Abstract
Interventional studies have demonstrated the impact of hyperglycemia on the development of vascular complications associated with type 2 diabetes, which underscores the importance of safely lowering glucose to as near-normal as possible. Among the current challenges to reducing the risk of vascular disease associated with diabetes is the management of body weight in a predominantly overweight patient population, and in which weight gain is likely with many current therapies. Exenatide is the first in a new class of agents termed incretin mimetics, which replicate several glucoregulatory effects of the endogenous incretin hormone, glucagon-like peptide-1 (GLP-1). Currently approved in the US as an injectable adjunct to metformin and/or sulfonylurea therapy, exenatide improves glycemic control through multiple mechanisms of action including: glucose-dependent enhancement of insulin secretion that potentially reduces the risk of hypoglycemia compared with insulin secretagogues; restoration of first-phase insulin secretion typically deficient in patients with type 2 diabetes; suppression of inappropriately elevated glucagon secretion to reduce postprandial hepatic output; and slowing the rate of gastric emptying to regulate glucose appearance into the circulation. Clinical trials in patients with type 2 diabetes treated with subcutaneous exenatide twice daily demonstrated sustained improvements in glycemic control, evidenced by reductions in postprandial and fasting glycemia and glycosylated hemoglobin (HbA1c) levels. Notably, improvements in glycemic control with exenatide were coupled with progressive reductions in body weight, which represents a distinct therapeutic benefit for patients with type 2 diabetes. Acute effects of exenatide on beta-cell responsiveness along with significant reductions in body weight in patients with type 2 diabetes may have a positive impact on disease progression and potentially decrease the risk of associated long-term complications.
Keywords: exenatide, type 2 diabetes, incretin, incretin mimetic
Type 2 diabetes and vascular disease
Vascular complications represent the leading cause of mortality and morbidity in patients with diabetes (Morrish et al 2001). Approximately 65% of deaths among patients with diabetes are due to cardiovascular and cerebrovascular diseases, including myocardial ischemia and stroke (Haffner et al 1998; Klein 1999; Erdmann 2005). Adverse outcomes are also associated with microvascular diseases such as nephropathy, neuropathy, and retinopathy, which contribute significantly to the development of end-stage renal disease, foot ulcers, and blindness (Klein 1999; Singleton et al 2003). Type 2 diabetes is the clinical manifestation of long-term metabolic abnormalities involving multiple organs and hormonal pathways that impair the body's ability to maintain glucose homeostasis (Aronoff et al 2004). Pancreatic beta-cell dysfunction and insulin resistance are widely recognized as the cardinal defects that lead to chronic hyperglycemia, which is a major contributor of diabetic vascular complications. At the cellular level, elevated glucose concentrations cause increased production of reactive oxidant species (ROS) that ultimately contribute to the deterioration of vascular health through multiple pathways (Brownlee 2005). Effects of hyperglycemia-induced damage include endothelial dysfunction, inflammation, and the promotion of atherogenic conditions (Ceriello 2001; Ahmad et al 2005).
In parallel with impaired glucose homeostasis, patients with type 2 diabetes often have an associated constellation of cardiovascular disease (CVD) risk factors including hypertension, abdominal obesity, atherogenic dyslipidemia (characterized by hypertriglyceridemia and low levels of high-density lipoprotein cholesterol [HDL-C]), chronic inflammation, and alterations in thrombolytic parameters (Kannel and McGee 1979; Reaven 1988; Beckman et al 2002; Erdmann 2005). It is not surprising that type 2 diabetes has been implicated as a coronary heart disease (CHD) “risk equivalent”, carrying an absolute risk for coronary heart events similar to that for nondiabetic individuals with established CHD (NCEP 2002).
Obesity is strongly associated with type 2 diabetes and is present in approximately 80%–95% of type 2 diabetes cases (Astrup 2001). Obesity also exacerbates the metabolic abnormalities of type 2 diabetes, particularly hyperglycemia, hyperlipidemia, and hypertension (Kannel and McGee 1979; Maggio and Pi-Sunyer 1997). Additionally, the risk of developing type 2 diabetes increases exponentially in relation to body mass index (BMI) (Mokdad et al 2000). This is particularly evident in children and adolescents, in whom obesity is on the rise and the incidence of type 2 diabetes is nearing that of type 1 (Kaufmann 2002). Management of weight gain in patients with type 2 diabetes, particularly in those already overweight, is important to improving glycemic control and reducing CV risk (Anderson et al 2003).
