Abstract
The development of stent has been a major advance in the treatment of obstructive coronary artery disease since the introduction of balloon angioplasty. However, neointimal hyperplasia occurring within the stent leading to in-stent restenosis is a main obstacle in the long-term success of percutaneous coronary intervention (PCI). The recent introduction of drug-eluting stents (DES) contributes a major breakthrough to interventional cardiology. Many large randomized clinical trials using DES have shown a remarkable reduction in angiographic restenosis and target vessel revascularization when compared with bare metal stents. The results of these trials also appear to be supported by evidence from everyday practice and noncontrolled clinical trials. However, the expanded applications of DES, especially in treating complex lesions such as left main trunk, bifurcation, saphenous vein graft lesions, or in-stent restenosis, are still under evaluation with ongoing studies. With the availability of different types of DES in the market, the issue of cost should not be a deterrent and DES will eventually be an economically viable option for all patients. The adoption of DES in all percutaneous coronary intervention may become a reality in the near future. In this review article, we summarize the recent development and progress of DES as well as compare and update the results of clinical trials.
Keywords: drug-eluting stent, percutaneous transluminal coronary angioplasty, in-stent restenosis
Introduction
Background of drug-eluting stent development
After the advent of cardiac catheterization in the late 1920s and the development of coronary angiographic technology in the late 1950s, balloon angioplasty (BA) was introduced in the mid 1960s. Balloon angioplasty was first applied to the revascularization of the femoral, popliteal, and renal arteries, and was adapted to the coronary arteries in the late 1970s (Forssmann 1929; Dotter and Judkins 1964; Hurst 1985, 1986). There were important limitations of coronary BA, including the risk of uncontrollable plaque disruption and vascular recoil that may lead to periprocedural coronary occlusion and myocardial infarction, and a 20%–40% incidence of restenosis within 6–12 months after successful revascularization, which compromises the longterm prognosis (Miller et al 1999). Various atherectomy techniques such as rotational atherectomy (rotablation), Excimer Laser Coronary Angioplasty (ELCA), and Directional Coronary Atherectomy (DCA) were developed in late 1980s and early 1990s, but these devices did not significantly improve the long-term outcome due to a lack of an impact on restenosis rate (Mueller et al 1995; Karthikeyan et al 2004). On the other hand, scaffolding metallic mesh, called stent, was developed during the same period to prevent restenosis after BA. The clinical efficacy of stent compared with conventional BA was studied in two landmark clinical trials. The North American Stent Restenosis Study (STRESS) showed a lower angiographic restenosis rate (31.6% vs 42.1%) and a lower target vessel revascularization (TVR) rate (10.2% vs 15.4%) in stent group than in BA group (Fischman et al 1994). The European comparison of balloon-expandable-stent implantation with BA in patients with coronary artery disease by the Benestent Study Group proved a similar, but more impressive, reduction of restenotic rate (22% vs 32%) (p = 0.02) and TVR rate (13.1% vs 22.9%) (p = 0.005) in stent group compared with BA group (Serruys et al 1994). Based on the result of these two studies, Palmaz-Schatz balloon-expandable stent (Cordis Corp; a Johnson and Johnson Company, Warren, NJ, USA) was approved as the first bare metal stent (BMS) for elective use by Food and Drug Administration (FDA) in 1994 after Gianturco-Roubin coil stent (Cook Inc, Bloomington, IN, USA) was approved as the first BMS for acute closure in 1993 (Mueller et al 1995). However, the sudden occlusion of vessel due to subacute stent thrombosis (SAT) and late in-stent restenosis (ISR) are two major complications that were initially encountered with the widespread use of BMS. Although the SAT rate has been reduced to approximately 1% with adequate antiplatelet therapy (ie, aspirin and clopidogrel), the incidence of ISR is still a hindrance to the long-term success of the stenting procedure (Schomig et al 1996, 1997). When the use of BMS was expanded in the high-risk restenosis groups of patients such as those with small vessel, long and bifurcation lesions, and diabetes mellitus, ISR and TVR escalated to the range of 50%–60% and 30%–50%, respectively (Yokoi et al 1996). Extensive research was carried out in the late 1990s to seek a solution to the problem of ISR. Brachytherapy with insertion of radioactive devices in the coronary artery was initially developed to prevent ISR (Raizner et al 2000). Despite its moderate success, brachytherapy had limitations such as late thrombosis, geographic mismatch, relatively high cost, and requirement of radiation oncologists, which made it unsuitable for widespread and routine clinical practice (Raizner et al 2000). During the period when the brachytherapy was becoming the treatment of choice of in-stent restenosis, clinical trials of drug-eluting stents (DES) demonstrated a pristine outcome with a very high success rate and very low in-stent restenosis rate. DES has now become the mainstream therapy of coronary artery stenosis due to the expected very low rate of in-stent restenosis and brachytherapy has become a thing of the past.
Pathophysiology of ISR and mechanism of action of DES to prevent ISR
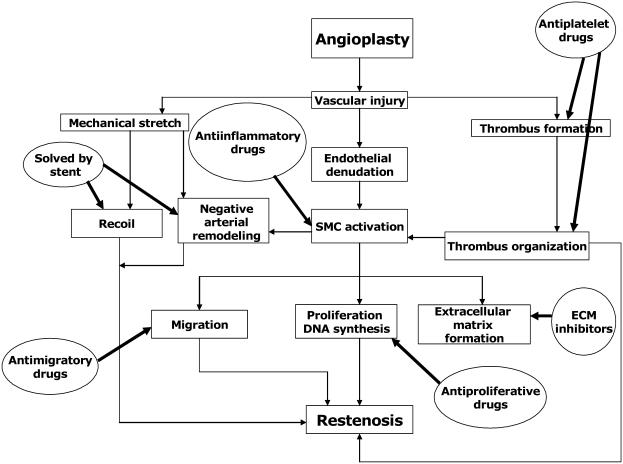
Three distinct processes are involved in the pathogenesis of ISR as depicted in Figure 1. These include: (1) immediate vessel recoil after stretch injury, (2) negative arterial remodeling, and (3) neointimal hyperplasia (Hoffmann et al 1996; Mintz et al 1996; Liu et al 2002; Muhlestein et al 2002). Elastic recoil is the immediate shrinkage of vessel after percutaneous coronary intervention (PCI) due to the elastic properties of arterial wall, which usually occurs within 24 hours after procedure. Negative remodeling is a process of local contraction of arterial wall and narrowing of the lumen at the injured vascular segment. It may be related to the healing process as well as the interaction between endothelial cells and nonlaminar blood flow (Liu et al 1989). Neointimal hyperplasia is the proliferation and migration of smooth muscle cells from the media, possibly circulating cells from bone marrow into the intima, and then encroach on the vascular lumen (Liu et al 1989). Negative remodeling and neointimal proliferation usually occur weeks to months after PCI (Liu et al 1989). The first two pathological processes were the main causes of restenosis in BA, but were basically eliminated by use of stent. The third mechanism, neointimal hyperplasia, becomes the only major mechanism in the pathogenesis of ISR (Virmani and Farb 1999).
Figure 1.
Pathophysiology of in-stent restenosis and the mechanisms of action of different therapeutic agents.
Abbreviations: ECM, extracellular matrix; SMC, smooth muscle cells.
Mechanism of neointimal hyperplasia
Immediately after stenting, the denuded endothelial surface and disrupted medial tissue of arterial wall due to mechanical trauma triggered an inflammatory process, which led to platelet adhesion, activation, and aggregation, and subsequently fibrin deposition and thrombus formation within the stent (thrombotic phase: day 0–3) (Liu et al 1989). These microthrombi as well as the stretch injury of the vessel wall attract inflammatory cells such as macrophages and lymphocytes, which demarginate from the bloodstream and also from vasa vasorum (recruitment phase: day 3–8). These inflammatory cells stimulate the production of various growth factors and cytokines locally, which activate the dormant (G0 phase) vascular smooth muscle cells in the media and also possibly recruit the circulating stem cells to re-enter into cell cycle and replicate. These proliferating smooth muscle cells subsequently migrate into the intima and thrombus in the stent lumen and eventually form a neointimal layer within the stent lumen (proliferative phase: day 8, healing) (Schwartz et al 2003). Even though cell proliferation ceases at 2 weeks after the initial injury, these smooth muscle cells continue to produce abundant extracellular matrix, which leads to increased neointimal volume. If this process of neointimal growth is exuberant and significantly encroaches on the vascular lumen, it will lead to ISR (Schwartz et al 2003). As a deeper vascular wall injury stimulates a higher degree of neointimal hyperplasia, the stent deployment injury actually induces more neointimal tissue growth than the BA injury (Schwartz et al 1992).
