Abstract
Warfarin sodium is an effective oral anticoagulant drug. However, warfarin has a narrow therapeutic window with significant risks of hemorrhage at therapeutic concentrations. Dosing is difficult and requires frequent monitoring. New oral anticoagulant agents are required to improve current anticoagulant therapy. Furthermore, while warfarin is effective in venous disease, it does not provide more than 60% risk reduction compared with placebo in venous thrombosis prophylaxis and considerably lower risk reduction in terms of arterial thrombosis. Ximelagatran is an oral pro-drug of melagatran, a synthetic small peptidomimetic with direct thrombin inhibitory actions and anticoagulant activity. As an oral agent, ximelagatran has a number of desirable properties including a rapid onset of action, fixed dosing, stable absorption, apparent low potential for medication interactions, and no requirement for monitoring of drug levels or dose adjustment. It has a short plasma elimination half-life of about 4 hours in cases of unexpected hemorrhage or need for reversal. Its main toxicity relates to the development of abnormal liver biochemistry and/or liver dysfunction with “long-term” use of the drug. This usually occurs within the first 6 months of commencing therapy, with a small percentage of patients developing jaundice. The biochemical abnormality usually resolves despite continuation of the drug. The cause of this toxicity remains unknown. Clinical studies to date have shown that ximelagatran is noninferior to warfarin in stroke prevention in patients with nonvalvular atrial fibrillation, noninferior to standard therapy as acute and extended therapy of deep vein thrombosis (DVT), and superior to warfarin for the prevention of venous thromboembolism post-major orthopedic surgery. It has also been shown to be more effective than aspirin alone for prevention of recurrent major cardiovascular events in patients with recent myocardial infarction.
Keywords: Ximelagatran, direct thrombin inhibitor, oral anticoagulants, thromboprophylaxis
Introduction
Oral anticoagulants have been used in clinical practice for more than 60 years. The most commonly prescribed oral anticoagulant has been warfarin sodium (either Coumadin® or Marevan®) or longer-acting coumarin preparations or indanedione derivatives. Warfarin is an effective anticoagulant, but has a narrow therapeutic window with significant risks of hemorrhage at therapeutic drug concentrations. This unpredictable and variable pharmacological response necessitates frequent monitoring of prothrombin time and reported as international normalized ratios (INR) and dose adjustments. The potential for drug interactions, the influence of lifestyle factors on INR (for example diet and alcohol consumption), and variable compliance by patients, contribute significantly to limiting warfarin's overall therapeutic benefit.
Thrombin has been recognized as having a principal role in the coagulation pathways, hence the quest for its specific inhibition. Ximelagatran (Exanta® AstraZeneca, Molndal, Sweden) is an oral pro-drug of melagatran, a synthetic small peptide direct inhibitor of thrombin with anticoagulant activity. Ximelagatran–melagatran has a number of properties, which make it an attractive alternative to warfarin sodium (see Table 1). It has predictable pharmacokinetics and pharmacodynamics with apparently no requirement for routine anticoagulant monitoring with a fixed twice-daily dose administration.
Table 1.
Comparison of ximelagatran–melagatran and warfarin sodium
| Property | Warfarin sodium | Ximelagatran–melagatran |
|---|---|---|
| Origin, source | Synthetic | Synthetic |
| Mechanism action | Reduced synthesis functional prothrombin and other clotting factors | Direct competitive and reversible thrombin inhibition |
| Rapid onset action | No | Yes |
| Effective anticoagulant | Yes | Yes (not inferior to well-controlled warfarin therapy in most studies) |
| Risk of hemorrhage | Significant | Equivalent to warfarin in most studies |
| Route administration | Oral, once daily | Oral, twice daily |
| Stable predictable pharmacokinetics | No | Yes |
| Interactions with diet and alcohol | Clinically significant | No |
| Interactions with other medications | Many | Possibly erythromycin |
| Dosing | Individualized to patient and target INR | Fixed dosing dependent on indication |
| Monitoring dose | INR every 1–2 weeks | No |
| Dose adjustments | Frequent | No |
| Use in severe liver disease | Problematic | No – excluded from clinical studies |
| Use in severe renal disease | Yes | No – drug renally excreted, excluded from clinical studies |
| Reversibility after cessation | Slow elimination and reversal antithrombotic effect | Rapid reversal dependent on elimination half-life (∼4 hours) |
| Antidote | Rapid reversal with factor replacement. Reversal with vitamin K | Possibly APCC and rFVIIa |
| Drug cost | Cheap | Marketed in Europe at €4 for 24 mg twice daily regimen |
Abbreviations: APCC, activated prothrombin complex concentrate; INR, international normalized ratios; rFVIIa, recombinant activated factor VII.
Ximelagatran has been investigated in several large randomized controlled studies for prophylaxis against venous thromboembolism occurring after major orthopedic surgery, therapy in vein thrombosis, stroke prevention in atrial fibrillation, and acute coronary syndromes. Ximelagatran is now registered in France and other European countries for the use in orthopedic prophylaxis. In 2004, the application to market ximelagatran in the USA was rejected by the Food and Drug Administration (FDA) mostly due to concerns over potential liver toxicity. It is timely to review the pharmacology and clinical experience with this new oral anticoagulant drug.
Pharmacology
Melagatran is a small synthetic peptide with low membrane permeability that is poorly absorbed after oral dosing. In order to provide an oral formulation, ximelagatran, a prodrug of melagatran, was developed. The oral bioavailability of ximelagatran is approximately 20% as measured by melagatran concentration with low inter-individual variability (coefficient of variation is 20%) (Eriksson et al 2003b, 2003d; Eriksson, Johansson, et al 2003; Johansson, Andersson, et al 2003). Absorption of ximelagatran is rapid and minimally influenced by food and other medications. The peak plasma melagatran concentration is observed 1.5–2 hours after oral ximelagatran, a peak anticoagulant effect equivalent to subcutaneous heparins. Bioavailability does not appreciably change with repeated administration (Eriksson et al 2003c, 2003e). After oral dosing, unabsorbed ximelagatran passes unchanged through the intestine.
