Abstract
Asian Indian dyslipidemia is characterized by: borderline high low-density lipoprotein (LDL) cholesterol and apolipoprotein (apo) B; high triglycerides, low high-density lipoprotein (HDL) cholesterol and apoA1; and high lipoprotein(a) (lp[a]). We performed a controlled multicentric trial in India to evaluate the efficacy and safety of a fixed dose combination of lovastatin and niacin extended release (niacinER) formulation in patients with moderate to severe dyslipidemia. Consecutive subjects that satisfied the selection criteria, agreed to an informed consent, and with no baseline presence of liver/renal disease or heart failure were enrolled in the study. After a 4-week run-in period there were 142 patients with LDL levels ≥130 mg/dL. Eleven patients were excluded because of uncontrolled hyperglycemia and 131 patients were recruited. After baseline evaluation of clinical and biochemical parameters all subjects were administered lovastatin (20 mg) and niacinER (500 mg) combination once daily. Dose escalation was done on basis of lipid parameters at 8 weeks and in 11 patients increased to lovastatin (20 mg) and niacinER (1000 mg). An intention-to-treat analysis was performed and data was analyzed using nonparametric Wilcoxon signed rank test. Thirteen patients (10%) were lost to follow-up and 4 (3%) withdrew because of dermatological adverse effects: flushing, pruritus, and rash. The mean values of various lipid parameters (mg/dL) at baseline, and at weeks 4, 12, and 24 respectively were: total cholesterol 233.9 ± 27, 206.3 ± 27, 189.8 ± 31, and 174.9 ± 27 mg/dL; LDL cholesterol 153.4 ± 22, 127.3 ± 21, 109.2 ± 27, and 95.1 ± 23 mg/dL; triglycerides 171.1 ± 72, 159.5 ± 75, 149.2 ± 45, and 135.2 ± 40 mg/dL; HDL cholesterol 45.6 ± 7, 48.9 ± 7, 51.6 ± 9, and 53.9 ± 10 mg/dL; lp(a) 48.5 ± 26, 40.1 ± 21, 35.4 ± 21, and 26.9 ± 19 mg/dL; and apoA1/apoB ratio 0.96 ± 0.7, 1.04 ± 0.4, 1.17 ± 0.5, and 1.45 ± 0.5 (p < 0.01). The percentage of decline in various lipids at 4, 12, and 24 weeks was: total cholesterol 11.8%, 18.8%, and 25.2%; LDL cholesterol 17.0%, 28.8%, and 38.0%; triglyceride 6.8%, 12.8%, and 21.0%; lp(a) 17.5%, 26.9%, and 44.5% respectively (p < 0.01). HDL cholesterol and apoA1/apoB increased by 7.2%, 13.1%, and 18.2%; and 7.9%, 21.9%, and 51.6% respectively (p < 0.01). Target LDL levels (<100 mg/dL in subjects with manifest coronary heart disease or diabetes; <130 mg/dL in subjects with >2 risk factors) were achieved in 92 (80.7%) patients. No significant changes were observed in systolic or diastolic blood pressure, blood creatinine, transaminases, or creatine kinase. A fixed dose combination of lovastatin and niacinER significantly improved cholesterol lipoprotein lipids as well as lp(a) and apoA1/apoB levels in Asian Indian dyslipidemic patients. Satisfactory safety and tolerability profile in this population was also demonstrated.
Keywords: Hypercholesterolemia, South Asians, coronary heart disease, lipid abnormalities, low HDL, lipoprotein(a)
Introduction
Asian Indian dyslipidemia is characterized by moderate to high low-density lipoprotein (LDL) cholesterol, raised small-dense LDL particles, low high-density lipoprotein (HDL) cholesterol, high triglyceride, and high lipoprotein(a) (lp [a]) (Bhatnagar 1998). This constellation of abnormalities is also known as atherogenic dyslipidemia and is part of the metabolic syndrome (Grundy 2004). Case-control studies within India have also reported that characteristic lipid abnormalities in patients with coronary heart disease are low HDL cholesterol and apolipoprotein (apo) A, and high basal and post-prandial triglycerides, lp(a), and apoB (Kumar et al 1976; Wasir et al 1987; Vashist et al 1990; Sahi et al 1993; Pais et al 1996; Dhanjal et al 2001; Gupta et al 2001), and abnormalities of apo E4 allele on genetic evaluation (Luthra et al 2002; Kumar et al 2003).
