Abstract
Objectives
Inflammation is known to be a major determinant of the progression of coronary artery disease (CAD). In the present study we have evaluated the plasma levels of cytokines – tumor necrosis factor-α(TNF), interleukin-1α(IL-1), interleukin-6 (IL-6), interferon-γ(IFN), and interleukin-10 (IL-10) – to examine the association between these cytokines and C-reactive protein (CRP) in patients with CAD.
Methods
Patients with acute coronary syndromes (ACS; n = 20) were compared with patients with stable angina (SA; n = 20) and with control volunteers (C; n = 20). Blood samples were collected at the time of admission from all patients and 15 and 30 days thereafter.
Results
CRP levels (20.8 ± 8.8 mg/L) (mean ± SEM) were higher at baseline in ACS than SA patients (4.1 ± 0.8 mg/L) or the control subjects (5.1 ± 1.8 mg/L) (p < 0.05). At admission, IL-6 was detected in 50% of the ACS patients and 5% of the SA patients or control subjects, while TNF was detected in 35% of the ACS and SA patients but only in 5% of control subjects. Subsequently, IL-6 levels declined and were no longer detectable, while TNF levels increased among ACS patients at all time periods tested when compared with other patients. The presence of IL-1 and IL-10 were not detectable in the blood samples examined, and IFN could only be detected in the ACS group. A significant correlation was observed between IL-6 and CRP levels (r = 0.4; p < 0.01) in all groups. There were no correlations among any of the other cytokines and CRP levels.
Conclusions
Our study demonstrates raised levels of TNF, IL6, IFN, and CRP in patients with ACS and a positive correlation between IL6 and CRP but not with the other cytokines.
Keywords: cytokines, tumor necrosis factor-α, interleukin-6, C-reactive protein, coronary artery disease
Introduction
Atherosclerosis can be considered an inflammatory disease as a number of ongoing inflammatory responses and immune reactions occur in the vessel wall (Ross 1999). Atherosclerotic lesions are filled with immune cells that coordinate and affect inflammatory responses. In fact, the initial lesions of atherosclerosis consist of macrophages and T cells, which both produce and secrete cytokines (Hansson et al 1997).
It is believed that the inflammatory responses related to atherosclerosis are, to a slight degree, caused by local inflammation in atherosclerotic tissue. Known, locally generated markers of inflammation are tumor necrosis factor-α(TNF) and interleukin-6 (IL-6) (Hansson et al 1997). Together, TNF and IL-1 stimulate the production of IL-6 by smooth muscle cells (Ng et al 1994). IL-6 gene transcripts are expressed in human atheromatous lesions (Rus et al 1996), and IL-6 is the main hepatic stimulus for C-reactive protein (CRP) production (Kulmatycki et al 2001).
Inflammation is also involved in the clinical manifestations of atherosclerosis (Biasucci et al 1996; Libby and Ridker 1999). Increased plasma levels of proinflammatory cytokines, such as IL-1, IL-6, and IL-8, have been reported in unstable but not in stable coronary artery disease (CAD) (Biasucci et al 1996; Fernandes et al 2004). The pattern and magnitude of release of inflammatory markers at the site of tissue injury is considered a critical determinant of the severity and duration of the response (Hansson et al 1997; Serrano Jr et al 1997; Ross 1999). In contrast, IL-10 appears to have an antiinflammatory (antiatherosclerotic) effect since it inhibits cytokine secretion (Hansson 2001).
Several reports have suggested that plasma CRP can predict increased risk of coronary heart disease (Danesh et al 2000), and concentration of CRP has been directly correlated with the presence and severity of coronary atherosclerosis (Haverkate et al 1997). Plasma concentration of CRP has been taken as an indication of overall inflammatory activity within the body (Danesh et al 2000). In addition, it has been suggested that CRP amplifies the underlying cytokine signal in the disease process (Haverkate et al 1997).