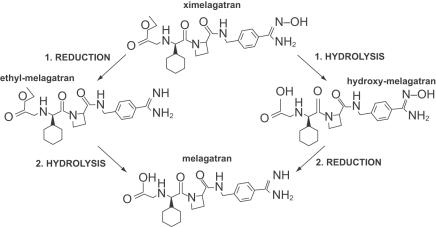
Ximelagatran undergoes rapid enzymatic conversion to melagatran via 2 intermediates, ethyl-melagatran (from the ethyl ester of melagatran formed by reduction of the hydroxyl group) and hydroxyl-melagatran (the hydroxyamidine of melagatran, formed by hydrolysis of the ethyl group) (see Figure 1) (Eriksson et al 2003d). This conversion in vivo probably occurs in several tissues. Enzymatic conversion in vitro occurs in microsomal preparations from the liver, intestinal membrane, and kidney. The highest activity was found in liver mitochondria. Cytochrome P450 (CYP) enzymes are unlikely to be involved in the bioconversion of ximelagatran as no reduction of hydroxyl-melagatran was found in preparations of 9 different CYP isoenzymes. The conversion of the intermediates to melagatran occurs rapidly in all ethnic groups including African, Asian, and Caucasian (Johansson, Andersson, et al 2003). Hydroxyl-melagatran, as with ximelagatran, is an ineffective thrombin inhibitor (potency ∼1% of melagatran) while ethyl-melagatran's anticoagulant activity is the same as melagatran (Gustaffson et al 2001).
Figure 1.
Chemical structure of ximelagatran and melagatran.
Pharmocokinetics
Melagatran exhibits first-order absorption kinetics with no significant protein binding (volume of distribution of 0.22 litres/kg). It is not significantly metabolized, with 80% renally excreted over 24 hours (Eriksson et al 2003a). Melagatran has a relatively short plasma elimination halflife of 1.5–2 hours in young healthy subjects. With increasing age, the plasma elimination half-life of melagatran increases to 4–5 hours as a consequence of age-related decrease in renal function.
In a small randomized crossover study (Eriksson et al 2003b) of 12 volunteers with severe renal impairment, the half-life and area under the plasma concentration–time curve of melagatran given in clinically relevant amounts was significantly higher. The optimal dose and schedule in patients with renal impairment is yet to be determined.
No significant difference was noted in the pharmacokinetics or pharmacodynamics in patients with mild to moderate hepatic impairment (Johansson, Wahlander, et al 2003).
Ximelagatran seemed to have a low potential for interaction with concomitant medications in clinical studies involving over 17 000 patients. Studies of possible interactions of ximelagatran–melagatran with alcohol, or other medications (including nifedipine, diazepam, diclofenac, acetylsalicylic acid, digoxin, and statins) have been negative. Therefore the metabolism of ximelagatran–melagatran is independent of CYP enzymes. Coadministration of ximelagatran and erythromycin has been shown to increase the area under the curve of melagatran by 80% (Dorani et al 2004). This interaction between ximelagatran and erythromycin may involve a transport protein such as P-glycoprotein. Dose adjustment for obesity (Body mass index [BMI] up to 39 kg/m2) has not been found to be necessary as evidenced by an open label, single dose of 24 mg ximelagatran in obese subjects (BMI 32–39 kg/m2) compared with sex and age-matched, nonobese subjects (BMI 21–26 kg/m2) (Sarich, Teng, et al 2003).
Antithrombotic activity of melagatran
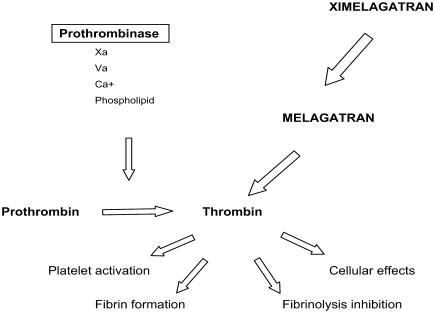
Melagatran is a potent, competitive, and reversible direct inhibitor binding to the active site of soluble and clot-bound α-thrombin. Ximelagatran has no pharmacodynamic effect. Melagatran's antithrombotic properties may be broadly categorized including inhibition of thrombin, inhibition of platelet aggregation, and other effects including augmented fibrinolysis (see Figure 2).
Figure 2.
Antithrombotic activity of ximelagatran is mediated by direct thrombin inhibition.
Effects on thrombin
Melagatran inhibits coagulation by antagonism of the thrombin-mediated cleavage of fibrinogen to fibrin as well as the cascade of interrelated events responsible for its anticoagulant activity, such as activation of clotting factors. This is evident by its prolongation of activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin clotting times at clinically relevant concentrations (doubling of clotting times at 0.59, 2.2, and 0.01 μmol/L, respectively). Capillary bleeding time was also prolonged by 36 seconds in 12 male volunteers after a single subcutaneous dose of melagatran and returned to baseline within 8 hours (Johansson, Wahlander, et al 2003). The effect on skin bleeding time has not been documented.
Effects on platelet aggregation
Several studies demonstrated that melagatran can inhibit thrombin-induced platelet activation and/or aggregation in a concentration-dependent manner (Gustaffson et al 1998; Soslau et al 2002; Nylander and Mattson 2003). Effects of melagatran on platelet activation appear to involve thrombin bound to glycoprotein Ib and also activation of protease activated receptor-4 (PAR-4) (Soslau et al 2002) and PAR-1 (Nylander and Mattson 2003).
