Abstract
Insulin glargine is an analogue of human insulin that is modified to provide a consistent level of plasma insulin over a long duration. Pharmacokinetic and pharmacodynamic studies show that a single injection of insulin glargine leads to a smooth 24-hour time–action profile with no undesirable pronounced peaks of activity. In clinical trials, this profile has been associated with at least equivalent, if not better, glycemic control than other traditional basal insulins and a significantly lower rate of overall and nocturnal hypoglycemia. The convenience of a once-daily injection, a lack of need for resuspension (insulin glargine is a clear solution when injected), and lower rates of hypoglycemia should translate into improvements in patient treatment satisfaction. This review appraises the evidence for the view that insulin glargine represents an advance in basal insulin therapy for both type 1 and type 2 diabetes patients.
Keywords: Insulin glargine, basal insulin therapy, diabetes, review
Introduction
While insulin remains the only antihyperglycemic therapy for type 1 diabetes, oral agents have been the mainstay of therapy for those with type 2 diabetes. Oral therapies are limited in their ability to maintain tight glycemic control in the long term (UKPDS 1995a, 1995b). There is increasing support for the earlier initiation of insulin in patients with type 2 diabetes to ensure tight glycemic control through the period of progressive β-cell failure and secondary failure of oral antidiabetes agents (Campbell and White 2002; Home et al 2003).
Since the first use of insulin over 80 years ago, a major aim of research has been the ever closer imitation of physiological insulin delivery. Basal insulin secretion is essential for the maintenance of fasting glucose levels, especially through inhibition of excessive glucose output from the liver. Advances in purification methods and genetic engineering have provided a range of short-, intermediate-, and long-acting insulin formulations (Feher and Bailey 2004). Insulin glargine was the first long-acting insulin analogue to become available and appears to provide a more physiological and convenient method of basal insulin replacement than older long-acting insulin formulations.
Tight glycemic control – an essential aim
Two landmark studies, UK Prospective Diabetes Study (UKPDS) in type 2 diabetes (UKPDS 1998a) and the Diabetes Control and Complications Trial (DCCT) in type 1 diabetes (DCCT 1993), definitively proved the benefits of tight glycemic control in reducing the risk of microvascular complications and suggested possible benefits for cardiovascular disease (CVD) outcomes in people with diabetes.
Microvascular complications
In the DCCT, 1441 patients with type 1 diabetes were randomized to receive intensive therapy (administered either with an external insulin pump or at least three daily insulin injections) or conventional therapy (one or two insulin injections per day) (DCCT 1993). After 3 months, and for the remainder of the 6.5-year study, the mean glycosylated hemaglobin (HbA1c) was significantly lower in the intensive group than in the conventional group (p < 0.001). The intensive group maintained a mean HbA1c of approximately 7.2% versus approximately 9.0% in the conventional group. This improvement in glycemic control reduced the risk of developing retinopathy by 76% (95% confidence interval [CI]; 67%–82%), of development of clinical neuropathy by 60% (95% CI; 38%–74%), and of microalbuminuria and albuminuria by 39% (95% CI; 21%–52%) and 54% (95% CI; 19%–74%), respectively (DCCT 1993). Furthermore, an observational follow-up of the original DCCT cohort, known as the Epidemiology of Diabetes Interventions and Complications (EDIC) study, reported maintenance of these benefits after a further 4 years (DCCT/EDIC 2000).
Similarly, in the UKPDS, 5102 patients with type 2 diabetes were randomized to receive either conventional dietary treatment or intensive blood glucose control using either insulin or a sulfonylurea (UKPDS 1998a). Over the 10-year study period, the mean HbA1c in the intensive group was reduced by 11% compared with those on conventional therapy (mean HbA1c of 7.9% [95% CI; 6.2%–8.2%] versus 7.0% [95% CI; 6.9%–8.8%] for conventional versus intensive therapy) This reduction in HbA1c was associated with a 25% reduction in the risk of microvascular complications (p = 0.0099) (UKPDS 1998a).