For the last ten years, statins or hydroxymethyl glutyrate co-enzyme A (HMG CoA) reductase inhibitors have been the mainstay of lipid management in patients with dyslipidemias and coronary heart disease (NCEP 2002; Pasternak et al 2002; Jones 2004). However, statins when used in large doses have significant side effects that include rhabdomyolysis and liver function abnormalities (Pasternak et al 2002; Botorrof 2004). The Third Adult Treatment Panel (ATP-III) of the American National Cholesterol Education Program (NCEP) has focused on LDL cholesterol as the primary treatment objective (NCEP 2002). The recent revision of ATP has, however, also highlighted other lipid abnormalities that are part of the metabolic syndrome and advocated combination therapy to achieve lipid targets (Deedwania et al 2004; Grundy et al 2004). Targets of LDL cholesterol have been set at <70 mg/dL (Cannon et al 2004; Grundy et al 2004). Similar stringent targets are on the anvil for other lipid fractions (triglycerides <100 mg/dL; non-HDL cholesterol <100 mg/dL; HDL cholesterol >60 mg/dL; and Lp(a) <20 mg/dL) (Gupta 2000; Deedwania et al 2004; Deedwania and Gupta 2005). Combination therapy is more relevant in the Indian context where a plethora of lipoprotein abnormalities exist (Bhatnagar 1998; Gupta 2000; Gupta et al 2001). One such combination with potential to reduce LDL cholesterol, non-HDL cholesterol, and lp(a), and increase HDL cholesterol is lovastatin with niacin (NCEP 2002; Deedwania et al 2004; Jones 2004). Previous studies have shown that usual doses of niacin are associated with significant vascular and dermatological side effects and a slow release formulation has been developed that is associated with low incidence of side effects of this type (Kashyap et al 2002; NCEP 2002). This combination has been reported to be safe and effective in Caucasian populations in many trials (Kashyap et al 2002; Jones 2004), however, no such study is available from India. Therefore, we performed a controlled multicentric trial to study the efficacy and safety of a fixed dose lovastatin and niacin extended release (niacinER) combination.
Methods
Study design
This was a 24-week open labeled, multicentric, noncomparative; nonrandomized study approved by the Drug Controller General of India and respective Institutional Ethics Committees and was conducted in accordance with the guideline of good clinical practice and the Helsinki Declaration. All patients were recruited after obtaining written informed consent before their participation. The study included men and women >21 years age willing to complete a 4-week run-in period. Participants were required to fulfill one of the following three criteria: (1) manifest coronary heart disease or diabetes and LDL cholesterol >130 mg/dL; (2) ≥2 coronary risk factors and LDL cholesterol >160 mg/dL in subjects with no preexisting coronary disease or diabetes; or (3) <2 coronary risk factors, LDL cholesterol >190 mg/dL and no evidence of coronary disease or diabetes. Exclusion criteria included pregnant women, serum triglycerides >800 mg/dL, HDL cholesterol >70 mg/dL, evident liver dysfunction or elevation in hepatic enzymes, recent myocardial infarction, stroke or bypass graft within 6 months, recent acute arterial hemorrhage, uncontrolled grade III hypertension, uncontrolled cardiac arrhythmia, uncontrolled diabetes, active gall bladder disease, peptic ulcer disease, history of recent gout attack, hypothyroidism not managed with stable thyroxin doses, concomitant use of other investigational agent or dyslipidemic medication, or concurrent therapy with agents having potential to cause drug-drug interactions (isotretinoin, cyclosporine, troglitazone, macrolide antibiotics, and azole-antifungal medications). Patients were informed that they were free to withdraw from the study at any time without stating the reason. The investigator could withdraw a subject from the study if he suffered from significant illness during the course of the study, experienced serious adverse effects, or withdrawal was in best interest of the patient. In case the patient did not come for follow up, he was treated as a drop out from the study. 142 patients were screened for the study and included for the initial run-in phase.
