Abstract
Calcium channel blockers (CCBs) are a widely used group of antihypertensive agents. CCBs are efficacious in the reduction of blood pressure but the extent to which they manifest beneficial effects on cardiovascular disease is variable. Clinical studies indicate that pleiotropic actions make significant contributions to the efficacy of agents aimed at preventing atherosclerosis. The “response to retention” hypothesis implicates the binding and retention of lipoproteins by glycosaminoglycan chains on proteoglycans as an initiating step in atherogenesis. Atherogenic factors act as agonists and several classes of drugs including peroxisome proliferating-activated receptor (PPAR)-α and -γ ligands act as antagonists in this model. Initial data have demonstrated that high concentrations of CCBs inhibit proteoglycan synthesis. Newer preliminary data show that the action is very modest at reasonable concentrations and appears to be independent of calcium channel blocking activity. We have reviewed the role of cardiovascular drugs acting on vascular smooth muscle proteoglycan synthesis and considered the potential action of CCBs in this model. We conclude that the inhibition of proteoglycan synthesis by CCBs does not play a role in the attenuation of atherosclerosis; however, the antihypertensive efficacy and alternative beneficial actions provide support for the use of CCBs in the therapy of cardiovascular disease.
Keywords: atherosclerosis, calcium channel blockers, cardiovascular disease, lipoprotein, proteoglycans
Introduction
Calcium channel blockers (CCBs) are efficacious and widely used drugs in the treatment of hypertension as a risk factor for cardiovascular disease (Hernandez et al 2003). They are chemically a heterogenous group of agents comprising three distinct classes: phenylalkylamines, dihydropyridines, and benzothiazepines (Fleckenstein 1990). CCBs are a group of agents used therapeutically in cardiovascular disease due to their hypotensive action. This hypotensive action is through the vasodilatation of blood vessels, which occurs via the antagonism of calcium entry on vascular smooth muscle cells (Antman et al 1980). CCBs classically block depolarization-mediated contraction of blood vessels in vitro at very low (nmol/L) concentrations, and this action is extended in vivo where they exhibit efficacious antihypertensive activity. At higher (μmol/L) concentrations, CCBs block vasoactive agonist induced increases in cellular calcium, although the contribution of this action to in vivo efficacy is unclear (Kohrogi et al 1985).
Hypertension is a driver of the development of atherosclerosis underlying cardiovascular disease, although the molecular mechanisms and associations have not been resolved (MacMahon et al 1990). Elevated blood pressure is a potent accelerating factor in cardiovascular disease and this is particularly so in the presence of diabetes (Lehto et al 1997). In the UK Prospective Diabetes Study (UKPDS), tight blood pressure control in patients with diabetes was more effective than improving hyperglycemia in reducing cardiovascular disease (UKDPS 1998a, 1998b).
Current therapeutic interventions for the prevention of cardiovascular disease are directed at the established risk factors such as hypertension, dyslipidemia (elevated low-density lipoprotein [LDL]-cholesterol and triglycerides and decreased high-density lipoprotein [HDL]-cholesterol), and hyperglycemia. The most recent classes of agents for the treatment of these risk factors, such as angiotensin-converting enzyme (ACE) inhibitors for the treatment of high blood pressure (UKDPS 1998b; Jensen 2000), statins for hypercholesterolemia (Bellosta et al 1998), and glitazones for hyperglycemia (Sidhu et al 2004) have demonstrated direct beneficial vascular effects that have been termed “pleiotropic” actions. These pleiotropic actions occurring directly in blood vessels are broadly vaso-protective or “antiinflammatory” and contribute significantly to the primary action on the target risk factor to alleviate the development of atherosclerosis and cardiovascular disease. While the primary action of CCBs is to reduce blood pressure via L-type calcium channel blockade, the role and contribution of direct antiatherogenic actions to the cardiovascular protection offered by this class of drug is unresolved.
Our knowledge of the factors involved in atherogenesis and development of life-threatening unstable atherosclerotic plaques has increased considerably in recent years and now extends well beyond the notion of “endothelial dysfunction” (Libby 2002). It is widely accepted that atherosclerosis commences with the retention, accumulation, and modification of lipids in the vessel wall. This process occurs due to the “trapping” role of highly negatively charged proteoglycans, utilizing ionic interactions to bind and retain positively charged amino acid residues on the apolipoprotein (Apo) moiety of an LDL particle. This retention of LDL within the vessel wall leads to the presentation of lipoproteins for metabolic alterations (Williams and Tabas 1995, 1998). Apolipoproteins bind to the glycosaminoglycan (GAG) chains on proteoglycans (Ballinger et al 2004; Tannock et al 2004). GAG chains on vascular proteoglycans are subject to pharmacological and possibly therapeutic modulation in a manner that may be pro- and antiatherogenic (Gustafsson and Boren 2004). Vasoactive hormones and growth factors modulate the properties of the GAG chains so that they bind more avidly to LDL (Figueroa and Vijayagopal 2002; Little et al 2002). Proteoglycan: LDL binding may be attenuated by a variety of pharmacological and metabolic agents (Little et al 2002; Tannock et al 2002, 2004; Nigro et al 2004). Thus, we have considered the role of CCBs in the regulation of proteoglycan biosynthesis in vascular smooth muscle and provide some preliminary data that impact on the possible mechanism of action of these agents. This review addresses the antiatherogenic actions of CCBs and considers whether or not antiatherogenic effects of CCBs relate to proteoglycan metabolism in the vasculature and if these actions are related to calcium channel blockade.
