Abstract
Lercanidipine is a lipophilic, dihydropyridine calcium antagonist with a long receptor half-life. Its slow onset of action helps to avoid reflex tachycardia associated with other dihydropyridines (DHPs). It produces even and sustained blood pressure lowering with once-daily dosing. It has equivalent antihypertensive efficacy to many other agents and is effective as initial monotherapy or in combination. Efficacy has been demonstrated in elderly as well as younger patients and also in the presence of other risk factors. Lercanidipine is well tolerated with DHP-associated adverse effects occurring early in treatment. The incidence of pedal edema and subsequent withdrawals has been found to be lower with lercanidipine than with amlodipine or nifedipine gastrointestinal transport system. Preclinical and preliminary clinical findings suggest lercanidipine may have beneficial effects on atherosclerosis and left ventricular hypertrophy. The efficacy and tolerability profiles of lercanidipine make it a suitable choice for treating hypertension in a wide range of affected patients.
Keywords: hypertension, calcium antagonists, lercanidipine
Introduction
Hypertension is a major contributor to global disease burden, occurring as an insidious accompaniment to aging populations (Kostis 2003; WHO 2003). It is estimated to have caused 7.1 million premature deaths in 2002 and is an ever-increasing worldwide problem (WHO 2003). It is a well recognized risk factor for cardiovascular disease (Chobanian et al 2003; European Society of Hypertension-European Society of Cardiology Guidelines Committee 2003), and is often combined with other risk factors such as smoking, obesity, and physical inactivity (WHO 2003), and it occurs commonly with diabetes (ADA et al 2004).
Antihypertensive drugs are well known to prevent cardiovascular morbidity and mortality. The risks of stroke and myocardial infarction decrease by about 40% and 15%, respectively, in individuals who experience long-term diastolic blood pressure reduction of 5–6 mmHg (Collins et al 1990). Hypertension management aims to reduce the long-term risk of cardiovascular complications, and involves lifestyle modifications, antihypertensive drug therapy, and treatment of comorbid conditions (European Society of Hypertension-European Society of Cardiology Guidelines Committee 2003). The major antihypertensive drug classes (diuretics, calcium antagonists, β-blockers, angiotensin converting enzyme [ACE] inhibitors, and angiotensin receptor blockers) are all effective at lowering blood pressure, such that drug choice may be governed by patient characteristics (risk profile, concomitant disease), drug tolerability, cost of drugs, and the growing realization of the requirement for combination therapy to achieve goal blood pressure (Cifkova et al 2003).
Calcium antagonists
Calcium antagonists are a heterogeneous group of established antihypertensive agents that includes the phenylalkylamine, verapamil, the benzothiazepine, diltiazem, and the dihydropyridines (DHPs). The landmark series of prospective trials comparing calcium antagonists with other antihypertensive medications showed that, as a class, calcium antagonists produce similar effects to diuretics, β-blockers, and ACE inhibitors on cardiovascular mortality and combined morbidity, and that they reduce stroke in elderly hypertensive patients with isolated systolic hypertension (ISH) (Hansson et al 1999, 2000; Brown et al 2000; ALLHAT Officers and Coordinators 2002).
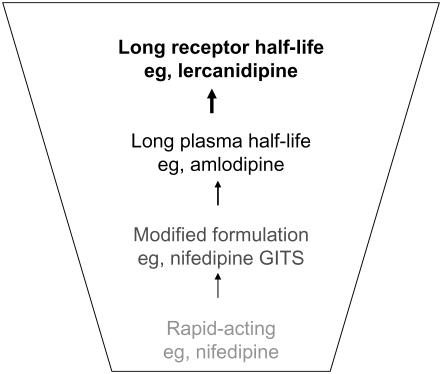
The DHPs work primarily as vasodilators and have evolved from the first generation, short-acting compounds (eg, nifedipine and felodipine), which produced unwanted reflex tachycardia. Modified formulations were introduced to extend duration of action and limit adverse effects; however, amlodipine was the first DHP with an inherently long plasma half-life. The latest advance is the introduction of agents with a prolonged receptor half-life (reviewed in Messerli 2002) (Figure 1). The present review will describe the clinical efficacy and potential benefits of one of these newer agents, lercanidipine.
Figure 1.
Evolution of dihydropyridine calcium antagonists for improved clinical efficacy and tolerability. Abbreviations: GITS, gastrointestinal therapeutic system.
Pharmacology
Pharmacokinetics
Lercanidipine has a plasma half-life of 8–10 hours (Bang et al 2003) but this does not relate to its duration of antihypertensive activity. Its high membrane partition coefficient provides a long-lasting effect at receptor and membrane levels (Herbette et al 1998) allowing for once-daily administration. Oral lercanidipine is maximally absorbed after 2 hours of administration, it exhibits high serum protein binding, and is rapidly accumulated in arteriolar cell membranes. It is metabolized by cytochrome P450 (CYP) 3A4 and the metabolites are eliminated in urine and feces (Bang et al 2003). As with other DHPs, the potential for interaction with drugs that induce or inhibit P450 (CYP) 3A4 exists and should be considered (see review by Bang et al 2003).
Pharmacodynamics
Preclinical studies show lercanidipine is highly selective for vascular tissue and produces smooth muscle relaxation through competitive binding to L-type calcium channels (Guarneri et al 1996; Wirtz and Herzig 2004). It is highly lipophilic and is stored within cell membranes, which explains its slow onset of action and persistent smooth muscle relaxant effect (Guarneri et al 1996; Sironi et al 1996a; Herbette et al 1998). The antihypertensive effect of lercanidipine primarily occurs by peripheral and coronary vasodilatation (Sironi et al 1996b). Lercanidipine has greater vascular selectivity and causes less negative inotropism in vitro than other DHPs including lacidipine, amlodipine, felodipine, and nitrendipine (Guarneri et al 1996; Angelico et al 1999). It does not cause significant reflex tachycardia or other signs of sympathetic activation when given at therapeutic doses to patients with hypertension (Omboni et al 1998; Barbagallo M and Barbagallo SG 2000; Cavallini and Terzi 2000; Fogari et al 2003; Grassi et al 2003). Lercanidipine (10 mg/day) produces a smooth antihypertensive effect without unfavorable hemodynamic or sympathetic effects (Omboni et al 1998; Macchiarulo et al 2001; Motero et al 2002; Millar-Craig et al 2003).