Cardiovascular disease
Results from the EDIC study, the long-term follow up of the DCCT, suggest a cardiovascular (CV) benefit of tight glycemic control in type 1 diabetes (Nathan et al 2003). Patients assigned to intensive (n = 618) or conventional (n = 611) therapy received ultrasound scans of the internal and common carotid arteries at year 1 and year 6 of the study. Progression of intima media thickness (IMT), a wellestablished marker of atherosclerosis, was significantly less in those assigned to intensive versus conventional therapy (p = 0.01 and p = 0.02 for internal and common carotid IMT, respectively). These studies suggest that tight glycemic control could reduce the risk of CV events, at least in part, through modifying the atherosclerotic disease process. Results from the DCCT/EDIC study published in December 2005 showed that of the 1375 volunteers continuing to participate in the DCCT/EDIC, the intensively treated patients had approximately half the number of CV events compared with those treated conventionally (Nathan et al 2005).
In the UKPDS, the improved glycemic control in the intensive group was associated with a 16% reduction in the risk of myocardial infarction (MI), although the result did not quite reach accepted statistical significance (p = 0.052) (UKPDS 1998a). In a sub-study of the UKPDS, 753 obese patients with type 2 diabetes were randomized to intensive therapy with metformin or conventional therapy. After a median follow-up of 10.7 years, metformin was associated with a 39% reduction in the risk of MI (p = 0.01). A 96% (p = 0.039) increase in diabetes-related death in a small subset of patients treated with metformin plus sulfonylurea, however, makes the interpretation of these results difficult (UKPDS 1998b).
Limitations of current insulin regimens
Advances in genetic engineering, formulation science, and medical device technology have brought the aim of tight glycemic control ever closer (Feher and Bailey 2004). In type 1 diabetes, complex regimens involving short-, intermediate-, or long-acting insulins aim to mimic physiological insulin delivery. These regimens are far from perfect, however, as shown by the fact that even in the highly motivated setting of the DCCT, only 5% of those assigned to intensive therapy maintained an average HbA1c below the target level of 6.5% throughout the study (DCCT 1993). Furthermore, the DCCT highlighted the difficulty in balancing tight glycemic control and hypoglycemia with those in the intensive therapy group being at 3-fold greater risk of severe hypoglycemia (p < 0.001) (DCCT 1993).
In type 2 diabetes, insulin therapy is usually reserved for those who are failing on oral therapy, but its use is often delayed in these patients. This is despite the fact that insulin is the only therapy that can control glycemia in the long term (UKPDS 1998a; Wright et al 2002). It is well established that there are practitioner and patient barriers to the initiation of insulin therapy in type 2 diabetes, such as perceived complexity, fear of injections, and the fear of hypoglycemia (Cryer 1999; Wallace and Matthews 2000).
In both type 1 and type 2 diabetes, complexity of regimen, the need for multiple dosing, and a fear of hypoglycemia underlie much of the failure to reach targets for glycemic control. Insulin glargine is an insulin analogue that has been available in the US and UK since 2001. This form of insulin has attributes that may contribute to overcoming some of the barriers to tight glycemic control.
Limitations of long-acting insulins
The aim of basal insulin replacement is to provide a constant level of insulin between meals without increasing the risk of hypoglycemia, particularly at night. Long-acting, basal insulins are modified to delay their absorption. The most frequently used basal insulins are insulins complexed with protamine (neutral protamine Hagedorn [NPH] insulins) or the hexamer-stabilizing agent zinc (lente and ultralente insulins) (Feher and Bailey 2004). These formulations fall short of providing an appropriate basal supply of insulin because of variable absorption, undesirable peaks in hypoglycemic action, and an insufficient duration of action. Basal NPH insulin produces a maximal insulin concentration 4–6 hours after injection, which can cause hypoglycemia and often necessitates twice-daily injections (Zinman et al 1999). Furthermore, subcutaneous absorption of these formulations varies considerably and undesirable plasma concentration peaks can lead to hypoglycemic episodes, especially during the night (Heinemann and Richter 1993). Conversely, the same unpredictable absorption can also lead to high fasting glucose levels in the morning (Gillies et al 2000). Ultralente formulations share many of these limitations and are also associated with wide inter- and intra-individual variability in pharmacodynamics and pharmacokinetics (Hirsch 1998, Rosskamp and Park 1999).
