Abstract
Diabetes mellitus is an important risk factor for cardiovascular morbidity and mortality. The metabolic abnormalities caused by diabetes mellitus induce vascular endothelial dysfunction that predisposes patients with diabetes mellitus to atherosclerosis. Two mega clinical trials showed that intensive glycemic control does not have favorable effects on reducing macrovascular events although it demonstrated significant reductions in microvascular complications. It is becoming worthwhile to clarify the beneficial effects of tight controls on blood pressure, serum lipids, and postprandial hyperglycemia to prevent atherosclerosis in patients with type 2 diabetes mellitus. Here, we focus on vascular endothelium as a target of the prostaglandin I2 analog beraprost sodium and the peroxisome proliferators-activated receptor α activator fenofibrate for the prevention and treatment of atherosclerosis in patients with type 2 diabetes mellitus. Beraprost sodium lowered circulating vascular cell adhesion molecule-1 (VCAM-1) concentration and prevented the progression of carotid atherosclerosis in type 2 diabetic patients, probably through inhibiting VCAM-1 expression in vascular endothelium. Fenofibrate up-regulated endothelial nitric oxide synthase expression, which may explain its effects to improve endothelium-dependent vasodilatation and to prevent the progression of coronary atherosclerosis. The approaches to target the molecules expressed in vascular endothelium will become important for preventing the atherosclerosis in type 2 diabetes mellitus.
Keywords: vascular cell adhesion molecule-1, endothelial nitric oxide synthase, peroxisome proliferators-activated receptor α, beraprost sodium, fenofibrate
Introduction
Type 2 diabetes mellitus is a syndrome of disordered metabolism with inappropriate hyperglycemia due to both an absolute deficiency of insulin secretion and a reduction in the biological effectiveness of insulin. The chronic exposure to high levels of blood glucose is an important factor leading to diabetic vascular complications. Diabetes mellitus is an independent risk factor for cardiovascular disease, cerebrovascular disease, and peripheral vascular disease. According to the Multiple Risk Factor Interventional Trial (MRFIT), patients with diabetes mellitus are known to be affected by cardiovascular disease three times as frequently as nondiabetic subjects, with adjustment for other risk factors (Stamler et al 1993). From the two mega trial studies with type 1 diabetes mellitus and type 2 diabetes mellitus, establishing that the intensive glycemic control decreases the incidence of macrovascular complications has proved much more elusive than the established beneficial effects on microvascular complications such as retinopathy or renal disease (Diabetes Control and Complications Trial Research Group 1993; UKPDS 1998a). Diabetic macrovascular complications occur by complex mechanisms consisting of atherosclerosis, thrombosis, and hemodynamic abnormalities. Among them, atherosclerosis is an important component for diabetic macrovascular complications. Thus, understanding the pathophysiology of atherosclerosis in diabetes mellitus and prevention of atherosclerotic vascular diseases are clinically important issues. Vascular endothelial dysfunction is an early event of diabetic macroangiopathies (Stehouwer et al 1992). A number of pharmacotherapeutic approaches for correcting impaired endothelial function, such as antioxidants, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, tetrahydrobiopterin, methytetrahydrofolic acid, and arginine, have been investigated (for a comprehensive review, see Woodman et al 2005). In this review, we focus on two kinds of reagents targeting vascular endothelium for the prevention and treatment of atherosclerosis in patients with type 2 diabetes mellitus: the prostaglandin (PG) I2 analog beraprost sodium, and the peroxisome proliferators-activated receptor (PPAR)α activator fenofibrate.
Hyperglycemia as a target of antiatherosclerotic therapy in type 2 diabetes mellitus
Macrovascular complications of diabetes mellitus are postulated as distinct from diabetic microvascular complications due to their pathophysiology and epidemiology. It has been shown that a 1% increase in glycosylated hemoglobin (HbA1c) resulted in a 70% increase in proliferative retinopathy, but only a 10% increase in cardiovascular events (Klein 1995). Thus, hyperglycemia is a dominant risk factor for diabetic retinopathy or diabetic kidney disease, but it showed not to be the only risk factor for atherosclerosis in diabetic patients.