In addition to its general antihypertensive activity, lercanidipine has a nephroprotective effect in spontaneously hypertensive rats (Sabbatini, Vitaioli, et al 2000) and dilates both afferent and efferent arterioles (Sabbatini, Leonardi, et al 2000; Sabbatini et al 2002). Lercanidipine may also have benefits in patients with hypertension and atherosclerotic disease where it is able to reside in cell membranes in the presence of high cholesterol levels (Herbette et al 1998). It has well described (in in vitro, animal, and clinical studies) antioxidant effects affecting vasodilatation and reducing oxidation of low-density lipoproteins (Bellosta and Bernini 2000; Digiesi et al 2000; Incandela et al 2001; Rachmani et al 2002; Taddei et al 2003; Versari et al 2004). Regression of left ventricular hypertrophy has been described with lercanidipine in patients with hypertension, with or without diabetes (Fogari, Mugellini, et al 2000; Seravalle et al 2002). Interestingly, lercanidipine exerted a prolonged vasodilatory action in the microcirculation of 19 patients with hypertension, where it might protect against target organ damage (Cesarone et al 2000).
Clinical studies
Efficacy in mild-to-moderate hypertension
The efficacy of lercanidipine has been demonstrated in several uncontrolled trials (10–20 mg/day) carried out in more than 20 000 patients with mild-to-moderate hypertension (Barrios et al 2002; Schwinger and Schmidt-Mertens 2002; Guillen et al 2003; Robles et al 2003; Luque et al 2004). In summary, systolic and diastolic blood pressure reductions of 19–26 mmHg and 13–15 mmHg, respectively, were found 3–6 months after starting therapy.
There have been several comparisons of lercanidipine with other antihypertensive agents (Table 1). In double-blind/crossover studies, lercanidipine (10–20 mg/day) was as effective as amlodipine 10 mg/day (De Giorgio et al 1999; Pedrinelli et al 2003), felodipine (10–20 mg/day) (Romito et al 2003), and nifedipine slow-release (40–80 mg/day) (Policicchio et al 1997) or gastrointestinal therapeutic system (GITS, 30–60 mg/day) formulations (Fogari, Malamani, et al 2000; Fogari et al 2003; Romito et al 2003). Compared with other antihypertensive drugs, lercanidipine (10–20 mg/day) showed efficacy equivalent to atenolol (50–100 mg/day) (Morisco and Trimarco 1997), captopril (50–100 mg/day) (Sangiorgi et al 1997), hydrochlorothiazide (12.5–25 mg/day) (Notarbartolo et al 1999), losartan (50–100 mg/day) (James et al 2002), telmisartan (80 mg/day) (Sarafidis et al 2004), and candesartan (Aranda et al 2000). In summary, lercanidipine reduced systolic and diastolic blood pressure in these studies, producing a high response rate and/or normalization of blood pressure in as little as 4 weeks of therapy (Table 1).
Table 1.
Efficacy and tolerability of lercanidipine compared with other antihypertensive drugs in patients with mild-to-moderate hypertension
| Study | Study design/duration (w) | N/Age (y) | Dosages (mg/day) | SBP/DBP at baseline (mmHg) | SBP/DBP at study end (mmHg) | Responding (normalized) at 4 weeks (%) | Overall efficacy | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Comparison with calcium antagonists | ||||||||
| Pedrinelli et al 2003 | sb, co/2 | 22/48 ± 5 | L 20 | L 146 ± 16/93 ± 13 | L 138 ± 9/86 ± 9a | NR | L≡A | L < A (leg edema)b |
| A 10 | A 148 ± 17/95 ± 11 | A 137 ± 13/84 ± 9a | ||||||
| De Giorgio et al 1999 | db, co/4 | 20/55 ± 9c | L 20 | L 162 ± 20/101 ± 5 | L 141 ± 24/88 ± 10d | L 86 (86) (33) | L≡A | L≡A |
| A 10 | A 166 ± 19/104 ± 6 | A 152 ± 16/94 ± 9d | A 56 | |||||
| Romito et al 2003 | db, p/8e | 325/31–74 | L 10 | L 155 ± 11/99 ± 3 | L 141 ± 13/87 ± 7f | L≡NG≡F | L≡NG≡F | L≡NG < Fg |
| NG 30 | NG 155 ± 12/99 ± 3 | NG 142 ± 10/86 ± 7f | ||||||
| F 10 | F 155 ± 12/99 ± 3 | F 138 ± 10/85 ± 7f | ||||||
| Policicchio et al 1997 | db, p/8e | 130/18–70 | L 10 | L 163 ± 12/101 ± 5 | L 151 ± 13/91 ± 9h | L 58 (51) | L≡NS | L≡NS |
| NS 40 | NS 163 ± 14/101 ± 4 | NS 151 ± 14/91 ± 8h | NS 63 (53) | |||||
| Fogari et al 2003 | db, p/48e | 60/30–65 | L 10 | L 159 ± 11/101 ± 6 | L 137/85a | NR | L≡NG | NRi |
| NG 30 | NG 159 ± 10/101 ± 5 | NG 21/15a | ||||||
| Fogari et al 2000a | db, p/12e | 60/36–70 | L 10 | L 163 ± 5/98 ± 4 | L 144 ± 5/86 ± 4a | NR | L≡NG | L < NG (ankle edema)j |
| NG 30 | NG 162 ± 6/97 ± 4 | NG 144 ± 5/86 ± 3a | ||||||
| Comparison with other antihypertensive drug classes | ||||||||
| Morisco et al 1997 | db, p/8e | 217/18–70 | L 10 | L 157 ± 11/100 ± 3 | L 145 ± 12/90 ± 8d | L 71 (65) | L≡AT | L≡AT |
| AT 50 | AT 157 ± 11/100 ± 4 | AT 142 ± 13/88 ± 7d | AT 78 (76) | |||||
| Sangiorgi et al 1997 | db, p/12e | 115/18–80 | L 10 | L 161 ± 10/100 ± 3 | L 147 ± 10/89 ± 7d | L 75 (81) | L≡C | L≡C |
| C 50 | C 159 ± 10/100 ± 3 | C 148 ± 11/91 ± 7d | C 73 (74) | |||||
| Notarbartolo et al 1999 | db, p/24e | 52/18–70 | L 10 | L 159 ± 13/105 ± 5 | L 143 ± 7/92 ± 7d | L 65 (54) | L≡H | L < H (lipid changes) |
| H 12.5 | H 158 ± 14/103 ± 5 | H 146 ± 10/93 ± 7d | H 58 (54) | |||||
| James et al 2002 | db, p/16e | 562/18–75 | L 10 | L 163 ± 15/101 ± 5 | L 148 ± 16/88 ± 7 | L ≈ 80 (71) (16w) | L≡LO | L≡LO |
| LO 50 | LO 162 ± 13/101 ± 5 | LO 144 ± 15/88 ± 8 | LO ≈ 80 (65) (16w) | |||||