On the basis of the hypothesis that coronary atherosclerosis is associated with signs of inflammation, the present study was undertaken to assess the changes over time of the plasma levels of cytokines and CRP in patients with stable and unstable CAD.
Methods
Study population
This study was approved by our institution's committee on human research. The informed consent to participate in the study was obtained from all of the subjects. The groups in the investigation included patients with acute coronary syndromes (ACS; n = 20), stable angina (SA; n = 20), and individuals without known cardiovascular disease (control; n = 20). The groups were age- and sex-matched.
Definitions
An ACS was defined either as an unstable angina (group IIIB of Braunwald classification) (Braunwald 1989) or as an acute myocardial infarction without ST segment elevation, which was diagnosed on the basis of the presence of chest pain and typical ischemic electrocardiographic changes. Stable angina was defined on the basis of the presence of typical and stable chest pain during effort, a positive treadmill exercise test, and obstructive coronary lesions as determined by coronary angiography (>75% stenosis). The control group consisted of volunteers without ACS.
Protocol
Blood samples for cytokines and CRP were obtained from an antecubital vein. In patients with ACS, blood samples were obtained at admission. Venous blood taken on admission or thereafter (at days 15 and 30) (Caligiuri et al 1998) were collected into a tube containing 0.5 mL of sodium citrate (0.13 mol/L, pH 7.5). All blood samples were immediately centrifuged at 3000 rpm for 10 minutes at room temperature, and the aliquots of plasma were stored at −80°C for enzymatic immunoassay (ELISA) assays.
Cytokine levels were measured using a commercially available ELISA kit (R&D Systems Inc, Minneapolis, USA): cytokines TNF, IL-1, IL-6, and interferon-γ(IFN); and IL-10, with sensitivities of 3.0 pg/mL for IL-6, IFN, and IL-10; and 0.5 pg/mL for IL-1 and TNF. The assays employed the quantitative sandwich enzyme immunoassay technique.
To avoid variation within an assay, measurements were performed in duplicate, simultaneously using the same ELISA kit to avoid variation within assay, and in a blind manner. Baseline plasma samples from each patient and control subject were assayed for CRP with the use of latex-enhanced immunonephelometric assays on a BN II analyzer (Dade Behring, Newark, Del, USA). The range of detection for CRP was 0.75–1100 mg/L.
Statistical analysis
Discrete variables are expressed as counts (number and percentage) and were compared by chi-square or Fisher's exact test as appropriate. Continuous variables are expressed as means ± SEM and compared using the Student t-test or the Mann-Whitney test when the distribution was abnormal. Continuous variables are also categorized according to the presence or absence of detection in the diagnostic test. The definiton of the cytokine being “present” is when it is above the lower limit of the assay's detection. Statistical significance of changes of plasma cytokine levels over time were evaluated by 1-way analysis of variance with repeated measures and Duncan's test (Shimomura et al 1998). Spearman's rank test was used for correlations. Probability levels < 0.05 were considered statistically significant.
Results
The baseline characteristics of matched controls, patients with ACS, and SA patients are shown in Table 1. There were no marked differences among the three groups in the following variables: age, gender, and the presence of coronary risk factors (smoking status, hypertension, diabetes mellitus, family history of premature CAD, and hypercholesterolemia). There were no significant differences between ACS and SA groups in medications being taken (statins, angiotensin-converting enzyme inhibitors, and aspirin) at the time of blood sampling.
Table 1.