Stent-based drug delivery system
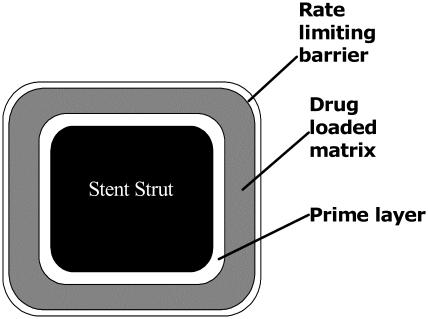
The main processes of ISR, smooth muscle cell activation and replication, occur locally at the site of injury. Therefore, one of the most logical approaches is a stent-based drug delivery system to locally deliver an appropriate concentration of an effective agent to stop this process without systemic toxicity. An effective system would consist of 3 components: (1) a metallic platform, (2) a drug carrier vehicle that stores a therapeutic agent as well as allows the agent to diffuse into the vascular tissue in a controlled fashion, and (3) an effective therapeutic agent that reduces the neointimal growth induced by stent implantation. The cross-section of a stent strut with typical coating configuration can be seen in Figure 2. Therefore, an ideal DES to achieve the greatest clinical efficacy and safety is one that requires an optimization of these three essential parameters.
Figure 2.
Cross-section of a stent strut with a drug-loaded polymeric coating.
Stent design in relation to even drug distribution to vessel wall
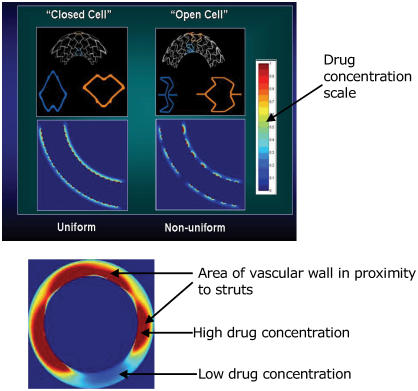
The effect of different stent designs on the drug distribution pattern has been scrutinized in experimental studies and also tested in clinical trials (Hwang et al 2001; Takebayashi et al 2004). Recent experimental data suggest that the stent strut configuration directly determines the pattern and degree of drug delivery achieved by DES (Hwang et al 2001). The simple proximity of stent struts to vascular tissue does not ensure adequate drug delivery and distribution because most nonuniform distribution has been found in the layers of the artery closest to the stent (Hwang et al 2001). After deployment of even highly lipid-soluble and rapidly diffusing agents, homogeneous drug delivery throughout the vessel with uniform concentration at various depths of the vessel wall was not achieved in their study. In the same study, the uniformity of drug distribution was found to be increased with the strut number as well as significantly dependent on the strut pattern of distribution. Therefore, a symmetric expansion of stents with homogeneous distribution of struts is essential for the optimization of drug distribution (Figure 3). The importance of this concept was further verified by a recent clinical study using Sirolimus-eluting stent (SES) (Takebayashi et al 2004). In the latter study, a nonuniform stent strut distribution and a greater gap distance between struts after stent implantation resulted in more neointimal hyperplasia (Takebayashi et al 2004). Although a large number of stent designs have been developed to date, only the multicellular design is currently most commonly used; they can be categorized into “closed cell” and “open cell” configurations (Rogers 2002). A closed cell stent has a uniform cell expansion and constant cell spacing when deployed in a curved vascular segment, which gives more uniform drug distribution (Rogers 2002). An open cell stent has a greater variation in the surface coverage between the inner and outer curvatures in the curved segment, but gives better conformability to curved surface at the expense of less uniform drug distribution (Figure 3) (Rogers 2002). The majority of current BMS use a closed cell design. In summary, the optimal stent design for drug delivery should have a large stent surface area, a small cell gap, and minimal strut deformation after deployment while maintaining conformability, radial support, and flexibility to reach the complex coronary lesions.
Figure 3.
Uniform vs nonuniform drug distribution in closed cell vs open cell stents was shown in the longitudinal sections of the vessel wall after a deployment of a drug-eluting stent. Drug concentration was shown in the color intensity in the column. Upper red-brown color is the highest and lower blue color is the lowest drug concentration. (Upper panel). A cross-section of vessel after a deployment of a drug-eluting stent depicts nonuniform strut spacing resulting in uneven drug distribution (Lower panel).
Coating matrix as a reservoir for drugs and controller of kinetic drug release
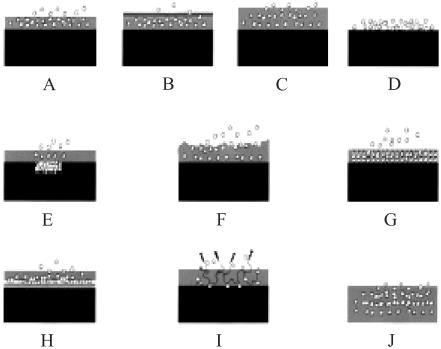
Many methods of coating stents with drugs have been developed for DES (Figure 4). Some drugs can be bonded directly to a metal stent (eg, prostacyclin, paclitaxel), but most of the agents must be bonded to a matrix polymer, which acts as a drug reservoir to ensure drug retention during deployment and a uniform distribution on the stent (Sousa et al 2003a). The types, compositions, and designs of the polymers coated on the stent dictate the eluting kinetic of the sustain time release of the drug over a period of weeks or months following the implantation in situ. The coating materials can be categorized as organic vs inorganic, bioerodable vs nonbioerodable, and synthetic vs naturally occurring substances (Sousa et al 2003a). Generally, for long-term application, a nonbioerodable polymer is used in order to prevent triggering an inflammatory process. The most successfully tested DESs to date have been coated with synthetic polymers; poly-n-butyl methacrylate and polyethylene-vinyl acetate with sirolimus and a poly (lactide-co-Σ-caprolactone) copolymer with paclitax-eleluting stents. All naturally occurring organic materials are both bio- and hemo-compatible (Ratner 1993; De Scheerder et al 2000). Fibrin, cellulose, and albumin have been tested in animal models, but only phosphorylcholine is used for clinical purposes. Phosphorylcholine is a naturally occurring phospholipid polymer with less potential to elicit inflammation and to interfere with re-endothelialization of the stent surface (Lewis et al 2002). BiodivYsio® stents are phosphorylcholine-coated stents currently available (Galli et al 2000). Inorganic substances have also been tested for coating on the stent surface to improve electrochemical properties. One example is a stent coated with a nonporous 300 μm ceramic layer containing tacrolimus-loaded nanocavities (Grube et al 2003).
Figure 4.
Different types of stent-based drug delivery system: (A) Drug released by diffusion from polymer, (B) Drug released by diffusion through ratelimiting coating, (C) Drug released by swelling of coating, (D) Drug release directly from coating, (E) Drug loaded in pore or reservoir in stent, (F) Drug release by erosion of polymer coating, (G) Drug loaded in nanoporous reservoir in coating, (H) Drug loaded between coating layers, (I) Drug released by hydrolysis or enzymatic action from polymer, (J) Bioerodable polymer coating stent.
Therapeutic agents to inhibit neointimal growth
Many agents with antiinflammatory or antiproliferative properties have been incorporated on the stent surface and tested clinically (Tables 1 and 2). Many of the agents listed in the tables have more than one mechanism of action. The general mechanism of action for most of these drugs is to stop cell cycle progression by inhibiting DNA synthesis. Everolimus, sirolimus, tacrolimus (FK-506), ABT-578, interferon, dexamethasone, and cyclosporine all fall into this category. In this group, sirolimus and its derivatives were shown to reduce intimal thickening (Sousa et al 2001; Sousa, Costa, et al 2003). Dexamethasone-coated BiodivYsio stent also showed a mild to moderate benefit in reducing restenosis (Liu et al 2002). Paclitaxel has been approved for clinical use and ABT-578 also appears promising (Meredith 2003). Angiopeptin and c-myc antisense also has been tested in clinical trials. Migration inhibitors (eg, batimastat) are aimed at preventing smooth muscle cell from migrating into the inside of the stent. If smooth muscle cells migrate to the luminal side of the stent, they can produce extracellular matrix and narrow the vascular lumen (Tanabe, Regar, et al 2004). Therefore, inhibition of smooth muscle cell migration may have therapeutic applications for preventing ISR. Examples of these compounds are batimastat and halofuginone (Tanabe, Regar, et al 2004). Batimastat inhibits matrix metalloproteinase enzymes and prevents the matrix degradation that is necessary for cells to free themselves to move and invade the stent area (Chevalier 2002). However, a clinical study using batimastat-coated stent failed to show a reduction in restenosis rate when compared with BMS (Chevalier 2002). Enhanced healing factors promote healing of the stent implantation site by reducing platelet aggregation and increasing the rate of re-endothelialization (Tanabe, Regar, et al 2004). Estradiols and nitric oxide donor compounds may also replicate this effect (Abizaid 2003; Constantini 2003). A unique approach of promoting the healing process has been the use of CD34 antibodies-coated stents in order to capture the circulating endothelial progenitor cells (Tanabe, Regar, et al 2004). In this experimental model, the surface of the stent was completely re-endothelialized within 48 hours following stent deployment (Tanabe, Regar, et al 2004). A first human clinical trial using CD34 antibodies-coated stent has been completed with a result of safety and feasibility in the treatment of de novo coronary artery disease (Aoki et al 2005). Therefore, an ideal agent should exert sufficient antirestenotic effects, but also allow re-endothelialization and adequate vessel healing at the site of implantation. It should also have negligible or no systemic effects (Marx et al 1995; Sousa et al 2003a, 2003b). Only 2 antiproliferative agents, sirolimus and paclitaxel, have been proved to be effective in clinical trials to date (Sousa et al 2003a, 2003b).