Assessments
Symptoms and demographic data were recorded at enrolment. Physical examination, 12-lead electrocardiography, urinalysis, hematological examinations (blood counts, sedimentation rate), and chest radiography were performed at the beginning and at 24 weeks (end of trial). Plasma lipids, total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, apoA1, apoB, and lp(a) were measured at baseline and after weeks 4, 12, and 24 of drug therapy. All the biochemical investigations were standardized across sites by using internal and external quality control measures. ApoA1, apoB, and lp(a) were estimated by nephelometry and immunoturbidimetry using Randox® kit. The intraassay and interassay coefficient of variations respectively were: apoA1 2.13%–2.78% and 2.65%–2.98%; apoB 1.82%–4.50% and 5.65%–6.69%; lp(a) 6.55%–10.34% and 13.61%–16.26%. Evaluation of hepatic functions (transaminases, alkaline phosphatase, prothrombin time), renal functions (creatinine, urea, uric acid), and creatine kinase, and vital signs (pulse and blood pressure) was also performed at baseline and weeks 4, 12, and 24. Patients were also advised to visit at the 16th week when additional adverse drug reaction monitoring was performed. Primary and secondary end points of the trial were evaluation of efficacy and safety of the drug combination. Efficacy was assessed by the achievement of goals according to the NCEP (2002) guidelines. Safety was evaluated as nonoccurrence of any drug related adverse effects using clinical and laboratory investigations.
Intervention
A fixed dose combination (FDC) of lovastatin (20 mg) and niacinER (500 mg) was administered once daily with a low fat snack at bedtime. The physicochemical characteristics of this extended release form of niacin were comparable with the internationally available niacin formulation: Niaspan® (Panacea-Biotec 2004). Dose escalation was done on the basis of LDL cholesterol levels at week 8 of therapy. In coronary disease or diabetic subjects who did not achieve LDL cholesterol of <100 mg/dL, or non-diabetic or noncoronary disease subjects with ≥2 risk factors and LDL cholesterol <130 mg/dL, the dose of niacin was increased. During first dose escalation FDC of lovastatin (20 mg) and niacinER (l000 mg) was used and dose greater than 40/2000 mg was not recommended. Use of aspirin was permitted 30 minutes before dosing to prevent flushing.
Statistical analysis
A sample size of 120 subjects was considered adequate for the study statistical power. Descriptive statistics (number, mean, standard deviation) have been presented. Pre- and post-intervention comparison was done using nonparametric Wilcoxon signed rank test. P < 0.05 was considered significant.
Results
Of the 142 subjects eligible for enrollment in the study, 11 were not initiated into the study due to poor glycemic control. The mean age of subjects was 52.6 ± 11.1 years, weight 67.6 ± 10.1 kg, and height 1.64 ± 0.1 m. Fifty-three (40.4%) subjects had body mass index ≥25 kg/m2, concomitant hypertension was in 53 (40.4%), diabetes in 28 (21.4%), coronary artery disease in 60 (45.8%), controlled hypothyroidism in 1 (0.8%), and 72 (55.0%) were on aspirin. Of these 131 patients, 4 withdrew from the study because of dermatological adverse effects, and 13 were lost to follow up. The baseline characteristics of the 13 patients who were lost to follow up did not differ significantly from those who continued in the study. The mean age of this group was 50.8 ± 9 years, weight 69.4 ± 13.6 kg, total cholesterol 227.6 ± 22 mg/dL, triglycerides 196.8 ± 84 mg/dL, HDL cholesterol 43.1 ± 10.1 mg/dL, LDL cholesterol 149.3 ± 14.8 mg/dL, lp(a) 51.3 ± 20.1 mg/dL and apoA1/apoB was 0.82 ± 0.39 (see Table 1 for comparison). Thus, at the end of the study a total of 114 subjects were available for efficacy and safety evaluations.
Table 1.