Results from the United Kingdom Prospective Diabetes Study (UKPDS) of intensively treated patients with type 2 diabetes demonstrated the beneficial impact of glycemic control for reducing microvascular complications, and implicated a role for hyperglycemia in macrovascular disease (UKPDS 1998). Notably, these data indicated that any improvement in glycemic control has a beneficial impact in reducing diabetes-related complications (Stratton et al 2000). Near-normalization of glycemia is possible with current diabetes therapies; however, patients continue to experience excessive diurnal glucose fluctuations (Monnier et al 2002; Hirsch 2005). In addition, a majority of therapies are typically accompanied by undesired weight gain and an increased risk of hypoglycemia, particularly when treatment includes insulin and several oral antihyperglycemic agents (DeFronzo 1999; Purnell and Weyer 2003). Therefore, pharmacological agents that provide glycemic control with a reduced risk of severe hypoglycemia and reductions in body weight would present a distinct treatment option compared with currently available therapies for patients with type 2 diabetes.
Exenatide, an incretin mimetic
Glucagon-like peptide-1 (GLP-1) is an insulinotropic gut hormone, or incretin, that is partly responsible for the more robust insulin secretory response elicited by enteral glucose administration compared with that measured after isoglycemic intravenous glucose infusion (Perley and Kipnis 1967; Creutzfeldt 1979; Kreymann et al 1987). Partly due to reduced postprandial secretion of GLP-1, patients with type 2 diabetes exhibit an impaired incretin response compared with individuals with normal glucose tolerance (Nauck et al 1986; Toft-Nielsen et al 2001). Exogenous administration of GLP-1 has been shown to elicit glucoregulatory effects in patients with type 2 diabetes (Nauck et al 1993; Zander et al 2002). More recently, preclinical and clinical studies have shown that GLP-1 also has salutary cardiovascular effects (Nystrom, Gonon, et al 2004; Bose et al 2005). In particular, clinical studies have demonstrated that GLP-1 ameliorates endothelial dysfunction in patients with type 2 diabetes with established CHD, and can improve left ventricular heart function in patients with acute myocardial infarction (Nikolaidis et al 2004; Nystrom, Gutniak, et al 2004). Despite these features, the therapeutic potential of GLP-1 is limited due to its rapid degradation by the ubiquitous enzyme, dipeptidyl peptidase-IV (DPP-IV) (Nauck et al 1993; Deacon et al 1995; Zander et al 2002). The circulating half-life of GLP-1 is approximately 1–2 minutes, which precludes the maintenance of therapeutic levels by subcutaneous dosing (Drucker 2003). Several approaches have been undertaken to develop agents that replicate or replace the actions of GLP-1, which are in various stages of clinical development (Holst 2002; Deacon 2004). The first of these GLP-1-like molecules to receive regulatory approval for the treatment of type 2 diabetes is the incretin mimetic, exenatide.
Exenatide (exendin-4) is a 39 amino acid peptide originally isolated from the salivary secretions of the lizard Heloderma suspectum (Gila monster) that is approved in the US as an adjunctive therapy to metformin and/or sulfonyureas in patients with type 2 diabetes (Eng et al 1992; Nielsen et al 2004; BYETTA® prescribing information [PI], Amylin Pharmaceuticals Inc, San Diego, CA, USA). Exenatide shares 53% amino acid sequence identity with human GLP-1 and has been shown in vitro to mediate its insulinotropic effects through binding to the GLP-1 receptor on pancreatic beta cells (Eng et al 1992; Goke et al 1993).
Exenatide exhibits a higher potency (ED50 [effective dose 50]; acute dose-response in animal models) and longer duration of action relative to GLP-1 largely due to its pharmacokinetic profile (Young et al 1999; Kolterman et al 2005). Exenatide lacks a penultimate alanine that directs DPP-IV cleavage, rendering it more proteolytically stable than GLP-1 (Thum et al 2002). Also, significant differences in clearance rates between GLP-1 and exenatide have been reported, with the plasma clearance of exenatide approximately 10% that of GLP-1 (Parkes et al 2001). The circulating half-life of exenatide is 2.4 hours and its effects are detectable for approximately 6–8 hours postadministration (Fineman et al 2003; Kolterman et al 2005; BYETTA PI).
