, Leddy JP. Inhibition of the lytic action of cell-bound terminal complement components by human high density lipoproteins and apoproteins. J Clin Invest. 1983;71:795–808. doi: 10.1172/JCI110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS, Lowe GD. Effects of lipids and lipoproteins on thrombosis and rheology. Atherosclerosis. 1998;140:271–80. doi: 10.1016/s0021-9150(98)00144-0. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Saxena U, Kulkarni NM, Ferguson E, et al. Lipoprotein lipase-mediated lipolysis of very low density lipoproteins increases monocyte adhesion to aortic endothelial cells. Biochem Biophys Res Commun. 1992;189:1653–8. doi: 10.1016/0006-291x(92)90267-o. [DOI] [PubMed] [Google Scholar]
- Silver DL, Jiang XC, Arai T, et al. Receptors and lipid transfer proteins in HDL metabolism. Ann N Y Acad Sci. 2000;902:103–11. doi: 10.1007/978-4-431-68424-4_20. discussion 111–12. [DOI] [PubMed] [Google Scholar]
- Speidel MT, Booyse FM, Abrams A, et al. Lipolyzed hypertriglyceridemic serum and triglyceride-rich lipoprotein cause lipid accumulation in and are cytotoxic to cultured human endothelial cells. High density lipoproteins inhibit this cytotoxicity. Thromb Res. 1990;58:251–64. doi: 10.1016/0049-3848(90)90095-t. [DOI] [PubMed] [Google Scholar]
- Spieker LE, Sudano I, Hurlimann D, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105:1399–402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]
- Stiko-Rahm A, Wiman B, Hamsten A, et al. Secretion of plasminogen activator inhibitor-1 from cultured human umbilical vein endothelial cells is induced by very low density lipoprotein. Arteriosclerosis. 1990;10:1067–73. doi: 10.1161/01.atv.10.6.1067. [DOI] [PubMed] [Google Scholar]
- Suc I, Escargueil-Blanc I, Troly M, et al. HDL and ApoA prevent cell death of endothelial cells induced by oxidized LDL. Arterioscler Thromb Vasc Biol. 1997;17:2158–66. doi: 10.1161/01.atv.17.10.2158. [DOI] [PubMed] [Google Scholar]
- Sugano M, Tsuchida K, Makino N. High-density lipoproteins protect endothelial cells from tumor necrosis factor-alpha-induced apoptosis. Biochem Biophys Res Commun. 2000;272:872–6. doi: 10.1006/bbrc.2000.2877. [DOI] [PubMed] [Google Scholar]
- Sugatani J, Miwa M, Komiyama Y, et al. High-density lipoprotein inhibits the synthesis of platelet-activating factor in human vascular endothelial cells. J Lipid Mediat Cell Signal. 1996;13:73–88. doi: 10.1016/0929-7855(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Kugiyama K, Matsumura T, et al. Lipoproteins regulate C-type natriuretic peptide secretion from cultured vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15:1968–74. doi: 10.1161/01.atv.15.11.1968. [DOI] [PubMed] [Google Scholar]
- Tamagaki T, Sawada S, Imamura H, et al. Effects of high-density lipoproteins on intracellular pH and proliferation of human vascular endothelial cells. Atherosclerosis. 1996;123:73–82. doi: 10.1016/0021-9150(95)05774-9. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res. 2001;88:877–87. doi: 10.1161/hh0901.090440. [DOI] [PubMed] [Google Scholar]
- Tournier JF, Bayard F, Tauber JP. Rapid purification and activity of apolipoprotein C1 on the proliferation of bovine vascular endothelial cells in vitro. Biochim Biophys Acta. 1984;804:216–20. doi: 10.1016/0167-4889(84)90152-6. [DOI] [PubMed] [Google Scholar]
- Uemura S, Matsushita H, Li W, et al. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res. 2001;88:1291–8. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A, Shaul PW, Yuhanna IS, et al. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–83. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- Unoki H, Fan J, Watanabe T. Low-density lipoproteins modulate endothelial cells to secrete endothelin-1 in a polarized pattern: a study using a culture model system simulating arterial intima. Cell Tissue Res. 1999;295:89–99. doi: 10.1007/s004410051215. [DOI] [PubMed] [Google Scholar]
- Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–8. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal AC, Das PK, Tigges AJ, et al. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am J Pathol. 1992;141:1427–33. [PMC free article] [PubMed] [Google Scholar]
- Viswambharan H, Ming XF, Zhu S, et al. Reconstituted high-density lipoprotein inhibits thrombin-induced endothelial tissue factor expression through inhibition of RhoA and stimulation of phosphatidylinositol 3-kinase but not Akt/endothelial nitric oxide synthase. Circ Res. 2004;94:918–25. doi: 10.1161/01.RES.0000124302.20396.B7. [DOI] [PubMed] [Google Scholar]
- von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2001;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- Xia P, Vadas MA, Rye KA, et al. High density lipoproteins (HDL) interrupt the sphingosine kinase signaling pathway. A possible mechanism for protection against atherosclerosis by HDL. J Biol Chem. 1999;274:33143–7. doi: 10.1074/jbc.274.46.33143. [DOI] [PubMed] [Google Scholar]
- Xu XP, Meisel SR, Ong JM, et al. Oxidized low-density lipoprotein regulates matrix metalloproteinase-9 and its tissue inhibitor in human monocyte-derived macrophages. Circulation. 1999;99:993–8. doi: 10.1161/01.cir.99.8.993. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kondratenko N, Green S, et al. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem J. 1998;334(Pt 1):9–13. doi: 10.1042/bj3340009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhanna IS, Zhu Y, Cox BE, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–7. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- Zeiher AM, Fisslthaler B, Schray-Utz B, et al. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995;76:980–6. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]