Abstract
Initially considered as a semipermeable barrier separating lumen from vessel wall, the endothelium is now recognised as a complex endocrine organ responsible for a variety of physiological processes vital for vascular homeostasis. These include the regulation of vascular tone, luminal diameter, and blood flow; hemostasis and thrombolysis; platelet and leucocyte vessel-wall interactions; the regulation of vascular permeability; and tissue growth and remodelling. The endothelium modulates arterial stiffness, which precedes overt atherosclerosis and is an independent predictor of cardiovascular events. Unsurprisingly, dysfunction of the endothelium may be considered as an early and potentially reversible step in the process of atherogenesis and numerous methods have been developed to assess endothelial status and large artery stiffness. Methodology includes flow-mediated dilatation of the brachial artery, assessment of coronary flow reserve, carotid intimamedia thickness, pulse wave analysis, pulse wave velocity, and plethysmography. This review outlines the various modalities, indications, and limitations of available methods to assess arterial dysfunction and vascular risk.
Keywords: endothelial function, vascular risk, vascular stiffness
The vascular endothelium
The endothelium has an important role in maintaining vascular homeostasis. Although once considered simply as a semipermeable membrane, endothelial cells transduce a wide range of physiological stimuli, and in response, produce a variety of signalling molecules that exert autocrine and paracrine effects. The endothelium can therefore be considered as an important endocrine organ (Vanhoutte et al 1989; Vane et al 1990), and is responsible for maintaining vasomotor tone, hemostasis and thrombosis, inflammatory processes, platelet and leucocyte vessel-wall interactions, and controlling vascular permeability. The equilibrium between vasodilatation and vasoconstriction in regional vascular beds is largely controlled by the interaction between endothelium-derived vasoactive mediators and the vascular smooth muscle layer. Endothelial nitric oxide (NO), produced by constitutive activity of nitric oxide synthase (eNOS) (Schini-Kerth 1999), is a potent vasodilator and one of the most important regulators of vascular tone. In addition, NO is antiatherogenic, inhibiting platelet aggregation, smooth muscle proliferation, expression of adhesion molecules, and neutrophil aggregation (Vanhoutte et al 1989; Vane et al 1990). Arterial endothelial dysfunction is a key, early, and potentially reversible, event in the process of atherogenesis and is characterised by impaired NO bioavailability (Healy 1990; Ross 1993; Berliner et al 1995). Endothelial dysfunction causes impaired vasomotor responses to various neurohumoral stimuli which may contribute to transient myocardial ischemia, plaque rupture, thrombosis, and myocardial infarction (Maseri et al 1978). Endothelial dysfunction has so far been described in association with many established cardiovascular risk factors such as active and passive smoking, hypertension, hypercholesterolemia, obesity, and type II diabetes (McVeigh et al 1992; Anderson, Meredith, et al 1995; Treasure et al 1995; Goodfellow et al 1996; Williams et al 1996; Koller 2002; Williams et al 2002). The extent of endothelial dysfunction and vasomotor responsiveness correlates with the rate of progression of atherosclerosis and cardiovascular events (Schachinger et al 2000; Widlansky et al 2003). As such, endothelial function has importance, not only in determining predisposition to atherosclerotic disease, but also in determining prognosis in clinically affected patients.
Clinical assessment of endothelial function
Endothelial function can be measured in a variety of ways using invasive and noninvasive techniques in the coronary and peripheral circulation. The clinical examination of endothelial function involves assessing the ability of the endothelium to release NO in response to various exogenous and endogenous stimuli. The quantity of NO released from endothelial cells determines the degree of vasodilatation detected in clinical studies, and thus arterial “health”. Ludmer and collegues (1986) initially tested this concept by infusing acetylcholine into coronary arteries at angiography and measuring subsequent changes in arterial diameter. In healthy arteries, infusion of acetylcholine caused vasodilation, whereas vasoconstriction occurred in the presence of a damaged endothelial lining. Further evidence that the observed vasodilation was secondary to NO release was derived from studies which blocked dilatation using inhibitors of the L-arginine-NO pathway (Hodgson and Marshall 1989; Lefroy et al 1993). Subsequent studies using vasoactive pharmacological and physiological agents have confirmed differences in dilatation and endothelial responsiveness between healthy vessels and atherosclerotic vessels (Gollino et al 1991; Yeung et al 1991).
Coronary angiography
Methods of assessment
Quantitative coronary artery angiography of the left anterior descending artery and intracoronary doppler techniques have been applied to measure coronary artery vaso-responsiveness in response to endothelium-dependent agonists such as bradykinin and mechanical stimuli such as increased flow. Quantitative angiography can assess coronary arterial luminal diameter following cardiac catheterization, whilst doppler probes evaluate blood flow velocity in response to infusion of vasoactive agents (Groves et al 1995).
The integrity of coronary arteries has recently been evaluated angiographically via stimulation of the sympathetic nervous system using exercise and cold pressor tests. Sympathetic neuronal stimulation using cold pressor testing induces vasodilatation in healthy vasculature and vasoconstriction in proatherogenic disease states and atherosclerotic coronary arteries (Zeiher et al 1991; Antony et al 1994; Nitenberg et al 1998).
Although the coronary angiography technique is undoubtedly a useful tool for assessing vascular risk, widespread use is not practical. Coronary angiography is invasive and unsuitable for studying early preclinical atherosclerosis in asymptomatic subjects, or for the serial evaluation of vascular physiology in response to potential antiatherogenic strategies. Furthermore, its use is limited as a consequence of serious adverse reactions reported following the intracoronary infusion of acetylcholine at angiography (Tio et al 2002).
Association with coronary artery disease and atherosclerotic risk factors
Impaired vascular reactivity in the coronary artery circulation is associated with traditional coronary risk factors such as type 2 diabetes, insulin resistance, hypertension, and dyslipidemia (Nitenberg et al 1998; Dagres et al 2004; Mokelke et al 2005), even in the absence of clinically overt atherosclerotic lesions. Impaired vascular reactivity may also serve as an index integrating the overall stress imposed by coronary risk factors (Vogel and Corretti 1998). Coronary endothelial vasodilator dysfunction persists after angiographically significant coronary atherosclerotic plaque is evident and has been shown to predict long-term disease progression and cardiovascular event rates in patients at risk of coronary disease (Schachinger et al 2000). However, disease modifying agents which reduce cholesterol and exhibit antioxidant qualities improve coronary artery endothelium-dependent dilatation (Anderson, Meredith, et al 1995; Treasure et al 1995) and may reflect the cardioprotective qualities of these agents.
Flow-mediated dilatation
Method of assessment
A noninvasive technique using high-resolution ultrasound to overcome the practical constraints of invasive coronary artery testing has been developed to assess endothelial function in the peripheral circulation (Celermajer et al 1992). Using this technique, changes in brachial artery diameter are measured by following the endothelium-dependent stimulus of increased blood flow and may be compared with changes in vessel diameter following the oral administration of endothelium-independent agonists such as glyceryl trinitrate (GTN) (Celemajer et al 1992). Since endothelial dysfunction is a generalized systemic process, it occurs in both the coronary and systemic circulation (Anderson, Gerhard, et al 1995). Indeed, a close relationship has been demonstrated between vasodilator responses in the brachial artery and those in the coronary circulation (Anderson, Uehata, et al 1995; Matsuo et al 2004). The sensitivity of the original technique developed by Celermajer et al (1992) has been improved by using a validated computerized vessel wall tracking system (Vadirec Medical Systems® [Ramsey et al 1995]) to follow changes in brachial artery diameter throughout the cardiac cycle.