| Sarafidis et al 2004 | NR/16 | 20/53 ± 9 | L 10 | L 165 ± 8/102 ± 2 | L 151 ± 9/97 ± 4k | L NR (20) (8w) | L≡T | NRl |
| T 80 | T 166 ± 8/103 ± 5 | T 152 ± 8/96 ± 4k | T NR (20) (8w) | |||||
| Aranda et al 2000 | open/32 | 338/55 | L 10 | L 162 ± 11/97 ± 9 | L 135/82m | NR | L≡CA | L≡CA |
| CA 16 | CA 161 ± 10/96 ± 7 | CA 133/82m | ||||||
| Comparative studies in the elderly | ||||||||
| Cherubini et al 2003 | db, p/24e | 324/≥65 | L 5 | L 167 ± 11/98 ± 5 | L 140/80f | L/NG > LA (24w)n | L≡LA≡NG | L≡LA/NGo |
| LA 2 | LA 168 ± 12/98 ± 4 | LA 142/81f | ||||||
| NG 30 | NG 167 ± 11/97 ± 4 | NG 138/79f | ||||||
| Leonetti et al 1999 | db, p/26–104e | 828/≥60 | L 10 | L 170 ± 10/97 ± 6 | L 140/83p | L≡LA≡A | L≡LA≡A | L≡LA < Aq |
| LA 2 | LA 170 ± 10/97 ± 6 | LA 141/83p | ||||||
| A 5 | A 171 ± 11/97 ± 7 | A 141/82p | ||||||
p < 0.001 versus baseline.
p = 0.006.
57 ± 10 years in other treatment arm.
Values for first 4-week treatment period (p < 0.01 versus baseline).
Dosages doubled after 4 weeks if unsatisfactory response (2 weeks in Cherubini et al, 8 weeks in James et al).
p < 0.01 versus baseline.
p < 0.05.
Values for first 4 weeks of treatment (p < 0.001 versus baseline).
Sustained sympathetic activation with chronic NG therapy (p < 0.05 versus baseline).
Ankle edema significantly greater with NG (p < 0.001 versus L).
Values for first 8 weeks of treatment (p < 0.001 versus baseline).
Neither drug affected insulin resistance.
Values for first 6 weeks of treatment.
p < 0.001 between treatment groups in numbers of responding and normalized patients.
Incidence of adverse drug reactions was lowest for L.
Values are at 6 months (p < 0.01 versus baseline). AT or enalapril (plus diuretic, if required) was added after 8 weeks in 22%–29% of patients with an unsatisfactory response.
Higher rates of edema, edema-related symptoms, and early edema-related discontinuation with A compared with L and LA (p < 0.001).
Abbreviations: A, amlodipine; AT, atenolol; BP, blood pressure; co, crossover; C, captopril; CA, candesartan; DBP, diastolic BP; db, double blind; H, hydrochlorothiazide; LA, lacidipine; L, lercanidipine; LO, losartan; NE, norepinephrine; NG, nifedipine gastrointestinal therapeutic system; NR, not reported; NS, nifedipine slow-release formulation; p, parallel; sb, single blind; SBP, systolic BP; T, telmisartan.
Efficacy in specific populations
Elderly patients
Lercanidipine is effective in elderly patients with mild-to-moderate hypertension. In a double-blind, placebo-controlled study of 144 elderly patients with hypertension (aged 60–85 years), lercanidipine 10 mg/day for 4 weeks reduced systolic and diastolic blood pressure to a greater extent than placebo (15 vs 7 mmHg and 10 vs 6 mmHg, respectively, p < 0.01 for diastolic blood pressure) and increased the response rate (59% vs 38%, p < 0.05) (Ninci et al 1997). In an open study of 756 patients, lercanidipine (10–20 mg/day for 8 weeks) reduced blood pressure to a similar extent in patients under and over 65 years (Poncelet et al 2004). Reports of 3 open studies in patients at least 60 years old show that lercanidipine produces significant reductions of systolic, diastolic, and pulse pressure in this patient population (Calvo et al 2002; Martell et al 2004; Roma et al 2004).
Two large controlled studies showed that lercanidipine (5–10 mg/day or 10–20 mg/day) is as effective as lacidipine (2–4 mg/day), amlodipine (5–10 mg/day), or nifedipine GITS (30–60 mg/day) at lowering blood pressure in elderly patients with hypertension (Table 1) (Leonetti et al 2002; Cherubini et al 2003). In elderly patients with ISH, lercanidipine was more effective than placebo in terms of reduction of systolic blood pressure and number of patients responding or being normalized after 4 and 8 weeks of therapy (p < 0.001 for all comparisons) (Barbagallo M and Barbagallo SG 2000). A similar well designed study in 290 patients with ISH found that lercanidipine (10 mg/day) and lacidipine (2 mg/day) were equivalent at reducing systolic blood pressure (24 versus 22 mmHg after 16 weeks) (Millar-Craig et al 2003).
Concomitant diseases
Several large-scale uncontrolled trials have shown that lercanidipine is effective in antihypertensive patients with concomitant diseases or risk factors. The ELYPSE study of lercanidipine in daily clinical practice comprised 9059 patients with mild-to-moderate hypertension and a range of additional risk factors such as obesity, hypercholesterolemia, smoking, and diabetes mellitus (Barrios et al 2002). The authors found that 3 months of treatment with lercanidipine was effective at reducing blood pressure across this broad range of patients. Similar findings were achieved in an observational 6-week study of 32 345 patients with mild-to-moderate hypertension and concomitant diseases such as lipid disorders, diabetes mellitus, coronary heart disease, and heart failure (Marx et al 2004). An open study in 3175 hypertensive patients with various levels of cardiovascular risk found that lercanidipine was effective across all risk levels and appeared most effective in those with the highest risk (Barrios et al 2004b). A further study in 2793 patients with hypertension who were also overweight showed lercanidipine efficacy is not adversely affected by this cardiovascular risk factor (Barrios et al 2004a).
