Abstract
Neovascularization is essential to the process of development and differentiation of tissues in the vertebrate embryo, and is also involved in a wide variety of physiological and pathological conditions in adults, including wound repair, metabolic diseases, inflammation, cardiovascular disorders, and tumor progression. Thanks to cumulative studies on vasculature, new therapeutic approaches have been opened for us to some life-threatening diseases by controlling angiogenesis in the affected organs. In cancer therapy, for example, modulation of factors responsible for tumor angiogenesis may be beneficial in inhibiting of tumor progression. Several antiangiogenic approaches are currently under preclinical trial. However, the mechanisms of neovascularization in tumors are complicated and each tumor shows unique features in its vasculature, depending on tissue specificity, angiogenic micromilieu, grades and stages, host immunity, and so on. For better understanding and effective therapeutic approaches, it is important to clarify both the general mechanism of angiogenic events and the disease-specific mechanism of neovascularization. This review discusses the general features of angiogenesis under physiological and pathological conditions, mainly in tumor progression. In addition, recent topics such as contribution of the endothelial progenitor cells, tumor vasculogenic mimicry, markers for tumor-derived endothelial cells and pericytes, and angiogenic/angiostatic chemokines are summarized.
Keywords: neovascularization, angiogenesis, tumor, endothelial cell, pericyte, chemokine
Introduction
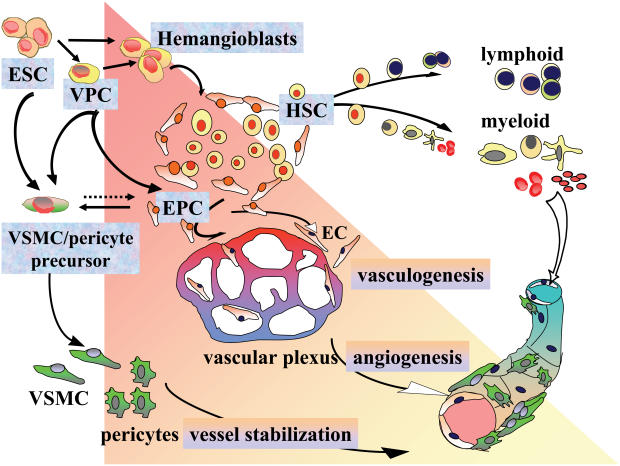
The vascular system is a functional organ that starts developing in the very early stage of embryogenesis (Sumpio et al 2002). Hemangioblasts, the progenitor cells derived from embryonic mesoderm, generate the blood-island composed of endothelial progenitor cells (also named angioblasts) and hematopoietic stem cells. This process of primary vascular plexus formation is called “vasculogenesis” (Risau 1997). During development and differentiation, vasculature is further differentiated by sprouting, intussusception, and bridging of endothelial cells from the preexisting plexus, and both cellular and structural maturation proceed. This step is called “angiogenesis” (Figure 1) (Carmeliet and Collen 1999). In embryogenesis, dynamics of neovascularization are finely controlled by numerous angiogenic and angiostatic factors (Distler et al 2003). In the postnatal stage, the constituent cells of blood vessels are generally quiescent in G0 phase of cell cycle, which means in a mature and differentiated state, whereas in certain organs/tissues such as ovary, endometrium, and placenta, vascular remodeling takes place actively under physiological controls (Shweiki et al 1993; Ferrara et al 1998).
Figure 1.
Vasculogenesis and angiogenesis during development and differentiation. The mesodermal progenitor cells derived from embryonic stem cells (ESC) differentiate into various vascular and hematopoietic cells. Vascular progenitor cells (VPC) are bi-potentials that can differentiate into either pericyte/vascular smooth muscle cells (VSMC) or endothelial cells (EC) lineage. Hemangioblasts generate the blood island composed of endothelial progenitor cells, also called angioblasts (EPC) and hematopoietic stem cells (HSC). EPC differentiate into EC and form vessel tubes, whereas HSC further differentiate into lymphoid/myeloid lineage. Nascent vessels are stabilized by vascular mural cell coverage and extracellular matrix.
Apart from the physiological angiogenesis, pathological angiogenesis occurs under specific conditions such as wound repair, diabetic retinopathy, rheumatoid arthritis, and tumors (Dvorak et al 1999). Such pathological angiogenesis plays an important role in the process of progression and/or healing of each disease (Carmeliet 2003). Newly formed vessels in adult tissues are generally thought to be derived from the neighboring cells of preexisting blood vessels (Hanahan and Folkman 1996). In addition, recent studies on progenitor cells have revealed another mechanism of neovascularization, ie, contribution of bone marrow-derived and circulating endothelial precursor cells to adult vasculogenesis/angiogenesis, which will be discussed later.
The present review focuses on features of neovascularization under physiological and pathological conditions, with an emphasis on tumor neovascularization.
Constituent cells of blood vessels
Constituent cells of blood vessels, endothelial cells (EC), pericytes, and vascular smooth muscle cells (VSMC) are differentiated from separate types of precursor cells, or from common vascular progenitor cells (Carmeliet 2000; Yamashita et al 2000). VSMC of great vessels are also derived from neural crest cells (Gittenberger-de Groot et al 1999). Furthermore, EC and endothelial precursor cells have been shown to transdifferentiate into pericytes or other mesenchymal cells under specific conditions (Paranya et al 2001; Badorff et al 2003).
Since EC form the inner lining of blood vessels and are directly exposed to blood flow, they stand as a primary gate for intravasation and extravasation of blood cells, plasma proteins, and various biochemical substances (Mitchell and Contran 1999). Blood vessels themselves may also synthesize and secrete bioactive substances (Cotran et al 1999). Vascular cells participate in blood pressure control, immune responses, and many other homeostatic regulations (Schoen and Cotran 1999). There are various receptors expressed on the surface of EC, and many kinds of intercellular junctional molecules mediate between EC–EC and EC–pericytes/VSMC interaction (Cleaver and Melton 2003). In response to cytokines, chemokines, and other vasoactive substances, these receptors and junctional molecules mediate several signalings that alter vascular tone and permeability, and/or cause neointimal formation through proliferation and migration of both EC and pericytes/VSMC (Dvorak et al 1999).
Molecules that regulate angiogenesis
Multiple steps are required for angiogenesis in adult tissues. At the onset of angiogenic sprouting, proangiogenic factors including vascular endothelial growth factor (VEGF) and related molecules act on EC and increase EC permeability. In response to the angiogenic switch, pericytes and VSMC are detached from the preexisting vessels (Carmeliet and Collen 1999; Dvorak et al 1999). In this process, degradation of the basement membrane and extracellular matrix (ECM) by proteolytic enzymes is required. Matrix metalloproteinases (MMPs), tissue inhibitors of MMPs (TIMPs), elastases, and cathepsins play important roles for matrix remodeling (Kalluri 2003). Activated EC then migrate into the perivascular space and proliferate to form tubular structures. In succession, pericytes/VSMC envelope these EC and thus nascent vessels are stabilized (Carmeliet 2000).
Representative growth factors and cytokines that control angiogenesis include VEGF, fibroblast growth factors (FGF), angiopoietins (Ang), transforming growth factor-β (TGF-β), and tumor necrosis factor-α (TNF-α). VEGF acts rather more selectively on EC than other cytokines do (eg, FGF, TGF-β, and TNF-α) and works as a master switch of angiogenesis in an early stage of both physiological and pathological conditions (Dvorak 2002). In addition to the factors listed above, there are numerous important factors that control vessel remodeling. Among them, two key molecules, VEGF and FGF, are highlighted in this review.