Other effects of thrombin inhibition
Melagatran appears to enhance endogenous fibrinolysis with no inhibitory effect on tissue plasminogen activator (t-PA)-induced fibrinolysis (Mattson et al 2000). This effect appears to be due to melagatran's inhibition of thrombin-mediated activation of TAFI (thrombin activable fibrinolysis inhibitor; pro-carboxypeptidase U) to activated TAFI. Two clinical studies have confirmed the absence of inhibition of fibrinolysis by therapeutic melagatran concentrations (Eriksson et al 1999; Eriksson, Wahlander, et al 2003). The melagatran effect on other thrombin-mediated effects including thrombomodulin and activation of endothelial cells is not clear.
Reversal of the antihemostatic effect
Whilst there are antidotes for heparin (protamine) and warfarin (vitamin K or prothrombin complex concentrate), no antidote is available for the rapid reversal of ximelagatran–melagatran. Prothrombin factor replacement, as plasma or concentrate, would not be expected to reverse the direct thrombin inhibition, which is dependent on plasma concentration. Removal of melagatran from plasma by dialysis is possible with some dialysis membranes. Overcoming the thrombin inhibition by administration of activated clotting factors seems the most promising method. Activated prothrombin complex concentrate (APCC) has been found to reduce in a dose dependent manner the bleeding time and blood loss produced by high concentrations of melagatran in rats and rabbits. Activated factor VII (FVIIa) appeared less effective than APCC in these models. However, human FVIIa binds poorly to rat tissue factor (TF) and activates rat factor X poorly in the absence of TF, and this may account for some the differences noted (Elg et al 2002). Clinical studies to define an effective strategy for managing unexpected hemorrhage are required.
Clinical studies
With the potential advantages of clinical efficacy and safety, ximelagatran–melagatran has been assessed in an expansive clinical study program (Table 2). The studies can be broadly categorized into: stroke prevention in atrial fibrillation; prophylaxis for venous thromboembolism after major orthopedic surgery; treatment of vein thrombosis; and in acute coronary syndromes. As with most clinical studies, patients with severe renal disease, hepatic disease, and pregnant patients were excluded from the ximelagatran clinical study program. Further studies will need to address the efficacy and safety of ximelagatran in these patient groups.
Table 2.
Summary of clinical development of ximelagatran–melagatran
| Study and patient group | Type of study | Number of subjects | Treatment allocation |
|---|---|---|---|
| Nonvalvular atrial fibrillation | |||
| (Stroke Prevention using an ORal Thrombin Inhibitor in atrial Fibrillation–SPORTIF) | |||
| SPORTIF II (Petersen et al 2003) | Dose finding | 254 | Ximelagatran (20, 40, or 60 mg twice daily) or warfarin to INR 2–3. |
| SPORTIF III (Olsson et al 2003) | RCT open label | 3407 | Ximelagatran (36 mg twice daily) or warfarin to INR 2–3 |
| SPORTIF IV | Dose finding | 167 | Ximelagatran (36 mg twice daily) or warfarin to INR 2–3 for 2–5 years |
| SPORTIF V (Halperin et al 2003) | RCT double-blind | 3922 | Ximelagatran (36 mg twice daily) or warfarin to INR 2–3 for 20 months |
| Prophylaxis for venous thromboembolism after major orthopedic surgery | |||
| (MElagatran as prophylaxis of THrombosis in ORthopedic surgery – METHRO) | |||
| METHRO I – Hip or knee surgery (Eriksson, Arfwidsson, et al 2002 | Randomized dose finding | 135 | Melagatran–ximelagatran (pre- and postoperative melagatran 1, 2, or 4 mg for 3 doses followed by ximelagatran 6, 12, or 24 mg twice daily for 8–11 days) or dalteparin 5000 IU once daily started preoperatively |
| METHRO II – Hip or knee surgery (Eriksson, Bergqvist, et al 2002) | Phase II randomized double-blind dose finding | 1916 | Melagatran–ximelagatran (preoperative melagatran 1, 1.5, 2.25 or 3 mg followed by postoperative melagatran or ximelagatran 8, 12, 18 or 24 mg twice daily for 8–11 days) or dalteparin 5000 IU once daily started preoperatively |
| METHRO III – Hip or knee surgery (Eriiksson et al 2003a) | Phase III randomized double-blind | 2788 | Melagatran–ximelagatran (postoperative melagatran 3 mg followed by ximelagatran 24 mg twice daily) or enoxaparin 40 mg once daily started pre-operatively |
| EXPRESS – Hip or knee surgery (Eriksson et al 2003b) | Phase III randomized double-blind | 2885 | Melagatran–ximelagatran (melagatran preoperatively 2 mg, melagatran 12 hours postoperatively 3 mg, then ximelagatran 24 mg twice daily) or enoxaparin 40 mg once daily |
| EXULT A – Knee surgery (Francis, Berkowitz, et al 2003) | Phase III randomized double-blind | 2301 | Ximelagatran (24 mg or 36 mg bd twice daily, initiated the morning after surgery) or warfarin initiated evening of the day of surgery for 7–12 days |
| EXULT B – Knee surgery (Colwell et al 2003) | Phase III randomized double-blind | 2303 | Ximelagatran (36 mg bd twice daily initiated the morning after surgery) or warfarin initiated evening of day of surgery for 7–12 days |
| Therapy: DVT without symptomatic PE | |||
| (THRombin Inhibitor in Venous thromboEmbolism – THRIVE) | |||
| THRIVE I – Acute DVT (Eriksson, Wahlander, et al 2003) | Randomized dose finding | 350 | Ximelagatran (24, 36, 48, 60 mg twice daily) or dalteparin + warfarin |
| THRIVE II and V – Acute DVT with or without PE (Francis, Ginsberg, et al 2003) | Phase III double-blind RCT | 2491 | Ximelagatran 36 mg twice daily for 6 months or enoxaparin 1 mg/kg/bid + warfarin to INR 2–3 for 6 months |
| THRIVE III – Extended anticoa gulation for DVT or PE (Schulman et al 2003) | Phase III | 1223 | Ximelagatran 24 mg twice daily or placebo |
| THRIVE IV – Acute PE (Wahlander et al 2001) | Limited dose finding in PE | 12 | Ximelagatran (48 mg twice daily) for 2 weeks with lung scanning days 1 and 7 and pharmacokinetic data |
| Long term treatment in high risk arterial thrombotic events – post-MI patients | |||
| ESTEEM study – acute MI (Wallentin et al 2003) | Randomized dose finding | 1883 | Ximelagatran (24, 36, 48, 60 mg twice daily) or placebo. All patients received aspirin 160 mg once daily. |
Abbreviations: DVT; deep vein thrombosis; INR, international normalized ratios; PE, pulmonary embolism; MI, myocardial infarction; RCT, randomized controlled trial.