Lercanidipine monotherapy (10 or 20 mg/day) was also effective in a randomized double-blind study of 40 patients with hypertension and type 2 diabetes mellitus where it did not adversely affect glucose homeostasis (Viviani 2002). In a smaller open study in 34 similar patients, lercanidipine (10 mg/day) replaced β-blocker therapy in patients not controlled by combination of an ACE inhibitor and β-blocker, and significantly reduced mean arterial pressure and glycosylated hemoglobin which the authors attributed to decreased vascular resistance (Cleophas et al 2001). Lercanidipine (10 mg/day) was found to produce a similar reduction in insulin resistance to telmisartan (80 mg/day) in a study of 20 patients with mild-to-moderate hypertension (Sarafidis et al 2004).
Lercanidipine (10 mg/day) did not adversely affect proteinuria in 42 patients with hypertension and diabetes who also had chronic renal failure when added to existing but inadequate therapy with an ACE inhibitor or angiotensin receptor blocker (Robles et al 2004). Significantly, lercanidipine (10–20 mg/day over 9–12 months) was found to reduce blood pressure and reduce albumin excretion rate in a randomized, double-blind study of 277 patients with hypertension, diabetes, and microalbuminuria, producing a similar result to ramipril 5–10 mg/day (Dalla Vestra et al 2004). Most recently, lercanidipine (10 mg/day for 6 months) produced significant reductions in blood pressure, cholesterol, and triglyceride levels in 203 patients with hypertension and chronic renal failure, previously treated with ACE inhibitors or angiotensin receptor blockers. Furthermore, creatinine clearance was increased and proteinuria reduced adding further support to a role for lercanidipine in improving renal function (Robles et al 2005).
Following unsuccessful therapies or used in combination therapy
Two daily practice studies of lercanidipine included a large proportion of patients (66% and 69%) who had achieved a poor response or complained of adverse events when treated with other antihypertensive medications (Barrios et al 2002; Schwinger and Schmidt-Mertens 2002). In one of the studies, lercanidipine was given as monotherapy and produced significant blood pressure reduction over 3 months (p < 0.001 versus baseline) with 64% achieving diastolic blood pressure lower than 90 mmHg (Barrios et al 2002). In the other study, it was equally effective when given as monotherapy or combined with other antihypertensive agents (Schwinger and Schmidt-Mertens 2002).
The efficacy of lercanidipine as add-on therapy has been shown in several open studies. In a study of 756 hypertensive patients, lercanidipine (10 mg/day) reduced blood pressure over 8 weeks as monotherapy or as combination therapy (Poncelet et al 2004). Lercanidipine (10mg/day) is also effective when combined with ACE inhibitor or angiotensin receptor blocker therapy (Guillen et al 2003; Robles et al 2004) and reduces blood pressure more than β-blocker plus ACE inhibitor combination therapy (Cleophas et al 2001) in patients with diabetes mellitus and hypertension. In one study of 80 patients with hypertension resistant to monotherapy (including β-blockers, ACE inhibitors, or diuretics), lercanidipine (10 mg/day) or nitrendipine (10 mg/day) as add-on therapy produced significant decreases in blood pressure after 4 weeks and approximately 90% of patients were normalized in each group at 12 weeks (Rengo and Romis 1997).
Several other antihypertensive drugs have been successfully added to lercanidipine monotherapy. For example, combining lercanidipine with hydrochlorothiazide or telmisartan increased the rate of blood pressure normalization in patients where an unsatisfactory response to lercanidipine monotherapy was observed after either 4 or 8 weeks therapy (Policicchio et al 1997; Sarafidis et al 2004). Similarly, lercanidipine plus candesartan therapy was found to be effective in 70% of patients who had not responded to 6 weeks of monotherapy with either drug (Aranda et al 2000).
Tolerability and patient compliance
A pooled analysis of 20 randomized, double-blind studies shows that adverse events were reported by 11.8% of 1317 lercanidipine-treated patients (10–20 mg/day) versus 7.0% in those who received placebo (227) for 1–52 weeks of treatment (Leonetti 1999). Most adverse events occurred within the first 4 weeks of treatment and there were only slight differences from placebo when lercanidipine was given at its starting dose of 10 mg/day and then titrated to higher dosages. Adverse events with lercanidipine 10 mg/day included flushing (1.1% vs 0.4% for placebo), ankle edema 0.9% vs 1.3%), palpitations (0.6% vs 0.4%), headache (2.3% vs 1.3%), vertigo (0.4% vs 0.4%), and asthenia (0.4% vs 0.4%). The incidence of adverse events in elderly patients was 5.70%, which compared well with younger patients (6.60%) (Leonetti 1999). An analysis of 14 placebo-controlled, double-blind trials (1850 patients with hypertension or stable angina treated for up to 18 weeks) confirmed that adverse events with lercanidipine (10–20 mg/day) are generally mild-to-moderate (Hollenberg 2002). Headache (5.6%), edema (2.4%, tachycardia (2.1%), flushing (2.0%), palpitations (1.7%), rhinitis (1.3%), and hypokalemia (1.2%) were the most frequently-reported treatment-emergent adverse events compared with headache (3.8%), hypokalemia (1.3%), and hyperuricemia (1.1%) with placebo.