Table 1.
Agents used in drug-eluting stent
| Antineoplastics and antiinflammatory immunomodulators | Antiproliferative | Migration inhibitors and ECM modulators | Enhanced healing and re-endothelialization factors |
|---|---|---|---|
| Sirolimus | QP-2, Taxol(paclitaxel) | Batimastat | BCP671 |
| Tacrolimus | Actinomycin | Prolyl hydroxylase inhibitors | VEGF |
| Everolimus | Methotraxate | Halofunginone | Estradiols |
| Leflunomide | Angiopeptin | C-proteinase inhibitors | NO donor compounds |
| M-Prednisolone | Vincristine | Probucol | EPC antibodies |
| Dexamethasone | Mitomycine | Biorest | |
| Interferon r-1b | Statins | ||
| Mycophenolic acid | C-myc antisense | ||
| Mizoribine | Abbott ABT-578 | ||
| Cyclosporine | RestenASE | ||
| Tranilast | 2-choloro-deoxyadenosine | ||
| PCNA ribozyme |
Abbreviations: ECM, extracellular matrix; EPC, endothelial progenitor cells; NO, nitric oxide; PCNA, proliferating cell nuclear antigen; VEGF, vascular endothelial growth factor; QP-2, 7-hexanoyltaxol.
Table 2.
Clinical trials using agents excluding sirolimus and paclitaxel
| Tacrolimus | PRESENT I–III | Preliminary safety evaluation of nanoporous tacrolimus-eluting stents |
| EVIDENT | The endovascular investigation determining the safety of new tacrolimus-eluting stent grafts | |
| Everolimus | FUTURE I–IV | First used to underscore reduction in restenosis with everolimus |
| SPIRITS-FIRST | ||
| M-Prednisolone implantation | IMPRESS | Immunosuppressive therapy for the prevention of restenosis after coronary artery stent |
| Dexamethasone | STRIDE | The study of antirestenosis with BiodivYsio dexamethasone-eluting stent |
| EMPEROR | Evaluation of 9α-F-16 methylprednisolone (dexamethasone)-eluting stent on the reduction of restenosis | |
| DESIRE | Dexamethasone-eluting stent Italian registry | |
| SAFE | Sorin and aspirin following elective stenting | |
| Mycophenolic acid | IMPACT | Inhibition with MPA of coronary restenosis trial |
| Batimastat | BATMAN | BiodivYsio batimastat SV stent versus balloon angioplasty for the reduction of restenosis in small coronary arteries |
| BRILLIANT | Batimastat (BB-94) antirestenosis trial utilizing the BiodivYsio local drug delivery PC stent | |
| Actinomycin hyperplasia | ACTION | Recruitment in the actinomycin-eluting stent improves outcomes by reducing neointimal |
| Angiopeptin | SWAN | Stent with angiopeptin |
| C-myc antisense | RESTEN-NG | |
| Medtronic ABT-578 | ENDEAVOR I–III | Randomized controlled trial to evaluate the safety and efficacy of the Medtronic AVE ABT-578-eluting driver™ coronary stent in de novo native coronary artery lesions |
| Abbott ABT-578 | Zomaxx 1 | Zomaxx coronary drug-eluting stent for de novo lesion in coronary arteries. |
| Estradiols | EASTER | Estrogen and stent to eliminate restenosis |
| NO donor compounds | NOBLESSE | Nitric oxide through biodegradable layer elective study for safety and efficacy |
| EPC antibodies | HEALING I–II | Healthy endothelial accelerated lining inhibits neointimal growth |
Abbreviations: MPA, mycophenolic acid; PC, phosphorylcholine; SV, small-vessel.
Sirolimus (rapamycin), a fermentation product of Streptomyces hygroscopicus, was discovered in 1977 as an antifungal macrolide antibiotic with potent immunosuppressive properties (Poon et al 1996; Marx et al 2001; Sousa, Sousa, et al 2003). Since sirolimus is a lipophilic molecule, it readily diffuses across the cell membranes of vascular smooth muscle cells and leukocytes. Once in the cytoplasm, it binds with high affinity to a specific intracellular protein (FKBP12), and the resultant complex inhibits a regulatory enzyme, called TOR (target of rapamycin). Ultimately it blocks cell cycle progression from G1 to S phase, and therefore limits smooth muscle replication and proliferation (Poon et al 1996; Marx et al 2001; Sousa, Sousa, et al 2003).
Paclitaxel is an antineoplastic agent originally isolated from the bark of the Pacific yew tree, Taxus brevifolia (Schiff et al 1979; Sollott et al 1995). It was approved by FDA for treatment of breast and ovarian cancer in 1992. It is also a lipophilic molecule that readily diffuses across cell membranes and has a potent stabilizing effect on microtubules (Schiff et al 1979; Sollott et al 1995). Since microtubule disassembly is essential for the progression of the G2 to M phase in the mitotic cell cycle, stabilization of microtubule inhibits the mitosis of smooth muscle cell as well as inhibits the cell migration. This reduces the infiltration of vascular smooth muscle cells and leukocytes into the zone of injury caused by stent (Schiff et al 1979; Sollott et al 1995).
Commercially available DES
The sirolimus-eluting Cypher™ stent (Cordis Corp, a Johnson and Johnson Company, Miami, FL, USA) was approved by FDA in April 2003. It is coated with a layer of nonerodable polymer, of 5 μm–10 μm thickness, which is incorporated with sirolimus (140 μg sirolimus/cm2 of stent surface area). An additional topcoat is placed on it as a diffusion barrier, which provides the vehicle for controlled release of the drug. It is designed as such that approximately 80% of the total dose of the agent is released in 4 weeks and the remainder over the course of the next 2 weeks (Wong and Chan 2004). The second commercially available DES is the Taxus™ stent (Boston Scientific, Natrick, MA, USA), which has a proprietary platform, the Express™ stent, and is coated with a proprietary polymer (Translute™) loaded with 1 μg of paclitaxel/mm2 of stent surface area. Although there are three drug-release formulations (slow, moderate, and fast), only moderate- and slow-release formulations have been tested in clinical trials (Sahatjian 2003; Waugh and Wagstaff 2004). The moderate-release (MR) form of Taxus stent allows for an initial bolus release over the first 48 hours after stenting followed by a low-level release over at least the next 10 days. In the initial 10 days of drug release, the slow-release (SR) formulation of Taxus stent has a drug release concentration of 8–10 times lower than that of the MR formulation. Only SR formulation is used in FDA approved Taxus stents. (Sahatjian 2003; Waugh and Wagstaff 2004).