Many wall tracking systems have been developed to determine flow-mediated dilatation (FMD). One such system comprises an adapted duplex colour flow echo machine, giving high axial resolution (Ramsey et al 1995). With this technique, the brachial artery is identified using an ultrasound transducer with a stand-off device containing ultrasound coupling gel placed between the transducer and the arm to prevent compression of the anterior wall of the artery. Vessel wall movements are tracked using the stored radio frequency signals to produce displacement waveforms of the anterior and posterior walls together with the distension waveform (diameter change as a function of time [Hoeks et al 1990]). The distension waveform allows measurement of end-diastolic diameter for each beat, providing a theoretical resolution of 3 m. Forearm blood flow is measured throughout the study using a continuous wave doppler probe positioned over the brachial artery (Smith et al 2002). Once baseline measurements of brachial artery diameter are established, a cuff placed at the wrist is inflated to suprasystolic pressure, causing relative hand ischemia. Release of the occluding cuff results in reactive hand hyperemia and an associated increase in blood flow through the brachial artery, which induces shear stress on the arterial wall and provides a stimulus for endothelium-dependent dilatation. Similar measurements can also be made using a NO donor, eg, glyceryl trinitrate (GTN) for an assessment of endothelial-independent vasodilatation (Celermajer et al 1992).
A degree of investigator expertise is required to determine brachial artery vasodilation using ultrasonography and no consensus exists regarding the degree of vasodilation which should be expected in individuals with healthy endothelial function (Faulx et al 2003). Significant changes in brachial artery reactivity have been reported within healthy subjects throughout the course of a day when measured by the same operators, suggesting that variability occurs between morning and evening measurements, in addition to variability between subjects examined on different days (De Roos et al 2003). However, this is disputed by others who have carefully controlled for confounding factors (ter Avest et al 2005). Despite this, FMD will continue to be an extensively used technique for assessing endothelial function. Improvements in available equipment and operator expertise will reduce variability in results.
Association with coronary artery disease and atherosclerotic risk factors
The FMD technique is now one of the most widely used noninvasive methods of assessing endothelial function and closely correlates with cardiovascular risk (Kuvin and Karas 2003). Impaired FMD is described in insulin resistant states and type 2 diabetes, dyslipidemia, hypertension, end stage renal disease, and smoking (Yildiz et al 2003; Esen et al 2004; Holay et al 2004; Thomas et al 2004). Subsequently, FMD has been used extensively to assess the potential antiatherogenic qualities of treatment options, and continues to be indispensable in determining endothelial integrity.
Carotid intimamedial thickness
Method of assessment
Another noninvasive method of assessing subclinical atherosclerosis involves measurements of carotid intimamedia thickness (IMT) with high resolution B-mode ultrasonography. This is a well established technique which has been extensively used to estimate coronary artery events and the extent of established atherosclerosis in central and peripheral vasculature (Bots et al 1993), with early increases in IMT possibly reflecting adaptation to elevated intravascular shear stress (Bots et al 1997). As a noninvasive imaging technique, quantitative carotid IMT is versatile for use in large populations with minimal risk to subjects (Barth 2004). However, accurate measurements, particularly of the near wall, require a high level of technical expertise. Consequently, some authors suggest the administration of contrast media during the examination period (Macioch et al 2004; Martin and Lekaris 2004).
A variety of techniques have been used in the determination of carotid IMT. Measurements of the common carotid, internal carotid, and carotid bifurcation are all technically acceptable, including combination measurements of 12 carotid arterial sites, eg, using Meijer's Arch (Bots et al 2003). In view of the diversity and lack of uniformity in determining carotid IMT, meta-analysis suggests that circumferential scanning of the carotid artery and calculation of the mean maximum carotid IMT provides a more accurate measurement of carotid atherosclerosis (Bots et al 2003). However, all sites of carotid IMT measurement appear to have equivalent value in predicting future coronary artery events (Iglesias del Sol et al 2002).
Association with coronary artery disease and atherosclerotic risk factors
Carotid IMT correlates with cardiovascular risk factors such as the ‘metabolic syndrome’ (McNeill et al 2004), insulin resistance in type 1 and 2 diabetics (Fujiwara et al 2003; Singh 2003), microalbuminuria (Jadhav and Kadam 2002), hypercholesterolemia (Wendelhag et al 1992), and atherogenesis (Salonen and Salonen 1993).
Carotid IMT and the progression of IMT correlates well with cardiovascular and cerebrovascular end-points (Bots et al 1997; Hodis et al 1998; O'Leary et al 1999). However, although extensive data supports the use of carotid IMT as a predictor of cardiovascular risk, endothelial dysfunction manifesting as impaired brachial artery reactivity may be an earlier predictor of coronary artery disease, with increased carotid IMT being evident at a later stage in the process of atherogenesis (Furumoto et al 2002).
Cardiovascular risk and vascular stiffness
Large artery stiffness
Arteriosclerosis is an integral part of the aging process (Pearson et al 1994) and is now also recognised as an important and independent risk factor for cardiovascular disease (Arnett et al 1994). As a result of vascular stiffening, the diastolic blood pressure decreases and pulse pressure widens, as occurs with advancing age (Franklin et al 1997). Consequently, brachial artery pulse pressure is a surrogate marker of vascular stiffness and is used to determine cardiovascular risk in normotensive and hypertensive subjects (Benetos et al 1997, 1998; Franklin et al 1999), having a higher predictive value of cardiovascular risk than mean arterial blood pressure (Domanski et al 1999; Miller et al 1999). Isolated systolic hypertension and elevated pulse pressure have also been identified as a major cardiovascular risk factor in the Systolic Hypertension in the Elderly Programme and the Systolic Hypertension in Europe trial (Frishman 2000).
Confirming the association between pulse pressure and cardiovascular risk, Philippe et al (2002) demonstrated a direct correlation between aortic pulse pressure, measured with intra-aortic balloons at coronary angiography, and the extent of atherosclerotic disease. Wave reflection within the vascular tree leads to a higher pulse pressure in central vessels than in the periphery (Pauca et al 1992; Nichols and O'Rourke 1998, 2005). The resulting increase in left ventricular after-load increases myocardial oxygen consumption and promotes left ventricular hypertrophy. In addition, increasing systolic pressure elevates arterial wall circumferential stress and predisposes to atherosclerotic plaque generation. Left ventricular hypertrophy that arises from increased aortic systolic pressure (Lakatta 1991) predisposes to coronary artery disease (MacMahon et al 1990), cerebrovascular events (Kannel et al 1981), and is an independent predictor of cardiovascular mortality (Levy et al 1990). Furthermore, increased arterial stiffening has also been demonstrated in subjects with increased cardiovascular risk including diabetes mellitus (Wahlqvist et al 1988, Salomaa et al 1995), hypertension (McVeigh et al 1991, Armentano et al 1991), hypercholesterolemia (Dart et al 1991, Giannattasio et al 1996), and end stage renal disease (Blacher et al 2002).