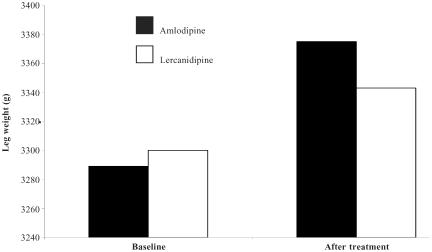
Lercanidipine was well tolerated in all of the uncontrolled studies and in the controlled studies reported in Table 1. It produced no significant heart rate changes, while most adverse events were the result of vasodilatation early in treatment. Lercanidipine also compared well against amlodipine, felodipine, and nifedipine GITS (Table 1). Specifically, objective assessments have shown that lercanidipine produces less pedal edema than nifedipine GITS (Fogari, Malamani, et al 2000) and amlodipine (Figure 2) (Leonetti et al 2002; Lund-Johansen et al 2003; Pedrinelli et al 2003). The incidence of edema was also low in a general practice study of 8981 hypertensive patients who received lercanidipine combined with other antihypertensive agents (the incidence was lowest when lercanidipine was given with an ACE inhibitor) (Mallion et al 2004). The comparative tolerability of lercanidipine and other DHPs were investigated in an open study of 125 patients with hypertension and drug-specific adverse events. Lercanidipine (10–20 mg/day for 4 weeks) produced significant reductions in edema, flushing, headache, rash, and dizziness when it was switched from previous treatment with amlodipine, nifedipine GITS, felodipine, or nitrendipine (p < 0.001) (Borghi et al 2003). Weir has commented that the 2.4% incidence of edema found with lercanidipine (10–20 mg/day) in a pooled analysis (Hollenberg 2002) contrasts well with incidences of 6%–29% found in studies of other DHPs (Hollenberg 2002; Weir 2003).
Figure 2.
Amlodipine produces significantly more leg edema than lercanidipine after 2 weeks therapy in patients with mild to moderate hypertension (p = 0.006) (Pedrinelli et al 2003).
Reported withdrawal rates with lercanidipine are low or similar to placebo (Barbagallo M and Barbagallo SG 2000; Barrios et al 2002; Schwinger and Schmidt-Mertens 2002). This is most probably related to its good tolerability – withdrawals due to edema are less with lercanidipine than with amlodipine (Leonetti et al 2002; Lund-Johansen et al 2003) and nifedipine GITS (Romito et al 2003).
Patient support/disease management programs
Official organizations have recently re-issued their guidelines on hypertension (Chobanian et al 2003; European Society of Hypertension-European Society of Cardiology Guidelines Committee 2003; WHO 2003) and readers may consult these documents to update on diagnosis, evaluation, and non-pharmacological approaches to treatment. There is general agreement for drug intervention if systolic/diastolic blood pressures are greater than 140/90 mmHg (high risk groups may be treated at lower levels) and that first-line therapy may comprise any of the 5 major drug classes (Stergiou and Salgami 2004), largely because of their equivalent blood pressure lowering ability that has the greatest benefit (European Society of Hypertension-European Society of Cardiology Guidelines Committee 2003).
Diuretics may be favored for their low cost and extent of outcome data (Collins et al 1990; ALLHAT Officers and Coordinators 2002; Chobanian et al 2003); even though the results of the ALLHAT study do not support their prevailing role in terms of prevention of coronary events or increased survival (ALLHAT Officers and Coordinators 2002). Moreover, diuretics have adverse metabolic effects and may affect male sexual function, which may be important limitations for their chronic use (Stergiou and Salgami 2004). Assuming equal blood pressure lowering efficacy across all drug classes, the decision on which drug to prescribe may come down to its likely adverse events and the individual patient's risk profile (eg, age, target organ damage, concomitant diseases). With regard to calcium antagonists, these are metabolically neutral and may be favored across a wide range of patients with hypertension, including the elderly, those with ISH, angina pectoris, diabetes mellitus, peripheral vascular disease, carotid atherosclerosis, and during pregnancy (European Society of Hypertension-European Society of Cardiology Guidelines Committee 2003).
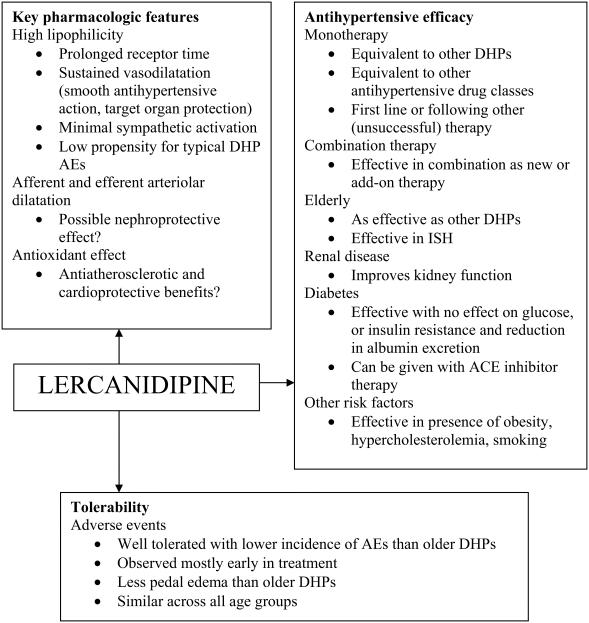
Combination therapy is appropriate in more than half of patients with hypertension (Stergiou and Salgami 2004), and calcium antagonists may be combined with any of the other 4 drug classes (Cifkova et al 2003). Combination therapy is most often required in elderly patients and those with diabetes (Cifkova et al 2003; ADA 2004); therefore, calcium antagonists offer a reliable choice in these patients and in others with concomitant cardiovascular risks. Third generation agents such as lercanidipine circumvent the problems faced with other calcium antagonists, such as uneven therapeutic effect and reflex sympathetic and vasodilator adverse effects (Kostis 2003), and this may improve patient compliance (Weir 2003). Figure 3 provides a clinical summary of lercanidipine based on its intrinsic properties, clinical trial results and applicability to latest guidelines.
Figure 3.
Clinical summary of lercanidipine in hypertension. All features and comments are derived from referenced information in the text. Abbreviations: ACE, angiotensin converting enzyme; AEs, adverse events; DHP, dihydropyridine; ISH, isolated systolic hypertension.
Conclusion
Lercanidipine exhibits a slow onset of action, which helps to avoid reflex tachycardia, and produces an even and sustained reduction in blood pressure. Preclinical and clinical findings suggest lercanidipine may have protective effects on the kidneys, cardiovascular system, and target organs. The antihypertensive action of lercanidipine is equivalent to many other antihypertensive agents, and it is equally effective in young and old patients and in the presence of other risk factors, including patients with renal dysfunction and/or type 2 diabetes. It is suitable for use as monotherapy or in combination with other agents, in line with current treatment guidelines. Lercanidipine is well tolerated in all age groups and DHP-associated adverse effects occur primarily in the first 4 weeks of treatment. The incidence of pedal edema and subsequent withdrawals has been found to be lower with lercanidipine than with amlodipine or nifedipine GITs. The favorable efficacy and safety profile of lercanidipine makes it a flexible choice for antihypertensive treatment across a broad range of patients.