The glucoregulatory effects of exenatide are achieved through a range of actions similar to native GLP-1, including the enhancement of glucose-dependent insulin secretion; suppression of inappropriately elevated postprandial glucagon secretion; slowing of gastric emptying; and reduction in food intake (Table 1) (reviewed in Nielsen et al 2004). In addition, exenatide restores insulin responses and secretory patterns typically deficient and abnormal in patients with type 2 diabetes (Fehse et al 2005). These mechanisms work in concert to reduce fasting and postprandial glucose concentrations by modulation of both glucose appearance (slowing of gastric emptying, suppression of glucagon secretion, reduction of food intake) and glucose disposal (enhancement of insulin secretion) in the circulation.
Table 1.
Primary glucoregulatory actions of exenatide
| • enhances glucose-dependent insulin secretion |
| • restores 1st phase insulin secretion |
| • slows gastric emptying |
| • suppresses inappropriate glucagon secretion |
| • reduces food intake |
Clinical studies with exenatide
Glycemic effects
Prospective epidemiological studies have demonstrated the relationship between the incidence of microvascular and macrovascular disease and hyperglycemia (Coutinho et al 1999; Stratton et al 2000). Increased risk of vascular disease is continuous and incrementally graded across fasting plasma glucose (FPG) concentrations, plasma glucose concentrations after an oral glucose challenge, and average levels of glycemia (HbA1c) (Stratton et al 2000). In the Norfolk cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk), levels of HbA1c were found to be continuously related to the increased risk of all-cause mortality, CVD, and ischemic disease, with a 1% increase in HbA1c associated with a 28% increase in the risk of death (Khaw et al 2001). Increasing evidence has emphasized the role of postchallenge hyperglycemia measured either 1–2 hours after a glucose load as a direct and independent risk factor for CVD beyond the risk associated with FPG alone (Bonora and Muggeo 2001, Ceriello 2005; Home 2005). In particular, the DECODE (Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe) and Framingham Offspring Study designated postchallenge hyperglycemia as an independent risk factor for CVD (DECODE 2001; Meigs et al 2002). Moreover, the prognostic significance of postchallenge hyperglycemia is further supported by studies indicating that impaired glucose tolerance (IGT), rather than impaired fasting glucose (IFG), is a critical determinant of fatal CVD (Barrett-Connor and Ferrara 1998; Tominaga et al 1999; Califf et al 2003).
Numerous studies have established the effective glucoregulatory actions of exenatide as evidenced by reductions in FPG concentrations, postprandial plasma glucose (PPG) concentrations, and HbA1c (Fineman et al 2003; Kolterman et al 2003; Buse et al 2004; DeFronzo et al 2005; Kendall, Kim, et al 2005; Poon et al 2005). The antihyperglycemic effects of exenatide are mediated by the key actions of enhancement of glucose-dependent insulin secretion, suppression of inappropriate glucagon secretion, and slowing of gastric emptying.
Acute glucoregulatory effects of exenatide were exemplified in a 28-day study in patients with type 2 diabetes treated with diet, sulfonylureas, and/or metformin. Compared with placebo, exenatide-treated patients showed significant mean reductions from baseline in HbA1c of approximately 0.9% (Fineman et al 2003). Reductions in HbA1c were observable after 1 month of exenatide treatment, a notable response given that HbA1c reflects sustained glycemic changes over the preceding 3 months. Exenatide treatment also resulted in marked reductions in postprandial glycemia (Fineman et al 2003). Glucose profiles during ingestion of a mixed meal showed significant postprandial glucose-lowering effects that were observable from the first day of exenatide treatment compared with placebo, and were sustained to day 28 (Fineman et al 2003). Since postprandial hyperglycemia may be a stronger predictor of vascular disease than elevated FPG concentrations in patients with diabetes (Ceriello 2005; Home 2005), pharmacological control of meal-time glucose concentrations with exenatide has the potential to reduce the incidence of long-term complications in patients with type 2 diabetes. Additional effects reported for exenatide include reductions in the magnitude of diurnal glucose excursions (Heine et al 2005) and reductions in postprandial triglyceride concentrations (Kolterman et al 2003).