Baseline clinical characteristics of controls and patients with ischemic heart disease
| ACS (n = 20) | SA (n = 20) | Control (n = 20) | |
|---|---|---|---|
| Age (years) | 58.9 ± 12.4 | 57.0 ± 9.1 | 57.6 ± 6.4 |
| Sex (male/female) | 14/6 | 13/7 | 13/7 |
| Risk factor | |||
| Hyperlipidemia | 10 (50) | 13 (65) | 9 (45) |
| Diabetes | 6 (30) | 3 (15) | 4 (20) |
| Hypertension | 8 (40) | 10 (50) | 14(70) |
| Family history | 7 (35) | 7 (35) | 8 (40) |
| Smokers | 10 (50) | 7 (35) | 3 (15) |
| Serum total cholesterol (mg/dL) | 221.6 ± 13.8 | 198.3 ± 14.0 | 194.5 ± 12.2 |
| Serum LDL cholesterol (mg/dL) | 153.2 ± 15.2 | 142.2 ± 14.1 | 137.1 ± 12.2 |
| Angiography | |||
| Single-vesssel disease | 6 (30) | 10 (50) | 0 (0) |
| Multivessel disease | 9 (45) | 7 (35) | 0 (0) |
note: Values are expressed as means ± SEM or numbers (percentages). Thirteen of 20 control subjects had undergone coronary angiography.
Abbreviations: ACS, acute coronary syndromes; SA, stable angina; LDL, low-density lipoproteins.
On admission, CRP levels of (20.8 ± 8.8 mg/L) at baseline in ACS patients were significantly (p < 0.05) higher than SA patients (4.1 ± 0.8 mg/L) or control subjects (5.1 ± 1.8 mg/L) (Table 2). A significant correlation was observed between IL-6 and CRP levels (r = 0.4; p < 0.01) in all groups. There were no correlations among other cytokines and CRP levels.
Table 2.
Baseline cytokines and C-reactive protein of controls and patients with ischemic heart disease
| ACS (n = 20) | SA (n = 20) | Control (n = 20) | |
|---|---|---|---|
| CRP (mg/L) | 20.8 ± 8.8a | 4.1 ± 0.8 | 5.1 ± 1.8 |
| IL-1 (pg/mL) | nd | nd | nd |
| IL-6 (pg/mL) | 7.5 ± 3.9 | (22.2)b | (9.1)b |
| IL-10 (pg/mL) | nd | nd | nd |
| IFN (pg/mL) | 304.1 ± 254.4 | nd | nd |
| TNF (pg/mL) | 4.3 ± 0.9c | 2.8 ± 0.9 | 1.2 ± 1.2 |
p < 0.05.
(one value, only).
p > 0.05.
note: Values are expressed as means ± SEM.
Abbreviations: ACS, acute coronary syndromes; SA, stable angina; CRP, C-reactive protein; IL-1, interleukin-lα; nd, not detectable; IL-6, interleukin-6; IL-10, interleukin-10; IFN, interferon-γ; TNF, tumor necrosis factor-α.
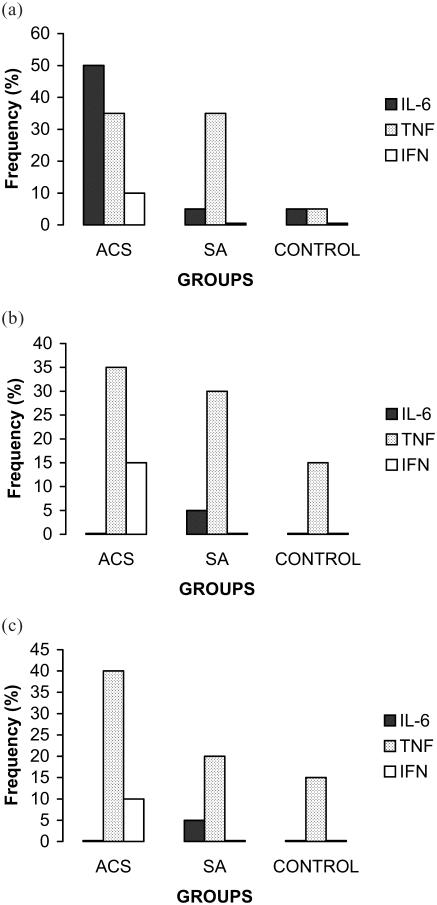
IL-6 was detected in 50% of the ACS patients and 5% of the SA patients and control subjects (Figure 1a). The levels of IL-6 in ACS patients was 7.5 ± 3.9 pg/mL (Table 2). TNF was detected in 35% of the ACS and SA patients, but only in 5% of control subjects (Figure 1a). It was possible to detect the presence of IFN in the ACS group but only in 10% of these patients (Figure 1a).