Clinical trials of DES
The initial feasibility study of sirolimus-eluting stent (SES), the First In Man (FIM) study, consisted of 45 patients with angina pectoris (Sousa et al 2001; Sousa, Costa, et al 2003). There were no major adverse cardiac events (MACE); ie, death, myocardial infarction, coronary bypass grafting, or target lesion revascularization (TLR) at 1- and 2-year clinical follow-up; only minimal neointimal hyperplasia within the stent on angiographic and intravascular ultrasound (IVUS) follow-up at 1 year; and 10% TVR rate for the entire cohort at 2 years (Sousa et al 2001; Sousa, Costa, et al 2003). The first randomized clinical trial of SES, the RAVEL (Randomized study with the sirolimus-eluting Bx Velocity balloon-expandable stent) trial, compared the Cypher stent with BMS-Bx Velocity stent in single, noncomplex lesion of native coronary arteries. The RAVEL study result remarkably showed 0% binary restenosis in the SES arm vs 26.6% in the BMS arm at 6-month follow-up, and 5.8% MACE rate in the SES arm vs 28.8% (p < 0.001) in the BMS arm at 1-year follow-up (Morice et al 2002; Serruys et al 2002). The effect of SES in more complex lesions was further confirmed in the larger American trial; SIRIUS (Sirolimus-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions). SIRIUS included a greater proportion of patients with high-risk features of restenosis such as diabetes, smaller vessels, and longer lesion requiring overlapping stents. This study showed a restenosis rate of 8.9% in the SES arm vs 36.3% (p < 0.001) in the BMS arm angiographically at 9-month follow-up (Moses et al 2003). Additional similar SIRIUS trials using SES in Europe and Canada (E- and C-SIRIUS) showed consistent, impressive results. These two studies included a total of 452 patients and had binary restenosis rates 5.9%, 2.3%, respectively, in the SES arm vs 42.3%, 52.3% (p = 0.0001, p < 0.001) in the BMS arm. The MACE rate was 8.0%, 2.0%, respectively, in the SES arm vs 22.6%, 19.0%, respectively (p = 0.0002, p = 0.0002), in the BMS arm. The TLR rate was 4.0%, 4.0% in the SES arm vs 20.9%, 18%, respectively (p < 0.0001, p = 0.05), in the control arm at 9-month follow-up (Schofer et al 2003; Schampaert et al 2004). Results of these clinical trials are summarized in Table 3.
Table 3.
Summary of randomized controlled trials of sirolimus-eluting stent vs bare metal stent
| RAVEL | SIRIUS | E-SIRIUS | C-SIRIUS | |||||
|---|---|---|---|---|---|---|---|---|
| Sirolimus (n = 120) | Control (n = 118) | Sirolimus (n = 533) | Control (n = 525) | Sirolimus (n = 175) | Control (n = 177) | Sirolimus (n = 50) | Control (n = 50) | |
| Mean lesion length (mm) | 9.56 | 9.61 | 14.4 | 14.4 | 14.9 | 15.1 | 14.5 | 12.6 |
| Mean RVD (mm) | 2.6 | 2.64 | 2.79 | 2.81 | 2.6 | 2.51 | 2.65 | 2.62 |
| Angiographic follow-up | 6 mon | 9 mon | 9 mon | 9 mon | ||||
| Mean late luminal loss* (mm) | −0.01 | 0.80 | 0.24 | 0.81 | 0.19 | 0.80 | 0.12 | 0.79 |
| Binary restenosis(%) | 0 | 26.6 | 8.9 | 36.3 | 5.9 | 42.3 | 2.3 | 52.3 |
| Subacute stent thrombosis (%) | 0 | 0 | 0.4 | 0.8 | 1.1 | 0 | 2.0 | 2.0 |
| TVR (%) | 0 | 26 | 3.4 | 4.8 | 4.0 | 20.9 | – | – |
| TLR (%) | 0 | 23.7 | 4.1 | 16.6 | 4.0 | 20.9 | 4.0 | 18.0 |
| Overall MACE (%) | 5.8 | 28.8 | 7.1 | 18.9 | 8.0 | 22.6 | 4.0 | 18.3 |
Abbreviations: RAVEL, Randomized study with sirolimus-eluting Velocity balloon-expandable stent in the treatment of patients with de novo native coronary artery lesion; SIRIUS, Sirolimus-coated BX Velocity stent in the treatment of patients with de novo coronary artery lesions; E- & C-SIRIUS, European- & Canadian-SIRIUS; MACE, major adverse cardiac events; mon, months; RVD, reference vessel diameter; TLR, target lesion revascularization; TVR, target vessel revascularization.
note: All mean late luminal loss were in-segment except for RAVEL which was in-stent.
Unlike SES, paclitaxel and its derivatives have been studied in different coatings and stent designs. There were mainly 3 types of paclitaxel coatings: (1) 7-hexanoyltaxol which used polyacrylate sleeves as a release mechanism tested in the SCORE (Study to compare restenosis rate between QueSt and QuaDS-QP2) trial; (2) Paclitaxel-eluting stent (PES), which used nonpolymer coating as platform tested in 3 other trials; ELUTES (European evaluation of paclitaxel-eluting stent), ASPECT (Asian paclitaxel-eluting stent clinical trial), and DELIVER (Paclitaxel-coated RX ACHIEVE coronary stent system [CSS] versus the RX ML PENTA stainless steel stent in the treatment of focal de novo coronary lesions); and (3) PES which used polymer coating as a platform tested in TAXUS I–VI trials (Grube et al 2002; Hong et al 2003; Gershlick et al 2004; Lansky et al 2004). Except for the polymer-coated-PES, the other two types of coating have not been very suitable or useful (Grube et al 2002; Hong et al 2003; Gershlick et al 2004; Lansky et al 2004). The initial feasibility study of polymer-based PES, TAXUS I, was performed in Europe where the feasibility of using a SR-PES to treat short (<15 mm) de novo lesions was demonstrated (Grube et al 2003). TAXUS II was the first randomized trial using both SR- and MR-PESs with a binary restenosis rate of 5.5% in SR and 8.6% in MR, respectively, vs 21.9% in BMS arm (Colombo et al 2003). TAXUS III was a feasibility trial of SR-PES in ISR with the result of feasible and safe use in the treatment of ISR in 28 patients with a MACE rate of 29% (Tanabe et al 2003). TAXUS IV, a major pivotal American trial of PES compared with BMS, showed significant reduction in the TLR rate (4.4% vs 15.1%, p < 0.0001), TVR rate (7.1% vs 17.1%, p < 0.0001), and composite MACE rate (10.8% vs 20.0%, p < 0.0001) (Stone et al 2004). TAXUS VI was a randomized trial using MR-PES in complex lesions such as very long lesions or lesions in small vessels. The results showed in-stent binary restenosis rate of 12.4% vs 35.7% (p < 0.0001), TLR rate of 6.8% vs 18.9% (p = 0.0001) and TVR rate of 9.1% vs 19.4% (p = 0.0027) when comparing PES with BMS arm at 9 months (Dawkins 2004a; Grube 2004). The results of published randomized controlled trials using PES are summarized in Table 4.
Table 4.
Summary of randomized controlled trials of paclitaxel-eluting stent vs bare metal stent
| TAXUS I | TAXUS II | TAXUS IV | TAXUS VI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 30) | SR (n = 31) | Control (n = 270) | SR (n = 131) | MR (n = 135) | Express (n = 652) | Taxus (n = 662) | Control (n = 227) | MR (n = 219) | |
| Lesion length (mm) | 11.9 | 10.7 | 10.6 | 10.6 | 10.2 | 13.4 | 13.4 | 20.32 | 20.94 |
| RVD (mm) | 2.94 | 2.99 | 2.78 | 2.8 | 2.7 | 2.75 | 2.75 | 2.77 | 2.81 |
| Late luminal loss (mm) | 0.71 | 0.36 | 0.78 | 0.31 | 0.3 | 0.61 | 0.23 | 0.99 | 0.39 |
| In-stent binary restenosis (%) | 10 | 0 | 21.9 | 5.5 | 8.6 | 26.6 | 7.9 | 32.9 | 9.1 |
| Stent thrombosis (%) | 0 | 0 | 0 | 1.5 | 0.7 | 0.8 | 0.6 | 0 | 0 |
| TLR or TVR (%) | 10 or 10 | 0 or 3.3 | 17.5 | 10.1 | 6.9 | 12.0 | 4.7 | 18.9 or 19.4 | 6.8 or 9.1 |
| Overall MACE (%) | 10 | 3.3 | 21.7 | 10.9 | 9.9 | 15.0 | 8.5 | 22.5 | 16.4 |
Abbreviations: MACE, major adverse cardiovascular events; MR, moderate-release; RVD, reference vessel diameter; SR, slow-release; TLR, target lesion revascularization; TVR, target vessel revascularization.
Other promising DES in clinical trials
Stents coated with rapamycin analogues, ABT-578, and everolimus were also studied in clinical trials. In the ENDEAVOR I (Randomized controlled trial to evaluate the safety and efficacy of the Medtronic AVE ABT-578-eluting driver™ coronary stent in de novo native coronary artery lesions) trial, ABT-578 (Abbott Laboratories, Abbott Park, IL, USA) coated cobalt-alloy stent (Driver stent, Medtronic Inc, Minneapolis, MN, USA) was used to treat 100 patients with de novo lesions. The result showed 0.33 mm of angiographic in-stent late lumen loss at 4 months and 0.58 mm at one-year follow-up. The target-vessel failure rate was comparable to the results of SIRIUS or TAXUS IV (Meredith 2003). ENDEAVOR II and III are large pivotal trials for ABT-578 and the results are pending. FUTURE (First used to underscore reduction in restenosis with everolimus) I and II trials were designed to demonstrate the safety and feasibility of the everolimus-eluting-stent (Novartis Pharma AG, Basel, Switzerland) in a small population with focal de novo coronary artery lesions with a in-stent late lumen loss of 0.12 mm, a low TLR rate (4.3%) and a very low MACE rate (6.4%) at 6-month follow-up (Costa et al 2003). A large clinical trial for everolimuseluting stent is ongoing.