Stroke prevention in atrial fibrillation
Nonvalvular atrial fibrillation (NVAF) affects 6% of people over the age of 65 years and about 10% of those over 80 years. These patients have an absolute risk of stroke and systemic embolism of around 5%, not including the effects of other risk factors. There is conclusive evidence to demonstrate that warfarin sodium, given to achieve a target INR of 2–3, is highly effective at preventing stroke with a risk reduction of 62%. However, this is associated with a cost of around 1% per year of fatal hemorrhage. Despite the published efficacy of warfarin, only 20%–40% of eligible patients actually receive warfarin (Bungard et al 2000).
Ximelagatran has been evaluated in at least 2 dose-finding exploratory studies (SPORTIF II and IV) and 2 large Phase III studies (SPORTIF [Stroke Prevention using an ORal Thrombin Inhibitor in atrial Fibrillation] III and V) recruiting about 8000 patients with atrial fibrillation.
An open label study (SPORTIF III) (Olsson 2003) of 3407 European patients with NVAF at moderate risk of stroke or systemic embolism was conducted while the SPORTIF V study (Halperin et al 2003) examined 3922 North American patients using a double-blind comparison The aims of these 2 studies were to demonstrate noninferiority of a fixed dose of ximelagatran of 36 mg twice daily compared with warfarin. The rates of stroke and systemic embolism for warfarin and ximelagatran were 2.3% per year and 1.6% per year in SPORTIF III with no significant difference between groups in rates of major bleeding, although there was less total bleeding with ximelagatran (37% vs 47% per year, 95% Confidence Index [CI] for the difference, −14% to −6.0% per year; p < 0.001). These studies, both separately and in pooled analysis, did not find any significant difference between warfarin and ximelagatran in treated groups and hence demonstrated the noninferiority of 36 mg of ximelagatran.
There was no significant difference in the rates of fatal/major bleeding between warfarin and ximelagatran groups (SPORTIF III 1.8% and 1.3%; SPORTIF V 3.1% and 2.4% for warfarin and ximelagatran, respectively), although there was a slight excess of total bleeding in warfarin-treated patients (SPORTIF III 29.5% and 25.5%, p = 0.007; SPORTIF V 47% and 37%, p < 0.0001). Alanaine transaminase (ALT) was above 3 times upper limit of normal (ULN) in 0.8% and 1% of warfarin-treated compared with 6% of ximelagatran-treated patients in both SPORTIF III and V. The conclusion of these studies is that ximelagatran 36 mg twice daily was not inferior to warfarin with target of 2–3.
A number of concerns have been raised concerning the use of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. This relates to trial designs (noninferiority), cost of the drug, and need for liver function monitoring. The noninferiority design makes it possible that ximelagatran was in fact a slightly inferior treatment than warfarin (Eikelboom and Hankey 2004). A detailed cost analysis of ximelagatran has been published (O'Brien and Gage 2005). The overall conclusions of this analysis were that, assuming equal effectiveness in stroke prevention and decreased hemorrhage risk, ximelagatran is not likely to be cost-effective in patients with atrial fibrillation unless they have a high risk of intracranial hemorrhage or low quality of life with warfarin.
Prophylaxis for venous thromboembolism occurring after major orthopedic surgery
Without prophylaxis, arthroplasty surgery of the knee and hip (range 40%–84%) carry high risks of post-operative vein thrombosis and death from pulmonary embolism (PE) (Geerts et al 2004). Standard prophylaxis generally includes early mobilization, compression stockings, and daily low-molecular-weight heparin (LMWH) for 5–10 days. With these general measures, 1%–2% of patients will develop symptomatic DVT or PE. Fatality rates over the 6-week postoperative period range from 0.1% to 0.5%, most clinical events occurring out of hospital. Recent studies using LMWH as prolonged prophylaxis for 4–6 weeks after hip surgery have shown significantly reduced symptomatic venous thromboembolism (Eikelboom et al 2001). In North America, about 50% of patients receive warfarin prophylaxis post knee replacement surgery.
Given the high rates of venous thromboembolism, major orthopaedic surgery provides an opportunity for more effective anticoagulants to improve clinical outcomes. Studies of standard prophylaxis with bilateral leg contrast venography performed 7–14 days after hip or knee replacement surgery find total DVT rates of 15%–30% respectively and proximal DVT rates of about 6% in both groups.