Regulation of large artery stiffness
Elastin and collagen are the major determinants of large artery stiffness, with smooth muscle originally thought to play a minor role (Wilkinson and McEniery 2004). Advancing age causes gradual destruction in arterial wall elasticity, with increasing demands placed on the collagenous element (O'Rourke 1976; Avolio et al 1983). This redistribution of the heterogeneous element in the vascular wall can be triggered by endothelial dysfunction (Nili et al 2002). However, recent work has highlighted the importance of smooth muscle in determining vessel stiffening. Vasoconstrictors such as noradrenaline can increase vascular stiffness and dilators such as hydrallazine and sodium nitroprusside have the opposite effect (Nichols and O'Rourke 2005). Such vasoactive pharmacological agents can alter vessel diameter by up to 30%, independent of changes in peripheral resistance or blood pressure (Latson et al 1988), demonstrating the important dynamic contribution of vascular smooth muscle to large vessel stiffness.
Whilst NO profoundly alters basal arterial tone, its effect on arterial stiffness remains unclear. However, the acute administration of a NO donor such as GTN produces changes in the peripheral pressure pulse that are consistent with a reduction in arterial stiffness (Cockcroft et al 1997). β-adrenergic drugs, in particular albuterol, act via the L-arginine-NO pathway to stimulate NO release and cause vasodilatation in resistance arteries (Cardillo et al 1997; Dawes et al 1997) via their action on β2-adrenoreceptors. β2-agonists have therefore been used to evaluate endothelial integrity in healthy controls (Hayward et al 2002).
Clinical assessment of arterial stiffness
Pulse wave analysis and pulse wave velocity
Method of assessment
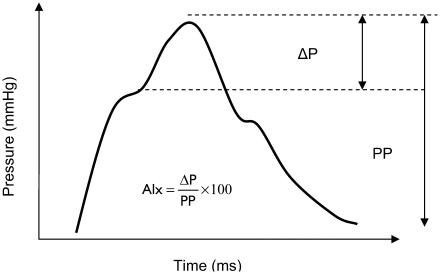
The peripheral pressure waveform can be useful in determining cardiovascular risk. However, an adequate assessment of central arterial pressure waveforms cannot be determined from peripheral pulse wave analysis (PWA) because changes in vessel stiffening throughout the vascular tree causes location-dependent changes in the pressure waveform. In addition, central arterial waveforms will be influenced by the reflective wave phenomenon as described by Nichols and O'Rourke (2005). The systolic waveform leaves the aortic root and travels to the periphery, where smaller arterioles provide multiple points of reflection. A resulting ‘reflective’ wave is generated and returns to central arteries (O'Rourke and Kelly 1993). In individuals with healthy compliant arteries, the reflective wave will return to the central vasculature in diastole and augment diastolic coronary arterial blood flow. The speed of the advancing wave is termed pulse wave velocity (PWV) (Lehmann et al 1997). With age, a combination of increased reflective capacity at peripheral sites and faster PWV within stiffened vessels causes premature augmentation of the systolic waveform, forming a ‘late systolic peak’ on waveform analysis (Figure 1). This explains the differences between the brachial and aortic pressure waveforms, which may be as high as 20 mmHg (Pauca et al 1992). The central pressure waveform is important in view of determining left ventricular workload, which is relatively independent of the brachial pressure.
Figure 1.
Representation of central arterial waveform.
Abbreviations: Alx; Augmentation Index; PP, pulse pressure.
In view of the above observations, a technique has been derived by O'Rourke and Gallagher (1996), which is able to noninvasively record the peripheral pulse pressure wave and generate a corresponding central arterial waveform. The technique involves the use of an applanation tonometer to record the radial pulse wave. Applanation tonometry causes partial flattening of the arterial wall and equilibration of intra-arterial circumferential pressure. The accuracy of arterial tonometry in recording peripheral waveforms has been described by previous investigators who evaluated waveforms derived from noninvasive tonometry and direct arterial puncture (Cohn et al 1995). The central arterial waveform can subsequently be derived from the peripheral waveform using a validated generalized transfer factor (Karamanoglu et al 1993; Chen et al 1997; Takazawa et al 1998; Fetics et al 1999). This is then expressed in terms of an Augmentation Index (AIx) which can be used to assess vascular stiffness and cardiovascular risk between study groups.
Pulse wave analysis has more recently been used as a noninvasive tool to assess endothelial function (Wilkinson, Hall, et al 2002). Administration of β-agonist therapy induces repeatable reductions in the AIx, which are inhibited by infusion of L-NG-monomethyl arginine (L-NMMA), suggesting that observed differences are endothelial- and NO-dependent (Wilkinson, Hall, et al 2002). Nitroglycerin administration induces further reductions in the AIx which are unaffected by L-NMMA and thus endothelial-independent (Wilkinson, Hall, et al 2002). Furthermore, the technique has been shown to correlate with FMD in healthy and type 2 diabetic subjects (Wilson et al 2004).
The analysis of PWV uses a similar system that calculates pulse wave propagation velocity between two sites, commonly the carotid and femoral pulses, or carotid and radial (Oliver and Webb 2003), although brachial-ankle PWV has been assessed by some (Katayama et al 2004; Igarashi et al 2005). Pulse wave velocity is inversely proportional to vessel stiffness and distensibility (Nichols and O'Rourke 2005). Waveform data is recorded from two sites using noninvasive tonometry and stored electronically. Following documentation of the distance between the two recording sites, determination of the pulse transit time allows calculation of PWV. In order to assess pulse transit time a correlation point is identified within the pressure waveform, which may be the foot of the pressure wave (using SphygmoCor system) or the point of maximal upstroke (using Complior system) (Millasseau et al 2005). If the two pressure waveforms are not recorded simultaneously, an R wave on the electrocardiograph can be used to calculate wave transit time. Elevation of PWV leads to augmentation of the ascending aortic systolic waveform as previously outlined, resulting in higher left ventricular afterload and amplification of pulse pressure (Nichols 2005).
Pulse wave analysis and PWV are both noninvasive simple techniques that can be used to assess vascular stiffness in research and clinical settings (O’Rourke and Gallagher 1996; Hayward et al 2002; Sutton-Tyrrell et al 2005). Both techniques are influenced by factors that may confound data. For example, elevation in pulse rate will lower the AIx as a result of a reduction in reflective wave amplitude, and does not represent a change in vascular stiffness (Wilkinson, Mohammed, et al 2002). Consequently, a correction factor has been suggested to standardize for variation in heart rate (Wilkinson et al 2000). In addition, an inverse relationship between AIx, PWV, and height has been described, which may result from shorter reflective wave propagation time in individuals with short stature (McGrath et al 2001). While some investigators consider this a confounding variable in data analysis, it may explain the elevation in cardiovascular risk observed in short individuals (Kannam et al 1994). Despite the lack of data to confirm correlation between noninvasive and invasive measurements of PWV (Chiu et al 1991; Davies and Struthers 2003), the evidence that tonometer-derived PWV is an important determinant of cardiovascular risk is difficult to dispute.
Association with coronary artery disease and atherosclerotic risk factors
Pulse wave analysis has revealed accelerated large artery stiffening and endothelial dysfunction in association with several well established cardiovascular risk factors such as obesity (Suh et al 2005), end-stage renal failure (Covic et al 2003), and hypercholesterolemia (Wilkinson, Prasad, et al 2002). Similarly, PWV is increased in microalbuminuria (Smith et al 2005), renal dysfunction (Haydar et al 2004), type 2 diabetes (Tsuchiya et al 2004), and insulin resistance (Sengstock et al 2005).
Using PWA and PWV, vascular stiffness has been assessed and identified as an independent risk marker for cardiovascular mortality (Laurent et al 2001; Meaume et al 2001) and cerebrovascular events (Laurent and Boutouyrie 2005), and has a prognostic value equivalent to currently available biomarkers.