Real world experience of DES
The “real world” registries are important because patients in clinical trials of DES are highly selected groups of patients and these clinical trials do not include patients with high-risk lesions for restenosis such as small-vessel, very long lesions, ostial lesions, left main lesions, bifurcation lesions, totally occluded and saphenous vein lesions, and lesions in acute myocardial infarction. However, these registries gather the clinical outcomes of DES use in unselected population to further validate its clinical efficacy. SES currently has 2 major registries, RESEARCH and E-Cypher. PES has 3 major registries, WISDOM, MILESTONE II, and ARRIVE. The RESEARCH (Rapamycin-eluting stent evaluated at Rotterdam Cardiology Hospital) registry is a single-center registry of an unrestricted use of SES in all patients with de novo lesions to evaluate the safety and efficacy in the daily practice at 1-year period compared with a control group who received BMS in the previous year. This registry had excellent results with lower TLR rate (5.1% vs 10.9%) and MACE rate (9.7% vs 14.8%) (Lemos, Serruys, et al 2004).
E-Cypher is an international internet-based registry of SES that enrolled all patients receiving one and more SES with current recruitment of more than 12 000 patients in 275 international sites and more than 80% eligible patients at 6-month follow-up. This registry showed absolutely stunning outcomes of 2.95% MACE rate, 1% TLR rate, and 0.3% SAT rate at 6 months (Urban 2003). WISDOM (Web-based Taxus intercontinental observational data transitional registry program) was a multicenter registry of 778 patients who received PES without strict clinical and angiographic inclusion criteria. The interim result of this registry showed a TLR rate of 1.8% and a MACE rate of 4.5% (Abizaid 2004). MILESTONE II was a European and intercontinental post-market registry of 3708 patients who received PES compared with the result of MILESTONE I which used BMS. MILESTONE II included the high-risk lesions and diabetic patients, especially insulin dependent diabetes, and it also had successful results of 7.1% MACE rate, 4.2% of TVR rate and 0.9% of stent thrombosis rate (acute and late angiographic restenosis) at 6 months with the diabetic population having 8.9% MACE rate and 5% TVR rate (Boston Scientific Corporation 2004). ARRIVE is a peri-approval Taxus stent registry of 2600 consecutive patients at 50 centers in USA and showed the result of 2.7% MACE rate and 1.3% of stent thrombosis rate at 30 days (Cox 2004). The clinical outcomes of these registries showed that the use of DES in the real world practice, ie, in a larger and unselected patient population, achieved similarly successful outcome as those of previous randomized trials.
Current issues of DES
Expanded indications
Although DES was proved to be a safe and effective method in the treatment of coronary artery stenosis by both randomized clinical trials and real world practice, its expanded indications in complex and high-risk lesions for restenosis such as totally occluded lesions, left main lesions, bifurcation lesions, ostial lesions, small and long lesions, saphenous vein graft lesion, ISR, and diabetes mellitus are still under evaluation with ongoing trials. The results of clinical trials for some expanded indications are now available.
Of the high-risk subsets, diabetes mellitus is the most common and important one. Subanalysis of diabetic patients in the many clinical trials such as SIRIUS, TAXUS IV, or E-Cypher registry showed a great efficacy of DES in these patients even though these trials were not designed to investigate the efficacy in diabetes mellitus. SIRIUS study showed excellent results with a MACE rate of 9.2% vs 25% (p < 0.001) and a TLR rate of 6.9% vs 22.3% (p < 0.001) in the SES arm compared with the BMS arm at 9-month follow-up (Moussa et al 2004). In the diabetic group of the E-Cypher registry, the results were 4.2% MACE, 1.4% TLR, and 0.5% SAT with similar result in insulin dependent diabetic subset; 5.9% MACE, 1.5% TLR, and 0.4% SAT (p < 0.01) (Gershlick 2003). In the TAXUS IV trial, similar results were shown in diabetic patients with a binary angiographic restenosis rate of 6.4% in PES arm vs 34.5% in BMS arm (p < 0.0001) at 9-month follow-up, the TLR rate of 7.4% vs 20.9% (p = 0.0008) and TVR rate of 11.3% vs 24% (p < 0.004), respectively, at 12-month follow-up. More specifically, in insulin dependent diabetic subset, a very similar rate of angiographic restenosis 7.7% vs 42.9% (p = 0.0065), and TLR rate of 6.2% vs 19.4% (p = 0.07), when compared with BMS was found to exist (Hermiller 2005). A large comparison study using DES in treating multi-vessel disease in diabetic patients with coronary bypass surgery is currently ongoing.
In the subset of the lesions in small vessels, the data from the RESEARCH registry showed that the larger vessels group (reference vessel diameter of 2.52 mm ± 0.57 mm) has a late loss of 0.03 mm ± 0.38 mm and binary restenosis rate of 3.9% whereas the small vessels group (reference vessel diameter of 1.88 mm ± 0.34 mm) has a late loss of 0.07 mm ± 0.48 mm and a restenosis rate of 10.7%. (Lemos, Arampatzis, et al 2004). In the SIRIUS trial, the small vessel (reverence vessel diameter [RVD] ≤2.3 mm) subset study showed an in-lesion restenosis rate of 18.6% in the SES group vs 42.9% in the control group (p < 0.001) (Guagliumi 2004). Moreover, with an improved deployment technique to cover the full length of lesions, a different trial, ie, Small vessels treated with the Cypher stent (SVELTE) was performed. The SVELTE trial was a prospective study using Cypher stent in small-vessel lesions (RVD of 2.25 mm–2.75 mm) while comparing the historical control from the SIRIUS trial. The findings indicated a better in-stent binary restenosis rate of 3.2% in SVELTE group vs 3.9% in SIRIUS-SES group and 38.0% in SIRIUS-control group with much improved in-lesion restenosis rate of 6.3% vs 11.6% and 39.0%, respectively (Sousa 2004).
For very long lesions, the study from the RESEARCH registry examining patients with long lesions with a SES stent length of 39 mm ± 29 mm (mean ± standard deviation) showed an in-stent late loss 0.13 mm ± 0.47 mm at 6-month follow-up and a TVR rate of 6.2% and a MACE rate of 8.3% at 320-day follow-up (Degertekin et al 2004). The TAXUS VI study for very long lesions (18 mm–40 mm in lesion length) showed a very impressive low TLR rate of 6.8% in the PES arm vs 18.9% in the BMS arm at 9 month follow-up (Dawkins 2004b).
In a totally occluded lesion substudy, the data from RESEARCH registry showed a reduction in the MACE rate (cumulative MACE-free survival rate of 96.4% in SES arm vs 82.1% in BMS arm) and a binary restenosis rate of 9.1% in the SES arm vs other BMS trials for total occluded lesions: 55% in TOSCA (Total occlusion study of Canada); 42% in STOP (Stents in total occlusion for restenosis prevention); 32% in GISSOC (Gruppo italiano di studio sullo stent nelle occlusioni coronariche) and 32% in SICCO (Stenting in Chronic Coronary Occlusion) (Hoye et al 2004). In addition, SICTO (Sirolimus-eluting stent in chronic total occlusion) was a multicenter, prospective and nonrandomized feasibility study of SES in treatment of patients with chronic total occlusion and showed a persistently pristine result of late loss (−0.1 mm ± 0.3 mm) and a TVR rate of 8% at 6-month follow-up (Lotan 2004).
For the treatment of in-stent restenosis, TAXUS III trial, a feasibility and safety study, showed a result of 21% TLR rate and a binary restenosis rate of 16% in the PES arm (Tanabe et al 2003). In RESEARCH registry, the SES group had the results of a MACE rate of 18.6% and a TVR rate of 4.7% similar to the results of the brachytherapy group, 20.9%, 4.7%, respectively, at 9-month follow-up (Saia et al 2004). But much more strikingly, TROPICAL (Sirolimus-eluting stent in the treatment of patients with in-stent restenotic native coronary artery lesion) was a multicenter, nonrandomized study that showed a much better result of stent thrombosis rate (0.6% vs 3.9%), binary restenosis rate (9.7% vs 40.3%), and MACE rate (3.7% vs 18.8%) in the SES arm when compared with the historic control from the brachytherapy trials (GAMMA I and II) (Neumann et al 2004). The ISAR-DESIRE (Intracoronary stenting and angiographic results: drug-eluting stent for in-stent restenosis) trial was a 3-arm study comparing the efficacy of SES, PES, and BA in patients with ISR. Both SES and PES were associated with a lower TVR rate and smaller late lumen loss than use of BA at 9 months (Kastrati 2004).