Clinical trial data on the regression or prevention of atherosclerosis by CCBs
CCBs have demonstrated antiatherogenic properties in various clinical studies, revealing slowed progression and decreased formation of new lesions in treated patients (Lichtlen et al 1990; Waters et al 1990; Hernandez et al 2003).
The International Nifedipine Trial on Anti-atherosclerotic Therapy (INTACT) study and its 6-year follow-up demonstrated an appreciable and statistically significant reduction of 28% in angiographically detected new coronary lesions in coronary heart disease (CHD) patients treated with nifedipine after a three-year interval. In the second threeyear period (from the second angiogram to the third one) a further reduction of new lesions was found (78% in the nifedipine group and 73% in the former placebo group) (Lichtlen et al 1990).
The three-year Prospective Randomized Evaluation of the Vascular Effect of Norvasc Trial (PREVENT) of 825 patients examined the effect of amlodipine on the progression of atherosclerosis. Amlodipine therapy was associated with a significant slowing of the progression of carotid artery atherosclerosis (p = 0.007) (Pitt et al 2000; Hernandez et al 2003). The Verapamil in Hypertension and Atherosclerosis Study (VHAS) showed that verapamil caused the regression of thicker carotid lesions in parallel with a reduction in the incidence of cardiovascular events (Zanchetti et al 1998).
In the Regression Growth Evaluation Statin Study (REGRESS), co-administration of the CCBs amlodipine or nifedipine with pravastatin resulted in a significant reduction in the appearance of new angiographic lesions (p = 0.0078) (Jukema et al 1996). In two subprotocols of the Intervention as a Goal in the Hypertension Treatment (INSIGHT) study, nifedipine treatment prevented an increase in intima-media thickness in the carotid artery and significantly (p < 0.05) slowed the progression of coronary calcification compared with the diuretic co-amilozide in hypertensive patients (Motro and Shemesh 2001; Simon et al 2001). In another subprotocol of the INSIGHT study, nifedipine was shown to be as effective as co-amilozide in reducing cardiovascular complications in patients with hypertension and diabetes; however, treatment with nifedipine was associated with a lower incidence of vascular and non-vascular deaths and new cases of type 2 diabetes mellitus (Mancia et al 2003). Preliminary results of the European Lacidipine Study on Atherosclerosis (ELSA) showed that the CCB lacidipine was more effective in reducing the intima-media thickness progression rate, compared with the β-blocker atenolol (Zanchetti et al 2004).
Important information on the relationship between blood pressure and vascular complications in people with diabetes has been derived from the UKPDS (1998b). This large study included calcium antagonists as add-on therapy to achieve targets after the use of ACE inhibitors and β-blockers. The original study demonstrated the efficacy of reducing blood pressure in reducing complications in people with type 2 diabetes, especially microvascular complications (UKPDS 1998b). Further analysis demonstrated that there is a direct relationship between blood pressure and complications in people with diabetes and, importantly, that there is no apparent threshold (Adler et al 2000). The study allowed for a conclusion that the effects of ACE inhibitors and β-blockers exceeded those predicted from their blood pressure lowering action and thus gave further evidence of the potential role of pleiotropic actions in generating the best outcome in patients with cardiovascular risk factors (Adler et al 2000).
Although there is evidence that CCBs reduce blood pressure and can inhibit the development and progression of atherosclerotic lesions in patients with cardiovascular risk factors, the mechanisms by which these effects occur remains questionable. That is, do the antiatherogenic effects of CCBs include an action on calcium channels, and do the actions extend beyond the reduction in blood pressure associated with CCB therapy?
Cellular mechanisms of atherosclerosis
The primary initiating factor in plaque formation is the retention and accumulation of low-density lipoproteins in the subendothelial matrix of a blood vessel (Skalen et al 2002). LDL is transported through the endothelium by small vesicular carriers, a process of transcytosis that is nonsaturable and augmented at high concentrations of LDL (Vasile et al 1983). The accumulation and retention of LDL within the vessel wall involves an interaction between the ApoB protein moiety of the LDL particle and matrix proteoglycans, synthesized by vascular smooth muscle cells (Lusis 2000). Native LDL is not phagocytozed by macrophages, therefore trapped LDL undergoes a process of modification including lipolysis, proteolysis, oxidation, aggregation, and glycation in the presence of hyperglycemia, before being taken up to form foam cells via scavenger receptors on these cells and smooth muscle cells (Kadar and Glasz 2001). Oxidized LDL is also chemoattractive to circulating monocytes, attracting monocytes to the vessel wall where they penetrate and differentiate into macrophages. The accumulation of oxidized LDL stimulates the overlying endothelial cells to secrete proinflammatory molecules such as monocyte chemoattractant protein (MCP-1) and macrophage colony stimulating factor (M-CSF) as well as adhesion molecules ICAM-1, P selectin, and E selectin, which leads to the recruitment of more monocytes and cells of the immune system (T cells) into the vascular wall. Thus, vascular proteoglycans that trap and retain atherogenic LDL particles have an initiating role in atherogenesis (Skalen et al 2002; Ballinger et al 2004).