A further practical problem that arises with NPH insulin is the need to mix thoroughly before injection. A study of 109 diabetes patients treated with NPH insulin found that inadequate suspension was common (only 9% of patients tipped and rolled their insulin pens more than 10 times) with a consequent wide variation in the dose of insulin administered (Jehle et al 2000).
These limitations have led to attempts to develop improved basal insulins with no pronounced peaks in insulin levels, reproducible antihyperglycemic efficacy, and once-daily administration.
Insulin glargine – an improved basal insulin?
Insulin glargine was developed as an improved long-acting, basal insulin. It is an analogue of human insulin that is produced in a non-pathogenic strain of Escherichia coli. Insulin glargine differs from human insulin by the addition of two arginine amino acids to the C-terminus of the B-chain and the replacement of asparagine at position A21 by glycine. These changes shift the isoelectric point so that the molecule is soluble at an acid pH, but less soluble at neutral physiological pH levels. This results in a clear solution (pH 4.0) that when injected forms a precipitate in the subcutaneous tissue, which delays absorption and prolongs duration of action (Bahr et al 1997). The absorption characteristics of insulin glargine are not affected by the site of injection (arm, leg, or abdominal regions). Furthermore, compared with NPH insulin, the absorption rate is significantly slower with approximately 50% of the injected dose of insulin glargine still detectable after 24 hours compared with approximately 20% of the NPH insulin dose (Owens et al 2000).
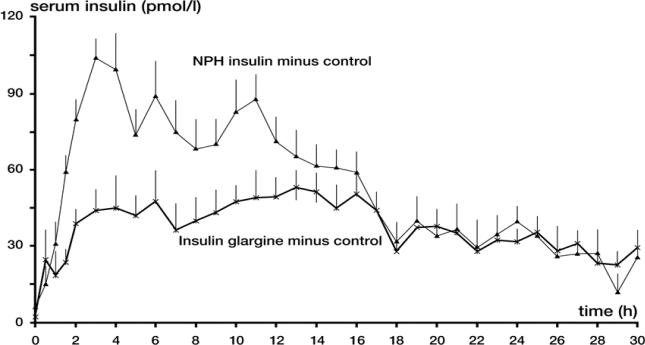
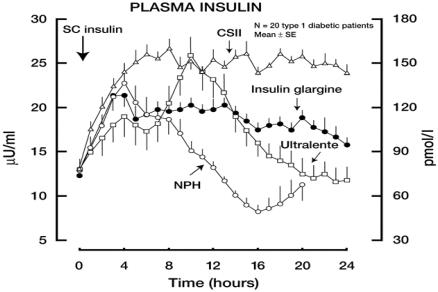
A potential major advantage of insulin glargine over NPH insulin and ultralente preparations is a lack of pronounced peaks in plasma insulin concentrations and a more constant delivery of insulin over a 24 hour period. This smooth profile was clearly shown in studies of insulin glargine versus NPH insulin in healthy volunteers (Figure 1) and of insulin glargine versus NPH and ultralente insulin in patients with type 1 diabetes (Figure 2) (Heinemann et al 2000; Lepore et al 2000). In fact, in this latter study, the delayed absorption of insulin glargine provided a consistent delivery of insulin that closely mimicked insulin delivery by continuous subcutaneous insulin infusion (CSII) (Figure 2) (Lepore et al 2000). Furthermore, in this study, interindividual variability in plasma insulin concentrations was lower with insulin glargine than with NPH or ultralente (Lepore et al 2000).
Figure 1.
Serum insulin profiles for insulin glargine and NPH insulin in healthy volunteers.