In patients with bifurcation lesions, RESEARCH registry showed encouraging TLR rate (8.6%) and binary restenosis rate (22.7%) in SES group (Tanabe et al 2004). Colombo et al (2004) described a safety and efficacy of SES in the treatment of bifurcation lesion with the result of TLR rate of 17.6% and binary restenosis rate of 25.7% but it was concluded that restenosis at the side branch remained a problem (Colombo et al 2004). The use of DES in left main lesion and the vein graft lesion is still under clinical investigation.
In terms of the superiority of DES, two large randomized clinical trials in head-to-head comparison of the efficacy of SES to PES in patients with de novo lesions, ie, REALITY (A prospective, randomized, multi-center comparison of the cypher sirolimus-eluting and the Taxus paclitaxel-eluting stent systems) and SIRTAX (Randomized comparison of a sirolimus- vs a paclitaxel-eluting (Taxus) stent for coronary revascularization) were recently completed. The result of REALTY trial showed no difference between SES and PES in binary angiographic in-lesion restenosis at 8 months (9.6% Cypher vs 11.1% TAXUS), but all angiographic parameters favored more robust inhibition of neointimal hyerplasia by Cypher (in-stent late loss: 0.09 mm vs 0.31 mm; diameter stenosis%: 23.1% vs 26.7%; in-stent minimal luminal diameter: 2.0 mm vs 1.85 mm) (Morice 2005). In the SIRTAX trial, SES showed a lower in-stent and in-lesion late luminal loss than PES (0.13 mm vs 0.25 mm, 0.19 mm vs 0.32 mm respectively) as well as a lower in-stent and in-lesion binary restenosis than PES (3.2% vs 7.6%, 6.7% vs 11.9%, respectively) (Windecker 2005).
SAT and late stent thrombosis
Subacute stent thrombosis is approximately 1% in patients on dual antiplatelet therapy. However, an IVUS study has revealed that inadequate stent expansion was related to SAT. An adequate stent expansion and IVUS guided stent implantation had been shown to reduce the SAT rate (Moussa et al 1997). However, there was a case report of an occurrence of late stent thrombosis in one patient who discontinued clopidogrel at 18 month after SES implantation (Virmani et al 2004). Subsequetly, a 2nd case report revealed four patients developed similar late stent thrombosis following the discontinuation of clopidogrel after 1 year of PES implantation. (McFadden et al 2004). This has raised the question of whether there is a need to increase the duration of antiplatelet therapy for patients receiving DES beyond the current recommended guidelines of practice.
Issue of stent-based delivery: incomplete stent apposition and uneven stent strut distribution
Incomplete stent apposition (ISA), defined as one or more stent struts not in contact with vascular wall on IVUS at any point in time after stent implantation, was found in 21% of the SES arm in RAVEL vs 4% in the BMS arm at 6-month follow-up (Serruys et al 2002). It is possible that this is due to either an initial incomplete deployment of stent during implantation or positive remodeling of vessel wall but other mechanisms like plaque regression, cell necrosis, apoptosis, and allergic reaction to sirolimus have been postulated (Lemos et al 2003; Takebayashi et al 2004). Uneven stent strut distribution and incomplete wall apposition has been considered to be the causes of ISR after the DES implantation in two clinical studies (Lemos et al 2003; Takebayashi et al 2004).
Economic burden
One of the thorniest issues regarding DES is their cost and reimbursement. In the USA, a BMS costs approximately $900–$1200 each while a DES costs approximately $3065–$3195. However, in the cost-effective analysis of SIRIUS trial, the difference of cost between the 2 groups were only about US $300 at 1 year, despite an initial $3000 difference after hospitalization (Cohen et al 2003). The advent of more varieties of DES in near future will minimize the cost issue and make DES available to all patients.
Conclusion
The recent introduction of DES in PCI is a major innovative advancement in interventional cardiology. DES dramatically reduces the ISR rate in all subgroups of patients in both randomized clinical trials and real-world practice. Continuing improvement in drug-delivery stent technologies and gradual reduction in cost would make DES an effective mainstay of therapy for coronary artery disease.
References
- Abizaid AC. Novel studies with drug-eluting stents: new drug-eluting trials EASTER, IMPACT, PISCES, BioRest, and more [online]. Presented at Transcatheter Cardiovascular Therapeutics; Sep 15–19 2003; Washington DC, USA. 2003. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=5901. [Google Scholar]
- Abizaid AC. WISDOM International Registry [online]. Presented at Drug Eluting Stent symposium at American College of Cardiology; New Orleans, LA, USA. 2004. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=6630. [Google Scholar]
- Aoki J, Serruys PW, van Beusekom H, et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol. 2005;45:1574–9. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Boston Scientific Corporation. MILESTONE II Registry. TAXUS Clinical Trials [online] 2004 Accessed 27 Dec 2004. URL: http://www.bostonscientific.com/templatedata/imports/multimedia/AboutBSC/media_taxusclinicaltrialsummary_04.pdf.
- Chevalier BR. Batimastat stent: BRILLANT I results and future directions [online]. Presented at Transcatheter Cardiovascular Therapeutics; 2002. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=3569. [Google Scholar]
- Cohen DJ, Bakhai A, Shi C, et al. Cost-effectiveness of sirolimus-eluting stents for the treatment of complex coronary stenosis: results from the randomized SIRIUS trial [abstract] J Am Coll Cardiol. 2003;41(Suppl):32A. [Google Scholar]
- Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–94. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- Colombo A, Moses JW, Morice MC, et al. Randomized study to evaluate sirolimus-eluting stents implanted at coronary bifurcation lesions. Circulation. 2004;109:1244–9. doi: 10.1161/01.CIR.0000118474.71662.E3. [DOI] [PubMed] [Google Scholar]
- Constantini CR. Results from NOBLESSE I trial. Nitric oxide through biodegradable layer elective study for safety and efficacy [online]. Presented at Transcatheter Cardiovascular Therapeutics; 2003. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expertpresentations/multi-slide.html?product_id=5902. [Google Scholar]
- Costa R, Lansky A, Mehran R, et al. Everolimus-eluting stent for the treatment of de novo coronary lesion: an angiographic follow-up of the FUTURE trial [abstract] Am J Cardio. 2003;92(Suppl 1):61L. [Google Scholar]
- Cox D. ARRIVE: TAXUS Peri-approval registry (Safety surveillance program) [online]. Presented at Drug-eluting stent symposium at ACC; 6 Mar 2004; New Orleans, LA, USA. 2004. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=6629. [Google Scholar]
- Dawkins K. TAXUS VI: 9-month angiographic results [online]. Presented at Paris Course on Revascularization; 25–28 May 2004; Paris, France. 2004a. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=7185. [Google Scholar]
- Dawkins KD. TAXUS VI-9 month result. In depth analysis of long lesions [online]. Presented at Paris Course on Revascularization; 25–28 May 2004; Paris, France. 2004b. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=7203. [Google Scholar]
- Degertekin M, Arampatzis CA, Lemos PA, et al. Very long sirolimus-eluting stent implantation for de novo coronary lesions. Am J Cardiol. 2004;93:826–9. doi: 10.1016/j.amjcard.2003.12.018. [DOI] [PubMed] [Google Scholar]
- De Scheerder I, Szilard M, Yanming H, et al. Evaluation of the biocompatibility of two new diamond-like stent coatings (Dylyn) in a porcine coronary stent model. J Invas Cardiol. 2000;12:389–94. [PubMed] [Google Scholar]
- Dotter CT, Judkins MP. Transluminal treatment of arteriosclerotic obstruction: description of a new technique and a preliminary report of its application. Circulation. 1964;30:654–70. doi: 10.1161/01.cir.30.5.654. [DOI] [PubMed] [Google Scholar]
- Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent lacement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- Forssmann W. The catheterization of the right side of the heart. Klin Wochenschr. 1929;8:2085–7. [Google Scholar]