Advanced atherosclerotic plaques consist of two main components: soft, lipid-rich atheromatous material and hard, collagen-rich sclerotic tissue (Yutani et al 1999). Hard, fibrous plaques are characterized by a growing mass of extracellular lipid and the accumulation of smooth muscle cells (SMCs). SMCs secrete an extracellular matrix which leads to the formation of a fibrous cap (Lusis 2000). The cap protects the deeper components of the plaque from contact with circulating blood (Shah 2003). Atherosclerotic lesions also contain significant amounts of collagen, which confers firmness and stability on the plaque. The stability of a plaque is also influenced by the amount of intimal calcification (Shanahan et al 1994). Unstable or “vulnerable” plaques have an eccentric, lipid-rich core with a thin overlying fibrous cap. The lipid core may confer a mechanical disadvantage to the plaque, as it redistributes stress to the shoulder regions of the plaque, which in nearly 60% of cases is the area at which the fibrous caps rupture (Shah 2003). Increased numbers of inflammatory cells and an increased expression of inflammatory mediators are observed at sites of plaque rupture (Lutgens et al 2003). Vulnerable plaques may produce less luminal stenosis due to outward remodeling. Once the atheromatous “gruel” comes into contact with blood from the lumen of the artery, there is activation of the clotting cascade and platelet activation, adhesion, and aggregation, leading to thrombosis, vessel occlusion, ischemia, vascular and myocardial cell death, and tissue necrosis (Shah 2003). This process has important implications for coronary and cerebral arterial occlusion as myocardial infarction and stroke will occur if a significant portion of the heart and brain tissue is affected.
Modulation of GAG synthesis as an initiating factor in atherosclerosis
The entrapment of lipoproteins in the vessel wall by matrix molecules, most prominently proteoglycans, is continuing to emerge as a critical step in atherogenesis (Williams and Tabas 1995; Skalen et al 2002). This phenomenon forms the basis of the “response-to-retention” hypothesis explaining the origin of atherosclerosis (Williams and Tabas 1995). The interaction of proteoglycans and lipoproteins sets the stage and is responsible for predisposing the arterial intima to the development of atherosclerotic cardiovascular disease (Chait and Wight 2000).
Mogen Faber first suggested the involvement of proteoglycans in the retention of LDL in the vessel wall in 1949 (Faber 1949). Arterial proteoglycans participate in adhesion, migration, and proliferation, important processes in homeostasis and lipid metabolism leading to atherogenesis. Therefore, any changes to proteoglycan structure, content, or concentration, could affect the behavior of vascular cells and the pathophysiological properties of blood vessels. Increased production of arterial wall proteoglycans is implicated in atherogenesis (Tao et al 1997). Injury to the arterial wall results in an increase in the production of proteoglycan variants that have enhanced binding to LDL (Srinivasan et al 1993). A number of studies have demonstrated that increasing the number of negatively charged residues on the GAG chain, by either an increase in proteoglycan synthesis or change in the length, composition, and sulfation pattern of the GAGs during atherogenesis leads to increased retention of atherogenic LDL in the vessel wall (Radhakrishnamurthy et al 1990; Williams and Tabas 1995, 1998; Hurt-Camejo et al 1997; Camejo et al 1998; Chait and Wight 2000; Little et al 2002). Thus, pharmacological and potentially therapeutic modulation of GAG synthesis on vascular proteoglycans is emerging as a potential pathway for the recognition of the beneficial “pleiotropic” actions of existing agents and potentially a source of new mechanistic agents for the prevention of atherosclerosis (Nigro et al 2005). Several important drug classes including CCBs are known to modulate proteoglycan synthesis in vascular smooth muscle (Schonherr et al 1997; Vijayagopal and Subramaniam 2001; Nigro et al 2004).
Pro- and antiatherogenic factors affecting proteoglycan biosynthesis and structure in vascular smooth muscle
Our extension of the response to retention hypothesis is that growth factors that modify the properties of the GAG chains on vascular proteoglycans, such that they bind more avidly to LDL, will be proatherogenic (Ballinger et al 2004). It follows that agents that can antagonize growth factor induced GAG chain modification would reduce GAG chain affinity for LDL and thus would be antiatherogenic. These interactions can and have been studied in vitro, and the validity of these observations depends upon the outcomes available from tightly controlled studies in animal models and from clinical trial data.
Agents that have been shown to modify the biosynthesis and structure of the GAG chains on proteoglycans produced by vascular smooth muscle include the growth factors platelet derived growth factor and transforming growth factor (TGF)-β(Schonherr et al 1991; Little et al 2002), vasoactive agents such as angiotensin II (Shimizu-Hirota et al 2001; Figueroa and Vijayagopal 2002), and metabolic agents such as free fatty acids (Olsson et al 1999) and oxidized LDL (Chang et al 2000). The most common alteration is an increase in the size of the proteoglycans, which occurs by an increase in the length of the GAG chains with no change in the proteoglycan core protein (Nigro et al 2004). Agonist-induced changes in the size of the proteoglycans have been demonstrated by decreased mobility on SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and, more importantly, by earlier elution using size exclusion chromatography techniques. Changes in the sulfation pattern and the carbohydrate composition of the GAG chains could theoretically be associated with increased LDL binding, but these have not been comprehensively described (Ballinger et al 2004). Proteoglycans showed enhanced ionic properties when examined by salt gradient elution from anion exchange columns resulting in increased proteoglycan binding to LDL. In vitro proteoglycan binding to LDL has been demonstrated by both LDL affinity columns and Gel Mobility Shift Assays (Ballinger et al 2004).
The actions of agonists on proteoglycan synthesis can be blocked by several pharmacological and therapeutic agents as well as by experimental agents that block signaling pathways. Peroxisome proliferator-activated receptor (PPAR) ligands have been extensively investigated for their ability to modify proteoglycan synthesis in vascular smooth muscle (Nigro et al 2002, 2004; Tannock et al 2004). Both of the clinically relevant PPAR-α ligands, gemfibrozil and fenofibrate, modify the structure of vascular proteoglycans by inhibition of GAG chain elongation and alteration of disaccharide composition, resulting in reduced binding to human LDL in vitro (Nigro et al 2002, 2004). Thiazolidinediones, the newest class of antidiabetic agents, which are PPAR-γ ligands and insulin sensitizers, also inhibit the synthesis and shorten the GAG chains on proteoglycans synthesized by primate aortic SMCs, with a consequential reduction in the binding affinity of proteoglycans to LDL (Tannock et al 2004). The only published exception to the paradigm of decreased GAG size and reduced binding to LDL occurs in the instance of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, the statins. Simvastatin and cerivastatin increase the size of GAGs but decrease LDL binding and this observation requires further investigation (Meyers et al 2003).