In our analysis of 125 type 2 diabetic patients without microvascular complications, 50% of the patients were diagnosed as having atherosclerosis of the carotid arteries based on ultrasonographic evaluation. Risk factors for early atherosclerosis of the carotid arteries were age, low-density lipoprotein (LDL)-cholesterol, hypertension, and diabetes treatment, but neither fasting plasma glucose, HbA1c, nor known diabetes duration (Goya, Kitamura, et al 2003). These results indicate that glycemic control estimated by fasting plasma glucose and HbA1c levels is poorly related to asymptomatic atherosclerosis in type 2 diabetic patients without diabetic microvascular complications.
Recent epidemiological studies suggest that postprandial hyperglycemia may be a risk factor of cardiovascular disease beyond and more powerful than fasting hyperglycemia (Bonora and Muggeo 2001). A possible mechanism is that hyperglycemia after meals causes an overproduction of free radicals and thrombin proportional to blood glucose levels in type 2 diabetic patients (Ceriello et al 2002). Moreover, acute elevation of glucose levels in healthy subjects reduces nitric oxide (NO) availability (Giugliano et al 1997) and increases the circulating levels of some cellular adhesion molecules (Marfella et al 2000). In subjects with normal glucose tolerance (NGT) and those with impaired glucose tolerance (IGT), acute hyperglycemia increases circulating cytokine concentrations by an oxidative mechanism (Esposito et al 2002). Oral glucose tolerance test (OGTT)-2 h glucose is an important predictor for plasma high-sensitivity C-reactive protein level, an associated marker of cardiovascular disease, in nondiabetic subjects (Hashimoto et al 2004). It has been reported that reduction of postprandial hyperglycemia in type 2 diabetic patients is associated with carotid atherosclerosis regression (Esposito et al 2004) and cardiovascular events (Hanefeld et al 2004).
Hypertension and dyslipidemia as targets of antiatherosclerotic therapy in type 2 diabetes mellitus
To prevent cardiovascular events in patients with type 2 diabetes mellitus, therapeutic strategies that have been shown by clinical trials include antihypertensive therapy, lipid-lowering therapy, and antiplatelet therapy.
The tight control of blood pressure has been shown to reduce the incidence of deaths related to diabetes, stroke, diabetic microvascular endpoints, progression of diabetic retinopathy, and deterioration in visual acuity (UKPDS 1998b). However, there was no significant reduction in all cause mortality and myocardial infarction in that study, but the study was not powered to detect moderate changes in these parameters. By contrast, subanalysis of the Hypertension Optimal Treatment (HOT) study showed that intensive lowering of diastolic blood pressure was associated with significant reductions in cardiovascular events in diabetic patients, although it failed to demonstrate the beneficial effects of tight blood pressure control on the prevention of myocardial infarction and stroke (Hansson et al 1998).