There have been extensive studies of the use of ximelagatran–melagatran after joint replacement surgery (see Table 2). METHRO I (Melagatran as prophylaxis of thrombosis in orthopaedic surgery), a dose-ranging pilot study, and METHRO II (Eriksson, Arfwidsson, et al 2002; Eriksson, Bergqvist, et al 2002), a phase II study, have found an overall dose-dependent decrease in venographic total and proximal DVT and/or symptomatic PE with increasing doses of ximelagatran–melagatran. The METHRO III study compared the use of ximelagatran–melagatran with enoxaparin in a randomized study of 2788 patients undergoing major orthopedic surgery. Doses of enoxaparin were 40 mg once daily commencing 12 hours preoperatively then daily for 1–10 days compared with melagatran 3 mg subcutaneously 4–12 hours postoperatively, then ximelagatran 24 mg orally twice daily commencing on day 1 or 2 postoperatively (Eriksson et al 2003a). Using bleeding or efficacy as endpoints, no difference was demonstrated in this study.
The EXPRESS study (EXpanded PRophylaxis Evaluation Surgery Study), similar in design to METHRO III with the exception that ximelagatran–melagatran-treated patients received 2 mg of melagatran preoperatively (Eriksson et al 2003b), demonstrated a total venographic DVT rate of 20.3% in the ximelagatran–melagatran group compared with 26.6% in enoxaparin-treated patients (p < 0.0001). There was no difference in the rates of venographic proximal DVT, symptomatic events or bleeding.
The EXULT (EXanta Used to Lessen Thrombosis) trials (Colwell et al 2003; Francis, Berkowitz, et al 2003), investigated 4604 patients in North America, randomized to receive either postoperative oral ximelagatran (24 mg or 36 mg twice daily) or warfarin (target INR 2.5). EXULT A study found that ximelagatran 36 mg twice daily was more effective than warfarin (total venographic vein thrombosis rate of 20.3% versus 27.6 % (p = 0.003). These results were confirmed in the EXULT B study (Collwell et al 2003). Bleeding events did not differ.
The overall conclusions of these studies are that ximelagatran with perioperative melagatran is a suitable prophylaxis regimen for patients undertaking arthroplasty surgery. Omission of the preop erative melagatran dose, while resulting in decreased surgical site bleeding, may compromise efficacy, particularly using ximelagatran 24 mg. Ximelagatran–melagatran was approved in October 2004 in several European countries for venous thromboemobolism (VTE) prophylaxis in hospitalized patients undergoing joint replacement surgery.
Therapy of vein thrombosis
Current acute therapy of venous thromboembolism, either DVT or PE, requires initial anticoagulation with parenteral heparin and subsequently vitamin K antagonists in most patients. Many patients can be treated safely out of hospital using LMWH, with warfarin usually initiated 24–48 hours after commencement, but usually requiring at least 1 week to establish therapeutic anticoagulation. Heparin and warfarin are overlapped for minimum of 5 days and for 2 days of consecutive INR readings in the target range of 2–3 (Geerts et al 2004). Optimal duration of warfarin therapy remains uncertain. Most patients with first provoked DVT or PE require 3–6 months of treatment; this is generally extended to 6–12 months if the vein thrombosis was unprovoked. Patients with unprovoked DVT and/or PE have a higher rate of recurrence of about 1 in 3 over 10 years. (Kearon et al 1999) Extension of warfarin therapy, with a target INR of 2.0–3.0, has been shown in a number of studies to be 90%–95% effective for prevention of recurrent thrombosis. Due to the unpredictable risk of major/fatal hemorrhage, most patients with first unprovoked DVT/PE cease warfarin after 6–12 months. Extension of therapy should be reserved for patients with recurrent DVT or significant thrombophilia.
The THRIVE (THRombin Inhibitor in Venous thrombo-Embolism) studies have evaluated ximelagatran compared with standard anticoagulation for acute treatment of DVT (THRIVE I, THRIVE II, and THRIVE V) (Eriksson, Wahlander, et al 2003; Francis, Ginsberg, et al 2003) or compared with placebo for extended secondary prevention (THRIVE III) (Schulman et al 2003).
THRIVE II and V (Francis, Ginsberg, et al 2003) were randomized double-blind studies in which 2489 patients with acute DVT (37% with asymptomatic PE), mean age of 57 years with 53% male, were allocated to ximelagatran 36 mg twice daily for 6 months or standard therapy with enoxaparin and warfarin targeted to INR 2–3 for 6 months. No coagulation monitoring was performed for ximelagatran-treated patients although sham INRs were performed. Intention to treat analysis found 2.1% and 2.0% of ximelagatran- and warfarin-treated patients developed recurrent symptomatic venous thrombembolism respectively (absolute difference 0.1%, 95% CI 1.0–1.3%). The conclusion of the THRIVE study was that ximelagatran was noninferior compared with standard therapy. There was no difference in major hemorrhage (1.3% vs 2.2%) or all-cause mortality (2.3% vs 3.4%) between ximelagatran- or warfarin-treated patients. Enrolled patients may have been at low risk for VTE as evidenced by the lower rate of recurrent VTE regardless of treatment assignment (1.5%–2.1%) compared with the typical expected rate of 3%–6% in patients receiving standard treatment with either unfractionated heparin (UFH) or LMWH.
The THRIVE III study (Schulman et al 2003) randomized 1233 patients in a double-blind fashion to receive placebo or ximelagatran 24 mg twice daily for 18 months after completion of 6 months' anticoagulant therapy for acute DVT or PE. The cumulative risk of symptomatic recurrent vein thrombosis was 2.8% in ximelagatran and 12.6% in the placebo-treated subjects (Risk Ratio [RR] 0.16, 95% CI 0.09–0.3, p < 0.001). Deaths occurred in 6 and 7 ximelagatran- and placebo-treated patients, respectively (0 and 3 from PE). Major, nonfatal hemorrhage occurred in 6 of 612 and 5 of 611 ximelagatran- and placebo-treated patients, respectively.