Much of the in vitro data cited above are supported by studies in animal models. Fenofibrate treatment of ApoE knock out (KO) mice reduces lipid loading in the vessel wall and the size of the atherosclerotic lesions (Duez et al 2002). Troglitazone treatment of LDL receptor KO mice or ApoE KO mice also markedly reduces the area of lipid lesions in the aorta of mice fed high-fat diets (Chen et al 2001; Collins et al 2001). Similarly, treatment of LDL receptor KO mice or ApoE KO mice with rosiglitazone showed a 30% and 18% reduction in aortic lesion area, respectively (Li et al 2000; Claudel et al 2001). Thus, the in vitro actions of agents are paralleled by observations in animal models of atherosclerosis but it has not been demonstrated that the alterations of proteoglycans occur in vivo and actually cause the reduced lipid binding and deposition in the vessel wall.
Antiatherogenic actions of CCBs based on inhibition of proteoglycan synthesis
CCBs have been reported to inhibit proteoglycan synthesis in human aortic SMCs (Vijayagopal and Subramaniam 2001) and in other cell types (Fagnen et al 1999), with a related reduction in proteoglycan size. Cultured human aortic SMC proteoglycan synthesis is inhibited by amlodipine and nifedipine with concentrations that are very high (up to 35 μmol/L amlodipine and 58 μmol/L nifedipine) compared with relevant therapeutic levels (Vijayagopal and Subramaniam 2001). In addition, treatment of aortic SMCs with amlodipine resulted in qualitative changes in the newly synthesized proteoglycans, which included reduced molecular size, decreased charge density, and reduced binding to LDL (Vijayagopal and Subramaniam 2001). The proteoglycans synthesized by SMCs in the presence of amlodipine bind LDL with a much lower affinity (Vijayagopal and Subramaniam 2001). Large effects on LDL binding affinity of proteoglycans after treatment with amlodipine may be due to the changes in the extent of sulfation (ionic charge) and size and number of GAG chains (Vijayagopal and Subramaniam 2001). These studies concluded that CCBs might be involved in proteoglycan biosynthesis presumably by modulating intracellular calcium ion levels. It is thus important to identify the precise mechanisms of how CCBs manifest their actions on proteoglycan synthesis.
Mechanism of action of CCBs on vascular proteoglycans: does the effect of calcium antagonists on proteoglycans involve calcium channel blockade?
It has been implied that the mechanism of action of CCBs on vascular proteoglycan synthesis involves calcium channel blockade (Vijayagopal and Subramaniam 2001). We have recently extended these studies with amlodipine by examining the actions of the stereoisomers of amlodipine on proteoglycan synthesis in human vascular smooth muscle. The (S−)-amlodipine enantiomer is a more active CCB than (R+)-amlodipine (Arrowsmith et al 1986; Goldmann et al 1992; Luksa et al 1997). Due to the difference in stereo-orientation of the amplodipine isomers and thus activity at calcium channels, it is expected that if the effects on proteoglycans occurred via calcium channel blockade activity, only the (S−)-amlodipine enantiomer would have inhibitory effects on proteoglycan synthesis. We examined the activity of the amlodipine stereoisomers against basal proteoglycan biosynthesis and synthesis stimulated by the calcium-dependent and calcium-independent vasoactive agents endothelin-1 and TGF-β, respectively. Both enantiomers of amlodipine resulted in similar concentration-dependent inhibitory effects on 35S-SO4 incorporation into proteoglycans (Figure 1, left panel). We also examined the effect on proteoglycan size as a surrogate for GAG size and the effects were identical for both amlodipine isomers (Figure 1). It should be noted that the concentration of amlodipine used in these experiments was considerably lower than the concentration used by Vijayagopal group (Vijayagopal and Subramaniam 2001). A lower concentration was used because in an earlier study of ours on related calcium blockers (Agrotis et al 1993) and in the present experiments, we observed toxicity when CCBs were used at concentrations above 10 μmol/L, which was manifest as cell detachment from the underlying matrix.
Figure 1.
Stereoisomers of the CCB amlodipine with or without CCB activity have equivalent effects on proteoglycan synthesis in human VSMCs. Human VSMCs were treated with (S−)-amlodipine (1–10 μmol/L, CCB inhibitor) and (R+)-amlodipine (1–10 μmol/L, no CCB activity) in the presence of TGF-β1 (top) and endothelin-1 (ET-1, bottom) and metabolically labeled with [35S]-SO4 for 24 h. Proteoglycans were quantitated by the CPC precipitation assay (left) and assessed by SDS-PAGE (right) as described previously (Nigro et al 2002). Abbreviations: CCB, calcium channel blockers; CPC, cetylpyridinium chloride; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; TGF, transforming growth factor; VSMCs, vascular smooth muscle cells.