Elevations of central systolic pressure and consequent proatherosclerotic effects can be modified by vasodilatory compounds which reduce PWV in peripheral vessels without altering brachial blood pressure. Such reductions in augmentation of central pressure may underlie the observed benefits of cardiovascular drugs which are not currently attributed to blood pressure modification (Nichols 2005). Vascular stiffness has consequently gained increasing importance as a therapeutic target.
Photophlesymographic assessment of pulse wave reflection
Method of assessment
Photophlethysmography involves measuring the digital volume pulse via infrared light transmission through the finger. Previous investigators have demonstrated that the digital volume pulse resembles the carotid pressure wave and alters in a similar way to vasoactive mediators (Takazawa et al 1998). Reflected peripheral waveforms cause a second peak in the digital volume pulse (DVP) in a similar fashion to that seen in the peripheral pressure waveform, as measured using PWA (Chowienczyk et al 1999). With this technique, the point at which the reflective wave meets the systolic waveform is termed the inflection point (IP). When suprasystolic pressure is applied to both lower limbs at thigh level, the reflective wave returns sooner and causes an expected elevation of the IP (Chowienczyk et al 1999). In a similar way to the peripheral pressure wave, the DVP undergoes changes in response to exogenous NO donors such as GTN (Morikawa 1967; Lund 1986), which is independent of changes in heart rate (Chowienczyk et al 1999). Using both techniques, the major change seen is a reduction in the diastolic component of the waveform and the preceding IP (Chowienczyk et al 1999). This has been demonstrated recently in vivo, where acetylcholine-induced endothelial-derived NO release resulted in lowering of the IP in the photophlethysmographic waveform recorded in cholesterol-fed rabbits. This response is diminished in cholesterol-fed rabbits when compared with healthy rabbits and antagonised by NOS inhibitors (Klemsdal et al 1994). As with PWA, pharmacological preparations which induce NO release, such as β-adrenergic drugs, will also cause a reduction in the IP of the pulse volume waveform (Chowienczyk et al 1999). Such effects are blunted by L-NMMA administration which suggests that this effect involves the L-arginine-NO pathway (Chowienczyk et al 1999).
Association with coronary artery disease and atherosclerotic risk factors
With evidence supporting the use of this technique to assess both endothelial-dependent and endothelial-independent vasodilatation, investigators have studied patient groups who are known to demonstrate marked endothelial dysfunction. Photophlethysmographic examination of type 2 diabetics has shown impairment of albuterol-induced responses with preservation of the endothelial-independent vasodilatation seen with GTN (Chowienczyk et al 1999). Vascular stiffness using this technique has also been described in cases of impaired glucose tolerance (Ohshita et al 2004) and hypertension in aging individuals, although some authors believe the technique to be inferior to PWV (Bortolotto et al 2000). However, this noninvasive technique provides a useful method of assessing vascular stiffening, in which both endothelial-dependent and independent responses can be determined (Millasseau et al 2002).
Biomarkers of endothelial function
As outlined previously, the antiatherogenic functions of the endothelium are complex. Several biochemical markers have been identified that correlate with coronary artery disease and conventional cardiovascular risk factors (Szmitko et al 2003). Further investigation of the inflammatory and thrombotic processes involved in atherogenesis will allow the assessment of potential biomarkers which may be incorporated into current cardiovascular risk stratification models. Such biomarkers include, oxidized low-density lipoprotein (oxLDL), high sensitivity C-reactive protein (CRP), endothelial progenitor cells (EPC), prothrombotic factors such as von Willebrand factor (VWF), and inflammatory markers including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and intracellular adhesion molecule-1 (ICAM-1) (Ridker et al 2001; Szmitko et al 2003). The clinical use of many of the biomarkers is restricted due to lack of end-point data (Tsimikas and Witztum 2001), with the exception of CRP which may correlate to a greater extent than traditional risk factors such as LDL-cholesterol (Ridker et al 2005). In consequence, CRP has already been incorporated into some risk stratification models.
Oxidized LDL
Oxidation of LDL occurs within the subendothelial cells of the vascular tissue which promotes the binding and transformation of monocytes to foam cells (Parhami et al 1993; Watson et al 1997). Mechanisms include enhancing chemotaxis and monocyte adhesion, upregulation of inflammatory genes and growth factors, causing endothelial cell dysfunction and apoptosis, enhancing platelet aggregation with thrombus formation, and inducing plaque destabilisation (Berliner et al 1995; Aikawa et al 1998; Norata et al 2002). Consequently, oxLDL levels are increased in cases of acute coronary syndrome and myocardial infarction (Tsimikas et al 2003).
Prothrombotic factors
The prerequisites for thrombogenesis in atheromatous plaques include activation of the coagulation cascade and platelet activation (Smith et al 2003). As such, prothrombotic factors are often evaluated in studies aimed at assessing cardiovascular risk. von Willebrand factor is a multimeric glycoprotein that is synthesised in endothelial cells and released following endothelial damage (Mannucci 1998). As such, levels can be indicative of the degree of endothelial injury and subsequent atherosclerotic potential (Mannucci 1998). Prospective trials suggest that elevated VWF may predict future cardiovascular events in patients with established coronary atherosclerosis (Jansson et al 1991; Thompson et al 1995), which may be a reflection of the role of VWF in initiating platelet aggregation and thrombus formation (Mannucci 1998). von Willebrand factor also has a role in stabilising factor VIII and the latter, together with fibrinogen, have been incorporated into cardiovascular risk profiles in numerous studies (Wilhelmsen et al 1984; Folsom et al 1999; Chambless et al 2003; Chaves et al 2004).
Endothelial progenitor cells
Endothelial progenitor cells have also gained importance as a potential surrogate marker of endothelial health (Schmidt-Lucke et al 2005). These are essentially stem cells which are recruited to sites of endothelial injury in order to perform a therapeutic function by differentiating into mature endothelial cells (Szmitko et al 2003). Depletion of circulating EPCs with impaired adhesion to vasculature may reflect repeated and enhanced demand for EPC mobilization from bone marrow, and indicate a state of endothelial dysfunction predisposing to enhanced cardiovascular risk (Hill et al 2003). In support of this, reduced EPC levels with impaired activity have been demonstrated in subjects known to have impaired endothelial function, such as hypertension and ischemic heart disease (Vasa et al 2001) and their use for therapeutic intervention of vascular dysfunction continues to be evaluated (Silva et al 2005).
Inflammatory markers
Several inflammatory markers have been described in association with cardiovascular risk, including TNF-α, IL-6, ICAM-1, and CRP, of which CRP has greatest prognostic value (Ridker et al 2001; Ridker 2002). CRP is not only a marker of cardiovascular risk, but may itself function as a proatherogenic molecule (Szmitko et al 2003). The acute phase reactant has been demonstrated to enhance proinflammatory cytokines, such as IL-6, TNF-α, and monocyte chemoattractant protein-1 (MCP-1), promoting chemotaxis and lipid accumulation (Verma, Li, et al 2002; Li and Fang 2004). In addition, CRP may interfere with NO synthesis, inhibit angiogenesis, and influence vascular remodelling (Verma, Wang, et al 2002; Wang et al 2003). Although CRP correlates well with atherogenesis, it is feasible that elevation reflects the impact of traditional risk factors on inflammatory processes, as opposed to the direct influence of CRP on endothelial function (Vita et al 2004; Verma et al 2004).