Vascular endothelial growth factor
Members of the VEGF family are VEGF-A, -B, -C, -D, -E, and placenta growth factor (PlGF). VEGF-A gene is alternatively spliced to form several isoforms such as VEGF-A121,VEGF-A165, and VEGF-A189 in humans (Dvorak 2002; Ruhrberg 2003). These isoforms differ in the presence or absence of two heparin-binding domains of VEGF-A. VEGF-A165 is considered to be the most predominant isoform that supports angiogenic growth. Studies on VEGF isoforms have shown the importance of different isoforms for vessel specification. For example, mice selectively expressing a single isoform VEGF-A164 (VEGF164/164) were healthy and had normal retinal angiogenesis, whereas mice which expressed VEGF188/188 selectively displayed normal venular outgrowth, but had impaired arterial development (Stalmans et al 2002). Mice that expressed VEGF120/120 showed impaired vessel outgrowth (Ng et al 2001; Stalmans et al 2002).
VEGF-A functions as a main ligand for receptors VEGFR-1 (fms-like tyrosine kinase-1 [Flt-1]), VEGFR-2 (fetal liver kinase-1 [Flk-1]/kinase insert domain protein receptor [KDR]) and neuropilin-1/-2 (NRP-1/-2) (Dvorak 2002). Angiogenic VEGF signaling is mediated mainly through VEGFR-1 and VEGFR-2: members of receptor tyrosine kinases (RTKs) that contain the cytoplasmic tyrosine kinase domain separated by an intervening, noncatalytic, circa 70 amino acid sequence (Podar and Anderson 2005). This structure is common among the related family members, such as platelet-derived growth factor receptor (PDGFR), c-Kit, and c-Fms (Sherr 1990). The binding ability of VEGF-A to VEGFR-1 is at least 10-fold stronger than that to VEGFR-2 (Shibuya 2001). In contrast, the kinase activity of VEGFR-2 is 10-fold higher than that of VEGFR-1. Furthermore, VEGFR-1 tyrosine kinase-deficient homozygous mice developed normal vessels and survived, whereas VEGFR-2−/− mice embryos died from a failure of blood island formation and vasculogenesis (Shalaby et al 1995; Hiratsuka et al 1998). Thus, VEGF signaling in angiogenesis may be mediated mainly by VEGFR-2. The role of VEGFR-1 seems to be more complex. A soluble form of VEGFR-1 suppressed EC proliferation (Kendall and Thomas 1993). VEGFR-1−/− mice died in utero with an overgrowth of EC-like abnormal cells (Fong et al 1995). These findings indicate that VEGFR-1 may negatively regulate angiogenesis (Fong et al 1999; Kearney et al 2002). On the other hand, VEGFR-1 was shown to contribute to vascular sprouting (Kearney et al 2004) and metastasis (Hiratsuka et al 2002). Therefore, the pathophysiological roles of VEGFR-1 may depend on the local milieu and the stage of angiogenesis. Studies on hematopoietic stem cells have elucidated another role of VEGFR-1 in the repopulation of bone marrow-resident progenitor cells, which will be discussed later. VEGFR-1 also serves as a receptor for PlGF and VEGF-B (Sawano et al 1996; Olofsson et al 1998). VEGF-C and -D interact with VEGFR-3 (fms-like tyrosine kinase-4 [Flt-4]) expressed mainly in lymphatic EC, and they seem to have a major role in lymphangiogenesis and tumor metastasis via lymphatic vessels (Jeltsch et al 1997). VEGF-C and -D also potentially bind to VEGFR-2 (Joukov et al 1996).
Fibroblast growth factors
The FGF family is composed of over 20 members and its effects are mediated by four RTKs, ie, FGF receptor-1 (FGFR-1), -2, -3, and -4. FGF are required for development and differentiation of various organs from the early stage of embryogenesis, as indicated by the fact that the mice deprived of either FGFR-1 or FGFR-2 die prior to gastrulation (Deng et al 1994; Xu et al 1998). In the context of angiogenesis, overexpression of FGF-2 (also named basic FGF [bFGF]) is known to increase angiogenic activity by inducing chemotaxis and migration of EC, through FGFR-1-mediated signaling (Tanghetti et al 2002). FGF-2 has also been shown to collaborate with other angiogenic molecules such as VEGF, plasminogen activator and hepatocyte growth factor (HGF) (Bikfalvi et al 1997; Onimaru et al 2002). FGF-2 may efficiently integrate angiogenic activities of several factors in a synergetic manner (Ribatti and Presta 2002).
With regard to antiapoptotic mechanism in EC, FGF-2 preferentially protects EC from intrinsic stress-mediated apoptosis such as serum starvation, whereas VEGF works against the extrinsic apoptotic pathways induced by death ligands such as TNF-α and Interferon-γ (IFN-γ) (Alavi et al 2003). Since both FGF-2 and VEGF are known to be produced abundantly in various angiogenic diseases, targeting both factors would be expected to result in better antiangiogenic effects.
Cross-talk between endothelial cells and pericytes/extracellular matrix
There are some angiogenic factors that control cross-talk among different types of cells in the vasculature. For example, tyrosine kinase with Ig and EGF homology domain-2 (Tie-2) is expressed in EC, whereas angiopoietin-1 (Ang-1), the ligand for Tie-2, is produced by pericytes, and they cooperatively work for vascular stabilization (Davis et al 1996; Suri et al 1996). On the other hand, Ang-2 is produced by activated EC and competitively inhibits the function of Ang-1 (Tanaka et al 1999). As another example, platelet-derived growth factor-B (PDGF-B) expressed in EC has essential roles in stabilization of nascent vessels by recruiting pericytes in which the receptor for PDGF-B (PDGFR-β) is expressed (Lindahl et al 1998; Hellstrom et al 2001). Studies using knockout mice deprived of the factors such as those mentioned above have shown that proangiogenic signalings in EC and regulatory systems in which pericytes/VSMC participate are indispensable for embryonic vascular development and maturation (Soriano 1994; Sato et al 1995; Ferrara et al 1996; Hellstrom et al 2001).
Signalings in vascular cells are also controlled by ECM and luminal blood flow (eg, shear stress and hemodynamic load) as well as by humoral factors (Li et al 1999; Kalluri 2003). Mechanical force is known to act on several sensors including PDGFR-β, integrins and ion channels in the vascular cells, and to modulate cellular morphology (Li and Xu 2000). Certain integrin members are specifically expressed in vasculature and interact with the corresponding ligands on ECM (Hodivala-Dilke et al 2003). Such integrinmatrix interaction mediates secretion and activation of proteolytic enzymes and contributes to matrix remodeling. Integrins αVβ3 and αVβ5, in particular, have been well characterized (Eliceiri and Cheresh 2001). These integrins are generally upregulated in angiogenic vessels, and are regarded to possess proangiogenic function (Brooks et al 1994; Dallabrida et al 2000; Camenisch et al 2002). However, studies using mice lacking β3 and β5 subunits revealed that integrins αVβ3 and αVβ5 are dispensable for embryonic vascular development (Reynolds et al 2002; Reynolds et al 2004). It is more likely that the real function of these integrins is more complex than first thought.
Some endogenous angiogenesis inhibitors, eg, tumstatin, endostatin, and canstatin, bind certain integrins on EC (Kalluri 2003). Tumstatin is known to induce EC-specific apoptosis in which the presence of integrin αVβ3 is necessary as the binding site for this molecule (Maeshima et al 2002). Currently, a monoclonal antibody against integrin αVβ3 (Vitaxin) is under preclinical investigation for antiangiogenic therapies against rheumatoid arthritis and malignant tumors. Further studies are required to clarify the effectiveness of such therapeutic approaches and in relation to the function of endogenous angiogenic inhibitors.