The role of intracellular calcium on proteoglycan synthesis
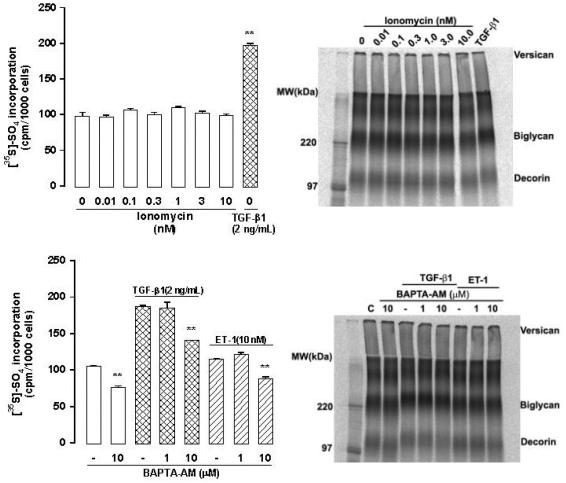
The calcium ion itself is an important second messenger for signaling events. Inositol (1,4,5) triphosphate produced from the enzymatic cleavage of phosphatidylinositol-bisphopshate by phopsholipase C, stimulates the release of calcium from intracellular stores. Calcium is able to activate a range of intracellular mediators, including protein kinase C (Guillon et al 1987). Therefore, we examined the potential involvement of calcium ions in proteoglycan biosynthesis. Ionomycin, an ionophore that raises the concentration of intracellular calcium, was used (Perlman et al 1980). It was found that raising the intracellular calcium concentration had no effect on the incorporation of sulfate into proteoglycans. An SDS-PAGE of these samples demonstrated that ionomycin had no effect on the electrophoretic mobilities of the proteoglycan bands, and hence no effect on their sizes (Figure 2).
Figure 2.
Intracellular calcium does not play a role in VSMC proteoglycan synthesis. Human VSMCs were treated with ionomycin (0.01–10 nmol/L, which increases intracellular calcium, upper panels) or BAPTA-AM (1–10 μmol/L, a calcium chelator, lower panels) in the presence of TGF-β1 and ET-1 and metabolically labeled with [35S]-SO4 for 24 h. Proteoglycans were quantitated by the CPC precipitation assay (left) and assessed by SDS-PAGE (right) as described previously (Nigro et al 2002). Abbreviations: CPC, cetylpyridinium chloride; ET-1, endothelin-1; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; TGF, transforming growth factor; VSMC, vascular smooth muscle cell.
Bis (2-aminophenyl)ethyleneglycol-N,N,N′,N′-tetraacetic acid, tetraacetoxymethyl ester (BAPTA-AM) is a chelator of calcium ions and prevents a rise in intracellular calcium concentration by acting as a calcium buffer (Tsien 1980). BAPTA-AM (10 μmol/L) had a significant effect on basal sulfate incorporation into proteoglycans, and had a significant effect to decrease sulfate incorporation stimulated by both TGF-β and endothelin-1; however, the effect of BAPTA-AM on the agonist stimulated sulfate incorporation was of a similar degree to the effect of BAPTA on basal sulfate incorporation. Thus, we conclude that effects seen on agonist stimulated sulfate incorporation were due to the effect of BAPTA-AM on basal sulfate incorporation.
Conclusions
Drugs for the prevention of cardiovascular disease currently target risk factors of blood pressure, lipid abnormalities, and hyperglycemia in people with diabetes. Clinical studies have shown the therapeutic agents that show the greatest efficacy in treating and preventing cardiovascular disease are those that have complementary, so called, pleiotropic actions directly on blood vessels to prevent some of the many pathways involved in the formation of atherosclerotic plaques (Barbier et al 2002). Current research in the area of the pathobiology of atherosclerotic plaque formation is likely to lead to mechanistic agents that directly target the blood vessel wall to prevent the development of or increase the stability of plaques (Staels 2002). In time, the combination of agents targeting risk factors with mechanistic agents targeting the vessel wall should reduce the resistant or residual disease which remains apparent in most clinical trials.
Perspective
CCBs remain a most interesting class of drug. These agents are efficacious in most situations for the lowering of blood pressure. Whether or not this translates to the extent of the blood pressure lowering or beyond to a reduction in atherosclerotic cardiovascular disease is unresolved.
The response to retention hypothesis of atherogenesis based on the binding and retention in the vessel wall of atherogenic lipoproteins by GAG chains of proteoglycans is emerging as a robust model in cell and animal studies. We have considered the action of CCBs from the perspective of their actions on vascular proteoglycans. Initial data have shown some potentially antiatherogenic actions that occurred at very high (>30 μmol/L) concentrations and which were ascribed to an action of calcium channels. We have offered some preliminary data that directly extend those studies using stereoisomers of amlodipine, which have different affinities for calcium channels. We found that at more modest concentrations (≤10 μmol/L), amlodipine has very small effects on proteoglycan synthesis and the stereoisomers have identical actions suggesting that the inhibitory action is not targeted at or dependent upon an action on calcium channels. This may not be surprising in that there are few theoretical possibilities for a relationship between calcium channel activity and antiatherosclerotic actions perhaps other than via intracellular calcium.
A well controlled study in ApoE KO mice induced with diabetes showed that an ACE inhibitor (irbesartan) and CCB (amlodipine) both reduced blood pressure but only the ACE inhibitor was associated with a reduction in atherosclerotic lesion formation (Candido et al 2004). Accordingly, from our in vitro and in vivo data like that of Candido et al (2004), we speculate that CCBs do not have an efficacious action on proteoglycan synthesis in vascular smooth muscle that makes any meaningful contribution to the prevention of atherosclerosis.