Figure 1.
Distribution of rates' frequency of cytokines detection on admission (baseline, a), at 15 days (b), and at 30 days (c) in patients with ACS, SA, and in individuals without cardiovascular disease (Control). Abbreviations: ACS, acute coronary syndromes; IFN, interferon; IL-6, interleukin-6; SA, stable angina; TNF, tumor necrosis factor-α.
On follow-up, at days 15 and 30, IL-6 was no longer detectable in the ACS patients or in control subjects, while it was still detectable in one patient in the SA group (Figures 1b, 1c). TNF could be detected in 35% and 40% of the ACS patients, 30% and 20% of the SA patients and 15% and 15% of control subjects, at days 15 and 30, respectively (Figures 1b, 1c).
ACS patients presented higher (though insignificantly so) plasma TNF concentration than SA patients and control individuals: 4.3 ± 0.9 vs 2.8 ± 0.9 vs 1.2 ± 1.2 pg/mL on admission (Table 3), with this difference persisting up to the 30-day follow-up. Plasma levels of TNF were similar among SA and control groups, and those of IFN for ACS group were 304.1 ± 254.4, 830.0 ± 368.5, and 351.4 ± 43.0 pg/mL on admission and at 15 and 30 days, respectively. The plasma levels of IFN reached their peak at day 15 and then declined. However, the peak level detected was not significantly different to the level measured at admission or at 30 days post-admission. The presence of IL-1 and IL-10 were not detectable in the blood samples examined.
Table 3.
Serial changes in plasma levels of tumor necrosis factor-α (TNF) of controls and patients with ischemic heart disease
| Period | ||||
|---|---|---|---|---|
| Groups | Admission | Day 15 | Day 30 | p |
| ACS (pg/mL) | 4.3 ± 0.9 | 3.8 ± 0.9 | 4.1 ± 0.9 | NS |
| SA (pg/mL) | 2.8 ± 0.9 | 2.7 ± 0.8 | 3.8 ± 2.1 | NS |
| C (pg/mL) | 1.2 ± 1.2 | 2.7 ± 0.9 | 4.4 ± 1.6 | NS |
| p | NS | NS | NS | |
note: Values are expressed as means ± SEM.
Abbreviations: ACS, acute coronary syndromes; C, control; NS, not significant (p > 0.05); p, probability; SA, stable angina.
Discussion
This study shows that in humans, various clinical forms of CAD affect the plasma levels of inflammatory cytokines differently. However, no more than 50% of subjects at any one point in time had elevated or detectable levels of cytokine concentrations. Thus, these findings call into question the clinical value of measuring cytokines as inflammatory markers in patients with CAD. Although several cytokines might be predictive of clinical disease, the laboratory tests to assess inflammation are limited to those that are detectable in clinical settings and that have standardized, commercially available assays.
The evidence from the present study indicates that baseline CRP, IL-6, and TNF increased in ACS patients as compared with SA patients or control subjects. Our present findings are in agreement with other reports (Maury and Teppo 1989; Liuzzo et al 1998). A possible explanation for the large variation (ie, SD) in the levels of CRP measurement in ACS patients in this investigation could be a lack of tight normal distribution within the population studied.
Other investigators have affirmed that IL-6 levels are markedly elevated in patients with unstable angina, although the cytokine was not detectable in all patients with this condition (Biasucci et al 1996; Tashiro et al 1997). Thus, our present findings support the view that IL-6 may be involved in ACS to a greater extent than in other stable forms of CAD, but that IL-6 levels are no longer detectable at 15 days following the acute phase of instability.