Tissue specificity in normal vasculature
Every tissue carries a tissue-specific signature in its vasculature (Ruoslahti and Rajotte 2000). Permeability of capillaries varies among organs. For example, liver sinusoidal EC are discontinuously lined without support of the basement membrane and have innumerable fenestrations. Liver sinusoidal EC express very low or undetectable levels of E-selectin, P-selectin, and CD31 (also named platelet endothelial adhesion molecule-1 [PECAM-1]), and they lack Factor VIII and Weibel-Palade bodies, sites of storage of von Willebrand factor (Lalor et al 2002). On the other hand, cerebral capillaries are particularly impermeable, and the system of tight barrier between microcirculation and brain parenchyma is named blood-brain barrier (BBB). EC in this vasculature lack fenestration and are connected to each other by adherence and tight junctions. Tight junctional molecules such as junctional adhesion molecule-1 (JAM-1), occludin, and claudins are well developed in cerebral EC, and they contribute to precise regulation of paracellular diffusion and ion flux (Hirase et al 1997; Morita et al 1999; Aurrand-Lions et al 2001; Brown and Davis 2002).
Recent development of genomics and proteomics has enabled us to address organ-specific EC markers, eg, lung-specific endothelial cell adhesion molecule (Lu-ECAM-1), dipeptidyl peptidase IV (DPP IV), and aminopeptidase-P in rodent lung EC (Zhu et al 1991; Johnson et al 1993; Oh et al 2004). Making use of such tissue-specific vessel markers may be of great advantage in efficient drug/gene delivery to the targeted organ (Arap et al 2002). However, if other unknown sites share these markers, such drug/gene delivery might induce accumulation of therapeutic substances in unexpected sites. Therefore, the application of tissue-specific EC markers for therapeutic strategies should be carefully considered.
Special features of tumor vessels
A landmark publication on tumor pathophysiology, Hallmarks of cancer by Hanahan and Weinberg (2000), defined seven critical features of cancer phenotype. One of the listed hallmarks, “sustained angiogenesis”, is explained as an acquired event during tumor development via an angiogenic switch from vascular quiescence. It is now well recognized that tumor vasculature is different from normal vasculature in the context of architecture and biological behavior. Progressive tumor tissues easily become hypoxic and necrotic because of rapid proliferation and insufficient blood supply (Pugh and Ratcliffe 2003). Host immune cells may infiltrate into the lesion, although the immune response generally fails to eradicate the tumors (Janeway et al 2001). Under such proinflammatory microenvironments, tumor angiogenic vessels show abnormal maturation. The conventional structure of peripheral blood vessels composed of arterioles, capillaries, and venules is abrogated. Instead, irregular sprouting and branching of angiogenic vessels with disordered coverage of pericytes are demonstrated in morphology (McDonald and Choyke 2003). Tumor vasculature frequently shows elevated permeability, increased proteolytic activities, and hemorrhage (Dvorak 2002; Hodivala-Dilke et al 2003).
Therapeutic approaches using antiangiogenic agents are currently clinically approved in some advanced cancers, and the possibilities for successful antiangiogenic therapies will be further opened to various angiogenic tumors (Sivakumar et al 2004). Tumor vessels are generally derived from preexisting resident vascular cells or recruited precursor EC (Hanahan and Folkman 1996; Ribatti 2004). Therefore, tumor vasculature is considered to be susceptible to therapeutic drugs, although tumor cells often circumvent chemotherapies and radiation (Blagosklonny 2004). It is expected that vascular targeting molecules in tumor therapies can be administered repetitively without inducing resistance and that such therapy keeps cancers in the state of dormancy, even though complete eradication may not be achieved (Boehm et al 1997). On the other hand, recent studies have demonstrated cytogenetic complexity of tumor vasculature; ie, EC aneuploidy (Hida et al 2004) and “tumor vasculogenic mimicry”, as discussed later (Maniotis et al 1999). In relation to possible resistance against antiangiogenic therapies, detailed studies on special features of tumor vessels, including chromosomal instability of tumor, will be required.
Tumor-derived endothelial cells
Tumor-derived endothelial cells (TEC) have been characterized intensively using highly reliable methods, such as endothelial purification from tumor tissues (St Croix et al 2000; Bussolati et al 2003) and immunoelectron microscopy (Sincock et al 1999). Comparison of molecular profiles of normal EC and TEC, by microarray analysis and proteomic mapping, has shown upregulation of various angiogenesis-related molecules (Shih et al 2002; Mutuberria et al 2004; Oh et al 2004). These molecules, highly expressed in TEC, although not specific in TEC, may be involved in increased permeability, proliferation, migration, and antiapoptosis of TEC, and also in matrix remodeling in tumor progression (St Croix et al 2000; Bussolati et al 2003). Currently, several molecules such as VEGFR-2, CD105 (also named endoglin), endothelial protein-disulfide isomerase (EndoPDI), and tumor endothelial markers (TEMs) are noted as representative surface markers of TEC because they are rarely expressed in the corresponding normal EC (Carson-Walter et al 2001; Duff et al 2003; Sullivan et al 2003; Fonsatti and Maio 2004). Under other conditions, including embryogenesis and inflammatory diseases, these markers may also be upregulated in nontumor EC (Gougos et al 1992; Carson-Walter et al 2001).
Clinical application of antiangiogenic therapy has some targets: membrane proteins that are specifically expressed in TEC (eg, VEGFR, CD105); intracellular signaling molecules that are highly activated in TEC (eg, mitogen-activated protein kinase [MAPK], Akt); and proangiogenic factors produced by tumor cells (eg, VEGF, FGF). When TEC markers are used for targeting, these markers should be localized specifically in TEC of the lesion but not expressed in normal EC of the other organs/tissues. For example, a recent study revealed that annexin A1 was highly expressed in TEC of rat lung tumors (Oh et al 2004), and neither in the corresponding normal lung nor in the other organs, which satisfied the requirement for therapeutic application in both organ specificity and TEC specificity. In the latter study, in comparison with annexin A1, other molecules were also found to be highly expressed in TEC; those such as VEGFR-1, VEGFR-2, NRP-1, and CD105 were detectable in heart, kidney, and liver. Detailed studies on TEC-specific molecules in each tumor will provide us with more effective antiangiogenic therapies.
Signal transduction in tumor-derived endothelial cells
The majority of TEC-specific molecules highlighted so far are present on the cell surface. In addition to these transmembrane molecules, cells in tumor vasculature also utilize various intracellular signaling cascades and transcription factors to sustain angiogenesis (Sato 2000; Shibuya 2003). Some key angiogenic factors stimulate RTKs such as VEGFR, Tie, and FGFR, and it is expected that these RTKs-mediated signalings are activated in angiogenic EC in vivo (Pintus et al 2002; Shu et al 2002; Deregibus et al 2003; Yilmaz et al 2003). The representative intracellular molecules involved in RTKs-mediated signaling pathways include: (1) Src homology and collagen (Shc) that binds to growth factor receptor bound protein 2 (Grb2)/son of sevenless (SOS) and consequently activates Ras/rapid fibrosarcoma virus (Raf)/MAPK pathways; (2) phosphoinositide-specific phospholipase C-γ (PLC-γ) that hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to generate inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) which activates protein kinase C (PKC); and (3) phosphatidylinositol 3-kinase (PI3K) that leads to activation of Akt (Hubbard et al 1998; Shibuya 2003). Signaling pathways through (1) and (2) induce increased permeability, proliferation, and migration of EC, and that, through (3), controls survival signals (Datta et al 1999; Pearson et al 2001). Limited information is currently available on the signaling processes that occur in TEC because many of the studies were carried out using non-TEC EC such as human umbilical vein endothelial cells (HUVEC), other primary cultured EC, or immortalized EC. TEC might preferentially take advantage of special signaling molecules under tumor microenvironment in vivo to sustain the aberrant angiogenesis, and to escape from apoptosis and host immunity (Wang et al 2002; Bussolati et al 2003). In the studies using TEC isolated from human tumors, PI3K/Akt was shown to be upregulated in renal cell carcinomas (Bussolati et al 2003), and colon carcinoma-derived TEC under hypoxia condition showed resistance to the antiangiogenic effects of IFN-γ by upregulating B cell lymphoma/leukemia-2 (Bcl-2) (Wang et al 2002). Further studies using TEC are necessary to clarify the detailed mechanisms of signalings and transcriptional regulation.