Various lipoprotein lipids (mean ± 1 standard deviation) at baseline and after 4-week, 12-week, and 24-week treatment with lovastatin and niacin combination
| Parameter (mg/dL) | Baseline (n = 131) | Week 4 (n = 120) | Week 12 (n = 115) | Week 24 (n = 114) |
|---|---|---|---|---|
| Total cholesterol | 233.9 ± 27.4 | 206.3 ± 27.4* | 189.8 ± 31.3* | 174.9 ± 27.2* |
| Triglycerides | 171.1 ± 72.2 | 159.5 ± 75.0* | 149.2 ± 45.0* | 135.2 ± 40.5* |
| HDL cholesterol | 45.6 ± 7.4 | 48.9 ± 7.4* | 51.6 ± 8.7* | 53.9 ± 9.5* |
| LDL cholesterol | 153.4 ± 21.9 | 127.3 ± 20.8* | 109.2 ± 26.8* | 95.1 ± 23.1* |
| ApoA1/B | 0.96 ± 0.67 | 1.04 ± 0.42* | 1.17 ± 0.49* | 1.45 ± 0.51* |
| Lipoprotein (a) | 48.5 ± 26.4 | 40.1 ± 21.2* | 35.4 ± 20.6* | 26.9 ± 19.2* |
p < 0.05.
Abbreviations: HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; Apo, apolipoprotein.
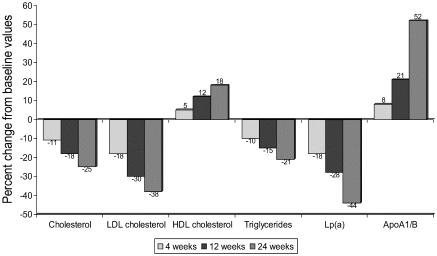
The mean values of various lipid parameters at baseline, and weeks 4, 12, and 24 are shown in Table 1. The values of various lipoprotein lipids respectively were: total cholesterol 233.9 ± 27, 206.3 ± 27, 189.8 ± 31, and 174.9 ± 27 mg/dL; LDL cholesterol 153.4 ± 22, 127.3 ± 21, 109.2 ± 27, and 95.1 ± 23 mg/dL; triglycerides 171.1 ± 72, 159.5 ± 75, 149.2 ± 45, and 135.2 ± 40 mg/dL; HDL cholesterol 45.6 ± 7, 48.9 ± 7, 51.6 ± 9, and 53.9 ± 10 mg/dL; lp(a) 48.5 ± 26, 40.1 ± 21, 35.4 ± 21, and 26.9 ± 19 mg/dL; and the ratio of apoA1/apoB were 0.96 ± 0.7, 1.04 ± 0.4, 1.17 ± 0.5, and 1.45 ± 0.5 (p < 0.01). The percentage of decline in various lipids at 4, 12, and 24 weeks were for total cholesterol 11.8%, 18.8%, and 25.2%; LDL cholesterol 17.0%, 28.8%, and 38.0%; triglyceride 6.8%,12.8%, and 21.0%; lp(a) 17.5%, 26.9%, and 44.5% respectively (p < 0.01). HDL cholesterol and apoA1/apoB increased by 7.2%, 13.1%, and 18.2%; and 7.9, 21.9 and 51.6% respectively (p < 0.01) (Figure 1). Of the 114 patients who were available at end of the trial, 92 patients (80.7%) achieved the target LDL cholesterol goals according to the NCEP ATP-III guidelines (NCEP 2002).
Figure 1.
Percent change in various lipid levels. There is significant change in all the lipid parameters when compared with baseline at weeks 4, 12, and 24 (p < 0.01).
Overall, treatment with lovastatin and niacinER FDC was well tolerated with no persistent or unexpected adverse drug reactions. Twenty-six patients (19.8%) reported minor adverse events; most were gastrointestinal (nausea, vomiting, dyspepsia) and dermatologic (pruritus, rash, flushing). Other adverse events were myalgia (2 subjects). Flushing caused 3 patients to withdraw from the study. One patient withdrew because of pruritus and rash; this patient was receiving the combination containing 1000 mg niacin. Clinical myopathy was not observed in any of the subjects. Haematological and biochemical changes are reported in Table 2.
Table 2.