The overall conclusions of these studies are that ximelagatran is not inferior to standard therapy for the acute therapy of DVT and is superior to placebo for extended secondary prevention of recurrent DVT and/or PE.
Treatment of acute coronary syndromes
Anticoagulation therapy in acute coronary artery disease depends on multiple factors, with selection of agents depending on the presence of acute coronary syndromes, need for bypass surgery, or planned percutaneous coronary intervention, or prophylaxis after a negative cardiovascular event. Anticoagulant therapies have long been thought to provide marginal or modest benefit in patients with acute coronary disease. Most recent studies have rekindled interest with warfarin or warfarin with low-dose aspirin providing about 20% relative risk reductions in recurrent ischemia, reinfarction, and death.
The ESTEEM (Efficacy and safety of the oral direct thrombin inhibitor ximelagatran in patients with recent myocardial damage) study (Wallentin et al 2003) was a phase II study assessing the efficacy and safety of ximelagatran in preventing recurrent acute coronary syndromes in 1833 patients within 14 days of myocardial infarction (MI). Patients were randomly assigned to receive aspirin 160 mg once daily or aspirin 160 mg once daily with ximelagatran (24, 36, 48, or 60 mg twice daily). The primary endpoint was a composite of recurrent nonfatal MI, recurrent ischemia, or death (from any cause). On an intention-to-treat analysis the primary endpoint occurred in 12.7% and 16.3% of ximelagatran plus aspirin- or aspirin alone-treated patients (RR 0.79, 95% CI 0.59–0.98, p = 0.036). Major bleeding occurred in 1.8% and 0.9% of patients (not statistically different). This trial suggests that the addition of ximelagatran to aspirin may reduce the rate of death and/or cardiovascular thrombotic outcomes. Larger phase III studies are needed to confirm this.
Tolerability and patient acceptability
Aside from hemorrhage, ximelagatran–melagatran has been remarkably free of significant adverse toxicities. Most concern has been placed on the dose-dependent increase in liver enzymes, predominantly elevated ALT, which is usually unaccompanied by clinical sequelae. This has been found in 6%–12% of patients in clinical studies with the biochemical abnormality typically occurring in 1–6 months of therapy, rarely developing after 6 months of treatment. In all clinical studies, patients required monthly screening of liver biochemistry in the first 6 months. The study medication was ceased if ALT exceeded 5 times ULN at any time. If ALT was 3–5 times ULN, more frequent monitoring was performed with 6% of patients overall of increased ALT to >3 times ULN. Only about one-half of patients discontinued the study as a result of this. With time, the ALT tends to decrease whether or not the drug is discontinued although a small percentage (data unpublished) still has persistent elevation. However, a small percentage of patients became jaundiced (0.4% in the ESTEEM study) or developed symptoms possibly attributable to liver dysfunction. The FDA analysis of longer-term exposure to ximelagatran suggests that signs of liver injury as reflected by elevations of ALT levels were typically observed after 1–2 months in approximately 6% of patients. The mechanism underlying the liver abnormality remains unclear, with further information from clinical studies required, particularly in view of the recent FDA concern which has resulted in nonapproval of ximelagatran. If ximelagatran is to be prescribed for more prolonged periods (other than short term prophylaxis), liver function should be monitored. This may offset the improved acceptability of the drug in terms of need for monitoring. In the short term, the lack of monitoring required should improve compliance, although twice daily dosing may be seen as more inconvenient than the once daily dosing required for warfarin. However, only fixed doses are required, compared with variable doses of warfarin.
The FDA also inferred a higher than expected increase in rate of MI from the orthopedic prophylaxis studies. This post-hoc analysis involved small numbers of events, the comparison between ximelagatran and standard therapy was not statistically significant, and MI was not an adjudicated endpoint. In the METHRO III study, 2 patients suffered MI during the treatment period and 1 succumbed to cardiogenic shock in the follow up period. No such event occurred in the control arm. Cardiomyopathy, ischemic heart disease, and congestive heart failure were causes of 3 deaths in the ximelagatran group in the METHRO II studies. No comments were made by the authors as to potential reasons for these adverse events or their significance. Similar findings regarding MI were not observed in the other clinical studies of ximelagatran.
Conclusion
The clinical studies of ximelagatran confirm that it is an effective antithrombotic agent in stroke prevention in nonvalvular atrial fibrillation, prevention, and therapy of venous thromboembolism, and possibly in preventing recurrent ischemia after acute MI. In most clinical indications, the conclusion from the studies is that ximelagatran is noninferior to well controlled warfarin therapy with respect to efficacy without increased bleeding propensity. In comparison with warfarin, ximelagatran has several desirable properties in terms of administration, dosing, and monitoring. Moreover, the minimal impact of diet and seeming lack of significant medication interactions make it a significantly more desirable therapeutic option over warfarin.
The major drawback of ximelagatran relates to its potential liver toxicity and need for monitoring of liver biochemistry for at least the first 6 months of treatment. Five percent to 10% of patients will develop ALT greater than 3 times ULN, leading to discontinuation of their medication. Unfortunately, the proposed Risk Minimization Action Plan (RiskMAP) submitted by AstraZeneca as part of its new drug application was felt to be inadequate by the FDA (http://www.fdaadvisorycommittee.com/). Furthermore, other similar monitoring programs using transaminase elevations as a marker of hepatotoxicity with medications such as bromfenac and troglitazone, failed to demonstrate effectiveness in prevention of severe drug-induced liver injury. Understanding the pathophysiology behind this predominantly biochemical adverse effect of ximelagatran–melagatran would thus be beneficial and necessary for the development of a useful and acceptable risk minimization program.