Contribution of endothelial progenitor cells, hematopoietic stem cells, and cells of the monocytic lineage to neovascularization
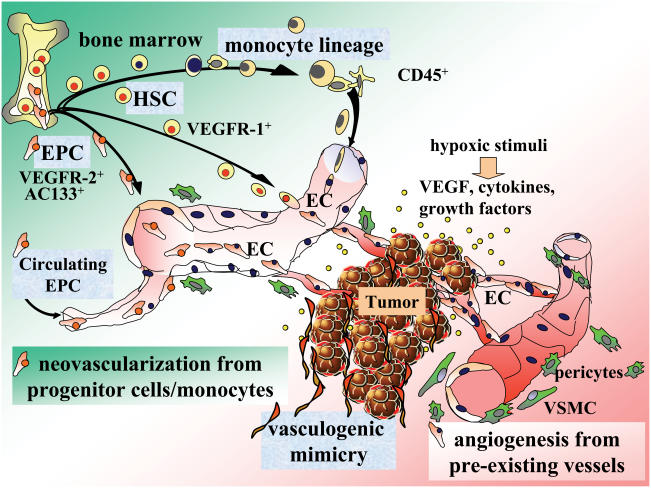
Recent studies have demonstrated the participation of endothelial progenitor cells (EPC), hematopoietic stem cells (HSC), and myeloid precursors in various pathological angiogenesis including tumor vasculature. EPC can be detected by the progenitor cell markers such as CD34 and AC133 (also named CD133) in addition to general EC markers (Ribatti 2004). Normally in adults, EPC are localized both in the bone marrow and in circulation (Asahara et al 1997; Patterson 2003). Although the number of circulating EPC is very small under normal conditions, they can be mobilized from the bone marrow and proliferate by hypoxic stimuli and VEGF upsurge (Aicher et al 2003; Rafii and Lyden 2003; Tepper et al 2005). Pathological neovascularization is now believed to occur not only from preexisting vasculature but also by the recruitment of circulating and bone marrow-mobilized EPC (Figure 2). Studies on the existence of EPC in the angiogenic vessels showed considerably varying results (Murayama et al 2002; Vajkoczy et al 2003). Contribution of EPC to tumor neovascularization may depend, at least in part, on aggressiveness of the tumor (Li et al 2004).
Figure 2.
Pathological neovascularization during tumor progression. Tumor tissue secretes various proinflammatory factors and vasoactive substances that sustain neovascularization. Tumor vasculature is supported not only by angiogenesis derived from preexisting vasculature, but also by vasculogenic mimicry and neovascularization from progenitor cells/monocytes. Endothelial progenitor cells (EPC) from the bone marrow and in circulation, hematopoietic stem cells (HSC), and further differentiated cells of the monocytic lineage such as macrophages and dendritic cells potentially contribute to pathological neovascularization; although the percentage of progenitor cells is generally low.
Abbreviations: EC, endothelial cells; EPC, endothelial progenitor cells; HSC, hematopoietic stem cells; VEGF, vascular endothelial growth factor; VEGFR-1+, fms-like tyrosine kinase-1; VEGFR-2+, fetal liver kinase-1/kinase insert domain protein receptor; VSMC, vascular smooth muscle cells.
In embryonic vasculogenesis, hemangioblasts are differentiated into EPC and HSC, and the latter is further subdivided into lymphoid and myeloid lineage (Figure 1). Detailed studies have revealed that HSC and myelomonocytic population are involved in adult vascular remodeling under pathological conditions such as myocardial infarction and liver regeneration (Wang et al 2003; Nygren et al 2004). Studies on tumor vasculature using Id-deficient mice revealed that bone marrow-derived VEGFR-1+ HSC, expressing both myeloid specific markers and EC markers, cooperated with VEGFR-2+ EPC and contributed to the development of tumor vasculature (Lyden et al 2001; Hattori et al 2002). It remains controversial as to whether HSC participate in angiogenesis and tissue regeneration by transdifferentiation or by cell fusion (Wagers et al 2002; Vassilopoulos et al 2003; Wang et al 2003). A very recent study, in patients who developed cancers after bone marrow transplantation from the opposite sex, supported the notion that HSC potentially produce EC, though transplanted HSC contributed to only 4.9% of the total TEC (Peters et al 2005). In the latter study, bone marrow-derived TEC were shown to have the diploid copy number of transplanted sex chromosome, indicating that cell fusion may not be the case (Peters et al 2005).
VEGFR-1+ HSC cells are known to possess myelomonocytic markers. Studies on peripheral blood monocytes revealed that further differentiated hematopoietic cells also contribute to neovascularization. Monocytes, macrophages, and dendritic cells (DC) in the peripheral blood that do not have a significant proliferative capacity (ie, the hallmark of progenitor cells) undergo EC-like specialization in certain pathological conditions (Rehman et al 2003; Zhao et al 2003; Conejo-Garcia et al 2004). Differentiation status of these myelomonocytic cells and their contribution to neovascularization may vary among diseases (Rehman et al 2003; Zhao et al 2003). In tumor vasculature in vivo, heterogeneity of EC may depend on both biological properties of tumor cells and host tissue environments (Conejo-Garcia et al 2004; Coukos et al 2005). Further investigation is required to clarify the contribution of these EC precursor cells in angiogenic diseases.
Tumor vasculogenic mimicry
Studies on the specific features of tumor progression have revealed another blood passage: fluid-conducting ECM meshwork lined by tumor cells instead of EC (Hendrix et al 2003). In several types of tumors, such as aggressive melanoma, ovarian, prostatic, and breast carcinomas, tumor cells themselves may sometimes display endothelial cell-like characteristics (Shirakawa et al 2001; Sood et al 2001; Hendrix et al 2002; Sharma et al 2002) known as “tumor vasculogenic mimicry” (Maniotis et al 1999). This alternative vascular network is composed of tumor cells that express some endothelial markers and embryonic vasculogenesis-related molecules such as VE-cadherin, CD34, and CD105 (Seftor et al 2002; Hendrix et al 2003). A similar process of vascular remodeling has been reported in physiological conditions. For example, during placenta formation, cytotrophoblasts establish blood pathways by invading uterine myometrium (Lyall et al 2001). In this process, some cytotrophoblasts were shown to undergo epithelial to EC transformation and replace EC lining of spiral arteries of maternal origin (Zhou et al 1997), and this has been referred to as “pseudo-vasculogenesis” (Damsky and Fisher 1998).
The detailed mechanism of tumor vasculogenic mimicry remains to be further elucidated, including responsible transcription factor(s) that alter tumor cell plasticity. At present it is considered that, under special microenvironments, tumor cells may dedifferentiate into a pluripotent, embryonic-like phenotype, or may transdifferentiate into EC-like phenotype during development (Hendrix et al 2003).
Tumor vasculogenic mimicry was reported to be resistant to angiogenesis inhibitors such as endostatin and TNP-470 in vitro (van der Schaft et al 2004). The biological properties of this special vasculature are also the subject of further studies; in particular, on whether and how this vascular network shows altered responsiveness or resistance to antiangiogenic drugs.
Pericytes and vascular smooth muscle cells
During embryogenesis, pericytes and VSMC are differentiated from smooth muscle progenitor cells, common vascular progenitor cells (VPC), and/or transdifferentiated from EC (Carmeliet 2000; Yamashita et al 2000; Jain 2003). These vascular mural cells are necessary for vessel stabilization and maturation, and they cooperate with EC for controlling vascular permeability, tone, and remodeling (Jain 2003). Representative markers for pericytes and VSMC include α-smooth muscle actin, desmin, and PDGFR-β (Morikawa et al 2002). These mural cells protect EC from apoptosis caused by VEGF-withdrawal and antivascular immune responses (Benjamin et al 1999; Reinmuth et al 2001; Gee et al 2003). Both PDGF-B−/− and PDGFR-β−/− mice die perinatally and show fragile vasculature without coverage by pericytes, indicating that PDGF is indispensable for vessel maturation (Soriano 1994; Levéen et al 1994).