Worsening beta-cell function is thought to be responsible for the progressive deterioration in glycemic control that occurs almost universally over time in patients with type 2 diabetes (Kahn 2000). Therefore, pharmacological agents that have the potential to reduce demand on residual beta-cell function and improve beta-cell responsiveness may positively impact long-term patient outcomes related to disease progression and development of vascular complications. One of the principal actions of exenatide is the enhancement of insulin secretion from beta cells (Egan et al 2002). Of clinical significance is that the insulinotropic effects of exenatide are glucose-dependent (Degn et al 2004). In an acute study, patients receiving varying doses of exenatide following an overnight fast showed dose-dependent reductions in plasma glucose concentrations during a subsequent 8-hour period while the prevailing glucose concentration was elevated (Kolterman et al 2003). As euglycemia was restored, basal insulinemia was re-established (Kolterman et al 2003). Thus, physiological glucose-sensing mechanisms assure that insulin secretion is coupled to glycemia, resulting in a reduced risk of hypoglycemia. This action of exenatide is in contrast to insulin secretagogues such as sulfonylureas, which increase insulin secretion regardless of glucose concentrations.
Early beta-cell dysfunction is manifested as a loss of the first phase insulin secretion, a rapid burst of insulin in response to an intravenous glucose bolus that is characteristically absent in patients with IGT and type 2 diabetes (Caumo and Luzi 2004). In a study comparing patients with type 2 diabetes with healthy individuals, both first-phase insulin secretion and second-phase insulin secretion were significantly increased in patients with type 2 diabetes treated with intravenous exenatide compared with saline (Fehse et al 2005). Further, exenatide restored insulin secretory patterns, typically lacking in patients with type 2 diabetes, to those observed in healthy subjects (Fehse et al 2005). Additional results from surrogate assays of beta-cell function, such as the proinsulin to insulin ratio, suggested improved beta-cell secretory function with exenatide compared with placebo, providing further evidence of exenatide-mediated improvements in beta-cell health (Fineman et al 2003; Buse et al 2004; DeFronzo et al 2005).
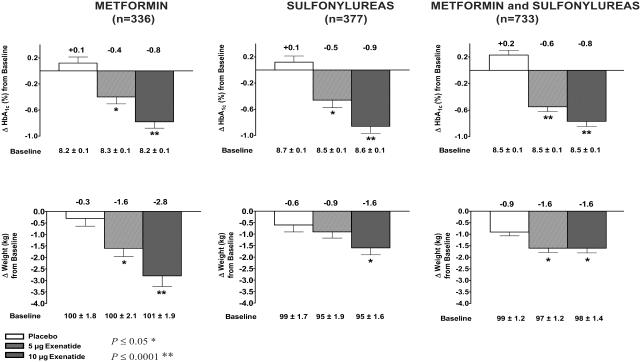
The safety and efficacy of exenatide were demonstrated in 3, 30-week clinical trials in approximately 1400 patients with type 2 diabetes unable to achieve glycemic control with metformin, a sulfonylurea, or both (Buse et al 2004; DeFronzo et al 2005; Kendall, Riddle, et al 2005). All three studies reported that 30 weeks of exenatide treatment, administered subcutaneously at 5 μg or 10 μg twice daily, resulted in significant reductions in HbA1c, FPG concentrations and postprandial plasma glucose concentrations compared with placebo (Buse et al 2004; DeFronzo et al 2005; Kendall, Riddle, et al 2005). Mean reductions from baseline in HbA1c ranged from −0.9 to −0.8% in the 10 μg exenatide groups compared with mean increases of +0.1 to +0.2% in the placebo groups (Figure 1). Consistent with these results, a cohort of 393 patients completing 82 weeks of exenatide treatment in open-label extensions of the placebo-controlled trials showed sustained reductions in HbA1c of approximately −1.1%, demonstrating the durability of the exenatide treatment response (Blonde et al 2005).
Figure 1.
Results from pivotal studies with exenatide. Mean ± SE changes from baseline in glycemia and body weight in patients with type 2 diabetes treated with exenatide or placebo for 30 weeks on a background of metformin, sulfonylureas or a combination of metformin and sulfonylureas.
In a phase 3, noninferiority study, exenatide was directly compared with basal insulin (insulin glargine) in patients with type 2 diabetes inadequately controlled with metformin or sulfonylureas (Heine et al 2005). Results from the 26-week, open-label study showed that exenatide achieved similar reductions in HbA1c to insulin glargine (exenatide: −1.0%, glargine: −1.1%). Comparison of self-monitored blood glucose profiles in treated patients showed improvements in postprandial glucose control and in 24-hour glucose profiles with exenatide, whereas glargine mainly lowered FPG concentrations, but did not alter the magnitude of postprandial glucose excursions throughout the day (Heine et al 2005). Results from this comparator study indicate that exenatide may have a role as an appropriate alternative to basal insulin in patients with type 2 diabetes failing to achieve treatment targets on combination oral medications.