Ximelagatran is a new oral antithrombotic drug, which will effectively benefit many patients and have a major role in clinical practice. Precaution concerning the risk of hepatotoxicity should be further investigated and not discourage the important and growing need for alternative oral anticoagulant therapies.
Disclosure
Dr Tim Brighton receives honoraria as an occasional speaker, clinical investigator, and consultant for AstraZeneca, Pfizer (formerly Pharmacia), and Sanofi-Aventis.
References
- Albers GW, Diener HC, Frison L, et al. SPORTIF Executive Steering Committee, SPORTIF V Investigators Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA. 2005;293:690–8. doi: 10.1001/jama.293.6.690. [DOI] [PubMed] [Google Scholar]
- Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Int Med. 2000;160:41–6. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- Colwell CW, Berkowitz SD, Comp PC, et al. Randomised doubleblind comparison of ximelagatran, an oral direct thrombin inhibitor, and warfarin to prevent venous thromboembolism after total knee replacement (Exult B study) Blood. 2003;102:14a. [Google Scholar]
- Dorani H, Schutzer KM, Sarich TC, et al. Effect of erythromycin on the pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor ximelagatran and its active form melagatran. Proceedings American Society for Clinical Pharmacology and Therapeutics; 20–24 Jun 2004; Miami Beach, FL, USA. 2004. [Google Scholar]
- Eikelboom JW, Hankey GJ. The beginning of the end of warfarin? Med J Aust. 2004;180:549–51. doi: 10.5694/j.1326-5377.2004.tb06088.x. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomized trials. Lancet. 2001;358:9–15. doi: 10.1016/S0140-6736(00)05249-1. [DOI] [PubMed] [Google Scholar]
- Elg M, Carlsson S, Gustafsson D. Prolonged bleeding time induced by a direct thrombin inhibitor is reversed by recombinant factor VIIa in anaesthetized rats pre-treated with lipopolysaccharide [abstract] Blood. 2002;98:44a. [Google Scholar]
- Eriksson BI, Agnelli G, Cohen AT, et al. Direct thrombin inhibitor melagatran followed by oral ximelagatran in comparison with enoxaparin for prevention of venous thromboembolism after total hip or knee replacement: the METHRO III study. Thromb Haemost. 2003a;89:288–96. [PubMed] [Google Scholar]
- Eriksson BI, Agnelli G, Cohen AT, et al. The direct thrombin inhibitor melagatran followed by oral ximelagatran compared with enoxaparin for the prevention of venous thromboembolism after total hip or knee replacement: the EXPRESS study. Thromb Haemost. 2003b;1:2490–6. doi: 10.1111/j.1538-7836.2003.00494.x. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Arfwidsson AC, Frison L, et al. A dose-ranging study of the oral direct thrombin inhibitor, ximelagatran, and its subcutaneous form, melagatran, compared with dalteparin in the prophylaxis of thromboembolism after hip or knee replacement: METHRO I. MElagatran for THRombin inhibition in Orthopaedic surgery. Thromb Haemost. 2002;87:231–7. [PubMed] [Google Scholar]
- Eriksson BI, Bergqvist D, Kalebo P, et al. Ximelagatran and melagatran compared with dalteparin for prevention of venous thromboembolism after total hip or knee replacement: the METHRO II randomised trial. Lancet. 2002;360:1441–7. doi: 10.1016/s0140-6736(02)11469-3. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Eriksson UG, Frison L, et al. Pharmacokinetics and pharmacodynamics of melagatran, a novel synthetic LMW thrombin inhibitor, in patients with acute DVT. Thromb Haemost. 1999;81:358–63. [PubMed] [Google Scholar]
- Eriksson H, Wahlander K, Gustafsson D, et al. A randomized, controlled, dose-guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. Thromb Haemost. 2003;1:41–7. doi: 10.1046/j.1538-7836.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Eriksson UG, Bredberg U, Gislen K, et al. Pharmacokinetics and pharmacodynamics of melagatran, a novel oral direct thrombin inhibitor, in young healthy male subjects. Eur J Clin Pharmacol. 2003c;59(1):35–43. doi: 10.1007/s00228-003-0565-7. [DOI] [PubMed] [Google Scholar]
- Eriksson UG, Bredberg U, Hoffman KJ, et al. Absorption, distribution, metabolism and excretion of ximelagatran, an oral direct thrombin inhibitor, in rats, dogs and humans. Drug Metab Dispos Mar. 2003d;31:294–305. doi: 10.1124/dmd.31.3.294. [DOI] [PubMed] [Google Scholar]
- Eriksson UG, Johansson S, Attman P, et al. Influence of severe renal impairment on the pharmacokinetics and pharmacodynamics of oral ximelagatran and subcutaneous melagatran. Clin Pharmacokinet. 2003;42:743–53. doi: 10.2165/00003088-200342080-00003. [DOI] [PubMed] [Google Scholar]
- Eriksson UG, Mandema JW, Karlsson MO, et al. Pharmacokinetics of melagatran and the effect of ex vivo coagulation time orthopaedic surgery patients receiving subcutaneous melagatran and oral ximelagatran: a population model analysis. Clin Pharmacokinet. 2003e;42:687–701. doi: 10.2165/00003088-200342070-00006. [DOI] [PubMed] [Google Scholar]
- Eriksson UG, Manderma JW, Karlsson MO, et al. Pharmacokinetics of melagatran and the effect of ex vivo coagulation time in orthopaedic surgery patients receiving subcutaneous melagatran and oral ximelagatran: a population model analysis. Clin Pharmocokinet. 2003f;42:687–701. doi: 10.2165/00003088-200342070-00006. [DOI] [PubMed] [Google Scholar]