The role of VSMC and pericytes in tumor vasculature remains less understood than those of TEC (Abramsson et al 2003; Gerhardt and Betsholtz 2003). In tumors, it is often seen that only a small fraction of vessels are covered by pericytes (Gee et al 2003), and that the pericytes are frequently altered in morphology and are loosely attached to TEC (Morikawa et al 2002). In rodent models, antivascular therapy resulted in selective loss of pericyte-negative vessels, indicating that proliferative vessels that are not covered by pericytes may be especially susceptible to angiogenic inhibitors (Gee et al 2003). On the other hand, there are some tumors in which vessels are predominantly covered with pericyte. Under such conditions, cross-talk between pericytes and EC may work in favor of tumor progression. Indeed, perturbation of PDGF signaling by a kinase inhibitor selective for PDGFR successfully blocked growth of insulinoma in RIP1Tag mice by impairing pericyte function (Bergers et al 2003).
Each part of the vessels in a tumor is in a different angiogenic status, with various degrees of contribution of pericytes and VSMC. This heterogeneity may depend largely on its developmental stage or its degree of maturation. The tumor vessels that are covered by abundant vascular mural cells may function as stable trunks of blood supply in tumor progression. If these stabilized vessels are more resistant to antiangiogenic approaches than those without pericytes, these mural cells would be particularly important as targets for antiangiogenic tumor therapies.
Regulator of G protein signaling 5 and vascular remodeling
Studies using either PDGF-B or PDGFR-β deficient mice embryo indicate that a regulator of G protein signaling 5 (RGS5) may be a useful marker for pericytes and VSMC (Bondjers et al 2003; Cho et al 2003). RGS5, a member of the RGS protein superfamily, serves as GTPase-activating proteins for Ga subunits of heterotrimeric G proteins and negatively regulates G protein-coupled receptor (GPCR) signaling. Expressions of RGS5 have been demonstrated in vascular mural cells; eg, brain pericytes in mouse embryos, smooth muscle cells in the medial wall of macaque aorta, and tumor angiogenic pericytes in RIP1Tag mice (Adams et al 2000; Bondjers et al 2003; Cho et al 2003; Berger et al 2005), as well as in visceral smooth muscle cells in the gut and bronchus. On the other hand, RGS5 is not expressed in cultured smooth muscle cells. It is detectable in HUVEC; although its expression in HUVEC is repressed during capillary morphogenesis (Bell et al 2001). The expression of RGS5 has also been demonstrated in EC of cerebral capillaries in stroke-prone spontaneously hypertensive rats and in TEC in human renal cell carcinomas (Kirsch et al 2001; Furuya et al 2004). Accumulating data indicate that RGS5 seems to be involved in the dynamics of pathological angiogenesis as well as play a regular role in the physiological cardiovascular system (Seki et al 1998; Cho et al 2003; Wieland and Mittmann 2003). Pathophysiological roles of RGS5 in angiogenesis should be further investigated, especially on the nature of GPCR signalings responsible for angiogenesis in each disease.
G protein-coupled receptor-mediated signalings in tumor vasculature
Several GPCR ligands are crucially involved in embryonic vascular development and/or tumor angiogenesis, eg, angiotensin II, endothelin-1, endocrine-gland-derived vascular endothelial growth factor (EG-VEGF), and sphingosine-1-phosphate (LeCouter et al 2001; Zhou et al 2001; Egami et al 2003; Chae et al 2004). In addition, several chemokines, whose signalings are also mediated by GPCRs, are known to play important roles in angiogenesis (Arya et al 2003; Distler et al 2003). The involvement of these GPCR ligands have been shown in various angiogenic disorders and tumor vasculature (Hla 2004; Romagnani et al 2004). It should be noted that signalings of GPCRs and RTKs may cross-communicate; eg, PDGF-B-triggered signaling depends in part on EDG-1 receptor signaling (Hobson et al 2001; Rosenfeldt et al 2001), and various GPCRs transactivate epidermal growth factor receptor (EGFR) (Prenzel et al 1999). Some intracellular regulators as RGS5 might alter not only GPCR-mediated but also RTK-mediated signalings in pathological angiogenesis in vivo (Kehrl 1998).
Chemokines in tumor angiogenesis
Some tumors have a significant background of chronic inflammation, eg, papilloma virus infection and chronic hepatitis (Villa 1997; Rehermann and Nascimbeni 2005). Even if a tumor is developed de novo without any inflammatory background, a profound leukocyte infiltration is frequently observed (Bukowski 1999; Coussens and Werb 2002). Furthermore, many tumors produce an array of cytokines and chemokines. Although chemokines were initially defined as factors that control leukocyte chemotaxis and migration at the sites of inflammation, involvement of chemokines have been demonstrated in various diseases other than inflammation, and it is now widely accepted that many types of cells, including tumor cells, potentially produce chemokines and/or express chemokine receptors (Coussens and Werb 2002; Vicari and Caux 2002). Chemokines are subgrouped into CC, CXC, C, and CX3C depending on the spacing or the presence of four N-terminal cysteine residues (Janeway et al 2001). CXC chemokines are known to possess pro- or antiangiogenic activities (Strieter et al 2004).
CXC chemokines with glutamic acid-leucine-arginine (Glu-Leu-Arg [ELR]) motif (ELR+), such as interleukin-8 (IL-8), neutrophil-activating protein-2 (NAP-2), granulocyte chemotactic protein-2 (GCP-2), epithelial-derived neutrophil-activating protein-78 (ENA-78), growth-related protein (GRO)-α, -β, and -γ, induce EC migration and proliferation as potent angiogenic factors (Strieter et al 1995; Arenberg et al 1998; Vicari and Caux 2002). The receptors for ELR+ chemokines are CXCR1 and CXCR2. Limited members of chemokines such as IL-8 and GCP-2 bind to CXCR1, whereas all ELR+ chemokines bind to CXCR2. Both receptors are inducible on EC surface, and CXCR2 is regarded as a primary functional receptor for ELR+ chemokines (Frederick and Clayman 2001).
IFN-γ-inducible CXC chemokines without ELR motif (ELR−), such as IFN-inducible T cell α chemoattractant (ITAC), monokine induced by IFN-γ (Mig) and IFN-γ-inducible protein-10 (IP-10), are considered to be antiangiogenic factors (Frederick and Clayman 2001). These chemokines share a common receptor, CXCR3, and inhibit endothelial migration and proliferation (Strieter et al 1995; Romagnani et al 2001). Another ELR− chemokine, platelet factor-4 (PF-4), interferes with EC proliferation and interacts with CXCR3-B, a variant of CXCR3 (Lasagni et al 2003). On the other hand, stromal cell derived factor-1 (SDF-1), one of ELR− CXC chemokines, binds to CXCR4 and positively induces angiogenesis (Arya et al 2003). CXCR4 is expressed abundantly in cultured EC lines (Salcedo et al 2000), and studies on CXCR4−/− mice indicate that SDF-1/CXCR4 signaling pathway is essential for embryonic blood vessel formation (Tachibana et al 1998).