CCBs may have a role as a primary or, perhaps more importantly, as an adjunct therapy for the reduction in blood pressure because hypertension is clearly one of the most prominent drivers of atherosclerosis. Hypertension is widely recognized as being undertreated in general practice (Gaede et al 2003) and in patients for which CCBs are well tolerated they may make an important contribution to the lowering of blood pressure towards the desired target levels. Other agents, some existing and having beneficial pleiotropic actions to prevent atherosclerosis and some agents that directly target mechanistic pathways of atherogenesis in the vessel wall, remain the most important pathways for targeting and preventing cardiovascular disease. The widening occurrence of obesity and the epidemic of Type 2 diabetes are driving an increase in atherosclerotic cardiovascular disease. New and efficacious agents and strategies are required to complement lifestyle changes in alleviating this wave of cardiovascular disease that threatens the health budgets of many countries and the health and longevity of individuals.
Acknowledgments
Research in the laboratory is supported by the National Health and Medical Research Council of Australia and the Alfred Hospital Foundation. The stereoisomers of amlodipine were kindly supplied by Pfizer Pty Ltd, West Ryde, Australia.
References
- Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–19. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrotis A, Little PJ, Saltis J, et al. Dihydropyridine Ca2+ channel antagonists inhibit the salvage pathway for DNA synthesis in human vascular smooth muscle cells. Eur J Pharmacol. 1993;244:269–75. doi: 10.1016/0922-4106(93)90152-y. [DOI] [PubMed] [Google Scholar]
- Antman EM, Stone PH, Muller JE, et al. Calcium channel blocking agents in the treatment of cardiovascular disorders. Part I: Basic and clinical electrophysiologic effects. Ann Intern Med. 1980;93:875–85. doi: 10.7326/0003-4819-93-6-875. [DOI] [PubMed] [Google Scholar]
- Arrowsmith JE, Campbell SF, Cross PE, et al. Long-acting dihyropryridine calcium antagonists. 1,2-alkoxymethyl derivatives incorporating basic substituents. J Med Chem. 1986;29:1696–702. doi: 10.1021/jm00159a022. [DOI] [PubMed] [Google Scholar]
- Ballinger M, Nigro J, Frontanilla K, et al. Regulation of glycosaminoglycan structure and atherogenesis. Cell Mol Life Sci. 2004;61:1296–306. doi: 10.1007/s00018-004-3389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier O, Torra IP, Duguay Y, et al. Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:717–26. doi: 10.1161/01.atv.0000015598.86369.04. [DOI] [PubMed] [Google Scholar]
- Bellosta S, Bernini F, Ferri N, et al. Direct vascular effects of HMG-CoA reductase inhibitors. Atherosclerosis. 1998;137(Suppl):S101–9. doi: 10.1016/s0021-9150(97)00319-5. [DOI] [PubMed] [Google Scholar]
- Camejo G, Hurt-Camejo E, Wiklund O, et al. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 1998;139:205–22. doi: 10.1016/s0021-9150(98)00107-5. [DOI] [PubMed] [Google Scholar]
- Candido R, Allen TJ, Lassila M, et al. Irbesartan but not amlodipine suppresses diabetes-associated atherosclerosis. Circulation. 2004;109:1536–42. doi: 10.1161/01.CIR.0000124061.78478.94. [DOI] [PubMed] [Google Scholar]
- Chait A, Wight TN. Interaction of native and modified low-density lipoproteins with extracellular matrix. Curr Opin Lipidol. 2000;11:457–63. doi: 10.1097/00041433-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Chang MY, Potter-Perigo S, Tsoi C, et al. Oxidized low density lipoproteins regulate synthesis of monkey aortic smooth muscle cell proteoglycans that have enhanced native low density lipoprotein binding properties. J Biol Chem. 2000;275:4766–73. doi: 10.1074/jbc.275.7.4766. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ishibashi S, Perrey S, et al. Troglitazone inhibits atherosclerosis in apolipoprotein E-knockout mice: pleiotropic effects on CD36 expression and HDL. Arterioscler Thromb Vasc Biol. 2001;21:372–7. doi: 10.1161/01.atv.21.3.372. [DOI] [PubMed] [Google Scholar]
- Claudel T, Leibowitz MD, Fievet C, et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc Natl Acad Sci U S A. 2001;98:2610–15. doi: 10.1073/pnas.041609298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR, Meehan WP, Kintscher U, et al. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:365–71. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- Duez H, Chao YS, Hernandez M, et al. Reduction of atherosclerosis by the peroxisome proliferator-activated receptor alpha agonist fenofibrate in mice. J Biol Chem. 2002;277:48051–7. doi: 10.1074/jbc.M206966200. [DOI] [PubMed] [Google Scholar]
- Faber M. The human aorta: sulfate-containing polyuronides and the deposition of cholesterol. Arch Pathol Lab Med. 1949;48:342–50. [PubMed] [Google Scholar]
- Fagnen G, Phamantu NT, Bocquet J, et al. Inhibition of transmembrane calcium influx induces decrease in proteoglycan synthesis in immature rat Sertoli cells. J Cell Biochem. 1999;76:322–31. doi: 10.1002/(sici)1097-4644(20000201)76:2<322::aid-jcb15>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]