References
- [ADA] American Diabetes Association. Arauz-Pacheco C, Parrott MA, et al. Hypertension management in adults with diabetes. Diabetes Care. 2004;27(Suppl 1):S65–7. doi: 10.2337/diacare.27.2007.s65. [DOI] [PubMed] [Google Scholar]
- ALLHAT Officers and Coordinators. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- Angelico P, Guarneri L, Leonardi A, et al. Vascular-selective effect of lercanidipine and other 1,4-dihydropyridines in isolated rabbit tissues. J Pharm Pharmacol. 1999;51:709–14. doi: 10.1211/0022357991772844. [DOI] [PubMed] [Google Scholar]
- Aranda P, Aranda FJ, Bianchi JL, et al. Therapeutic efficacy and tolerability of lercanidipine versus candesartan, alone or in combination, in mild-moderate essential hypertensives. J Hypertens. 2000;18(Suppl 2):S152. [Google Scholar]
- Bang LM, Chapman TM, Goa KL. Lercanidipine: a review of its efficacy in the management of hypertension. Drugs. 2003;63:2449–72. doi: 10.2165/00003495-200363220-00013. [DOI] [PubMed] [Google Scholar]
- Barbagallo M, Barbagallo SG. Efficacy and tolerability of lercanidipine in monotherapy in elderly patients with isolated systolic hypertension. Aging (Milano) 2000;12:375–9. doi: 10.1007/BF03339863. [DOI] [PubMed] [Google Scholar]
- Barrios V, Calderon A, Navarro A, et al. Lercanidipine effectiveness and tolerability profile is not influenced by overweight or body fat increase. The LERZAMIG study. J Hypertens. 2004a;22(Suppl 2):S258–9. [Google Scholar]
- Barrios V, Calderon A, Navarro A, et al. Lercanidipine is an effective and well tolerated drug in essential hypertension, independently of the cardiovascular risk. The Laura study. J Hypertens. 2004b;22(Suppl 2):S235. [Google Scholar]
- Barrios V, Navarro A, Esteras A, et al. Antihypertensive efficacy and tolerability of lercanidipine in daily clinical practice. The ELYPSE Study. Eficacia de Lercanidipino y su Perfil de Seguridad. Blood Press. 2002;11:95–100. doi: 10.1080/08037050211265. [DOI] [PubMed] [Google Scholar]
- Bellosta S, Bernini F. Lipophilic calcium antagonists in antiatherosclerotic therapy. Curr Atheroscler Rep. 2000;2:76–81. doi: 10.1007/s11883-000-0098-9. [DOI] [PubMed] [Google Scholar]
- Borghi C, Prandin MG, Dormi A, et al. Improved tolerability of the dihydropyridine calcium-channel antagonist lercanidipine: the lercanidipine challenge trial. Blood Press. 2003;(Suppl 1):14–21. doi: 10.1080/08038020310000087. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) Lancet. 2000;356:366–72. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- Calvo C, Hermida RC, Navarro A. Treatment of elderly hypertensive patients. preliminary results from the zanycal study [abstract] Am J Hypertens. 2002;15:110A. [Google Scholar]
- Cavallini A, Terzi G. Effects of antihypertensive therapy with lercanidipine and verapamil in cardiac electrical activity in patients with hypertension: a randomised, double-blind pilot study. Curr Ther Res. 2000;61:477–87. [Google Scholar]
- Cesarone MR, Incandela L, Ledda A, et al. Pressure and microcirculatory effects of treatment with lercanidipine in hypertensive patients and in vascular patients with hypertension. Angiology. 2000;51:S53–63. doi: 10.1177/000331970005100807. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Fabris F, Ferrari E, et al. Comparative effects of lercanidipine, lacidipine, and nifedipine gastrointestinal therapeutic system on blood pressure and heart rate in elderly hypertensive patients: the ELderly and LErcanidipine (ELLE) study. Arch Gerontol Geriatr. 2003;37:203–12. doi: 10.1016/s0167-4943(03)00047-5. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Cifkova R, Erdine S, Fagard R, et al. Practice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelines. J Hypertens. 2003;21:1779–86. doi: 10.1097/01.hjh.0000084773.37215.1b. [DOI] [PubMed] [Google Scholar]
- Cleophas TJ, van Ouwerkerk BM, van der MJ, et al. Diabetics with hypertension not controlled with ACE inhibitors: alternate therapies. Angiology. 2001;52:469–75. doi: 10.1177/000331970105200705. [DOI] [PubMed] [Google Scholar]
- Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- Dalla Vestra M, Pozza G, Mosca A, et al. Effect of lercanidpine compared with ramipril on albumin excretion rate in hypertensive type 2 diabetic patients with microalbuminuria: DIAL study (Diabete, Ipertensione, Albuminuria, Lercanidipina) Diab Nutr Metab. 2004;17:259–66. [PubMed] [Google Scholar]
- De Giorgio LA, Orlandini F, Malasoma P, et al. Double-blind, crossover study of lercanidipine versus amlodipine in the treatment of mild-to-moderate essential hypertension. Curr Ther Res. 1999;60:511–20. [Google Scholar]
- Digiesi V, Fiorillo C, Cosmi L, et al. Reactive oxygen species and antioxidant status in essential arterial hypertension during therapy with dihydropyridine calcium channel antagonists. Clin Ter. 2000;151:15–18. [PubMed] [Google Scholar]
- European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension – European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–53. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- Fogari R, Malamani GD, Zoppi A, et al. Comparative effect of lercanidipine and nifedipine gastrointestinal therapeutic system on ankle volume and subcutanous interstitial pressure in hypertensive patients: a double-blind, randomised, parallel-group study. Curr Ther Res Clin Exp. 2000;61:850–62. [Google Scholar]
- Fogari R, Mugellini A, Corradi L, et al. Efficacy of lercanidipine vs losartan on left ventricular hypertrophy in hypertensive type 2 diabetic patients. J Hypertens. 2000;18(Suppl 2):s65. [Google Scholar]