There is a substantial group of type 2 diabetes patients in whom the levels of both plasma triglyceride and LDL-cholesterol are elevated and with low high-density lipoprotein (HDL) cholesterol. Treatment with the statin class of lipid-lowering drugs (HMG-CoA reductase inhibitors) efficiently corrects the elevated LDL-cholesterol level. In the Anglo-Scandinavian Cardiac Outcomes Trail-Lipid Lowering Arm (ASCOT-LLA), atorvastatin could not reduce the risk of nonfatal myocardial infarction and coronary artery heart disease death in patients with diabetes and hypertension who had no preexisting coronary heart disease, although LDL-cholesterol level in the atorvastatin group was 1.2 mmol/L lower than the placebo group (Sever et al 2003). The Heart Protection Study (HPS), which included 5963 patients with diabetes who were prospectively randomized to treatment with simvastatin or placebo, demonstrated 27% decrease (p = 0.0007) in major coronary events with active treatment (Collins et al 2003). In the Collaborative Atorvastatin Diabetes Study (CARDS) (Colhoun et al 2004), 2838 patients with type 2 diabetes mellitus were studied. A significant 37% reduction in cardiovascular events was shown in the atorvastatin group. Overall, some but not all studies have reported significant reduction in coronary heart disease risk with statin therapy in diabetic patients (Garg 2004). Statin therapy is shown to have the ability to improve insulin resistance in dyslipidemic type 2 diabetic patients (Paolisso et al 2000) and endothelial function through increasing the bioavailability of NO (Wolfrum et al 2003). Improvement of flow-mediated dilatation (FMD) after atorvastatin treatment for 6 months has been demonstrated in type 2 diabetic patients (Tan et al 2002), although there have been reports of controversial results with the use of atorvastatin or simvastatin (Sheu et al 1999; van Etten et al 2002). Thus, the beneficial effect of statins on cardiovascular diseases may be at least in part mediated by improving vascular function, in addition to the effect of controlling dyslipidemia.
The fibric acid derivative fenofibrate significantly reduced the angiographic progression of coronary artery disease in type 2 diabetes mellitus (Diabetes Atherosclerosis Intervention Study Investigators 2001). The favorable effect of fenofibrate on coronary artery disease is indicated to be exerted by increasing the expression of ApoA-I and ApoA-II as well as by increasing LDL particle size (Vakkilainen et al 2003). Additionally, since fenofibrate has the ability to activate PPARα more specifically than other fibrates such as gemfibrozil and bezafibrate (Keating and Ormrod 2002), the effects of fenofibrate also may be mediated through pleiotropic effects improving endothelial function, which will be shown later.
Multifactorial intervention that targeted hyperglycemia, hypertension, dyslipidemia, and microalubuminuria, along with secondary prevention of cardiovascular disease with aspirin, in patients with type 2 diabetes and microalubuminuria, has been recently shown to reduce the risk of cardiovascular and microvascular events by about 50% (Gaede et al 2003).
Adhesion molecules expressed in endothelium as targets of antiatherosclerotic therapy in type 2 diabetes mellitus
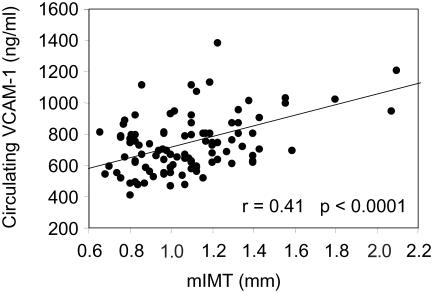
Atherosclerosis is characterized by endothelial cell injury, which in turn leads to mononuclear cell adhesion to the endothelium, the initial migration and proliferation of smooth muscle cells, and extracellular matrix deposition (Ross 1999). Various adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), E-selectin, and platelet endothelial cell adhesion molecule (PECAM) have been shown to be highly expressed in atherosclerotic lesions, which might be involved in mononuclear cell adhesion to the vascular endothelium (Cybulsky and Gimbrone 1991; O'Brien et al 1993; Bevilacqua et al 1994). It has been shown that circulating VCAM-1 level is correlated with early atherosclerosis in patients with type 2 diabetes mellitus (Figure 1) (Otsuki et al 1997). The Hoorn study demonstrated that elevated circulating VCAM-1 level is independently associated with an increased future risk of cardiovascular mortality in type 2 diabetes mellitus (Jager et al 2000). Thus, to inhibit the VCAM-1 expression on the vascular endothelium may be a strategy for preventing occurrence and progression of atherosclerosis in patients with type 2 diabetes.
Figure 1.
Relationship between serum circulating vascular cell adhesion molecule (VCAM)-1 and mean intima-medial thickness (mIMT) of carotid arteries in 101 patients with type 2 diabetic mellitus.