Effect of lovastatin – niacin combination tablet on selected laboratory parameters
| Baseline* (n = 131) | Week 4 | Week 8 | Week 12 | Week 24* (n = 114) | Normal range | |
|---|---|---|---|---|---|---|
| Hemoglobin (gm/dl) | 11.7 ± 1.6 | – | – | – | 11.8 ± 1.4 | 14 g%–18 g% |
| White cell count (cells/cmm) | 7461 ± 1426 | – | – | – | 6998 ± 1323 | 4300–10800/mm3 |
| ESR (mm 1st hour) | 24.5 ± 12.3 | – | – | – | 17.2 ± 10.2 | 0–20 mm in 1st hr |
| SGOT (IU/l) | 29.9 ± 10.0 | 29.3 ± 9.3 | 29.5 ± 12.5 | 30.1 ± 14.4 | 24.5 ± 7.4 | 0–35 IU/L |
| SGPT (IU/l) | 31.5 ± 12.7 | 29.2 ± 9.5 | 28.1 ± 8.8 | 28.0 ± 9.5 | 23.0 ± 8.0 | 0–35 IU/L |
| Alkaline Phosphatase (IU/l) | 113.3 ± 51.9 | 107.1 ± 43.7 | 101.2 ± 38.7 | 94.5 ± 30.2 | 91.1 ± 34.6 | 60–306 IU/L |
| Creatinine (mg/dL) | 0.93 ± 0.2 | 0.96 ± 0.7 | 1.01 ± 6.5 | 1.06 ± 0.7 | 1.01 ± 0.6 | <1.5 mg/dL |
| Uric acid (mg/dL) | 4.5 ± 1.1 | 4.4 ± 0.9 | 4.5 ± 0.9 | 4.4 ± 0.8 | 4.4 ± 1.0 | 2.5–8.0 mg/dL |
| Creatine kinase (mg/dL) | 146.3 ± 70.5 | 134.7 ± 62.5 | 130.0 ± 55.5 | 125.6 ± 50.7 | 115.3 ± 40.5 | 24–190 IU/L |
| Glucose fasting (mg/dL) | 90.3 ± 14.7 | – | – | – | 81.1 ± 23.5 | <110 mg/dL |
Mean ± 1 standard deviation
Abbreviations: ESR, erythrocyte sedimentation rate; SGOT, serum aspartate aminotransferase; SGPT, serum alanine aminotransferase.
Discussion
This study shows that a combination of lovastatin and low-dose niacin is effective in ameliorating the Asian Indian type of dyslipidemia. The pattern of dyslipidemia seen in Asian Indians is different from observed in other ethnic groups and is also the dyslipidemia of metabolic syndrome (Grundy 2004). Lovastatin and niacin in low doses used in the present study resulted in a significant decline in LDL cholesterol, triglycerides, and lp(a) along with an increase in HDL cholesterol and apoA1/apoB ratio.
Combination therapy for the treatment of dyslipidemias associated with coronary artery disease, diabetes, and hypertension is a widely used strategy to promote effective treatment in patients with disturbances in more than one lipid parameter (Deedwania et al 2004). Combination therapy allows the physician to take advantage of the independent effects of the therapies selected. An additive and possibly synergistic LDL cholesterol lowering effect may be achieved by using drugs that individually lower LDL cholesterol, but by different mechanisms. Additionally, different lipid parameters such as HDL cholesterol and triglycerides may also be corrected by using combination therapies. The combination of lovastatin and niacinER is the first hypolipidemic combination approved by the American Federal Drug Administration. Various studies have demonstrated that the efficacy of this combination is better than either of the two agents used alone (Guyton et al 1998; Chong and Bachenheimer 2000; Gupta and Ito 2002; Kashyap 2002; NCEP 2002).