In contrast, it was possible to detect some TNF levels after at least 30 days of the onset of ACS. Ridker et al (2000) have reported that plasma concentration of TNF remains elevated many months after myocardial infarction and that very high levels are associated with an increased risk of recurrent coronary events (Ridker et al 2000). The source of persistently elevated levels of TNF is uncertain (Marx et al 1997; Irwin et al 1999). Additional studies will be required to determine the source of the TNF elevations observed in these data.
In our study, IFN was detected only in patients with ACS at baseline. Since IFN is produced by T-lymphocytes which, among their functions inhibit collagen production by smooth muscle cells (Amento et al 1991; Irwin et al 1999), IFN could also have a fundamental role in the instabilization of atherosclerotic plaques. Therefore, it is understandable that IFN is only detectable in ACS patients, at admission, and not in SA patients and control individuals.
We did not find IL-1α in the plasma of patients tested and this is consistent with reports by other investigators (Tashiro et al 1997; Smith et al 1999). On the other hand, IL-1β plasma levels are elevated in patients with unstable angina (Simon et al 2000). This could be explained by the fact that IL-β is secreted as a soluble protein and might be more easily detected in the blood collected from the peripheral circulation than IL-1α.
These prospective data indicate that baseline CRP concentration could be a better marker for inflammation than cytokines in patients with CAD. One potential limitation of this study is that the control group included individuals with coronary risk factors. We did, however, confirm the absence of CAD through a normal coronary angiography. Furthermore, plasma levels of cytokines might have been below the detection levels of the assay used. Needless to say, a more sensitive assay system may have revealed abnormal cytokine levels.
A comparison of the various studies reveals substantial heterogeneity in the results that could be explained by methodological differences. Clearly, the heterogeneity of the present data could be related to the time of sampling, the study design, or the exclusion criteria employed, which is also a potential limitation of the study (Whiteside 1994).
Accurate biological levels of the cytokines are difficult to obtain, since these mediators are involved in complex molecular and cellular cascades and the only accessible compartment is the circulation, in which such proteins have short half-lives. Furthermore, assays for cytokines are technically difficult and generally unreliable. By contrast, immunoassays that cover the normal ranges as well as being reliable, sensitive, and robust have been developed.
It is possible that investigators are less likely to submit studies with negative results for publication; even if an investigator chooses to submit a report with negative findings, publication of such a report is less likely (Gibaldi 1993). Therefore, evaluation of only published material may lead to a bias in favor of positive results.
The assays that measure markers of inflammation in cardiovascular disease are becoming an important part of the clinical laboratory repertoire. The appropriate type of laboratory blood tests for the purpose of risk stratification and therapeutic and clinical monitoring remains to be established in patients at risk of coronary events. Current evidence supports the use of CRP as the analyte of choice (Pearson et al 2003). Although based on current findings, it is evident that the variation in CRP measurements is quite high, and it seems that Danesh et al (2004), analyzing data from the Reykjavik Study, have suggested that CRP may be not as good a predictor of future cardiac events as was previously thought. Furthermore, our data indicates that the measurement of cytokines as alternative analytes cannot be recommended at this time and further research is needed.
Acknowledgments
This study was supported in part by funding from the Research Grant from the “Fundação de Ciência e Tecnologia de Santa Catarina” (FUNCITEC).