Summary
As the number of individuals suffering from the ‘metabolic syndrome’ escalates, the cardiovascular morbidity and mortality rates of future generations may continue to rise despite advances in pharmaceutical interventions. Disruption of endothelial function is multifactorial and complex, and precedes clinically apparent coronary and cerebrovascular disease.
Therefore, the evaluation of reproducible, noninvasive techniques for assessing endothelial function should enable screening of large populations and may guide interventions designed specifically to reduce the individual's vascular risk.
References
- Aikawa M, Rabkin E, Okada Y, et al. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilisation. Circulation. 1998;97:2433–44. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Meredith IT, Yeung AC, et al. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Eng J Med. 1995;332:488–93. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Gerhard MD, Meredith IT, et al. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulation. J Am Coll Cardiol. 1995;26:1235–41. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Antony I, Aptecar E, Lerebours G, et al. Coronary artery constriction caused by the cold pressor test in human hypertension. Hypertension. 1994;24:212–19. doi: 10.1161/01.hyp.24.2.212. [DOI] [PubMed] [Google Scholar]
- Armentano R, Simon A, Levenson J, et al. Mechanical pressure versus intrinsic effects of hypertension on large arteries in humans. Hypertension. 1991;18:657–64. doi: 10.1161/01.hyp.18.5.657. [DOI] [PubMed] [Google Scholar]
- Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor. Am J Epidemiol. 1994;140:669–82. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- Avolio AP, Chen SG, Wang RP, et al. Effects of ageing on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–8. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- Barth JD. Carotid intima media thickness and beyond. Curr Drug Targets Cardiovasc Hematol Disord. 2004;4:129–45. doi: 10.2174/1568006043336339. [DOI] [PubMed] [Google Scholar]
- Benetos A, Rudnichi A, Safar M, et al. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension. 1998;32:560–4. doi: 10.1161/01.hyp.32.3.560. [DOI] [PubMed] [Google Scholar]
- Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–15. doi: 10.1161/01.hyp.30.6.1410. [DOI] [PubMed] [Google Scholar]
- Berliner J, Navab M, Fogelman A, et al. Atherosclerosis: basic mechanisms. Circulation. 1995;91:2488–96. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- Blacher J, Safar ME, Pannier B, et al. Prognostic significance of arterial stiffness measurements in end-stage renal disease patients. Curr Opin Nephrol Hypertens. 2002;11:629–34. doi: 10.1097/00041552-200211000-00010. [DOI] [PubMed] [Google Scholar]
- Bortolotto LA, Blacher J, Kondo T, et al. Assessment of vascular aging and atherosclerosis in hypertensive subjects: second derivative of photophlethysmogram versus pulse wave velocity. Am J Hypertens. 2000;13:165–71. doi: 10.1016/s0895-7061(99)00192-2. [DOI] [PubMed] [Google Scholar]
- Bots ML, Evans GW, Riley WA, et al. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations; a point of view. Stroke. 2003;34:2985–94. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- Bots ML, Hoes AW, Koudstaal PJ, et al. Common carotid intimamedia thickness and risk of stroke and myocardial infarction: the Rotterdam study. Circulation. 1997;96:1432–7. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- Bots ML, Witteman JC, Grobbee DE. Carotid intima-media wall thickness in elderly women with and without atherosclerosis of the abdominal aorta. Atherosclerosis. 1993;102:99–105. doi: 10.1016/0021-9150(93)90088-c. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Kilcoyne CM, Quyyumi AA, et al. Decreased vasodilator response to isoproterenol during nitric oxide inhibition in humans. Hypertension. 1997;30:918–21. doi: 10.1161/01.hyp.30.4.918. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch V, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–15. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Chambless LE, Folsom AR, Sharrett AR, et al. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–90. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- Chaves PH, Kuller LH, O'Leary DH, et al. Subclinical cardiovascular disease on older adults: insights from the Cardiovascular Health Study. Am J Geriatr Cardiol. 2004;13:137–51. doi: 10.1111/j.1076-7460.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Circulation. 1997;95:1827–36. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Arand PW, Shroff SG, et al. Determination of pulse wave velocities with computerised algorithms. Am Heart J. 1991;121:1460–70. doi: 10.1016/0002-8703(91)90153-9. [DOI] [PubMed] [Google Scholar]
- Chowienczyk P, Kelly R, MacCallum H, et al. Photophlesymographic assessment of pulse wave reflection: blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol. 1999;34:2007–14. doi: 10.1016/s0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- Cockcroft JR, Wilkinson IB, Webb DJ. The Trevor Howell Lecture. Age, arterial stiffness and the endothelium. Age Ageing. 1997;26(Suppl 4):53–60. doi: 10.1093/ageing/26.suppl_4.53. [DOI] [PubMed] [Google Scholar]
- Cohn J, Finkelstein S, McVeigh G, et al. Non-invasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26:503–8. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- Covic A, Goldsmith DJ, Gusbeth-Tatomir P, et al. Successful renal transplantation decreases aortic stiffness and increases vascular reactivity in dialysis patients. Transplantation. 2003;76:1573–7. doi: 10.1097/01.TP.0000086343.32903.A8. [DOI] [PubMed] [Google Scholar]
- Dagres N, Saller B, Haude M, et al. Insulin sensitivity and coronary vasoreactivity: insulin sensitivity relates to adenosine-stimulated coronary flow response in human subjects. Clin Endocrinol (Oxf) 2004;61:724–31. doi: 10.1111/j.1365-2265.2004.02156.x. [DOI] [PubMed] [Google Scholar]
- Dart AM, Lancombe F, Yeoh JK, et al. Aortic distensibility in patients with isolated hypercholesterolaemia, coronary artery disease, or cardiac transplantation. Lancet. 1991;338:270–3. doi: 10.1016/0140-6736(91)90415-l. [DOI] [PubMed] [Google Scholar]
- Davies JI, Struthers A. Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens. 2003;21:463–72. doi: 10.1097/00004872-200303000-00004. [DOI] [PubMed] [Google Scholar]
- Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the L-arginine/nitric oxide pathway on vasodilation caused by beta-adrenergic agonists in human forearm. Circulation. 1997;95:2293–7. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- De Roos NM, Bots ML, Schouten E, et al. Within subject variability of flow mediated vasodilation of the brachial artery in healthy men and women: implications for experimental studies. Ultrasound Med Biol. 2003;29:401–6. doi: 10.1016/s0301-5629(02)00709-3. [DOI] [PubMed] [Google Scholar]
- Domanski MJ, Davis BR, Pfeffer MA, et al. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34:375–80. doi: 10.1161/01.hyp.34.3.375. [DOI] [PubMed] [Google Scholar]
- Esen AM, Barutcu I, Acar M, et al. Effect of smoking on endothelial function and wall thickness of brachial artery. Circ Res. 2004;68:1123–6. doi: 10.1253/circj.68.1123. [DOI] [PubMed] [Google Scholar]