VCAM-1 expression is enhanced by various inflammatory molecules such as tumor necrosis factor (TNF)-α and interleukin (IL)-1. The expression of VCAM-1 is known to be inhibited by various reagents such as glucocorticoids (Simoncini et al 2000), estrogen (Caulin-Glaser et al 1996; Simoncini et al 1999), progestins (Otsuki et al 2001), retinoic acid (Gille et al 1997), and fibric acid derivatives (Marx et al 1999; Xu et al 2001).
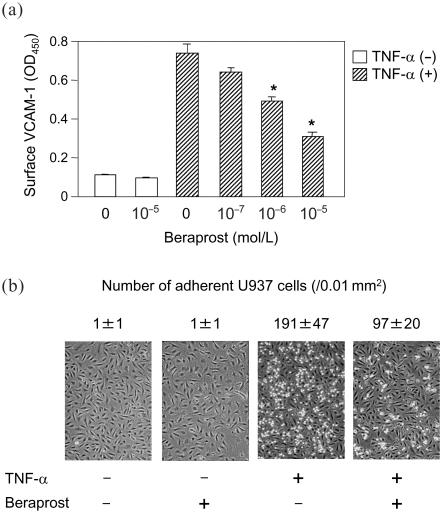
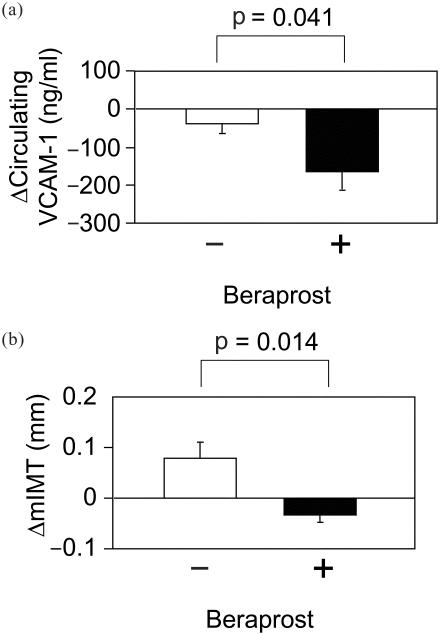
We recently found that beraprost sodium, orally active PG I2 analog, also efficiently inhibited TNF-α-induced VCAM-1 expression in human vascular endothelial cells (Figure 2a) (Goya, Otsuki, et al 2003). Beraprost also decreased the number of human monocytoid cells adhesion to vascular endothelial cells (Figure 2b). In our clinical study on 25 type 2 diabetic patients who had atherosclerotic change of carotid arteries, the 3-year changes of circulating VCAM-1 level were significantly lower in patients receiving beraprost sodium than those not receiving it (Figure 3). The 3-year changes of common carotid artery (CCA) intima-medial thickness (IMT) were also significantly lower in those with the beraprost treatment. Studies of 10 patients who could be followed up for 8 years with beraprost treatment showed that their CCA IMT was not significantly changed from 1.17 ± 0.24 mm (SE) to 1.13 ± 0.19 mm after 8 years (Figure 4). During this course, body mass index, fasting glucose, HbA1c and total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides were not significantly changed in these patients. Based upon the data of the Insulin Resistance Atherosclerosis Study (Wagenknecht et al 2003), the average CCA IMT progression reported 0.0072 mm per year in persons with diagnosed diabetes, corresponding to the increases of 0.056 mm in 8 years. Although these data cannot be simply compared with our results shown in Figure 4, beraprost may be effective long-term for the inhibition of atherosclerosis progression in type 2 diabetes. The sum total of these observations provides the potential of beraprost sodium as an antiatherosclerotic drug in type 2 diabetic patients, at least partly through inhibiting VCAM-1 expression in vascular endothelium.
Figure 2.