- Figueroa JE, Vijayagopal P. Angiotensin II stimulates synthesis of vascular smooth muscle cell proteoglycans with enhanced low density lipoprotein binding properties. Atherosclerosis. 2002;162:261–8. doi: 10.1016/s0021-9150(01)00714-6. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Peter Harris Award lecture. History and prospects in calcium antagonist research. J Mol Cell Cardiol. 1990;22:241–51. doi: 10.1016/0022-2828(90)91458-j. [DOI] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- Goldmann S, Stoltefuss J, Born L. Determination of the absolute configuration of the active amlodipine enantiomer as (−)-S: a correction. J Med Chem. 1992;35:3341–4. doi: 10.1021/jm00096a005. [DOI] [PubMed] [Google Scholar]
- Guillon G, Balestre MN, Mouillac B, et al. Mechanisms of phospholipase C activation: a comparison with the adenylate cyclase system. Biochimie. 1987;69:351–63. doi: 10.1016/0300-9084(87)90026-5. [DOI] [PubMed] [Google Scholar]
- Gustafsson M, Boren J. Mechanism of lipoprotein retention by the extracellular matrix. Curr Opin Lipidol. 2004;15:505–14. doi: 10.1097/00041433-200410000-00003. [DOI] [PubMed] [Google Scholar]
- Hernandez RH, Armas-Hernandez MJ, Velasco M, et al. Calcium antagonists and atherosclerosis protection in hypertension. Am J Ther. 2003;10:409–14. doi: 10.1097/00045391-200311000-00006. [DOI] [PubMed] [Google Scholar]
- Hurt-Camejo E, Olsson U, Wiklund O, et al. Cellular consequences of the association of apoB lipoproteins with proteoglycans. Potential contribution to atherogenesis. Arterioscler Thromb Vasc Biol. 1997;17:1011–17. doi: 10.1161/01.atv.17.6.1011. [DOI] [PubMed] [Google Scholar]
- Jensen T. The HOPE study and diabetes. Heart Outcomes Prevention Evaluation. Lancet. 2000;355:1181. doi: 10.1016/s0140-6736(00)02076-6. [DOI] [PubMed] [Google Scholar]
- Jukema JW, Zwinderman AH, van Boven AJ, et al. Evidence for a synergistic effect of calcium channel blockers with lipid-lowering therapy in retarding progression of coronary atherosclerosis in symptomatic patients with normal to moderately raised cholesterol levels. The REGRESS Study Group. Arterioscler Thromb Vasc Biol. 1996;16:425–30. doi: 10.1161/01.atv.16.3.425. [DOI] [PubMed] [Google Scholar]
- Kadar A, Glasz T. Development of atherosclerosis and plaque biology. Cardiovasc Surg. 2001;9:109–21. doi: 10.1016/s0967-2109(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Kohrogi H, Horio S, Ando M, et al. Nifedipine inhibits human bronchial smooth muscle contractions induced by leukotrienes C4 and D4, prostaglandin F2 alpha, and potassium. Am Rev Respir Dis. 1985;132:299–304. doi: 10.1164/arrd.1985.132.2.299. [DOI] [PubMed] [Google Scholar]
- Lehto S, Ronnemaa T, Haffner SM, et al. Dyslipidemia and hyperglycemia predict coronary heart disease events in middle-aged patients with NIDDM. Diabetes. 1997;46:1354–9. doi: 10.2337/diab.46.8.1354. [DOI] [PubMed] [Google Scholar]
- Li AC, Brown KK, Silvestre MJ, et al. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–31. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lichtlen PR, Hugenholtz PG, Rafflenbeul W, et al. Retardation of angiographic progression of coronary artery disease by nifedipine. Results of the International Nifedipine Trial on Antiatherosclerotic Therapy (INTACT) Lancet. 1990;335:1109–13. doi: 10.1016/0140-6736(90)91121-p. [DOI] [PubMed] [Google Scholar]
- Little PJ, Tannock L, Olin KL, et al. Proteoglycans synthesized by arterial smooth muscle cells in the presence of transforming growth factor-beta1 exhibit increased binding to LDLs. Arterioscler Thromb Vasc Biol. 2002;22:55–60. doi: 10.1161/hq0102.101100. [DOI] [PubMed] [Google Scholar]
- Luksa J, Josic D, Podobnik B, et al. Semi-preparative chromatographic purification of the enantiomers S-(−)-amlodipine and R-(+)-amlodipine. J Chromatogr B Biomed Sci Appl. 1997;693:367–75. doi: 10.1016/s0378-4347(97)00069-8. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens E, van Suylen RJ, Faber BC, et al. Atherosclerotic plaque rupture: local or systemic process? Arterioscler Thromb Vasc Biol. 2003;23:2123–30. doi: 10.1161/01.ATV.0000097783.01596.E2. [DOI] [PubMed] [Google Scholar]
- MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–74. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- Mancia G, Brown M, Castaigne A, et al. Outcomes with nifedipine GITS or Co-amilozide in hypertensive diabetics and nondiabetics in Intervention as a Goal in Hypertension (INSIGHT) Hypertension. 2003;41:431–6. doi: 10.1161/01.HYP.0000057420.27692.AD. [DOI] [PubMed] [Google Scholar]
- Meyers CD, Tannock LR, Wight TN, et al. Statin-exposed vascular smooth muscle cells secrete proteoglycans with decreased binding affinity for LDL. J Lipid Res. 2003;44:2152–60. doi: 10.1194/jlr.M300252-JLR200. [DOI] [PubMed] [Google Scholar]
- Motro M, Shemesh J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertension. 2001;37:1410–13. doi: 10.1161/01.hyp.37.6.1410. [DOI] [PubMed] [Google Scholar]
- Nigro J, Ballinger M, Dilley R, et al. Fenofibrate modifies human vascular smooth muscle proteoglycans and reduces LDL binding. Diabetologia. 2004;47:2105–13. doi: 10.1007/s00125-004-1588-z. [DOI] [PubMed] [Google Scholar]
- Nigro J, Ballinger ML, Osman N, et al. Anti-atherogenic role of peroxisome proliferator activated receptor ligands. Curr Cardiol Rev. 2005 In press. [Google Scholar]
- Nigro J, Dilley RJ, Little PJ. Differential effects of gemfibrozil on migration, proliferation and proteoglycan production in human vascular smooth muscle cells. Atherosclerosis. 2002;162:119–29. doi: 10.1016/s0021-9150(01)00704-3. [DOI] [PubMed] [Google Scholar]