Weight effects
Excess weight causes deleterious effects on glucose metabolism due predominantly to insulin resistance and elevated levels of free fatty acids. Numerous studies have demonstrated that even modest weight loss (5% to 10%) markedly improves glycemic control in patients with type 2 diabetes and reduces the severity of vascular risk factors and comorbidities (Goldstein 1992; Williamson et al 2000; Vidal 2002). It is well recognized that the cornerstone of managing diabetes is diet and exercise modification to achieve significant and sustained weight loss. Managing body weight gain becomes increasingly challenging given that intensive glucose control with most therapies may be accompanied by substantial weight gain (Purnell and Weyer 2003).
Studies with exenatide demonstrate that improvements in glycemic control in patients with type 2 diabetes are accompanied by significant reductions in body weight (Buse et al 2004; DeFronzo et al 2005; Kendall, Riddle, et al 2005). In the placebo-controlled, 30-week trials, mean reductions from baseline in body weight were observed, ranging from −2.8 kg to −1.6 kg in the 10 μg dosing group after 30 weeks of exenatide treatment, compared with +0.9 kg to +0.3 kg in the placebo group (Figure 1) (Buse et al 2004; DeFronzo et al 2005; Kendall, Riddle, et al 2005). Positive effects of exenatide on body weight were further evident when treatment effects were directly compared with those with insulin glargine in the 26-week noninferiority study. Similar reductions in HbA1c of approximately −1% were demonstrated with each treatment, however, exenatide treatment resulted in a mean reduction of −2.3 kg in body weight, whereas glargine treatment resulted in a mean increase of +1.8 kg in body weight (Heine et al 2005).
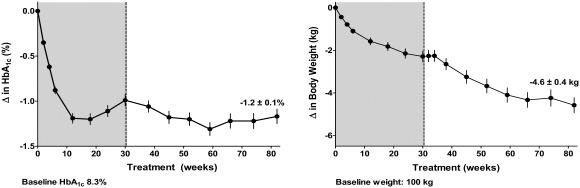
An interim assessment of ongoing, open-label extensions of the 30-week, placebo-controlled trials, a subgroup of patients completing 82 weeks of exenatide treatment (n = 265) showed sustained mean reductions in HbA1c of −1.2% and progressive reductions of body weight of −4.6 kg (Figure 2) (Kendall, Kim, et al 2005). It is notable that this body weight loss was observed without any protocol specified diet and exercise advice, and with a concurrent HbA1c reduction of approximately 1%. The impact of reductions in HbA1c and body weight over 82 weeks was further evaluated using several risk markers of CVD (Table 2). Significant improvements from baseline to Week 82 in circulating concentrations of triglycerides, HDL-C, and diastolic blood pressure were observed (Table 2) (Kendall, Kim, et al 2005). Additional analyses explored parameters by quartiles wherein the cohort was subdivided into 4 equal-sized groups based on their absolute change in weight. Findings from this analysis showed that the quartile with the greatest reduction in weight showed the greatest improvements in cardiovascular risk factors (Kendall, Kim, et al 2005). Therefore, sustained improvements in glycemic control and progressive reductions in body weight with exenatide treatment were associated with shifts toward a more favourable cardiovascular risk profile, particularly in patients that experienced the greatest weight loss. Whether exenatide exhibits direct cardiovascular effects similar to GLP-1 is currently unknown.
Figure 2.
Mean ± SE change from baseline in glycemia (HbA1c) (%) and body weight over 82 weeks of 10 μg exenatide treatment in open-label extension studies of placebo-controlled trials (grey) (n = 265).
Table 2.