(a) Effect of beraprost on cell surface VCAM-1 level in vascular endothelial cells. Vascular endothelial cells were treated for 15 minutes with or without the indicated concentrations of beraprost, and then the cells were stimulated with (hatched bars) or without (open bars) 0.1 ng/mL TNF-α for 2 hours. Cell surface VCAM-1 was determined by whole cell ELISA. Data are means ± SE. *p < 0.001 vs TNF-α only, by ANOVA.
(b) Effects of beraprost on U937 cell adhesion to TNF-α-stimulated vascular endothelial cells. Vascular endothelial cells were treated for 15 minutes with or without 10−5 mol/L beraprost and then stimulated with or without 0.1 ng/mL TNF-α for 2 hours. Data are means ± SE. Abbreviations: ANOVA, analysis of variance; ELISA, enzyme-linked immunoabsorbent assay; OD, optical density; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule.
Figure 3.
Changes in circulating vascular cell adhesion molecule (VCAM-1) level (a) and mean intima-medial thickness (mIMT) of common carotid arteries (b) in 25 patients with type 2 diabetes mellitus receiving (n = 11) or not receiving (n = 14) beraprost for 3 years. Data are means ± SE.
Figure 4.
Changes in mean intima-medial thickness (mIMT) of common carotid arteries in 10 patients with type 2 diabetes mellitus treated with beraprost for 8 years. Data are means ± SE. p = 0.44, by ANOVA (analysis of variance).
It has been shown that PPARα activators fenofibrate and WY14643 inhibited cytokine-induced VCAM-1 expression, resulting in inhibition of leukocyte adherence on vascular endothelial cells (Marx et al 1999). These observations indicate implications regarding the clinical use of the fibrate class of lipid-lowering agents. Although one clinical study of 11 patients with type 2 diabetes showed no significant change in plasma VCAM-1 concentrations by fenofibrate treatment for 6 weeks (Empen et al 2003), a larger cohort study should be investigated.
Endothelial nitric oxide synthase (eNOS) expressed in endothelium as a target for antiatherosclerotic therapy in type 2 diabetes mellitus
Endothelium-derived NO is a potent mediator with antiatherogenic properties such as stimulation of vasorelaxation and repression of endothelial leukocyte adhesion molecules, platelet aggregation, and smooth muscle cell proliferation. Endothelial dysfunction due to impaired production and/or action of NO occurs in the early stages of atherosclerosis caused by various risk factors such as hypercholesterolemia (Creager et al 1990), hypertension (Panza et al 1990), cigarette smoking (Celermajer et al 1993), aging (Celermajer, Sorensen, Spiegelhalter, et al 1994), male sex (Celermajer, Sorensen, Bull, et al 1994), and hyperhomocystinemia (Woo et al 1997). Impairment of NO production and its action has also been demonstrated in patients with diabetes mellitus (Jonhnstone et al 1993; Williams et al 1996; Beckmann et al 2002). NO is produced from L-arginine by eNOS. Endothelium-derived NO is responsible for FMD (Joannides et al 1995). The FMD of the brachial artery, a parameter of endothelium-dependent vasodilatation, is significantly lower in patients with type 2 diabetes than normal control subjects (Williams et al 1996; Meeking et al 1999). Thus, to increase eNOS expression in endothelium may be a potential antiatherogenic target in diabetic patients.