- Faulx MD, Wright AT, Hoit BD. Detection of endothelial dysfunction with brachial artery ultrasound scanning. Am Heart J. 2003;145:943–51. doi: 10.1016/S0002-8703(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Fetics B, Nevo E, Chen CH, et al. Parametric model derivation of transfer function for non-invasive estimation of aortic pressure by radial tonometry. IEEE Trans Biomed Eng. 1999;46:698–706. doi: 10.1109/10.764946. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Rosamond WD, Shahar E, et al. Prospective study of markers of hemostatic function with risk of ischaemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1999;100:736–42. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation. 1999;100:354–60. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Gustin W, 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure: The Framingham Heart Study. Circulation. 1997;96:308–15. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- Frishman WH. Increased vascular compliance/decreased cardiovascular risk: what the studies tell us. Heart Dis. 2000;2:384–8. [PubMed] [Google Scholar]
- Fujiwara S, Emoto M, Komatsu M, et al. Arterial wall thickness is associated with insulin resistance in type 2 diabetic patients. J Atheroscler Thromb. 2003;10:246–52. doi: 10.5551/jat.10.246. [DOI] [PubMed] [Google Scholar]
- Furumoto T, Fujii S, Saito N, et al. Relationships between brachial artery flow mediated dilation and carotid artery intima-media thickness in patients with suspected coronary artery disease. Jpn Heart J. 2002;43:117–25. doi: 10.1536/jhj.43.117. [DOI] [PubMed] [Google Scholar]
- Giannattasio C, Mangonic AA, Failla M, et al. Impaired radial artery compliance in normotensive subjects with familial hypercholesterolemia. Atherosclerosis. 1996;124:249–60. doi: 10.1016/0021-9150(96)05834-0. [DOI] [PubMed] [Google Scholar]
- Gollino P, Piscione F, Willerson JT, et al. Divergent effects of serotonin on coronary artery dimensions and blood flow in patients with coronary atherosclerosis and control patients. N Engl J Med. 1991;324:641–8. doi: 10.1056/NEJM199103073241001. [DOI] [PubMed] [Google Scholar]
- Goodfellow J, Ramsey MW, Luddington LA, et al. Endothelium and inelastic arteries: an early marker of vascular dysfunction in non-insulin dependent diabetes. Br Med J. 1996;312:744–5. doi: 10.1136/bmj.312.7033.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves P, Kurz S, Just H, et al. Role of endogenous bradykinin in human coronary vasomotor control. Circulation. 1995;92:3424–30. doi: 10.1161/01.cir.92.12.3424. [DOI] [PubMed] [Google Scholar]
- Haydar AA, Covic A, Colhoun H, et al. Coronary artery calcification and aortic pulse wave velocity in chronic kidney disease patients. Kidney Int. 2004;65:1790–4. doi: 10.1111/j.1523-1755.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- Hayward CS, Kraidly M, Webb CM, et al. Assessment of endothelial function using peripheral waveform analysis: a clinical application. J Am Coll Cardiol. 2002;40:521–8. doi: 10.1016/s0735-1097(02)01991-5. [DOI] [PubMed] [Google Scholar]
- Healy B. Endothelial cell dysfunction: an emerging endocrinopathy linked to coronary disease. J Am Coll Cardiol. 1990;16:357–8. doi: 10.1016/0735-1097(90)90585-d. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function and cardiovascular risk. N Eng J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Hodgson JM, Marshall JJ. Direct vasoconstriction and endothelium-dependent vasodilation: mechanisms of acetylcholine effects on coronary flow and arterial diameter in patients with nonstenotic coronary arteries. Circulation. 1989;79:1043–51. doi: 10.1161/01.cir.79.5.1043. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- Hoeks A, Brands P, Smeets F, et al. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990;16:121–8. doi: 10.1016/0301-5629(90)90139-4. [DOI] [PubMed] [Google Scholar]
- Holay MP, Paunikar NP, Joshi PP, et al. Effect of passive smoking on endothelial function in healthy adults. J Assoc Physicians India. 2004;52:114–17. [PubMed] [Google Scholar]
- Igarashi Y, Chikamori T, Tomiyama H, et al. Diagnostic value of simultaneous brachial and ankle blood pressure measurements for the extent and severity of coronary artery disease as assessed by myocardial perfusion imaging. Circ J. 2005;69:237–42. doi: 10.1253/circj.69.237. [DOI] [PubMed] [Google Scholar]
- Iglesias del Sol A, Bots ML, Grobbee DE, et al. Carotid intimamedia thickness at different sites: relation to incident myocardial infarction; The Rotterdam Study. Eur Heart J. 2002;23:916–18. doi: 10.1053/euhj.2001.2965. [DOI] [PubMed] [Google Scholar]
- Jadhav UM, Kadam NN. Association of microalbuminuria with carotid intima-media thickness and coronary artery disease – a cross sectional study in Western India. 2002. pp. 1124–9. [PubMed]
- Jansson JH, Nilsson TK, Johnson O. von Willebrand factor in plasma: a novel risk factor for recurrent myocardial infarction and death. Br Heart J. 1991;66:351–5. doi: 10.1136/hrt.66.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannam JP, Levy D, Larson M, et al. Short stature and risk for mortality and cardiovascular disease events. The Framingham Heart Study. Circulation. 1994;90:2241–7. doi: 10.1161/01.cir.90.5.2241. [DOI] [PubMed] [Google Scholar]
- Kannell WB, Wolf PA, McGee DL, et al. Systolic blood pressure, arterial rigidity, and risk of stroke. JAMA. 1981;245:1225–9. [PubMed] [Google Scholar]
- Karamanoglu M, O'Rourke MF, Avolio AP, et al. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–7. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Shige H, Yamamoto A, et al. Oral vitamin C ameliorates smoking-induced arterial wall stiffness in healthy volunteers. J Atheroscler Thromb. 2004;11:354–7. doi: 10.5551/jat.11.354. [DOI] [PubMed] [Google Scholar]
- Klemsdal TO, Andersson TL, Matz J, et al. Vitamin E restores endothelium dependent vasodilatation in cholesterol fed rabbits: in vivo measurements by photophlesymography. Cardiovasc Res. 1994;28:1397–1402. doi: 10.1093/cvr/28.9.1397. [DOI] [PubMed] [Google Scholar]
- Koller A. Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation. 2002;9:277–94. doi: 10.1038/sj.mn.7800142. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Karas RH. Clinical utility of endothelial function testing: ready for prime time? Circulation. 2003;107:3243–7. doi: 10.1161/01.CIR.0000075928.54461.33. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Similar myocardial effects of aging and hypertension. Eur Heart J. 1991;11:29–38. doi: 10.1093/eurheartj/11.suppl_g.29. [DOI] [PubMed] [Google Scholar]
- Latson TW, Hunter WC, Katoh N, et al. Effect of nitroglycerin on aortic impedance, diameter, and pulse-wave velocity. Circ Res. 1988;62:884–90. doi: 10.1161/01.res.62.5.884. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P. Arterial stiffness and stroke in hypertension; therapeutic implications for stroke prevention. CNS Drugs. 2005;19:1–11. doi: 10.2165/00023210-200519010-00001. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Lefroy DC, Crake T, Uren NG, et al. Effect of inhibition of NO synthesis on epicardial coronary artery caliber and coronary blood flow in humans. Circulation. 1993;88:43–54. doi: 10.1161/01.cir.88.1.43. [DOI] [PubMed] [Google Scholar]
- Lehmann ED, Riley WA, Clarkson P, et al. Non-invasive assessment of cardiovascular disease in diabetes mellitus. Lancet. 1997;350(Suppl 1):SI14–19. doi: 10.1016/s0140-6736(97)90023-4. [DOI] [PubMed] [Google Scholar]
- Levy D, Garrison R, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;332:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- Li JJ, Fang CH. C-reactive protein is not only an inflammatory marker but also a direct cause of cardiovascular diseases. Med Hypotheses. 2004;62:499–506. doi: 10.1016/j.mehy.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–51. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- Lund F. Digital pulse plethysmography (DPG) in studies of the hemodynamic responses to nitrates – a survey of recording methods and principles of analysis. Acta Pharmacol Toxicol (Copenh) 1986;59(Suppl 6):79–96. doi: 10.1111/j.1600-0773.1986.tb02551.x. [DOI] [PubMed] [Google Scholar]