Another role of CXC chemokines that lack Glu-Leu-Arg motif in tumor progression
Although most of ELR− CXC chemokines are considered to be antiangiogenic factors, some studies have revealed that the role of ELR− chemokines are not limited to antiangiogenesis, supported by the fact that CXCR3 is expressed in a wide variety of cells including tumor cells (Romagnani et al 1999; Robledo et al 2001; Goldberg-Bittman et al 2004). It has been demonstrated in in vitro studies that ELR− chemokines could induce migration of CXCR3+ tumor cells such as melanomas and lung carcinomas (Robledo et al 2001; Soejima and Rollins 2001). Furthermore, some CXC chemokine receptors in tumor cells, eg, CXCR4 in breast carcinoma cells, are suggested to play key roles in metastasis of the tumor to particular organs/tissues such as regional lymph nodes, bone marrow, and lung (Mueller et al 2001; Helbig et al 2003). Recent studies showed that CXCR3+ melanoma cells preferentially metastasize to lymph nodes that highly express Mig and IP-10 (Kawada et al 2004). On the other hand, in studies on CXCR3+ neuroblastomas, administration of ELR− chemokines did not resulted in increased cell migration or proliferation (Goldberg-Bittman et al 2005). These results indicate that interaction of ELR− chemokines with CXCR3 may potentially work for tumor progression in some, if not all, CXCR3+ tumors. It should be further investigated as to whether immune therapies such as IL-12 and IFN-γ administration, originally expected to elicit antiangiogenic effects of ELR− chemokines (Bukowski et al 1999; Portielje et al 2003), can be safely applied to CXCR3+ tumors or actually lead to tumor progression.
Conclusions and future prospects
The results of preclinical studies using antiangiogenic agents have warranted the importance of vascular targeting for better outcomes in human cancer therapies, although the results vary among the cases (Sivakumar et al 2004). Prospective antiangiogenic therapies, combined with conventional chemotherapy and other strategies including immune- and gene-therapies, may provide successful approaches for treatment of malignant tumors. Even if complete eradication cannot be achieved, such combined therapies may well keep tumors in a state of dormancy. Further investigation on pathophysiology of neovascularization in the entire course of tumor progression will help us to improve antiangiogenic approaches against various types and stages of human malignancies.
Besides tumor vasculature, angiogenesis plays crucial roles in many other pathological conditions, eg, rheumatoid arthritis, diabetic retinopathy, and myocardial infarction. Characteristic features of the vasculature in each condition may be explained at least in part by the disease-specific background, such as chronic inflammation, disrupted matrix remodeling, malfunctioned immunity, and tissue hypoxia. It is necessary to control both angiogenic factors and tissue environments that support vessel remodeling for better management of these pathological conditions. If the mechanism of pathophysiological neovascularization in various diseases, including tumors, is fully elucidated, it will certainly provide more precise disease- and tissue-specific strategies, and contribute to more effective and safer therapies in future.
Abbreviations
- Ang
angiopoietins
- EC
endothelial cells
- ECM
extracellular matrix
- ELR
glutamic acid-leucine-arginine
- EPC
endothelial progenitor cells
- FGF
fibroblast growth factors
- FGFR
fibroblast growth factor receptor
- GPCR
G protein-coupled receptor
- HGF
hepatocyte growth factor
- HSC
hematopoietic stem cells
- HUVEC
human umbilical vein endothelial cells
- IFN-γ
Interferon-γ
- IP-10
IFN-γ-inducible protein-10
- I-TAC
IFN-inducible T cell α chemoattractant
- MAPK
mitogen-activated protein kinase
- Mig
monokine induced by IFN-γ
- MMPs
matrix metalloproteinases
- NRP
neuropilin
- PDGF-B
platelet-derived growth factor-B
- PDGFR
platelet-derived growth factor receptor
- PI3K
phosphatidylinositol 3-kinase
- PlGF
placenta growth factor
- RGS5
regulator of G protein signaling 5
- RTKs
receptor tyrosine kinases
- SDF-1
stromal cell-derived factor-1
- TEC
tumor-derived endothelial cells
- TGF-β
transforming growth factor-β
- Tie-2
tyrosine kinase with Ig and EGF homology domain-2
- TIMPs
tissue inhibitors of MMPs
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- VPC
vascular progenitor cells
- VSMC
vascular smooth muscle cells
References
- Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–51. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams LD, Geary RL, McManus B, et al. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res. 2000;87:623–31. doi: 10.1161/01.res.87.7.623. [DOI] [PubMed] [Google Scholar]
- Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–9. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- Alavi A, Hood JD, Frausto R, et al. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–6. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- Arap W, Haedicke W, Bernasconi M, et al. Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci U S A. 2002;99:1527–31. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenberg DA, Keane MP, DiGiovine B, et al. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest. 1998;102:465–72. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya M, Patel HR, Williamson M. Chemokines: key players in cancer. Curr Med Res Opin. 2003;19:557–64. doi: 10.1185/030079903125002216. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Aurrand-Lions M, Johnson-Leger C, Wong C, et al. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- Badorff C, Brandes RP, Popp R, et al. Transdifferentiation of bloodderived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–32. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- Bell SE, Mavila A, Salazar R, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–73. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, et al. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–65. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Bergers G, Arnold B, et al. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105:1094–101. doi: 10.1182/blood-2004-06-2315. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikfalvi A, Klein S, Pintucci G, et al. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5:13–17. doi: 10.1016/s1535-6108(03)00336-2. [DOI] [PubMed] [Google Scholar]
- Boehm T, Folkman J, Browder T, et al. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–7. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- Bondjers C, Kalen M, Hellstrom M, et al. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162:721–9. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Calcium modulation of adherens and tight junction function: a potential mechanism for blood-brain barrier disruption after stroke. Stroke. 2002;33:1706–11. doi: 10.1161/01.str.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- Bukowski RM, Rayman P, Molto L, et al. Interferon-gamma and CXC chemokine induction by interleukin 12 in renal cell carcinoma. Clin Cancer Res. 1999;5:2780–9. [PubMed] [Google Scholar]
- Bussolati B, Deambrosis I, Russo S, et al. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003;17:1159–61. doi: 10.1096/fj.02-0557fje. [DOI] [PubMed] [Google Scholar]
- Camenisch G, Pisabarro MT, Sherman D, et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277:17281–90. doi: 10.1074/jbc.M109768200. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Collen D. Role of vascular endothelial growth factor and vascular endothelial growth factor receptors in vascular development. Curr Top Microbiol Immunol. 1999;237:133–58. doi: 10.1007/978-3-642-59953-8_7. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carson-Walter EB, Watkins DN, Nanda A, et al. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–55. [PubMed] [Google Scholar]
- Chae SS, Paik JH, Furneaux H, et al. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114:1082–9. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Kozasa T, Bondjers C, et al. Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J. 2003;17:440–2. doi: 10.1096/fj.02-0340fje. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–8. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Benencia F, Courreges MC, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–8. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- Cotran RS, Kumar V, Collins T. Acute and chronic inflammation. In: Cotran RS, editor. Pathologic basis of disease. 6th ed. Philadelphia: WB Saunders; 1999. pp. 50–88. [Google Scholar]
- Coukos G, Benencia F, Buckanovich RJ, et al. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182–7. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallabrida SM, De Sousa MA, Farrell DH. Expression of antisense to integrin subunit beta 3 inhibits microvascular endothelial cell capillary tube formation in fibrin. J Biol Chem. 2000;275:32281–8. doi: 10.1074/jbc.M001446200. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Fisher SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol. 1998;10:660–6. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Deng CX, Wynshaw-Boris A, Shen MM, et al. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–57. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- Deregibus MC, Buttiglieri S, Russo S, et al. CD40-dependent activation of phosphatidylinositol 3-kinase/Akt pathway mediates endothelial cell survival and in vitro angiogenesis. J Biol Chem. 2003;278:18008–14. doi: 10.1074/jbc.M300711200. [DOI] [PubMed] [Google Scholar]
- Distler JH, Hirth A, Kurowska-Stolarska M, et al. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47:149–61. [PubMed] [Google Scholar]