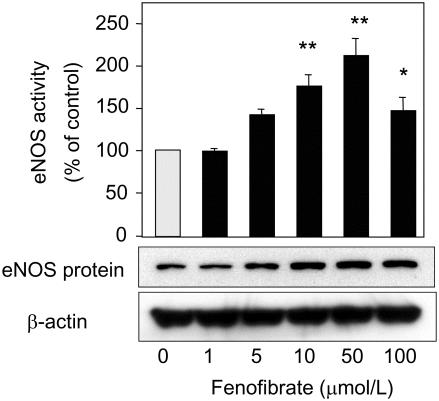
Recent clinical studies have shown that the fibric acid derivative fenofibrate improved vasodilator function. Playford et al (2002) showed that fenofibrate significantly improved brachial artery FMD, but not nitroglycerin-mediated dilatation in patients with type 2 diabetes. Fenofibrate significantly increased forearm blood flow in response to acetylcholine, nitroprusside, or verapamil in patients with hypertriglyceridemia (Capell et al 2003). These results indicate that fenofibrate can improve both endothelium-dependent and endothelium-independent vasodilatation. We have shown that fenofibrate and WY14643, also able to activate PPARα, up-regulated eNOS expression in vascular endothelial cells (Goya et al 2004). Bezafibrate, a fibrate drug that potentially activates three types of PPAR (PPARα, -γ, -δ), also increased eNOS expression, whereas rosiglitazone, a specific ligand for PPARγ, failed to increase eNOS expression. Taken together with our result that RU486, shown to have a potential to antagonize PPARα, inhibited the fenofibrate-induced up-regulation of eNOS expression, the fenofibrate-induced up-regulation of eNOS expression was mediated through PPARα. Analysis of the human eNOS promoter sequence (−1600 to +22 nucleotides) did not contain any discernible PPAR response element, and transient transfection of the eNOS promoter construct showed that fenofibrate failed to increase its promoter activity. In addition, the experiments using the transcriptional inhibitor actinomycin D showed that fenofibrate stabilized eNOS mRNA (Figure 5). These data also suggest direct effects of the fibrates on vessel wall for cardiovascular protection besides lipid-lowering effects. We will see in the near future the results of a large trial that is currently examining the effect of fenofibrate on cardiovascular mortality in patients with type 2 diabetes mellitus (Fenofibrate Intervention and Event Lowering in Diabetes [FIELD] study).
Figure 5.
Effects of fenofibrate on endothelial nitric oxide synthase (eNOS) activity and its protein levels in vascular endothelial cells. Vascular endothelial cells were treated for 48 hours with the indicated concentrations of fenofibrate. Data are means ± SE. *p < 0.005, **p < 0.0001 vs control, by ANOVA (analysis of variance).
Conclusion
Atherosclerosis in type 2 diabetes mellitus has been hard to overcome. Multifactorial therapies targeting hyperglycemia, blood pressure, and lipids have proven efficient for reducing cardiovascular events. However, it still remains unclear whether such combination therapy is the best of antiatherosclerotic therapies for type 2 diabetes mellitus, in terms of safety or cost-effectiveness. Here, we demonstrated the potentials of the PGI2 analog beraprost and the PPARα activator fenofibrate to target VCAM-1 and eNOS, respectively, in vascular endothelium. These drugs may be added to the lists of antiatherosclerotic modalities for type 2 diabetes.
References
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Nelson RM, Mannori G, et al. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev Med. 1994;45:361–78. doi: 10.1146/annurev.med.45.1.361. [DOI] [PubMed] [Google Scholar]
- Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107–14. doi: 10.1007/s001250100020. [DOI] [PubMed] [Google Scholar]
- Capell WH, DeSouza CA, Poirier P, et al. Short-term triglyceride lowering with fenofibrate improves vasodilator function in subjects with hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2003;23:307–13. doi: 10.1161/01.atv.0000046230.02211.b4. [DOI] [PubMed] [Google Scholar]