References
- Amento EP, Ehsani N, Palmer H, et al. Cytokines positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1223–30. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- Biasucci LM, Vitelli A, Liuzzo G, et al. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996;94:874–7. doi: 10.1161/01.cir.94.5.874. [DOI] [PubMed] [Google Scholar]
- Braunwald E. Unstable angina: a classification. Circulation. 1989;80:410–14. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- Caligiuri G, Liuzzo G, Biasucci LM, et al. Immune system activation follows inflammation in unstable angina: pathogenetic implications. J Am Coll Cardiol. 1998;32:1295–304. doi: 10.1016/s0735-1097(98)00410-0. [DOI] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JL, Mamoni RL, Oxford JL, et al. Increased TH1 activity in patients with coronary artery disease. Cytokine. 2004;26:131–7. doi: 10.1016/j.cyto.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Gibaldi M. Meta-analysis. A review of its place in therapeutic decision making. Drugs. 1993;46:805–18. doi: 10.2165/00003495-199346050-00002. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–90. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Stemme V, Yokota T. Cytokines and the cardiovascular system. In: Remick DG, Friedland JS, editors. Cytokines in health and disease. New York: Marcel Dekker; 1997. pp. 507–18. [Google Scholar]
- Haverkate F, Thompson SG, Pyke SDM, et al. Production of Creactive protein and risk of coronary events in stable and unstable angina. Lancet. 1997;349:462–6. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- Irwin MW, Mak S, Mann DL, et al. Tissue expression and immunolocalization of tumor necrosis factor-α in postinfarction dysfunctional myocardium. Circulation. 1999;99:1492–8. doi: 10.1161/01.cir.99.11.1492. [DOI] [PubMed] [Google Scholar]
- Kulmatycki KM, Jamali F. Therapeutic relevance of altered cytokine expression. Cytokine. 2001;14:1–10. doi: 10.1006/cyto.2000.0827. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM. Novel inflammatory markers of coronary risk: theory versus practice. Circulation. 1999;1000:1148–50. doi: 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Buffon A, Biasucci LM, et al. Enhanced inflammatory response to coronary angioplasty in patients with severe unstable angina. Circulation. 1998;98:2370–6. doi: 10.1161/01.cir.98.22.2370. [DOI] [PubMed] [Google Scholar]
- Marx N, Neumann F-J, Ott I, et al. Induction of cytokine expression in leukocytes in acute myocardial infarction. J Am Coll Cardiol. 1997;30:165–70. doi: 10.1016/s0735-1097(97)00116-2. [DOI] [PubMed] [Google Scholar]
- Maury CPJ, Teppo AM. Circulating tumor necrosis factor-α (cachectin) in myocardial infarction. J Intern Med. 1989;225:333–6. doi: 10.1111/j.1365-2796.1989.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Ng SB, Tan YH, Guy GR. Differential induction of the interleukin-6 gene by tumor necrosis factor and interleukin-1. J Biol Chem. 1994;269:19021–7. [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease – application to clinical and public health practice – a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Pfeffer M, et al. Elevation of tumor necrosis factor-α and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rus HG, Vlaicu R, Niculescu F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis. 1996;127:263–71. doi: 10.1016/s0021-9150(96)05968-0. [DOI] [PubMed] [Google Scholar]
- Serrano CV, Jr, Ramires JAF, Venturinelli M. Coronary angioplasty results in leukocyte and platelet activation with adhesion molecule expression. Evidence of inflammatory responses in coronary angioplasty. J Am Coll Cardiol. 1997;29:1276–83. doi: 10.1016/s0735-1097(97)00070-3. [DOI] [PubMed] [Google Scholar]
- Shimomura H, Ogawa H, Arai H, et al. Serial changes in plasma levels of soluble P selectin in patients with acute myocardial infarction. Am J Cardiol. 1998;81:397–400. doi: 10.1016/s0002-9149(97)00945-4. [DOI] [PubMed] [Google Scholar]
- Simon AD, Yazdani S, Wang W, et al. Circulating levels of IL-1 beta, a prothrombotic cytokine, are elevated in unstable angina versus stable angina. J Thromb Thrombolysis. 2000;9:217–22. doi: 10.1023/a:1018758409934. [DOI] [PubMed] [Google Scholar]
- Smith JK, Dykes R, Douglas JE, et al. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–7. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- Tashiro H, Shimokawa H, Yamamoto K, et al. Altered plasma levels of cytokines in patients with ischemic heart disease. Coron Art Dis. 1997;8:143–7. doi: 10.1097/00019501-199703000-00004. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Cytokines and cytokine measurements in a clinical laboratory. Clin Diagn Lab Immunol. 1994;1:257–60. doi: 10.1128/cdli.1.3.257-260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]