- Macioch JE, Katsamakis CD, Robin J, et al. Effect of contrast enhancement on measurement of carotid artery intimal medial thickness. Vasc Med. 2004;9:7–12. doi: 10.1191/1358863x04vm522oa. [DOI] [PubMed] [Google Scholar]
- MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for regression dilution bias. Lancet. 1990;335:765–74. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- Mannucci PM. von Willebrand factor: a marker of endothelial damage? Arteriscler Thromb Vasc Biol. 1998;18:1359–62. doi: 10.1161/01.atv.18.9.1359. [DOI] [PubMed] [Google Scholar]
- Martin RP, Lerakis S. Contrast for vascular imaging. Cardiol Clin. 2004;22:313–20. doi: 10.1016/j.ccl.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Maseri A, L'Abbate A, Baroldi G, et al. Coronary vasospasm as a possible cause of myocardial infarction: a conclusion derived from the study of “preinfarction” angina. N Eng J Med. 1978;229:1271–7. doi: 10.1056/NEJM197812072992303. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Matsumoto T, Takashima H, et al. The relationship between flow mediated brachial artery vasodilation and coronary vasomotor responses to bradykinin: comparison with those to acetylcholine. J Cardiovasc Pharmacol. 2004;44:164–70. doi: 10.1097/00005344-200408000-00004. [DOI] [PubMed] [Google Scholar]
- McGrath BP, Liang YL, Kotsopoulos D, et al. Impact of physical and physiological factors on arterial function. Clin Exp Pharmacol Physiol. 2001;28:1104–7. doi: 10.1046/j.1440-1681.2001.03591.x. [DOI] [PubMed] [Google Scholar]
- McNeill AM, Rosamond WD, Girman CJ, et al. Prevalence of coronary heart disease and carotid arterial thickening in patients with the metabolic syndrome (The ARIC study) Am J Cardiol. 2004;94:1249–54. doi: 10.1016/j.amjcard.2004.07.107. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Johnston GD, et al. Impaired endothelium-dependent and independent vasodilatation in patients with type 2 (non-insulin dependent) diabetes mellitus. Diabetologia. 1992;35:771–6. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Burns DE, Finkelstein SM, et al. Reduced vascular compliance as a marker for essential hypertension. Am J Hypertens. 1991;4:245–51. doi: 10.1093/ajh/4.3.245. [DOI] [PubMed] [Google Scholar]
- Meaume S, Benetos A, Henry OF, et al. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 yrs of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–50. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- Miller JA, Lever AF, Burke V. Pulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension Trial. J Hypertens. 1999;17:1065–72. doi: 10.1097/00004872-199917080-00004. [DOI] [PubMed] [Google Scholar]
- Millasseau SC, Stewart AD, Patel SJ, et al. Evaluation of carotid-femoral pulse wave velocity: influence of timing algorithm and heart rate. Hypertension. 2005;45:222–6. doi: 10.1161/01.HYP.0000154229.97341.d2. [DOI] [PubMed] [Google Scholar]
- Millasseau SC, Kelly RP, Ritter JM, et al. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (Lond) 2002;103:371–7. doi: 10.1042/cs1030371. [DOI] [PubMed] [Google Scholar]
- Morikawa Y. Characteristic pulse wave caused by organic nitrates. Nature. 1967;213:841–2. doi: 10.1038/213841a0. [DOI] [PubMed] [Google Scholar]
- Mokelke EA, Dietz NJ, Eckman DM, et al. Diabetic dyslipidaemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol. 2005;288:H1233–41. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- Nichols WW. Clinical measurement of arterial stiffness obtained from non-invasive pressure waveforms. Am J Hypertens. 2005;18:3–10. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O'Rourke MF. McDonald's blood flow in arteries: theoretical, experimental and clinical principles. London: Edward Arnold; 1998. [Google Scholar]
- Nichols WW, O'Rourke MF. McDonald's blood flow in arteries: theoretical, experimental and clinical principles. 5th Ed. London: Edward Arnold; 2005. [Google Scholar]
- Nili N, Zhang M, Strauss BH, et al. Biochemical analysis of collagen and elastin synthesis in the balloon injured rat carotid artery. Cardiovasc Pathol. 2002;11:272–6. doi: 10.1016/s1054-8807(02)00119-9. [DOI] [PubMed] [Google Scholar]
- Nitenberg A, Paycha F, Ledoux S, et al. Coronary artery responses to physical stimuli are improved by deferoxamine but not by L-arginine in non-insulin-dependent diabetic patients with angiographically normal coronary arteries and no other risk factors. Circulation. 1998;97:736–43. doi: 10.1161/01.cir.97.8.736. [DOI] [PubMed] [Google Scholar]
- Norata GD, Tonti L, Roma P, et al. Apoptosis and proliferation of endothelial cells in early atherosclerotic lesions: possible role of oxidised LDL. Nutr Metab Cardiovasc Dis. 2002;12:297–305. [PubMed] [Google Scholar]
- Ohshita K, Yamane K, Ishida K, et al. Post-challenge hyperglycaemia is an independent risk factor for arterial stiffness in Japanese men. Diab Med. 2004;21:636–9. doi: 10.1111/j.1464-5491.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Group. N Eng J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- Oliver J, Webb D. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–66. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens. 1996;14(Suppl 5):147–57. [PubMed] [Google Scholar]
- O'Rourke MF, Kelly RP. Wave reflection in the systemic circulation and its implications in ventricular function. J Hypertens. 1993;11:327–37. doi: 10.1097/00004872-199304000-00001. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF. Pulsatile arterial haemodynamics in hypertension. Aust N Z J Med. 1976;6(S2):40–8. doi: 10.1111/j.1445-5994.1976.tb03322.x. [DOI] [PubMed] [Google Scholar]
- Parhami F, Fang ZT, Fogelman AM, et al. Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest. 1993;92:471–8. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauca AL, Wallenhaupt ST, Kon ND, et al. Does radial artery pressure accurately reflect aortic pressure? Chest. 1992;102:1193–8. doi: 10.1378/chest.102.4.1193. [DOI] [PubMed] [Google Scholar]
- Pearson AC, Guo R, Orsinelli DA, et al. Transesophageal echocardiographic assessment of the effects of age, gender, and hypertension on thoracic aortic wall size, thickness, and stiffness. Am Heart J. 1994;128:344–51. doi: 10.1016/0002-8703(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Philippe F, Chemaly E, Blacher J, et al. Aortic pulse pressure and the extent of coronary artery disease in percutaneous transluminal coronary angioplasty candidates. Am J Hypertens. 2002;15:672–7. doi: 10.1016/s0895-7061(02)02961-8. [DOI] [PubMed] [Google Scholar]
- Ramsey M, Goodfellow J, Jones C, et al. Endothelial control of arterial distensibility is impaired in chronic heart failure. Circulation. 1995;92:3212–19. doi: 10.1161/01.cir.92.11.3212. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:73–5. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Ridker PM. High-sensitive C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2002;103:1813–18. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis; a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Salomaa V, Riley W, Kark J, et al. Non-insulin dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–43. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;87:II56–65. [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Schini-Kerth VB. Dual effects of insulin-like growth factor-I on the constitutive and inducible (NO) synthase-dependent formation of NO in vascular cells. J Endocrinol Invest. 1999;22(5 Suppl):82–8. [PubMed] [Google Scholar]
- Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- Sengstock DM, Vaitkevicius PV, Supiano MA. Arterial stiffness is related to insulin resistance in nondiabetic hypertensive older adults; J Clin Endocrinol Metab. 2005;90:2823–7. doi: 10.1210/jc.2004-1686. [DOI] [PubMed] [Google Scholar]