Mean (±SE) change from baseline to 82 weeks in lipids and sitting blood pressure (n=265) (Kendall, Kim, et al 2005)
| Parameter | Baseline (±SE) | Δ From baseline (±SE) | 95% confidence interval |
|---|---|---|---|
| TC (mmol/L) | 4.82 ± 0.06 | −0.07 ± 0.05 | −0.17, +0.04 |
| HDL-C (mmol/L) | 0.98 ± 0.01 | +0.12 ± 0.01 | +0.09, +0.14 |
| LDL-C (mmol/L) | 2.98 ± 0.06 | −0.04 ± 0.05 | −0.13, +0.06 |
| Apo B (g/L) | 0.92 ± 0.02 | −0.01 ± 0.01 | −0.04, +0.01 |
| Triglycerides (mmol/L) | 2.70 ± 0.13 | −0.42 ± 0.11 | −0.63, −0.20 |
| Systolic blood pressure (mmHg) | 128.59 ± 0.84 | −1.48 ± 1.01 | −3.46, +0.51 |
| Diastolic blood pressure (mmHg) | 78.66 ± 0.48 | −3.24 ± 0.58 | −4.37, −2.10 |
Abbreviations: Apo B, apolipoprotein B; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol.
Safety
Across the pivotal clinical trials, exenatide was generally well tolerated. Adverse events were mostly gastrointestinal in nature (nausea and vomiting) (Buse et al 2004; DeFronzo et al 2005; Kendall, Riddle, et al 2005; BYETTA 2005). Nausea, the most common treatment-emergent adverse event (exenatide 44%, placebo 18%), was mostly mild to moderate in intensity and diminished over time. Withdrawal due to nausea was approximately 4%.
Consistent with its glucose-dependent mechanism of action, most episodes of hypoglycemia were mild to moderate in intensity with one severe event that did not require medical assistance. Hypoglycemia was rarely observed in patients treated with the combination of exenatide and metformin and was similar in incidence to patients treated with placebo and metformin (exenatide 10 μg, 5%; placebo, 5%) (DeFronzo et al 2005). In contrast, the incidence of hypoglycemia was increased over that of placebo when exenatide was used in combination with a sulfonylurea (exenatide 10 μg, 32%; placebo, 8%) (Buse et al 2004; Kendall, Riddle, et al 2005). Initial reduction of sulfonylurea dosage may limit the risk of hypoglycemia associated with such therapies upon initiation of exenatide treatment (Kendall, Riddle, et al 2005).
Approximately 40% of exenatide-treated patients developed anti-exenatide antibodies irrespective of background therapy (Buse et al 2004; DeFronzo et al 2005; Kendall, Riddle, et al 2005). The majority of these antibodies were of low-titer with no apparent clinical consequence. A small proportion of exenatide-treated patients (6%) developed high-titer antibodies (≥1/625), of which 3% showed attenuated glycemic effects and 3% did not (BYETTA 2005). These findings, along with those from recent trials, indicate that the presence of antibodies is not predictive of the magnitude of clinical response, incidence, or type of adverse events.
Summary
Exenatide is a first in class incretin mimetic agent currently approved in the US as an adjunct to metformin and/or sulfonylureas for the treatment of type 2 diabetes. Exenatide improves glycemic control in patients with type 2 diabetes by significantly reducing HbA1c, and both fasting and postprandial hyperglycemia, through multiple mechanisms of action. The actions of exenatide include acute beta-cell effects (enhancement of glucose-dependent insulin secretion; restoration of first-phase insulin secretion), regulation of inappropriately elevated glucagon secretion, slowing of gastric emptying, and reduction of food intake. Mounting evidence implicates post-meal hyperglycemia as a stronger predictor of the development of vascular complications than FPG. Since aberrant first-phase insulin secretion and impaired suppression of endogenous glucose production are major contributors to postprandial hyperglycemia, the effects of exenatide to target these defects, and normalize glucose excursions in patients with type 2 diabetes are likely to be clinically significant.
Obesity, the development of type 2 diabetes, and vascular risk are strongly linked. There is increasing evidence that weight loss can be beneficial in both the prevention and treatment of type 2 diabetes, but it is also recognized that this is difficult to achieve in a majority of patients. Improvements in glycemic control with exenatide were coupled with progressive weight reductions, which represents a distinct therapeutic benefit for patients with type 2 diabetes not adequately controlled with metformin and/or sulfonylurea therapy. In particular, potential reductions in body weight in patients with type 2 diabetes may have a positive impact on the development of vascular complications and long-term patient outcomes.
Disclosure
Catherine A Schnabel, Matthew Wintle, and Orville Kolterman are employees of Amylin Pharmaceuticals, Inc.