Niacin inhibits hepatic triglyceride synthesis and results in reduced production of apoB-containing lipoproteins. Niacin is considered an excellent choice in increasing HDL cholesterol and is effective in reducing lp(a); an important emerging risk factor. Lovastatin is known to upregulate LDL receptors, which results in increased LDL and apoB catabolism. Therefore, this lipid modifying drug combination is ideal for improvement of the multiple coexistent lipid abnormalities frequently encountered in Indian population (Deedwania and Gupta 2005). The combined administration of a statin and nicotinic acid was first described over 15 years ago (Henkin et al 1991). A year-long open label study showed that concomitant administration of lovastatin, pravastatin, or simvastatin with niacin decreased LDL cholesterol to a greater extent than niacinER alone in hypercholesterolemic patients (−32% vs −18%) without unduly increasing the frequency of adverse events (Guyton et al 1998). Drug withdrawal was observed in 5% subjects. This is similar to the present study where LDL cholesterol decreased by 38% over a 6-month period and drug withdrawal was observed in 3% of cases. Ten percent dropped out of the study due to unspecified reasons and it is not clear whether this was due to adverse events or other issues of compliance.
There are studies that have compared the effects of statinniacin combination therapy with statin alone. Three were placebo controlled randomized trials (Davignon et al 1994; O'Keefe et al 1995; Vacek et al 1995), one was an open label study (Illingworth and Bacon 1989) and one was a retrospective analysis (Wolfe et al 2001). In these five studies, the combination of nicotinic acid with a statin resulted in greater decreases in LDL cholesterol and triglycerides, and greater increase in HDL cholesterol than occurred with statin monotherapy. Decreases in LDL cholesterol and increases in HDL cholesterol tended to be marked with high doses of niacin. At higher doses of niacin, the frequency of adverse events and drop-out rate was greater in those with combination therapy than those on statin alone, although statin use was associated with greater incidence of abnormal transaminases. As we did not use a control group with statins only, we cannot comment on this observation. Published studies also report that LDL lowering effects of statin-niacin combination were only slightly greater (10%) than achieved with statins alone and the combination was not recommended for LDL cholesterol lowering (Thompson 2004). In patients with raised triglycerides and low HDL cholesterol, which is the typical dyslipidemia of Asian Indians, and the metabolic syndrome, there are major benefits of this combination as decreases in triglycerides and increases in HDL approach 25%, which more than compensates for the side effects of niacin. The present study also demonstrates that this fixed drug combination causes a significant decline in lp(a) (−44%) and increase in the apoA1/apoB ratio (−52%), which are major benefits not reported previously. This study also shows that there is increasing efficacy of this drug combination and a trend towards a continued decline in LDL cholesterol, triglycerides, and lp(a), and an increase in HDL cholesterol and apoA1. Similar trends have been previously reported in short term studies (Chong and Bachenheimer 2000). In long term studies, it has been reported that the initial large decline tends to attenuate with time (Warnica 2004).
In the present study this combination in a lower dose was used to generate data in the Asian Indian population. At the end of weeks 4, 12, and 24, statistically significant changes were observed in the levels of LDL cholesterol, HDL cholesterol, triglycerides, apoA1/apoB ratio, and lp(a). The maximum change was observed towards end of the study. The target LDL cholesterol levels, as recommended by NCEP (2002) were achieved in more than 80% of the patients evaluated in the study. Dose escalation was needed on basis of serum lipid parameters in 11 patients only. In our study, the number of female patients was low and they were not analyzed separately. There are several limitations in the study. We used an open-labeled design with no blinded placebo group. This can lead to biased physician recruitment, such as the noninclusion of patients with severe hypercholesterolemia leading to more patients achieving the lipid targets, as well as greater reporting of niacin-related side effects. However, the present study design was important, as this was a regulatory post-marketing trial. All the laboratory investigations were blinded. Also the focus of the study was the amelioration of typical Asian Indian dyslipidemia, which is characterized by low HDL cholesterol, high triglycerides, and lp(a). Statins have been reported to be of insignificant benefit in these lipid abnormalities; therefore no control group with statins was included. It is also possible that dietary changes and more exercise were performed in the study patients leading to large benefits as seen in the present study. The diet – exercise advice was given uniformly to all the patients. In a subgroup of patients where dietary patterns and exercise were evaluated (Bhargava 2004) in detail it was noted that the change in diet was the greatest in the first two weeks, followed by a gradual reversal to baseline levels with time, hence, the influence of lifestyle changes appears minimal. The number of subjects in the present study is also small and larger studies are needed to more definitively answer the study question.