- Francis CW, Berkowitz SD, Comp PC, et al. EXULT A Study Group Comparison of ximelagatran with warfarin for the prevention of venous thromboembolism after total knee replacement. N Engl J Med. 2003;349:1703–12. doi: 10.1056/NEJMoa035162. [DOI] [PubMed] [Google Scholar]
- Francis CW, Ginsberg JS, Berkowitz SD, et al. THRIVE Treatment Study Investigators Efficacy and safety of the oral direct thrombin inhibitor ximelagatran compared with current standard therapy for acute, symptomatic deep vein thrombosis, with or without pulmonary embolism: The THRIVE Treatment Study. Blood. 2003;102:6a. [Google Scholar]
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism. The 7th ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- Gustafsson D, Antonsson T, Bylund R, et al. Effect of melagatran, a new low molecular weight thrombin inhibitor, on thrombin and fibrinolytic enzymes. Thromb Haemost. 1998;79:110–18. [PubMed] [Google Scholar]
- Gustafsson D, Nyström JE, Carlsson S, et al. The direct thrombin inhibitor melagatran and its oral prodrug H 376/95: intestinal absorption properties, biochemical and pharmacodynamic effects. Thromb Res. 2001;101:171–81. doi: 10.1016/s0049-3848(00)00399-6. [DOI] [PubMed] [Google Scholar]
- Halperin JL, Executive Steering Committee, SPORTIF III and V Study Investigators Ximelagatran compared with warfarin for prevention of thromboembolism in patients with nonvalvular atrial fibrillation: Rationale, objectives, and design of a pair of clinical studies and baseline patient characteristics – SPORTIF III and V. Am Heart J. 2003;146:431–8. doi: 10.1016/S0002-8703(03)00325-9. [DOI] [PubMed] [Google Scholar]
- Halperin JL, Executive Steering Committee, SPORTIF V Investigators Stroke prevention using the oral direct thrombin inhibitor ximelagatran in patients with nonvalvular atrial fibrillation – SPORTIF V. Circulation. 2003;108:2723. [Google Scholar]
- Johansson LC, Andersson M, Fager G, et al. No influence of ethnic origin on the pharmacokinetics and pharmacodynamics of melagatran following oral administration of ximelagatran, a novel oral direct thrombin inhibitor, to healthy male volunteers. Clin Pharmacokinet. 2003;42:475–84. doi: 10.2165/00003088-200342050-00005. [DOI] [PubMed] [Google Scholar]
- Johansson S, Wahlander K, Larson G, et al. Pharmacokinetics and anticoagulant effect of the direct thrombin inhibitor melagatran following subcutaneous administration to healthy young men. Blood Coagul Fibrinolysis. 2003;14:677–84. doi: 10.1097/00001721-200310000-00010. [DOI] [PubMed] [Google Scholar]
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–7. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- Mattson C, Berntsson P. Melagatran 2000. The active form of the oral direct thrombin inhibitor J376/95 enhances fibrinolysis via inhibition of thrombin-induced activation of pro-CPU [abstract] Blood. 2000;96:98–9. [Google Scholar]
- Nylander S, Mattson C. Thrombin-induced platelet activation and its inhibition by anticoagulants with different modes of action. Blood Coagul Fibrinolysis. 2003;14:159–67. doi: 10.1097/00001721-200302000-00007. [DOI] [PubMed] [Google Scholar]
- O'Brien CL, Gage BF. Cost and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA. 2005;293:699–706. doi: 10.1001/jama.293.6.699. [DOI] [PubMed] [Google Scholar]
- Olsson SB, Executive Steering Committee on behalf of the SPORTIF III Investigators Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with nonvalvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362:1691–8. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- Petersen P, Grind M, Adler J, SPORTIF II Investigators Ximelagatran versus warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. SPORTIF II: a dose-guiding, tolerability, and safety study. J Am Coll Cardiol. 2003;41:1445–51. doi: 10.1016/s0735-1097(03)00255-9. [DOI] [PubMed] [Google Scholar]
- Sarich TC, Teng R, Eriksson UG, et al. No influence of obesity on the pharmacokinetics and pharmacodynamics of melagatran, the active form of the oral direct thrombin inhibitor ximelagatran. Clin Pharmacokinet. 2003;42:485–92. doi: 10.2165/00003088-200342050-00006. [DOI] [PubMed] [Google Scholar]
- Schulman S, Wahlander K, Lundstrom T, et al. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med. 2003;349:1713–21. doi: 10.1056/NEJMoa030104. [DOI] [PubMed] [Google Scholar]
- Soslau G, Goldenberg SJ, Navas E, et al. (Xi)melagatran inhibition of alpha-thrombin interaction between digoxin and ximelagatran, an oral direct thrombin inhibitor [abstract] Blood. 2002;100:255a. [Google Scholar]
- Wahlander K, Lapidus L, Olsson C, et al. THRIVE IV: An openlabel, pilot study of the treatment of pulmonary embolism with the oral direct thrombin inhibitor ximelagatran [abstract] Blood. 2001;98:268a. [Google Scholar]
- Wallentin L, Wilcox RG, Weaver WD, et al. Oral ximelagatran for secondary prophylaxis after myocardial infarction: the ESTEEM randomized controlled trial. Lancet. 2003;362:789–97. doi: 10.1016/S0140-6736(03)14287-0. [DOI] [PubMed] [Google Scholar]