- Galli M, Bartorelli A, Bedogni F, et al. Italian BiodivYsio open registry (BiodivYsio PC-coated stent): study of clinical outcomes of the implant of a PC-coated coronary stent. J Invasive Cardiol. 2000;12:452–8. [PubMed] [Google Scholar]
- Gershlick A, De Scheerder I, Chevalier B, et al. Inhibition of restenosis with a paclitaxel-eluting, polymer-free coronary stent: the European evaLUation of pacliTaxel Eluting Stent (ELUTES) trial. Circulation. 2004;109:487–93. doi: 10.1161/01.CIR.0000109694.58299.A0. [DOI] [PubMed] [Google Scholar]
- Gershlick T. E-Cypher registry: Subgroup analyses and follow-up results. [online]. Presented at Drug Eluting Stent summit at Transcatheter Cardiovascular Therapeutics; 2003. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=6028. [Google Scholar]
- Grube E. Final tacrolimus outcomes in native coronary arteries and saphenous vein graft: PRESENT and EVIDENT [online]. Presented at the scientific session of American College of Cardiology, Drug-Eluting Stent Symposium; 29 March 2003; Chicago. 2003. Accessed 27 Dec 2004. URL: http://www.tctmd.com/csportal/appmanager/tctmd/descoe?_nfpb=true&_pageLabel=DESCenterContent&hdCon=958624. [Google Scholar]
- Grube E. TAXUS VI: 9-month results. Insights into diabetics [online]. Presented at Paris Course on Revascularization; 25–28 May 2004; Paris, France. 2004. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=7204. [Google Scholar]
- Grube E, Hauptmann K, Colombo A, et al. SCORE trial interim safety results: despite efficacy, late stent thrombosis with the QuaDDSQP2 stent [abstract] J Am Coll Cardiol. 2002;39(Suppl A):38A. [Google Scholar]
- Grube E, Silber S, Hauptmann KE, et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release peclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107:38–42. doi: 10.1161/01.cir.0000047700.58683.a1. [DOI] [PubMed] [Google Scholar]
- Guagliumi G. Cypher in small vessels: from SVELTE to EVOLUTION [online]. Presented at Transcatheter Cardiovascular Therapeutics on 1 Oct 2004; 2004. [online] Accessed on 2 Jan 2005. URL: http://www.tctmd.com/csportal/appmanager/tctmd/main?_nfpb=true&_pageLabel=TCTMDContent&_windowLabel=P450010&_450010_disContParam_displayMode=maximized&_state=maximized. [Google Scholar]
- Hermiller JB, Raizner A, Cannon L, et al. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol. 2005;45:1172–9. doi: 10.1016/j.jacc.2004.10.075. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94:1247–54. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- Hong MK, Mintz GS, Lee CW, et al. Paclitaxel coating reduces instent intimal hyperplasia in human coronary arteries: a serial volumetric intravascular ultrasound analysis from the Asian Paclitaxel-Eluting Stent Clinical Trial (ASPECT) Circulation. 2003;107:517–20. doi: 10.1161/01.cir.0000054163.42072.d4. [DOI] [PubMed] [Google Scholar]
- Hoye A, Tanabe K, Lemos PA, et al. Significant reduction in restenosis after the use of sirolimus-eluting stents in the treatment of chronic total occlusions. J Am Coll Cardiol. 2004;43:1954–8. doi: 10.1016/j.jacc.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Hurst JW. History of cardiac catheterization. In: King SB III, Douglas JS, et al., editors. Coronary arteriography and angioplasty. New York: McGraw-Hill; 1985. pp. 5–6. [Google Scholar]
- Hurst JW. The first coronary angioplasty as described by Andreas Gruentzig. Am J Cardiol. 1986;57:185–6. doi: 10.1016/0002-9149(86)90981-1. [DOI] [PubMed] [Google Scholar]
- Hwang CW, Wu D, Edelman ER. Physiological transport forces govern drug distribution for stent-based delivery. Circulation. 2001;104:600–5. doi: 10.1161/hc3101.092214. [DOI] [PubMed] [Google Scholar]
- Karthikeyan G, Bhargava B. Prevention of restenosis after coronary angioplasty. Curr Opin Cardiol. 2004;19:500–9. doi: 10.1097/01.hco.0000133658.77024.59. [DOI] [PubMed] [Google Scholar]
- Kastrati A. ISAR-DESIRE (Intracoronary stenting and angiographic results: drug-eluting stent for in-stent restenosis [online]. Presented at European Society of Cardiology Congress; 2004. Accessed 27 Dec 2004. URL: http://www.clinicaltrialresults.org/Files/shows/isar%20desire.ppt. [Google Scholar]
- Lansky AJ, Costa RA, Mintz GS, et al. Non-polymer-based paclitaxel-coated coronary stents for the treatment of patients with de novo coronary lesions: angiographic follow-up of the DELIVER clinical trial. Circulation. 2004;109:1948–54. doi: 10.1161/01.CIR.0000127129.94129.6F. [DOI] [PubMed] [Google Scholar]
- Lemos PA, Arampatzis CA, Saia F, et al. Treatment of very small vessels with 2.25-mm diameter sirolimus-eluting stents (from the RESEARCH registry) Am J Cardiol. 2004;93:633–6. doi: 10.1016/j.amjcard.2003.11.037. [DOI] [PubMed] [Google Scholar]
- Lemos PA, Saia F, Ligthart JM, et al. Coronary restenosis after sirolimus-eluting stent implantation: morphological description and mechanistic analysis from a consecutive series of cases. Circulation. 2003;108:257–60. doi: 10.1161/01.CIR.0000083366.33686.11. [DOI] [PubMed] [Google Scholar]
- Lemos PA, Serruys PW, van Domburg RT, et al. Unrestricted utilization of sirolimus-eluting stents compared with conventional bare stent implantation in the “real world”: the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry. Circulation. 2004;109:190–5. doi: 10.1161/01.CIR.0000109138.84579.FA. [DOI] [PubMed] [Google Scholar]
- Lewis AL, Tolhurst LA, Stratford PW. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantation. Biomaterials. 2002;23:1697–706. doi: 10.1016/s0142-9612(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Liu MW, Parks JM, Cox DA, et al. Vascular biology of mechanical intervention. 2nd ed. Philadelphia: Churchill Livingstone; 2002. Interventional cardiovascular medicine, principles and practice. [Google Scholar]
- Liu MW, Roubin GS, King SB., III Restenosis following coronary angioplasty potential biological determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–87. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- Liu X, Huang Y, Hanet C, et al. Study of antirestenosis with the BiodivYsio dexamethasone-eluting stent (STRIDE): a first-in-human multicenter pilot trial. Catheter Cardiovasc Interv. 2003;60:172–9. doi: 10.1002/ccd.10636. [DOI] [PubMed] [Google Scholar]
- Lotan C. The SICTO study (Sirolimus-eluting stent In Chronic Total Occlusion) [online]. Presented at Paris Course on Revascularization; Paris, France. 2004. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=7212. [Google Scholar]
- Marx SO, Jayaraman T, Go L, et al. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–17. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- Marx SO, Marks AR. Bench to bedside: development of rapamycin and its application to stent restenosis. Circulation. 2001;104:852–5. doi: 10.1161/01.cir.104.8.852. [DOI] [PubMed] [Google Scholar]
- McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drugeluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–21. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- Meredith I. ENDEAVOR I: 4-month angiographic and IVUS result [online]. Presented at Transcatheter Cardiovascular Therapeutic; 16–21 Sept 2003; Washington DC, USA. 2003. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multislide.html?product_id=5356. [Google Scholar]
- Miller JM, Ohman EM, Moliterno DJ, et al. Restenosis: the clinical issues. In: Topol EJ, editor. Textbook of interventional cardiology. 3rd ed. Philadelphia: WB Saunders; 1999. pp. 379–415. [Google Scholar]
- Mintz GS, Popma JJ, Pichard AD, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation. 1996;94:35–43. doi: 10.1161/01.cir.94.1.35. [DOI] [PubMed] [Google Scholar]
- Morice MC. REALITY: a prospective, randomized, multi-center comparison study of the CYPHER Sirolimus-eluting and TAXUS Paclitaxel-eluting stent systems [online]. Presented at American College of Cardiology 2005; 2005. Accessed on 1 Apr 2005. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=9463&largeimagep=0&start_idx=1. [Google Scholar]
- Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–80. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- Moussa I, Di Mario C, Reimers B, et al. Subacute stent thrombosis in the era of intravascular ultrasound-guided coronary stenting without anticoagulation: frequency, predictors and clinical outcome. J Am Coll Cardiol. 1997;29:6–12. doi: 10.1016/s0735-1097(96)00452-4. [DOI] [PubMed] [Google Scholar]