Overall, this study demonstrates that the low dose combination offers an effective control of LDL cholesterol besides providing additional benefits in terms of reduced triglycerides, lp(a), and increased HDL cholesterol and apoA1/apoB ratio. Latter findings have special relevance in context of Asian Indian dyslipidemia as well as insulin resistance syndrome and the metabolic syndrome.
Funding source
The study was funded and supported by Panacea-Biotec Ltd, New Delhi, India.
References
- Bhargava S. Evaluation of efficacy of non-drug therapy (dietary) and drug therapy in hypercholesterolemic subjects. Jaipur: University of Rajasthan; 2004. MSc Dissertation. [Google Scholar]
- Bhatnagar D. The metabolic basis of increased coronary risk attributed to people from the Indian subcontinent. Current Science. 1998;74:1087–94. [Google Scholar]
- Botorrof MB. Statin safety: what to know. Am J Geriatr Cardiol. 2004;13:34–8. [PubMed] [Google Scholar]
- Cannon CP, Braunwald E, McCabe C, et al. Pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction-22 investigators (PROVE-IT TIMI-22). Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- Chong PH, Bachenheimer BS. Current, new and future treatments in dyslipidemia and atherosclerosis. Drugs. 2000;60:55–93. doi: 10.2165/00003495-200060010-00005. [DOI] [PubMed] [Google Scholar]
- Davignon J, Roederer G, Montigny M, et al. Comparative efficacy and safety of pravastatin, nicotinic acid and two combined in patients with hypercholesterolemia. Am J Cardiol. 1994;73:339–45. doi: 10.1016/0002-9149(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Hunninghake DB, Bays H. Effects of lipid altering treatment in diabetes mellitus and the metabolic syndrome. Am J Cardiol. 2004;93(Suppl):18C–26C. doi: 10.1016/j.amjcard.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Gupta R. East Asians and South Asians, and Asian and Pacific Islander Americans. In: Wong ND, Black HR, Gardin JM, editors. Preventive Cardiology. 2nd Edition. New York: McGraw Hill; 2005. pp. 456–72. [Google Scholar]
- Dhanjal TS, Lal M, Haynes R, Lip G. A comparison of cardiovascular risk factors among Indo-Asian and Caucasian patients admitted with acute myocardial infarction in Kuala Lumpur, Malaysia and Birmingham, England. Int J Clin Pract. 2001;55:665–8. [PubMed] [Google Scholar]
- Grundy SM. Obesity, metabolic syndrome and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman N, Bairey-Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- Gupta EK, Ito MK. Lovastatin and extended-release niacin combination product: The first drug combination for the management of hyperlipidemia. Heart Disease. 2002;4:124–37. doi: 10.1097/00132580-200203000-00010. [DOI] [PubMed] [Google Scholar]
- Gupta R. Preventing coronary heart disease in India: focus on primary prevention. J Indian Med Assoc. 2000;98:703–9. [PubMed] [Google Scholar]
- Gupta R, Kaul V, Prakash H, et al. Lipid abnormalities in coronary heart disease: a population based case-control study. Indian Heart J. 2001;53:332–36. [PubMed] [Google Scholar]
- Guyton JR, Goldberg CA, Kreisberg RA, et al. Effectiveness of once-nightly dosing of extended-release niacin alone and in combination for hypercholesterolemia. Am J Cardiol. 1998;82:737–43. doi: 10.1016/s0002-9149(98)00448-2. [DOI] [PubMed] [Google Scholar]
- Henkin Y, Oberman A, Hurst DC, et al. Niacin revisited: clinical observations on an important but underutilized drug. Am J Med. 1991;91:239–46. doi: 10.1016/0002-9343(91)90122-e. [DOI] [PubMed] [Google Scholar]
- Illingworth DR, Bacon S. Treatment of heterozygous familial hypercholesterolemia with lipid-lowering drugs. Atherosclerosis. 1989;9(Suppl 1):1121–34. [PubMed] [Google Scholar]