- Caulin-Glaser T, Watson CA, Pardi R, et al. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996;98:36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–55. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Bull C, et al. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–74. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, et al. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–6. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Quagliaro L, Catone B, et al. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25:1439–43. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, et al. Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–34. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–91. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Diabetes Atherosclerosis Intervention Study Investigators. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–10. [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Empen K, Frost RJ, Geiss HC, et al. Differential effects of fenofibrate versus atorvastatin on the concentrations of E-selectin and vascular cellular adhesion molecule-1 in patients with type 2 diabetes mellitus and mixed hyperlipoproteinemia: a randomized cross-over trial. Cardiovasc Diabetol. 2003;2:17. doi: 10.1186/1475-2840-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Giugliano D, Nappo F, et al. Campanian Postprandial Hyperglycemia Study Group Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–19. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- Garg A. Statins for all patients with type 2 diabetes: not so soon. Lancet. 2004;364:641–2. doi: 10.1016/S0140-6736(04)16907-9. [DOI] [PubMed] [Google Scholar]
- Gille J, Paxton LL, Lawley TJ, et al. Retinoic acid inhibits the regulated expression of vascular cell adhesion molecule-1 by cultured dermal microvascular endothelial cells. J Clin Invest. 1997;99:492–500. doi: 10.1172/JCI119184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano D, Marfella R, Coppola L, et al. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation. 1997;95:1783–90. doi: 10.1161/01.cir.95.7.1783. [DOI] [PubMed] [Google Scholar]
- Goya K, Kitamura T, Inaba M, et al. Risk factors for asymptomatic atherosclerosis in Japanese type 2 diabetic patients without diabetic microvascular complications. Metabolism. 2003;52:1302–6. doi: 10.1016/s0026-0495(03)00197-5. [DOI] [PubMed] [Google Scholar]
- Goya K, Otsuki M, Xu X, et al. Effects of the prostaglandin I2 analogue, beraprost sodium, on vascular cell adhesion molecule-1 expression in human vascular endothelial cells and circulating vascular cell adhesion molecule-1 level in patients with type 2 diabetes mellitus. Metabolism. 2003;52:192–8. doi: 10.1053/meta.2003.50025. [DOI] [PubMed] [Google Scholar]
- Goya K, Sumitani S, Xu X, et al. Peroxisome proliferator-activated receptor α agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:658–63. doi: 10.1161/01.ATV.0000118682.58708.78. [DOI] [PubMed] [Google Scholar]
- Hanefeld M, Cagatay M, Petrowitsch T, et al. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10–16. doi: 10.1016/s0195-668x(03)00468-8. [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kasayama S, Yamamoto H, et al. Strong association of C-reactive protein with body mass index and 2-h post-challenge glucose in non-diabetic, non-smoker subjects without hypertension. Diabet Med. 2004;21:581–5. doi: 10.1111/j.1464-5491.2004.01212.x. [DOI] [PubMed] [Google Scholar]
- Jager A, van Hinsbergh VW, Kostense PJ, et al. Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes. 2000;49:485–91. doi: 10.2337/diabetes.49.3.485. [DOI] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–19. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- Johnstone MT, Creager SJ, Scales KM, et al. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–16. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- Keating GM, Ormrod D. Micronised fenofibrate: an updated review of its clinical efficacy in the management of dyslipidaemia. Drugs. 2002;62:1909–44. doi: 10.2165/00003495-200262130-00013. [DOI] [PubMed] [Google Scholar]
- Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18:258–68. doi: 10.2337/diacare.18.2.258. [DOI] [PubMed] [Google Scholar]
- Marfella R, Esposito K, Giunta R, et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101:2247–51. doi: 10.1161/01.cir.101.19.2247. [DOI] [PubMed] [Google Scholar]
- Marx N, Sukhova GK, Collins T, et al. PPARα activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–31. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeking DR, Cummings MH, Thorne S, et al. Endothelial dysfunction in type 2 diabetic subjects with and without microalbuminuria. Diabet Med. 1999;16:841–7. doi: 10.1046/j.1464-5491.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- O'Brien KD, Allen MD, McDonald TO, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–51. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki M, Hashimoto K, Morimoto Y, et al. Circulating vascular cell adhesion molecule-1 (VCAM-1) in atherosclerotic NIDDM patients. Diabetes. 1997;46:2096–101. doi: 10.2337/diab.46.12.2096. [DOI] [PubMed] [Google Scholar]