- Fogari R, Mugellini A, Zoppi A, et al. Differential effects of lercanidipine and nifedipine GITS on plasma norepinephrine in chronic treatment of hypertension. Am J Hypertens. 2003;16:596–9. doi: 10.1016/s0895-7061(03)00901-4. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Turri C, et al. Short-versus long-term effects of different dihydropyridines on sympathetic and baroreflex function in hypertension. Hypertension. 2003;41:558–62. doi: 10.1161/01.HYP.0000058003.27729.5A. [DOI] [PubMed] [Google Scholar]
- Guarneri L, Angelico P, Ibba M, et al. Pharmacological in vitro studies of the new 1,4-dihydropyridine calcium antagonist lercanidipine. Arzneimittelforschung. 1996;46:15–24. [PubMed] [Google Scholar]
- Guillen VFG, Abellan J, Llisterri JL, et al. Efficacy and safety of lercanidipine in combination with enalapril in HBP. Preliminary results of ZANYCONTROL study group. Am J Hypertens. 2003;16:115A. [Google Scholar]
- Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and betablockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356:359–65. doi: 10.1016/s0140-6736(00)02526-5. [DOI] [PubMed] [Google Scholar]
- Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354:1751–6. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- Herbette LG, Vecchiarelli M, Sartani A, et al. Lercanidipine: short plasma half-life, long duration of action and high cholesterol tolerance. Updated molecular model to rationalize its pharmacokinetic properties. Blood Press Suppl. 1998;2:10–17. [PubMed] [Google Scholar]
- Hollenberg NK. Observations on the safety of lercanidipine: adverse event data from placebo-controlled trials. Am J Hypertens. 2002;15:58A–59A. [Google Scholar]
- Incandela L, Belcaro G, Cesarone MR, et al. Oxygen-free radical decrease in hypertensive patients treated with lercanidipine. Int Angiol. 2001;20:136–40. [PubMed] [Google Scholar]
- James IGV, Jones A, Davies P. A randomised, double-blind, double-dummy comparison of the efficacy and tolerability of lercanidipine tablets and losartan tablets in patients with mild to moderate essential hypertension. J Hum Hypertens. 2002;16:605–10. doi: 10.1038/sj.jhh.1001430. [DOI] [PubMed] [Google Scholar]
- Kostis JB. Treatment of hypertension in older patients: an updated look at the role of calcium antagonists. Am J Geriatr Cardiol. 2003;12:319–27. doi: 10.1111/j.1076-7460.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- Leonetti G. The safety profile of antihypertensive drugs as the key factor for the achievement of blood pressure control: current experience with lercanidipine. High Blood Press. 1999;8:92–101. [Google Scholar]
- Leonetti G, Magnani B, Pessina AC, et al. Tolerability of long-term treatment with lercanidipine versus amlodipine and lacidipine in elderly hypertensives. Am J Hypertens. 2002;15:932–40. doi: 10.1016/s0895-7061(02)03000-5. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen P, Stranden E, Helberg S, et al. Quantification of leg oedema in postmenopausal hypertensive patients treated with lercanidipine or amlodipine. J Hypertens. 2003;21:1003–10. doi: 10.1097/00004872-200305000-00026. [DOI] [PubMed] [Google Scholar]
- Luque M, Ruilope LM, Tamargo J, et al. Drug surveillance study in patients with mild to moderate hypertension treated with lercanidipine: the ZANYTEN study. Am J Hypertens. 2004;20(Suppl 4):S163. [Google Scholar]
- Macchiarulo C, Pieri R, Mitolo DC, et al. Antihypertensive effects of 6 calcium antagonists: evidence from Fourier analysis of 24-hour ambulatory blood pressure recordings. Curr Ther Res. 2001;62:236–53. [Google Scholar]
- Mallion JM, Allaert FA, Scart-Gres C, et al. Variations in the frequency of lower limb oedema in patients recieving lercanidipine in adjunction to their antihypertensive treatment. J Hypertens. 2004;22(Suppl 2):S269. [Google Scholar]
- Martell N, Lopez-Eady MD, Castro P, et al. Modifications of the pulse pressure in elderly hypertensives treated with lercanidipine. J Hypertens. 2004;22(Suppl 2):S121. [Google Scholar]
- Marx A, Lichtenthal A, Milbredt C, et al. Effect of anthypertensive therapy with a new third generation calcium antagonist lercanidipine on patients with concomitant diseases. J Hypertens. 2004;22(Suppl 2):S236. [Google Scholar]
- Messerli FH. Calcium antagonists in hypertension: from hemodynamics to outcomes. Am J Hypertens. 2002;15:94S–97S. doi: 10.1016/s0895-7061(02)02950-3. [DOI] [PubMed] [Google Scholar]
- Millar-Craig M, Shaffu B, Greenough A, et al. Lercanidipine vs lacidipine in isolated systolic hypertension. J Hum Hypertens. 2003;17:799–806. doi: 10.1038/sj.jhh.1001614. [DOI] [PubMed] [Google Scholar]
- Morisco C, Trimarco B. Efficacy and tolerability of lercanidipine in comparison to and in combination with atenol in patients with mild to moderate hypertension in a double-bind controlled study. J Cardiovasc Pharmacol. 1997;29:S26–30. [Google Scholar]
- Motero J, Marques E, Eugenio JM, et al. Comparative study of the antihypertensive efficacy of lercanidipine in the treatment of mild to moderate high blood pressure in patients under and over 65 years old [abstract] J Hypertens. 2002;20(Suppl 4):S162. [Google Scholar]
- Ninci MA, Magliocca R, Malliani A. Efficacy and tolerability of lercanidipine in elderly patients with mild to moderate hypertension in a placebo-controlled, double-blind study. J Cardiovasc Pharmacol. 1997;29:S40–4. [Google Scholar]
- Notarbartolo A, Rengo F, Scafidi V, et al. Long-term effects of lercanidipine on the lipoprotein and apolipopprotein profile of patients with mild-to-moderate essential hypertension. Curr Ther Res. 1999;60:228–36. [Google Scholar]
- Omboni S, Zanchetti A, On behalf of the Multicenter Study Investigators Antihypertensive efficacy of lercanidipine at 2.5, 5 and 10 mg in mild to moderate essential hypertensives assessed by clinic and ambulatory blood pressure measurements. J Hypertens. 1998;16:1831–8. doi: 10.1097/00004872-199816120-00017. [DOI] [PubMed] [Google Scholar]