References
- Ahmad FK, He Z, King GL. Molecular targets of diabetic cardiovascular complications. Curr Drug Targets. 2005;6:487–94. doi: 10.2174/1389450054021990. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003;22:331–9. doi: 10.1080/07315724.2003.10719316. [DOI] [PubMed] [Google Scholar]
- Aronoff SL, Berkowitz K, Schreiner B, et al. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectrum. 2004;17:183–90. [Google Scholar]
- Astrup A. Healthy lifestyles in Europe: prevention of obesity and type 2 diabetes by diet and physical activity. Public Health Nutr. 2001;4:499–515. doi: 10.1079/phn2001136. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men: The Rancho Bernardo Study. Diabetes Care. 1998;21:1236–9. doi: 10.2337/diacare.21.8.1236. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–80. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- Blonde L, Han J, Mac S, et al. Exenatide (exendin-4) reduced A1C and weight over 82 weeks in overweight patients with type 2 diabetes. Diabetes. 2005;54:A118, 477-P. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107–14. doi: 10.1007/s001250100020. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, et al. Glucagon-like peptide1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–51. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications. A unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- BYETTA®. San Diego, CA, USA: Amylin Pharmaceuticals Inc; 2005. Prescribing information [online] Accessed on 1 July 2005. URL: http://www.byetta.com/ [Google Scholar]
- Califf R, Holman R, on behalf of the NAVIGATOR Trial Group 2003 People at increased risk of cardiovascular disease screenedfor the NAVIGATOR trial frequently have undiagnosed diabetes or impaired glucose tolerance [Abstract and poster]. 52nd annual scientific session of the American College of Cardiology; March 30 – April 2; Chicago. pp. 1169–51. [Google Scholar]
- Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab. 2004;287:E371–E385. doi: 10.1152/ajpendo.00139.2003. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Mechanisms of tissue damage in the postprandial state. Int J Clin Pract Suppl. 2001;Sep(123):7–12. [PubMed] [Google Scholar]
- Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- Coutinho M, Gerstein HC, Wang Y, et al. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–40. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Nauck MA, Toft-Nielsen M, et al. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2–terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–31. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- Deacon CF. Therapeutic strategies based on glucagon-like peptide 1. Diabetes. 2004;53:2181–9. doi: 10.2337/diabetes.53.9.2181. [DOI] [PubMed] [Google Scholar]
- [DECODE] DECODE study group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Inter Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Pharmacological therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- Degn KB, Brock B, Juhl CB, et al. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes. 2004;53:2397–403. doi: 10.2337/diabetes.53.9.2397. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929–40. doi: 10.2337/diacare.26.10.2929. [DOI] [PubMed] [Google Scholar]
- Egan JM, Clocquet AR, Elahi D. The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab. 2002;87:282–90. doi: 10.1210/jcem.87.3.8337. [DOI] [PubMed] [Google Scholar]
- Eng J, Kleinman WA, Singh L, et al. Isolation and characterization of exendin-4, an exendin-3 analogue from Heloderma suspectum venom. J Biol Chem. 1992;267:7402–5. [PubMed] [Google Scholar]
- Erdmann E. Diabetes and cardiovascular risk markers. Curr Med Res Opin. 2005;21:S21–8. doi: 10.1185/030079905X36459. [DOI] [PubMed] [Google Scholar]
- Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first and second phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:5991–7. doi: 10.1210/jc.2005-1093. [DOI] [PubMed] [Google Scholar]
- Fineman MS, Bicsak TA, Shen LZ, et al. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370–7. doi: 10.2337/diacare.26.8.2370. [DOI] [PubMed] [Google Scholar]
- Goke R, Fehmann HC, Linn T, et al. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650–5. [PubMed] [Google Scholar]
- Goldstein G. Beneficial health effects of modest weight loss. In J of Obes. 1992;16:397–415. [PubMed] [Google Scholar]
- Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in sugjects with type 2 diabetes and in nondiaetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143:559–69. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- Hirsch IB. Intensifying insulin therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118:21S–6S. doi: 10.1016/j.amjmed.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Holst JJ. Therapy of type 2 diabetes mellitus based on the action of glucagon-like peptide-1. Diabetes/Metab Res Rev. 2002;18:430–41. doi: 10.1002/dmrr.328. [DOI] [PubMed] [Google Scholar]
- Home P. Contributions of basal and post-prandial hyperglycemia to micro- and macrovascular complication in people with type 2 diabetes. Curr Med Res Opin. 2005;21:989–98. doi: 10.1185/030079905x49662. [DOI] [PubMed] [Google Scholar]
- Kahn SE. The importance of the beta cell in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108:2S–8S. doi: 10.1016/s0002-9343(00)00336-3. [DOI] [PubMed] [Google Scholar]
- Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham Study. JAMA. 1979;241:2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- Kaufman FR. Type 2 diabetes in children and young adults: A “new epidemic”. Clin Diabetes. 2002;20:217–18. [Google Scholar]
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–91. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- Kendall DM, Kim D, Poon T, et al. Improvements in cardiovascular risk factors accompanied sustained effects on glycemia and weight reduction in patients with type 2 diabetes treated with exenatide for 82 wk. Diabetes. 2005;54:A4–5, 16–OR. [Google Scholar]
- Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Perspective Investigation of Cancer and Nutrition (EPIC-Norfolk) BMJ. 2001;322:1–6. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1999;18:258–68. doi: 10.2337/diacare.18.2.258. [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–9. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Sys Pharmacy. 2005;62:173–81. doi: 10.1093/ajhp/62.2.173. [DOI] [PubMed] [Google Scholar]
- Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–4. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- Maggio CA, Pi-Sunyer FX. The prevention and treatment of obesity. Application to type 2 diabetes. Diabetes Care. 1997;20:1744–66. doi: 10.2337/diacare.20.11.1744. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Nathan DM, D'Agostino RB, et al. Fasting and postchallenge glycemia and cardiovascular disease risk. The Framingham Offspring Study. Diabetes Care. 2002;10:1845–9. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, et al. Diabetes trends in the US: 1990–1998. Diabetes Care. 2000;22:1278–83. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- Monnier L, Colette C, Rabasa-Lhoret R, et al. Morning hyperglycemic excursions: a constant failure in the metabolic control of non-insulin-using patients with type 2 diabetes. Diabetes Care. 2002;25:737–41. doi: 10.2337/diacare.25.4.737. [DOI] [PubMed] [Google Scholar]
- Morrish NJ, Wang SL, Stevens LK, et al. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44:S14–21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- [NCEP] National Cholesterol Education Program. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- Nauck M, Stockmann F, Ebert R, et al. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–4. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- Nielsen LL, Young AA, Parkes D. Pharmacology of exenatide (synthetic exendin-4): A potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117:77–88. doi: 10.1016/j.regpep.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- Nystrom T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary heart disease. Am J Physiol Endocrinol Metab. 2004a;287:E1209–15. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- Nystrom T, Gonon AT, Sjoholm A, et al. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2004b;125:173–7. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Parkes DG, Pittner R, Jodka C, et al. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism. 2001;50:583–9. doi: 10.1053/meta.2001.22519. [DOI] [PubMed] [Google Scholar]
- Perley M, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest. 1967;46:1954–62. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon T, Nelson P, Shen L, et al. Exenatide improves glycemic control and reduces body weight in subjects with type 2 diabetes: a doseranging study. Diabetes Technol Ther. 2005;7:467–77. doi: 10.1089/dia.2005.7.467. [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Weyer C. Weight effects of current and experimental drugs for diabetes mellitus. Treat Endocrin. 2003;2:33–47. doi: 10.2165/00024677-200302010-00004. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Banting Lecture: Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Singleton JR, Smith AG, Russell JW, et al. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–73. doi: 10.2337/diabetes.52.12.2867. [DOI] [PubMed] [Google Scholar]
- Stratton IM, Adler AI, Neil HA, et al. Association of glycemia with mascrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum A, Hupe-Sodmann K, Goke R, et al. Endoproteolysis by isolated membrane peptidases reveal metabolic stability of glucagon-like peptide-1 analogs, exendins-3 and -4. Exp Clin Endocrinol Diabetes. 2002;110:113–18. doi: 10.1055/s-2002-29087. [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Eguchi H, Manaka H, et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose: The Funagata Study. Diabetes Care. 1999;22:920–4. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- [UKPDS] United Kingdom Prospective Diabetes Study Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- Vidal J. Updated review on the benefits of weight loss. Int J Obes. 2002;26:S25–8. doi: 10.1038/sj.ijo.0802215. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Thompson TJ, Thun M, et al. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care. 2000;23:1499–504. doi: 10.2337/diacare.23.10.1499. [DOI] [PubMed] [Google Scholar]
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta) Diabetes. 1999;48:1026–34. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–30. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]