- Duff SE, Li C, Garland JM, et al. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984–92. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Nagy JA, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- Egami K, Murohara T, Shimada T, et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest. 2003;112:67–75. doi: 10.1172/JCI16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh DA. Adhesion events in angiogenesis. Curr Opin Cell Biol. 2001;13:563–8. doi: 10.1016/s0955-0674(00)00252-0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Chen H, Davis-Smyth T, et al. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med. 1998;4:336–40. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, et al. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Fong GH, Zhang L, Bryce DM, et al. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–25. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- Fonsatti E, Maio M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J Transl Med. 2004;2:18. doi: 10.1186/1479-5876-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick MJ, Clayman GL. Chemokines in cancer. Expert Rev Mol Med. 2001;2001:1–18. doi: 10.1017/S1462399401003301. [DOI] [PubMed] [Google Scholar]
- Furuya M, Nishiyama M, Kimura S, et al. Expression of regulator of G protein signalling protein 5 (RGS5) in the tumour vasculature of human renal cell carcinoma. J Pathol. 2004;203:551–8. doi: 10.1002/path.1543. [DOI] [PubMed] [Google Scholar]
- Gee MS, Procopio WN, Makonnen S, et al. Tumor vessel development and maturation impose limits on the effectiveness of antivascular therapy. Am J Pathol. 2003;162:183–93. doi: 10.1016/S0002-9440(10)63809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, et al. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999;19:1589–94. doi: 10.1161/01.atv.19.7.1589. [DOI] [PubMed] [Google Scholar]
- Goldberg-Bittman L, Neumark E, Sagi-Assif O, et al. The expression of the chemokine receptor CXCR3 and its ligand, CXCL10, in human breast adenocarcinoma cell lines. Immunol Lett. 2004;92:171–8. doi: 10.1016/j.imlet.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Goldberg-Bittman L, Sagi-Assif O, Meshel T, et al. Cellular characteristics of neuroblastoma cells: regulation by the ELR—CXC chemokine CXCL10 and expression of a CXCR3-like receptor. Cytokine. 2005;29:105–17. doi: 10.1016/j.cyto.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Gougos A, St Jacques S, Greaves A, et al. Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. Int Immunol. 1992;4:83–92. doi: 10.1093/intimm/4.1.83. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hattori K, Heissig B, Wu Y, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–9. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, et al. NFkappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–8. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–53. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor RE, Seftor EA, et al. Transendothelial function of human metastatic melanoma cells: role of the microenvironment in cell-fate determination. Cancer Res. 2002;62:665–8. [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, et al. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–21. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Hida K, Hida Y, Amin DN, et al. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–55. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- Hirase T, Staddon JM, Saitou M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110:1603–13. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Minowa O, Kuno J, et al. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–54. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Nakamura K, Iwai S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–20. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Hobson JP, Rosenfeldt HM, Barak LS, et al. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–3. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, Reynolds AR, Reynolds LE. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res. 2003;314:131–44. doi: 10.1007/s00441-003-0774-5. [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Mohammadi M, Schlessinger J. Autoregulatory mechanisms in protein-tyrosine kinases. J Biol Chem. 1998;273:11987–90. doi: 10.1074/jbc.273.20.11987. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, et al. Immunobiology. 5th ed. New York: Garland Publishing; 2001. [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–5. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Zhu D, Augustin-Voss HG, et al. Lung endothelial dipeptidyl peptidase IV is an adhesion molecule for lung-metastatic rat breast and prostate carcinoma cells. J Cell Biol. 1993;121:1423–32. doi: 10.1083/jcb.121.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–8. [PMC free article] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Kawada K, Sonoshita M, Sakashita H, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–17. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- Kearney JB, Ambler CA, Monaco KA, et al. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99:2397–407. doi: 10.1182/blood.v99.7.2397. [DOI] [PubMed] [Google Scholar]
- Kearney JB, Kappas NC, Ellerstrom C, et al. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–35. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- Kehrl JH. Heterotrimeric G protein signaling: roles in immune function and fine-tuning by RGS proteins. Immunity. 1998;8:1–10. doi: 10.1016/s1074-7613(00)80453-7. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–9. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T, Wellner M, Luft F.C, et al. Altered gene expression in cerebral capillaries of stroke-prone spontaneously hypertensive rats. Brain Res. 2001;9:106–15. doi: 10.1016/s0006-8993(01)02670-1. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Shields P, Grant A, et al. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80:52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- Lasagni L, Francalanci M, Annunziato F, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–49. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouter J, Kowalski J, Foster J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–84. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- Levéen P, Pekny M, Gebre-Medhin S, et al. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–87. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Li C, Hu Y, Mayr M, et al. Cyclic strain stress-induced mitogenactivated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J Biol Chem. 1999;274:25273–80. doi: 10.1074/jbc.274.36.25273. [DOI] [PubMed] [Google Scholar]
- Li C, Xu Q. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal. 2000;12:435–45. doi: 10.1016/s0898-6568(00)00096-6. [DOI] [PubMed] [Google Scholar]
- Li H, Gerald WL, Benezra R. Utilization of bone marrow-derived endothelial cell precursors in spontaneous prostate tumors varies with tumor grade. Cancer Res. 2004;64:6137–43. doi: 10.1158/0008-5472.CAN-04-1287. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Hellstrom M, Kalen M, et al. Paracrine PDGF-B/PDGFRbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–22. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- Lyall F, Bulmer JN, Duffie E, et al. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol. 2001;158:1713–21. doi: 10.1016/S0002-9440(10)64127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bonemarrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Sudhakar A, Lively JC, et al. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science. 2002;295:140–3. doi: 10.1126/science.1065298. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–25. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- Mitchell RN, Contran RS. Hemodynamic disorders, thrombosis and shock. In: Cotran RS, editor. Pathologic basis of disease. 6th ed. Philadelphia: WB Saunders; 1999. pp. 113–38. [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, et al. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, et al. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–94. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Murayama T, Tepper OM, Silver M, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30:967–72. doi: 10.1016/s0301-472x(02)00867-6. [DOI] [PubMed] [Google Scholar]
- Mutuberria R, Satijn S, Huijbers A, et al. Isolation of human antibodies to tumor-associated endothelial cell markers by in vitro human endothelial cell selection with phage display libraries. J Immunol Methods. 2004;287:31–47. doi: 10.1016/j.jim.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Ng YS, Rohan R, Sunday ME, et al. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220:112–21. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- Oh P, Li Y, Yu J, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–35. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- Olofsson B, Korpelainen E, Pepper MS, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:11709–14. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru M, Yonemitsu Y, Tanii M, et al. Fibroblast growth factor-2 gene transfer can stimulate hepatocyte growth factor expression irrespective of hypoxia-mediated downregulation in ischemic limbs. Circ Res. 2002;91:923–30. doi: 10.1161/01.res.0000043281.66969.32. [DOI] [PubMed] [Google Scholar]
- Paranya G, Vineberg S, Dvorin E, et al. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and nontransforming growth factor-beta-mediated transdifferentiation in vitro. Am J Pathol. 2001;159:1335–43. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C. The Ponzo effect: endothelial progenitor cells appear on the horizon. Circulation. 2003;107:2995–7. doi: 10.1161/01.CIR.0000074241.91121.70. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Peters BA, Diaz LA, Polyak K, et al. Contribution of bone marrowderived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–2. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- Pintus G, Tadolini B, Posadino AM, et al. Inhibition of the MEK/ERK signaling pathway by the novel antimetastatic agent NAMI-A down regulates c-myc gene expression and endothelial cell proliferation. Eur J Biochem. 2002;269:5861–70. doi: 10.1046/j.1432-1033.2002.03307.x. [DOI] [PubMed] [Google Scholar]