- Moussa I, Leon MB, Baim DS, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: A SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) Substudy. Circulation. 2004;109:2273–8. doi: 10.1161/01.CIR.0000129767.45513.71. [DOI] [PubMed] [Google Scholar]
- Mueller RL, Sanborn TA. The history of interventional cardiology: cardiac catheterization, angioplasty, and related interventions. Am Heart J. 1995;129:146–72. doi: 10.1016/0002-8703(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Muhlestein JB, Zidar JP, Blazing MA. Interventional cardiovascular medicine, principles and practice. 2nd ed. Philadelphia: Churchill Livingstone; 2002. The vascular biology of restenosis: an overview. [Google Scholar]
- Neumann FJ, Desmet W. A multicenter, non-randomized study of Cypher Sirolimus-eluting stent in the TReatment Of Patients with Instent restenotic native Coronary Artery Lesion (TROPICAL) [online]. Presented at Paris Course on Revascularization; 25–28 May 2004; 2004. Accessed 27 Dec 2004. URL: http://www.medscape.com/viewarticle/482835. [Google Scholar]
- Poon M, Marx SO, Gallo R, et al. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2277–83. doi: 10.1172/JCI119038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizner AE, Oesterle SN, Waksman R, et al. Inhibition of restenosis with beta-emitting radiotherapy: report of the Proliferation Reduction with Vascular Energy Trial (PREVENT) Circulation. 2000;102:951–8. doi: 10.1161/01.cir.102.9.951. [DOI] [PubMed] [Google Scholar]
- Ratner BD. The blood compatibility catastrophe. J Biomed Mater Res. 1993;27:283–7. doi: 10.1002/jbm.820270302. [DOI] [PubMed] [Google Scholar]
- Rogers CDK. Drug-eluting stents: role of stent design, delivery vehicle, and drug selection. Rev Cardiovasc Med. 2002;3(Suppl 5):S10–15. [PubMed] [Google Scholar]
- Sahatjian R. TAXUS: a polymer-based Paclitaxel-eluting stent-technology overview [online]. Presented at BEMA meeting on sciencebased assessment; 22–23 Apr 2003; Boston Scientific Corp, MA, USA. 2003. Accessed 27 Dec 2004. URL: http://216.239.63.104/search?q=cache:2DpKUQKeiDgJ:books.nap.edu/html/science_based/Ronald%2520A.%2520Sahatjian.pdf+TAXUS:+A+polymerbased+Paclitaxel-eluting+stent-Technology+overview&hl=en. [Google Scholar]
- Saia F, Lemos PA, Hoye A, et al. Clinical outcomes for sirolimus-eluting stent implantation and vascular brachytherapy for the treatment of in-stent restenosis. Catheter Cardiovasc Interv. 2004;62:283–8. doi: 10.1002/ccd.20068. [DOI] [PubMed] [Google Scholar]
- Schampaert E, Cohen EA, Schluter M, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS) J Am Coll Cardiol. 2004;43:1110–15. doi: 10.1016/j.jacc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schofer J, Schluter M, Gershlick AH, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomized controlled trial (E-SIRIUS) Lancet. 2003;362:1093–9. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084–9. doi: 10.1056/NEJM199604253341702. [DOI] [PubMed] [Google Scholar]
- Schomig A, Neumann FJ, Walter H, et al. Coronary stent placement in patients with acute myocardial infarction: comparison of clinical and angiographic outcome after randomization to antiplatelet or anticoagulant therapy. J Am Coll Cardiol. 1997;29:28–34. doi: 10.1016/s0735-1097(96)00450-0. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Henry TD. Pathophysiology of coronary artery restenosis. Rev Cardiovasc Med. 2003;3(Suppl 5):S4–9. [PubMed] [Google Scholar]
- Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19:267–74. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Degertekin M, Tanabe K, et al. Intravascular ultrasound findings in the multicenter, randomized, double-blind RAVEL (Randomized study with the sirolimus-eluting velocity balloon-expandable stent in the treatment of patients with de novo native coronary artery lesions) trial. Circulation. 2002;106:798–803. doi: 10.1161/01.cir.0000025585.63486.59. [DOI] [PubMed] [Google Scholar]
- Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. BENESTENT Study Group. N Eng J Med. 1994;331:489–95. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- Sollott SJ, Cheng L, Pauly RR, et al. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995;95:1869–76. doi: 10.1172/JCI117867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa E. 8-month data from the multi-center, non-randomized, historical controlled study in patients with De Novo coronary artery lesions in small vessels treated with Cypher stent (SVELTE trial) [online]. Presented at Paris Course of Revascularization; 2004. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multislide.html?product_id=7213. [Google Scholar]
- Sousa JE, Costa MA, Abizaid AC, et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation. 2001;104:2007–11. doi: 10.1161/hc4201.098056. [DOI] [PubMed] [Google Scholar]
- Sousa JE, Costa MA, Sousa AG, et al. Two-year angiographic and intravascular ultrasound follow-up after implantation of sirolimus-eluting stents in human coronary arteries. Circulation. 2003;107:381–3. doi: 10.1161/01.cir.0000051720.59095.6d. [DOI] [PubMed] [Google Scholar]
- Sousa JE, Serruys PW, Costa MA. New frontiers in cardiology: drug-eluting stents: part I. Circulation. 2003a;107:2274–9. doi: 10.1161/01.CIR.0000069330.41022.90. [DOI] [PubMed] [Google Scholar]
- Sousa JE, Serruys PW, Costa MA. New frontiers in cardiology: drug-eluting stents part II. Circulation. 2003b;107:2383–9. doi: 10.1161/01.CIR.0000069331.67148.2F. [DOI] [PubMed] [Google Scholar]
- Sousa JE, Sousa AG, Costa MA, et al. Use of rapamycin-impregnated stents in coronary arteries. Transplant Proc. 2003;35:5165–70. doi: 10.1016/s0041-1345(03)00215-x. [DOI] [PubMed] [Google Scholar]
- Stone GW, Ellis SG, Cox DA, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109:1942–7. doi: 10.1161/01.CIR.0000127110.49192.72. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Mintz GS, Carlier SG, et al. Nonuniform strut distribution correlates with more neointimal hyperplasia after Sirolimus-eluting stent implantation. Circulation. 2004;110:3430–4. doi: 10.1161/01.CIR.0000148371.53174.05. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Hoye A, Lemos PA, et al. Restenosis rates following bifurcation stenting with sirolimus-eluting stents for de novo narrowings. Am J Cardiol. 2004;94:115–18. doi: 10.1016/j.amjcard.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Regar E, Lee CH, et al. Local drug delivery using coated stents: new developments and future perspectives. Curr Pharm Des. 2004;10:357–67. doi: 10.2174/1381612043453289. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Serruys PW, Grube E, et al. TAXUS III Trial: in-stent restenosis treated with stent-based delivery of paclitaxel incorporated in a slow-release polymer formulation. Circulation. 2003;107:559–64. doi: 10.1161/01.cir.0000048184.96491.8a. [DOI] [PubMed] [Google Scholar]
- Urban P. E-Cypher registry: Study design, demographics and complications [online]. Presented at Drug-eluting stent summit at Transcatheter Cardiovascular Therapeutics; 18 Sept 2003; Washington DC, USA. 2003. Accessed 27 Dec 2004. URL: http://www.tctmd.com/expert-presentations/multi-slide.html?product_id=6027&large_image_p=0&start_idx=1. [Google Scholar]
- Virmani R, Farb A. Pathology of in-stent restenosis. Curr Opin Lipidol. 1999;10:499–506. doi: 10.1097/00041433-199912000-00004. [DOI] [PubMed] [Google Scholar]
- Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–5. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- Waugh J, Wagstaff AJ. The paclitaxel (Taxus)-eluting stent: a review of its use in the management of de novo coronary artery lesions. Am J Cardiovas Drugs. 2004;4:257–68. doi: 10.2165/00129784-200404040-00006. [DOI] [PubMed] [Google Scholar]
- Windecker S. SIRTAX: randomized comparison of a sirolimus- vs a paclitaxel-eluting stent for coronary revascularization [online]. Presented at American College of Cardiology, 2005; 2005. Assessed on 1 Apr 2005 URL: http://www.medscape.com/viewarticle/501392. [Google Scholar]
- Wong A, Chan C. Drug-eluting stents: the end of restenosis? Ann Acad Med Singapore. 2004;33:423–31. [PubMed] [Google Scholar]
- Yokoi H, Kumura T, Nakagawa Y, et al. Long-term clinical and quantitative angiographic follow-up after the Palmaz-Schatz stent restenosis [abstract] J Am Coll Cardiol. 1996;27:224A. [Google Scholar]