- Singh TP, Groehn H, Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 2003;41:661–5. doi: 10.1016/s0735-1097(02)02894-2. [DOI] [PubMed] [Google Scholar]
- Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischaemia model. Circulation. 2005;111:150–6. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- Smith A, Karalliedde J, De Angelis L, et al. Aortic pulse wave velocity and albuminuria in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16:1069–75. doi: 10.1681/ASN.2004090769. [DOI] [PubMed] [Google Scholar]
- Smith JC, Evans LM, Wilkinson I, et al. Effects of GH replacement on endothelial function and large-artery stiffness in GH-deficient adults: a randomized, double-blind, placebo-controlled study. Clin Endocrinol (Oxf) 2002;56:493–501. doi: 10.1046/j.1365-2265.2002.01514.x. [DOI] [PubMed] [Google Scholar]
- Smith JC, Lane HA, Lewis J, et al. Endothelial function and coagulant factors in growth hormone-treated hypopituitary adults receiving desmopressin. J Clin Endocrinol Metab. 2003;88:2152–6. doi: 10.1210/jc.2002-021618. [DOI] [PubMed] [Google Scholar]
- Suh HS, Park YW, Kang JH, et al. Vascular endothelial dysfunction tested by blunted response to endothelium-dependent vasodilation by salbutamol and its related factors in uncomplicated pre-menopausal obese women. Int J Obes (Lond) 2005;29:217–22. doi: 10.1038/sj.ijo.0802642. [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Najjar SS, Boudreeau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- Szmitko PE, Wang CH, Weisel RD, et al. New markers of inflammation and endothelial cell activation. Circulation. 2003;108:1917–23. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- Takazawa K, Tanaka N, Fujita M, et al. Assessment of vasoactive agents and vascular ageing by the second derivative of photophlesysmograph waveform. Hypertension. 1998;32:365–70. doi: 10.1161/01.hyp.32.2.365. [DOI] [PubMed] [Google Scholar]
- ter Avest E, Holewijn S, Stalenhoef AF, et al. Variation in non-invasive measurements of vascular function in healthy volunteers during daytime. Clin Sci. 2005;108:425–31. doi: 10.1042/CS20040300. [DOI] [PubMed] [Google Scholar]
- Thomas GN, Chook P, Oiao M, et al. Deleterious impact of “high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: the CATHAY study. Arteriscler Thromb Vasc Biol. 2004;24:739–43. doi: 10.1161/01.ATV.0000118015.26978.07. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Kienast J, Pyke SD, et al. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Eng J Med. 1995;332:635–41. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- Tio RA, Monnink SH, Amoroso G, et al. Safety evaluation of routine intracoronary acetylcholine infusion in patients undergoing a first diagnostic coronary angiogram. J Investig Med. 2002;50:133–9. doi: 10.2310/6650.2002.31305. [DOI] [PubMed] [Google Scholar]
- Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–7. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41:360–70. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Witztum J. Measuring circulating oxidized LDL to evaluate coronary risk. Circulation. 2001;103:1930–2. doi: 10.1161/01.cir.103.15.1930. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M, Suzuki E, Egawa K, et al. Stiffness and impaired blood flow in lower-leg arteries are associated with severity of coronary artery calcification among asymptomatic type 2 diabetic patients. Diabetes Care. 2004;27:2409–15. doi: 10.2337/diacare.27.10.2409. [DOI] [PubMed] [Google Scholar]
- Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelium and control of vascular function. Hypertension. 1989;13:658–67. doi: 10.1161/01.hyp.13.6.658. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Verma S, Wang CH, Lonn E, et al. Cross-sectional evaluation of brachial artery blood flow-mediated dilatation and C-rective protein in healthy individuals. Eur Heart J. 2004;25:1754–60. doi: 10.1016/j.ehj.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–19. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- Verma S, Li SH, Badiwala MV, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the pro-atherogenic effects of C-reactive protein. Circulation. 2002;105:1890–6. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- Vita JA, Keaney JF, Jr, Larson MG, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004;110:3604–9. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- Vogel RA, Corretti MC. Estrogens, progestins, and heart disease: can endothelial function divine the benefit? Circulation. 1998;97:1223–6. doi: 10.1161/01.cir.97.13.1223. [DOI] [PubMed] [Google Scholar]
- Wahlqvist M, Lo C, Myers K, et al. Putative determinants of arterial wall compliance in NIDDM. Diabetes Care. 1988;11:787–90. doi: 10.2337/diacare.11.10.787. [DOI] [PubMed] [Google Scholar]
- Watson AD, Leitinger N, Navab M, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;23:13597–607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- Wang CH, Li SH, Weisel RD, et al. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation. 2003;107:1783–90. doi: 10.1161/01.CIR.0000061916.95736.E5. [DOI] [PubMed] [Google Scholar]
- Wendelhag I, Wiklund O, Wikstrand J. Arterial wall thickness in familial hypercholesterolemia. Ultrasound measurement of intimamedia thickness in the common carotid artery. Arterioscler Thromb. 1992;12:70–7. doi: 10.1161/01.atv.12.1.70. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, et al. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, et al. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984;311:501–5. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- Williams IL, Wheatcroft SB, Shah AM, et al. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese individuals. Int J Obes Relat Metab Disord. 2002;26:754–64. doi: 10.1038/sj.ijo.0801995. [DOI] [PubMed] [Google Scholar]
- Williams SB, Cusco JA, Roddy MA, et al. Impaired nitric oxide-mediated vasodilatation in patients with non-insulin dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–73. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Hall IR, McCallum H, et al. Pulse wave analysis: clinical evaluation of a non-invasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vasc Biol. 2002;22:147–52. doi: 10.1161/hq0102.101770. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, MacCallum H, Flint L, et al. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–80. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson IB, McEniery CM. Arterial stiffness, endothelial function and novel pharmacological approaches. Clin Exp Pharmacol Physiol. 2004;31:795–9. doi: 10.1111/j.1440-1681.2004.04074.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Mohammad NH, Tyrrell S, et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15:24–30. doi: 10.1016/s0895-7061(01)02252-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Prasad K, Hall IR, et al. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002;39:1005–11. doi: 10.1016/s0735-1097(02)01723-0. [DOI] [PubMed] [Google Scholar]
- Wilson AM, O'Neal D, Nelson CL, et al. Comparison of arterial assessments in low and high vascular disease risk groups. Am J Hypertens. 2004;17:285–91. doi: 10.1016/j.amjhyper.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Yeung AC, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–6. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- Yildiz A, Oflaz H, Pusuroglu H, et al. Left ventricular hypertrophy and endothelial dysfunction in chronic hemodialysis patients. Am J Kidney Dis. 2003;41:616–23. doi: 10.1053/ajkd.2003.50123. [DOI] [PubMed] [Google Scholar]
- Zeiher AM, Drexler H, Wollschlager H, et al. Modulation of coronary vasomotor tone in humans: progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]