- Jones PH. Statins as the cornerstone of drug therapy for dyslipidemia: monotherapy and combination therapy options. Am Heart J. 2004;148:S9–S13. doi: 10.1016/j.ahj.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Kashyap ML, McGovern ME, Berra K, et al. Long term safety and efficacy of a once daily niacin-lovastatin formulation for patients with dyslipidemia. Am J Cardiol. 2002;89:672–78. doi: 10.1016/s0002-9149(01)02338-4. [DOI] [PubMed] [Google Scholar]
- Kumar M, Chakravarti RN, Singh A, et al. Serum lipid profiles in patients of myocardial infarction in Chandigarh area (Northern India) Atherosclerosis. 1976;24:355–61. doi: 10.1016/0021-9150(76)90127-1. [DOI] [PubMed] [Google Scholar]
- Kumar P, Luthra K, Dwivedi M, et al. Apolipoprotein E gene polymorphism in patients with premature myocardial infarction: a case-control study in northern Asian Indians. Ann Clin Biochem. 2003;40:382–6. doi: 10.1258/000456303766477020. [DOI] [PubMed] [Google Scholar]
- Luthra K, Bhargava B, Chhabra S, et al. Apolipoprotein E polymorphism in northern Indian patients with coronary heart disease: phenotype distribution and relation to serum lipids and lipoproteins. Mol Cell Biochem. 2002;232:97–102. doi: 10.1023/a:1014869827322. [DOI] [PubMed] [Google Scholar]
- [NCEP] National Cholesterol Education Program. Third report of the National Cholesterol Education Program expert panel on detection, evaluation and treatment of high blood cholesterol in adults (ATP-III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- O'Keefe JH, Harris WS, Nelson J, et al. Effects of pravastatin with niacin or magnesium on lipid levels ans postprandial lipemia. Am J Cardiol. 1995;6:480–4. doi: 10.1016/s0002-9149(99)80134-9. [DOI] [PubMed] [Google Scholar]
- Pais P, Pogue S, Gerstein H, et al. Risk factors for acute myocardial infarction in Indians: a case-control study. Lancet. 1996;348:358–63. doi: 10.1016/s0140-6736(96)02507-x. [DOI] [PubMed] [Google Scholar]
- Panacea-Biotec. Niacin extended release tablet pharmacokinetics. Data on file. 2004.
- Pasternak RC, Smith SC, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40:567–72. doi: 10.1016/s0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- Sahi N, Pahlajani DB, Sainani GS. Apolipoproteins Al and B as predictors of angiographicaIly assessed coronary artery disease. J Assoc Physicians India. 1993;41:713–15. [PubMed] [Google Scholar]
- Thompson GR. Combination therapy with statins. In: Gaw A, Packard CJ, Shepherd J, editors. Statins. 2nd edition. London: Martin Dunitz; 2004. pp. 189–99. [Google Scholar]
- Vacek JL, Dittmeir G, Chiarelli T, et al. Comparison of lovastatin (20 mg) and nicotinic acid (1.2 g) with either drug alone for type II hyperlipoprotenemia. Am J Cardiol. 1995;76:182–4. doi: 10.1016/s0002-9149(99)80056-3. [DOI] [PubMed] [Google Scholar]
- Vashist S, Narula J, Awtade A, et al. Lipids and lipoproteins in normal controls and clinically documented coronary heart disease patients. Ann Natl Acad Med Sci (India) 1990;26:57–66. [Google Scholar]
- Warnica JW. The CARE trial: the effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. In: Gaw A, Packard CJ, Shepherd J, editors. Statins. 2nd edition. London: Martin Dunitz; 2004. pp. 123–43. [Google Scholar]
- Wasir HS, Bharani A, Bhatia ML. Correlation of risk factors with coronary angiographic findings in patients of ischemic heart disease. J Assoc Physicians India. 1987;35:483–7. [PubMed] [Google Scholar]
- Wolfe ML, Vartianen SF, Ross JL, et al. Safety and effectiveness of Niaspan when added sequentially to a statin for treatment of dyslipidemia. Am J Cardiol. 2001;87:476–9. doi: 10.1016/s0002-9149(00)01410-7. [DOI] [PubMed] [Google Scholar]