- Olsson U, Bondjers G, Camejo G. Fatty acids modulate the composition of extracellular matrix in cultured human arterial smooth muscle cells by altering the expression of genes for proteoglycan core proteins. Diabetes. 1999;48:616–22. doi: 10.2337/diabetes.48.3.616. [DOI] [PubMed] [Google Scholar]
- Perlman RL, Cossi AF, Role LW. Mechanisms of ionophore-induced catecholamine secretion. J Pharmacol Exp Ther. 1980;213:241–6. [PubMed] [Google Scholar]
- Pitt B, Byington RP, Furberg CD, et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation. 2000;102:1503–10. doi: 10.1161/01.cir.102.13.1503. [DOI] [PubMed] [Google Scholar]
- Radhakrishnamurthy B, Srinivasan SR, Vijayagopal P, et al. Arterial wall proteoglycans – biological properties related to pathogenesis of atherosclerosis. Eur Heart J. 1990;11(Suppl E):148–57. doi: 10.1093/eurheartj/11.suppl_e.148. [DOI] [PubMed] [Google Scholar]
- Schonherr E, Jarvelainen HT, Sandell LJ, et al. Effects of platelet-derived growth factor and transforming growth factor-beta 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991;266:17640–7. [PubMed] [Google Scholar]
- Schonherr E, Kinsella MG, Wight TN. Genistein selectively inhibits platelet-derived growth factor-stimulated versican biosynthesis in monkey arterial smooth muscle cells. Arch Biochem Biophys. 1997;339:353–61. doi: 10.1006/abbi.1996.9854. [DOI] [PubMed] [Google Scholar]
- Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41:15S–22S. doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- Shanahan CM, Cary NR, Metcalfe JC, et al. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Hirota R, Sasamura H, Mifune M, et al. Regulation of vascular proteoglycan synthesis by angiotensin II type 1 and type 2 receptors. J Am Soc Nephrol. 2001;12:2609–15. doi: 10.1681/ASN.V12122609. [DOI] [PubMed] [Google Scholar]
- Sidhu JS, Kaposzta Z, Markus HS, et al. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol. 2004;24:930–4. doi: 10.1161/01.ATV.0000124890.40436.77. [DOI] [PubMed] [Google Scholar]
- Simon A, Gariepy J, Moyse D, et al. Differential effects of nifedipine and co-amilozide on the progression of early carotid wall changes. Circulation. 2001;103:2949–54. doi: 10.1161/01.cir.103.24.2949. [DOI] [PubMed] [Google Scholar]
- Skalen K, Gustafsson M, Rydberg EK, et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–4. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- Srinivasan SR, Xu JH, Vijayagopal P, et al. Injury to the arterial wall of rabbits produces proteoglycan variants with enhanced low-density lipoprotein-binding property. Biochim Biophys Acta. 1993;1168:158–66. doi: 10.1016/0005-2760(93)90120-x. [DOI] [PubMed] [Google Scholar]
- Staels B. Cardiovascular biology: a cholesterol tether. Nature. 2002;417:699–701. doi: 10.1038/417699a. [DOI] [PubMed] [Google Scholar]
- Tannock L, Little PJ, Wight TN, et al. Arterial smooth muscle cell proteoglycans synthesized in the presence of glucosamine demonstrate reduced binding to LDL. J Lipid Res. 2002;43:149–57. [PubMed] [Google Scholar]
- Tannock LR, Little PJ, Tsoi C, et al. Thiazolidinediones reduce the LDL binding affinity of non-human primate vascular cell proteoglycans. Diabetologia. 2004;47:837–43. doi: 10.1007/s00125-004-1358-y. [DOI] [PubMed] [Google Scholar]
- Tao Z, Smart FW, Figueroa JE, et al. Elevated expression of proteoglycans in proliferating vascular smooth muscle cells. Atherosclerosis. 1997;135:171–9. doi: 10.1016/s0021-9150(97)00158-5. [DOI] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- [UKPDS] UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998a;352:837–53. [PubMed] [Google Scholar]
- [UKPDS] UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J. 1998b;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983;96:1677–89. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayagopal P, Subramaniam P. Effect of calcium channel blockers on proteoglycan synthesis by vascular smooth muscle cells and low density lipoprotein-proteoglycan interaction. Atherosclerosis. 2001;157:353–60. doi: 10.1016/s0021-9150(00)00742-5. [DOI] [PubMed] [Google Scholar]
- Waters D, Lesperance J, Francetich M, et al. A controlled clinical trial to assess the effect of a calcium channel blocker on the progression of coronary atherosclerosis. Circulation. 1990;82:1940–53. doi: 10.1161/01.cir.82.6.1940. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–61. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of atherogenesis reinforced. Curr Opin Lipidol. 1998;9:471–4. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Yutani C, Imakita M, Ishibashi-Ueda H, et al. Coronary atherosclerosis and interventions: pathological sequences and restenosis. Pathol Int. 1999;49:273–90. doi: 10.1046/j.1440-1827.1999.00861.x. [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Bond MG, Hennig M, et al. Absolute and relative changes in carotid intima-media thickness and atherosclerotic plaques during long-term antihypertensive treatment: further results of the European Lacidipine Study on Atherosclerosis (ELSA) J Hypertens. 2004;22:1201–12. doi: 10.1097/00004872-200406000-00022. [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Rosei EA, Dal Palu C, et al. The Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of long-term randomized treatment with either verapamil or chlorthalidone on carotid intima-media thickness. J Hypertens. 1998;16:1667–76. doi: 10.1097/00004872-199816110-00014. [DOI] [PubMed] [Google Scholar]