- Otsuki M, Saito H, Xu X, et al. Progesterone, but not medroxyprogesterone, inhibits vascular cell adhesion molecule-1 expression in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:243–8. doi: 10.1161/01.atv.21.2.243. [DOI] [PubMed] [Google Scholar]
- Panza JA, Quyyumi AA, Brush JE, Jr, et al. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Barbagallo M, Petrella G, et al. Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non-insulin dependent diabetic patients. Atherosclerosis. 2000;150:121–7. doi: 10.1016/s0021-9150(99)00352-4. [DOI] [PubMed] [Google Scholar]
- Playford DA, Watts GF, Best JD, et al. Effect of fenofibrate on brachial artery flow-mediated dilatation in type 2 diabetes mellitus. Am J Cardiol. 2002;90:1254–7. doi: 10.1016/s0002-9149(02)02847-3. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- Sheu WH, Juang BL, Chen YT, et al. Endothelial dysfunction is not reversed by simvastatin treatment in type 2 diabetic patients with hypercholesterolemia. Diabetes Care. 1999;22:1224–5. doi: 10.2337/diacare.22.7.1224. [DOI] [PubMed] [Google Scholar]
- Simoncini T, De Caterina R, Genazzani AR. Selective estrogen receptor modulators: different actions on vascular cell adhesion molecule-1 (VCAM-1) expression in human endothelial cells. J Clin Endocrinol Metab. 1999;84:815–18. doi: 10.1210/jcem.84.2.5570. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Maffei S, Basta G, et al. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ Res. 2000;87:19–25. doi: 10.1161/01.res.87.1.19. [DOI] [PubMed] [Google Scholar]
- Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Nauta JJ, Zeldenrust GC, et al. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in noninsulin-dependent diabetes mellitus. Lancet. 1992;340:319–23. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- Tan KC, Chow WS, Tam SC, et al. Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2002;87:563–8. doi: 10.1210/jcem.87.2.8249. [DOI] [PubMed] [Google Scholar]
- [UKPDS] UK Prospective Diabetes Study Group. Intensive bloodglucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998a;352:837–53. [PubMed] [Google Scholar]
- [UKPDS] UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998b;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- Vakkilainen J, Steiner G, Ansquer JC, et al. Relationships between low-density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: the Diabetes Atherosclerosis Intervention Study (DAIS) Circulation. 2003;107:1733–7. doi: 10.1161/01.CIR.0000057982.50167.6E. [DOI] [PubMed] [Google Scholar]
- van Etten RW, de Koning EJ, Honing ML, et al. Intensive lipid lowering by statin therapy does not improve vasoreactivity in patients with type 2 diabetes. Arterioscler Thromb Vasc Biol. 2002;22:799–804. doi: 10.1161/01.atv.0000015330.64968.c4. [DOI] [PubMed] [Google Scholar]
- Wagenknecht LE, Zaccaro D, Espeland MA, et al. Diabetes and progression of carotid atherosclerosis: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol. 2003;23:1035–41. doi: 10.1161/01.ATV.0000072273.67342.6D. [DOI] [PubMed] [Google Scholar]
- Williams SB, Cusco JA, Roddy MA, et al. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23:729–36. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- Woo KS, Chook P, Lolin YI, et al. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation. 1997;96:2542–4. doi: 10.1161/01.cir.96.8.2542. [DOI] [PubMed] [Google Scholar]
- Woodman RJ, Chew GT, Watts GF. Mechanisms, significance and treatment of vascular dysfunction in type 2 diabetes mellitus: focus on lipid-regulating therapy. Drugs. 2005;65:31–74. doi: 10.2165/00003495-200565010-00003. [DOI] [PubMed] [Google Scholar]
- Xu X, Otsuki M, Saito H, et al. PPARα and GR differentially downregulate the expression of nuclear factor-κB-responsive genes in vascular endothelial cells. Endocrinology. 2001;142:3332–9. doi: 10.1210/endo.142.8.8340. [DOI] [PubMed] [Google Scholar]