- Pedrinelli R, Dell'Omo G, Nuti M, et al. Heterogeneous effect of calcium antagonists on leg oedema: a comparison of amlodipine versus lercanidipine in hypertensive patients. J Hypertens. 2003;21:1969–73. doi: 10.1097/00004872-200310000-00026. [DOI] [PubMed] [Google Scholar]
- Policicchio D, Magliocca R, Malliani A. Efficacy and tolerability of lercanidipine in patients with mild to moderate essential hypertension: a comparative study with slow release nifedipine. J Cardiovasc Pharmacol. 1997;29:S31–5. [Google Scholar]
- Poncelet P, Ribstein J, Goullard L, et al. Efficacy and acceptability of lercanidipine are not age dependent in patients with essential hypertension: the AGATE study. Ann Cardiol Angeiol (Paris) 2004;53:123–30. doi: 10.1016/j.ancard.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Rachmani R, Levi Z, Zadok BS, et al. Losartan and lercanidipine attenuate low-density lipoprotein oxidation in patients with hypertension and type 2 diabetes mellitus: a randomized, prospective crossover study. Clin Pharmacol Ther. 2002;72:302–7. doi: 10.1067/mcp.2002.127110. [DOI] [PubMed] [Google Scholar]
- Rengo F, Romis L. Activity of lercanidipine in double-blind comparison with nitrendipine in combination treatment of patients with resistant essential hypertension. J Cardiovasc Pharmacol. 1997;29:S54–8. [Google Scholar]
- Robles NR, Canelada JA, Iglesias M, et al. Evaluation of lercanidipine in the general practice setting. Ann Med Interna. 2003;20:282–6. [PubMed] [Google Scholar]
- Robles NR, Pastor L, Manjon M, et al. Lercanidipine in diabetic patients with renal failure. Nefrologia. 2004;24:338–43. [PubMed] [Google Scholar]
- Robles NR, Ocon J, Gomez CF, et al. Lercanidipine in patients with chronic renal failure: The ZAFRA study. Ren Fail. 2005;27:73–80. [PubMed] [Google Scholar]
- Roma J, Sobrino J, Soler-Amigo J, et al. Treatment with lercanidipine during six months in hypertensive elderly patients (more than sixty years) J Hypertens. 2004;20(Suppl 4):S391. [Google Scholar]
- Romito R, Pansini MI, Perticone F, et al. Comparative effect of lercanidipine, felodipine, and nifedipine GITS on blood pressure and heart rate in patients with mild to moderate arterial hypertension: the Lercanidipine in Adults (LEAD) Study. J Clin Hypertens (Greenwich) 2003;5:249–53. doi: 10.1111/j.1524-6175.2003.01960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini M, Leonardi A, Testa R, et al. Effect of calcium antagonists on glomerular arterioles in spontaneously hypertensive rats. Hypertension. 2000a;35:775–9. doi: 10.1161/01.hyp.35.3.775. [DOI] [PubMed] [Google Scholar]
- Sabbatini M, Leonardi A, Testa R, et al. Effects of dihydropyridine-type Ca2+ antagonists on the renal arterial tree in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2002;39:39–48. doi: 10.1097/00005344-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Sabbatini M, Vitaioli L, Baldoni E, et al. Nephroprotective effect of treatment with calcium channel blockers in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2000b;294:948–54. [PubMed] [Google Scholar]
- Sangiorgi GB, Putignano E, Calcara L, et al. Efficacy and tolerability of lercanidipine vs captopril in patients with mild to moderate hypertension in a double controlled study. J Cardiovasc Pharmacol. 1997;29(Suppl 2):s36–9. [Google Scholar]
- Sarafidis P, Lasaridis A, Hatzistavri L, et al. The effect of telmisartan and lercanidipine on blood pressure and insulin resistance in hypertensive patients. Rev Clin Pharmacol Pharmacokinet Int. 2004;18:60–6. [Google Scholar]
- Schwinger RHG, Schmidt-Mertens A. The new lipophilic calcium channel blocker lercanidipine combines high antihypertensive efficacy with low side effects. Dtsch Med Wochenschr. 2002;127(Suppl 1):s13. [Google Scholar]
- Seravalle G, Stella ML, Foglia G, et al. Temporal profile of antihypertensive drug-induced regression of cardiac and vascular structural alterations in hypertension [abstract] J Hypertens. 2002;20:S190. [Google Scholar]
- Sironi G, Montagna E, Greto L, et al. Antihypertensive effects of lercanidipine in experimental hypertensive rats and dogs. Arzneimittelforschung. 1996a;46:145–52. [PubMed] [Google Scholar]
- Sironi G, Montagna E, Greto L, et al. Haemodynamic effects of lercanidipine in anaesthetized open-chest dogs. Arzneimittelforschung. 1996b;46:256–61. [PubMed] [Google Scholar]
- Stergiou GS, Salgami EV. New European, American and International guidelines for hypertension management: agreement and disagreement. Expert Rev Cardiovasc Ther. 2004;2:359–68. doi: 10.1586/14779072.2.3.359. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, et al. Calcium antagonist treatment by lercanidipine prevents hyperpolarization in essential hypertension. Hypertension. 2003;41:950–5. doi: 10.1161/01.HYP.0000063361.70525.3C. [DOI] [PubMed] [Google Scholar]
- Versari D, Virdis A, Ghiadoni L, et al. Lercanidipine restores nitric oxide availability in the forearm of essential hypertensive patients. Am J Hypertens. 2004;15(Part 2):42A. [Google Scholar]
- Viviani GL. Lercanidipine in type II diabetic patients with mild to moderate arterial hypertension. J Cardiovasc Pharmacol. 2002;40:133–9. doi: 10.1097/00005344-200207000-00016. [DOI] [PubMed] [Google Scholar]
- Weir MR. Incidence of pedal edema formation with dihydropyridine calcium channel blockers: issues and practical significance. J Clin Hypertens (Greenwich) 2003;5:330–5. doi: 10.1111/j.1524-6175.2003.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Herzig S. Molecular mechanisms of vasoselectivity of the 1,4-dihydropyridine lercanidipine. Br J Pharmacol. 2004;142:275–84. doi: 10.1038/sj.bjp.0705786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [WHO] World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–92. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]