- Podar K, Anderson KC. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood. 2005;105:1383–95. doi: 10.1182/blood-2004-07-2909. [DOI] [PubMed] [Google Scholar]
- Portielje JE, Lamers CH, Kruit WH, et al. Repeated administrations of interleukin (IL)-12 are associated with persistently elevated plasma levels of IL-10 and declining IFN-gamma, tumor necrosis factor-alpha, IL-6, and IL-8 responses. Clin Cancer Res. 2003;9:76–83. [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- Rehman J, Li J, Orschell CM, et al. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Jung YD, et al. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15:1239–41. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Reynolds LE, Nagel TE, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64:8643–50. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Presta M. The role of fibroblast growth factor-2 in the vascularization of the chick embryo chorioallantoic membrane. J Cell Mol Med. 2002;6:439–46. doi: 10.1111/j.1582-4934.2002.tb00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D. The involvement of endothelial progenitor cells in tumor angiogenesis. J Cell Mol Med. 2004;8:294–300. doi: 10.1111/j.1582-4934.2004.tb00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;6:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Robledo MM, Bartolome RA, Longo N, et al. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Beltrame C, Annunziato F, et al. Role for interactions between IP-10/Mig and CXCR3 in proliferative glomerulonephritis. J Am Soc Nephrol. 1999;10:2518–26. doi: 10.1681/ASN.V10122518. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Annunziato F, Lasagni L, et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107:53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani P, Lasagni L, Annunziato F, et al. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–9. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt HM, Hobson JP, Maceyka M, et al. EDG-1 links the PDGF receptor to Src and focal adhesion kinase activation leading to lamellipodia formation and cell migration. FASEB J. 2001;15:2649–59. doi: 10.1096/fj.01-0523com. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C. Growing and shaping the vascular tree: multiple roles for VEGF. Bioessays. 2003;25:1052–60. doi: 10.1002/bies.10351. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Rajotte D. An address system in the vasculature of normal tissues and tumors. Annu Rev Immunol. 2000;18:813–27. doi: 10.1146/annurev.immunol.18.1.813. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Resau JH, Halverson D, et al. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 2000;14:2055–64. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Sato Y. Molecular mechanism of angiogenesis transcription factors and their therapeutic relevance. Pharmacol Ther. 2000;87:51–60. doi: 10.1016/s0163-7258(00)00067-x. [DOI] [PubMed] [Google Scholar]
- Sawano A, Takahashi T, Yamaguchi S, et al. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996;7:213–21. [PubMed] [Google Scholar]
- Seftor EA, Meltzer PS, Schatteman GC, et al. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol. 2002;44:17–27. doi: 10.1016/s1040-8428(01)00199-8. [DOI] [PubMed] [Google Scholar]
- Seki N, Sugano S, Suzuki Y, et al. Isolation, tissue expression, and chromosomal assignment of human RGS5, a novel G-protein signaling regulator gene. J Hum Genet. 1998;43:202–5. doi: 10.1007/s100380050071. [DOI] [PubMed] [Google Scholar]
- Schoen FJ, Cotran RS. Blood Vessels. In: Cotran RS, editor. Pathologic basis of disease. 6th ed. Philadelphia: WB Saunders; 1999. pp. 493–541. [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sharma N, Seftor RE, Seftor EA, et al. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002;50:189–201. doi: 10.1002/pros.10048. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Colony-stimulating factor-1 receptor. Blood. 1990;75:1–12. [PubMed] [Google Scholar]
- Shibuya M. Structure and dual function of vascular endothelial growth factor receptor-1 (Flt-1) Int J Biochem Cell Biol. 2001;33:409–20. doi: 10.1016/s1357-2725(01)00026-7. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Vascular endothelial growth factor receptor-2: its unique signaling and specific ligand, VEGF-E. Cancer Sci. 2003;94:751–6. doi: 10.1111/j.1349-7006.2003.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih SC, Robinson GS, Perruzzi CA, et al. Molecular profiling of angiogenesis markers. Am J Pathol. 2002;161:35–41. doi: 10.1016/S0002-9440(10)64154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa K, Tsuda H, Heike Y, et al. Absence of endothelial cells, central necrosis, and fibrosis are associated with aggressive inflammatory breast cancer. Cancer Res. 2001;61:445–51. [PubMed] [Google Scholar]
- Shu X, Wu W, Mosteller RD, et al. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–68. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Neufeld G, et al. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest. 1993;91:2235–43. doi: 10.1172/JCI116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincock PM, Fitter S, Parton RG, et al. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J Cell Sci. 1999;112:833–44. doi: 10.1242/jcs.112.6.833. [DOI] [PubMed] [Google Scholar]
- Sivakumar B, Harry LE, Paleolog EM. Modulating angiogenesis: more vs less. JAMA. 2004;292:972–7. doi: 10.1001/jama.292.8.972. [DOI] [PubMed] [Google Scholar]
- Soejima K, Rollins BJ. A functional IFN-gamma-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J Immunol. 2001;167:6576–82. doi: 10.4049/jimmunol.167.11.6576. [DOI] [PubMed] [Google Scholar]
- Sood AK, Seftor EA, Fletcher MS, et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158:1279–88. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–96. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–36. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Belperio JA, Phillips RJ, et al. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Sullivan DC, Huminiecki L, Moore JW, et al. EndoPDI, a novel protein-disulfide isomerase-like protein that is preferentially expressed in endothelial cells acts as a stress survival factor. J Biol Chem. 2003;278:47079–88. doi: 10.1074/jbc.M308124200. [DOI] [PubMed] [Google Scholar]
- Sumpio BE, Riley JT, Dardik A. Cells in focus: endothelial cell. Int J Biochem Cell Biol. 2002;34:1508–12. doi: 10.1016/s1357-2725(02)00075-4. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Mori M, Sakamoto Y, et al. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J Clin Invest. 1999;103:341–5. doi: 10.1172/JCI4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanghetti E, Ria R, Dell'Era P, et al. Biological activity of substratebound basic fibroblast growth factor (FGF2): recruitment of FGF receptor-1 in endothelial cell adhesion contacts. Oncogene. 2002;21:3889–97. doi: 10.1038/sj.onc.1205407. [DOI] [PubMed] [Google Scholar]
- Tepper OM, Capla JM, Galiano RD, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068–77. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Blum S, Lamparter M, et al. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med. 2003;197:1755–65. doi: 10.1084/jem.20021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaft DW, Seftor RE, Seftor EA, et al. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst. 2004;96:1473–7. doi: 10.1093/jnci/djh267. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–4. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13:143–54. doi: 10.1016/s1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]
- Villa LL. Human papillomaviruses and cervical cancer. In: Vande Woude GF, Klein G, editors. Advances in Cancer Research. Vol 71. San Diego Academic Press; 1997. pp. 321–43. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, et al. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Wang JH, Wu QD, Bouchier-Hayes D, et al. Hypoxia upregulates Bcl-2 expression and suppresses interferon-gamma induced antiangiogenic activity in human tumor derived endothelial cells. Cancer. 2002;94:2745–55. doi: 10.1002/cncr.10520. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Wieland T, Mittmann C. Regulators of G-protein signalling: multifunctional proteins with impact on signalling in the cardiovascular system. Pharmacol Ther. 2003;97:95–115. doi: 10.1016/s0163-7258(02)00326-1. [DOI] [PubMed] [Google Scholar]
- Xu X, Weinstein M, Li C, et al. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125:753–65. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Kliche S, Mayr-Beyrle U, et al. p38 MAPK inhibition is critically involved in VEGFR-2-mediated endothelial cell survival. Biochem Biophys Res Commun. 2003;306:730–6. doi: 10.1016/s0006-291x(03)01064-7. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Glesne D, Huberman E. A human peripheral blood monocytederived subset acts as pluripotent stem cells. Proc Natl Acad Sci U S A. 2003;100:2426–31. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Moroi K, Nishiyama M, et al. Characterization of RGS5 in regulation of G protein-coupled receptor signaling. Life Sci. 2001;68:1457–69. doi: 10.1016/s0024-3205(01)00939-0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–51. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DZ, Cheng CF, Pauli BU. Mediation of lung metastasis of murine melanomas by a lung-specific endothelial cell adhesion molecule. Proc Natl Acad Sci U S A. 1991;88:9568–72. doi: 10.1073/pnas.88.21.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]