Mean serum insulin concentrations ± SEM after subcutaneous injections of 0.4 U/kg body weight of insulin glargine and NPH insulin on three different study days in 15 healthy volunteers, corrected for serum insulin concentrations seen with placebo. Reproduced from Heinemann et al 2000. Copyright © American Diabetes Association. From Diabetes Care, 23: 644–9. Reprinted with permission from The American Diabetes Association.
Abbreviations: NPH, neutral protamine Hagedorn; SEM, standard error of the mean.
Figure 2.
Serum insulin profiles for four different basal insulins in patients with type 1 diabetes.
Free plasma insulin concentrations after subcutaneous injection of insulin glargine, NPH insulin, Ultralente and continuous subcutaneous infusion of insulin lispro. Reproduced from Lepore et al 2000. Copyright © 2000 American Diabetes Association. From Diabetes, 49:2142–8. Reprinted with permission from The American Diabetes Association.
Abbreviations: NPH, neutral protamine Hagedorn.
These pharmacokinetic studies highlight the potential of insulin glargine to be an improved basal insulin for patients with diabetes.
Insulin glargine – balancing tight glycemic control and hypoglycemia
Much evidence now supports the suggestion that the pharmacokinetics and pharmacodynamics of insulin glargine can translate into effective glycemic control with a reduced risk of hypoglycemia in patients with type 1 and type 2 diabetes. While a single dose of insulin glargine will achieve 24-hour coverage in the large majority of patients, a minority of patients may experience a premature decline in activity that typically occurs between 18 and 24 hours. In these cases, the appropriate insulin glargine dose can be divided between two doses 12 hours apart. Alternatively, the decline in activity can be compensated for by the addition of 1–2 units of regular insulin mixed with preprandial fast acting insulin before dinner. This ‘pseudo-basal’ dose will maintain insulinization, but eliminate the need to split the insulin glargine dose and increase the number of injections (Bohannon 2003).
An appraisal from the UK National Institute of Clinical Excellence (NICE) has recommended insulin glargine as a treatment option for all type 1 diabetes patients and for patients with type 2 diabetes who require assistance from a carer or healthcare professional to administer insulin, whose life is restricted by recurrent episodes of symptomatic hypoglycemia and for those who would otherwise need twice-daily basal insulin injections in combination with oral antidiabetic drugs (NICE 2002).
Type 1 diabetes
The first indications that insulin glargine could offer improved glycemic control came in two short-term trials. Rosenstock et al (2000) assigned 256 patients with type 1 diabetes to receive insulin glargine or NPH insulin. Insulin glargine produced more stable fasting plasma glucose (FPG) levels and reduced mean FPG levels by 2.2 mmol/L compared with patients taking NPH insulin (p = 0.0001). The advantage of insulin glargine over NPH insulin was seen mostly in patients who had previously been on NPH insulin once-daily. In this short study, the rates of hypoglycemia were higher in the insulin glargine group than those taking NPH (p = 0.03), although the authors state that the difference was not clinically meaningful. In a further study of similar design in 333 patients, insulin glargine produced significantly lower FPG (−1.88 mmol/L, p = 0.0005), fasting self-monitored blood glucose levels (−0.80 mmol/L, p = 0.002), and HbA1c levels (−0.14%, p = 0.03) versus NPH insulin (Pieber et al 2000). Although the overall frequency of hypoglycemia was similar in both treatment groups, nocturnal hypoglycemia over the treatment period as a whole was significantly lower among patients treated with insulin glargine than in patients treated with once daily NPH (36% versus 55%, p = 0.0037) although an analysis of rates of nocturnal hypoglycemia for the final week of the trial did not show any significant differences (Pieber et al 2000).
A longer-term study of 16 weeks (n = 310) also showed significant improvements in FPG levels (1.63 mmol/L versus 0.66 mmol/L, p = 0.0001) and more consistent FPG levels with insulin glargine compared with NPH insulin (median decrease in variability at study end of 3.44 mmol/L versus 0.79 mmol/L, p = 0.0124). Furthermore, a greater proportion of patients in the insulin glargine group reached a target fasting glucose level of 6.6 mmol/L than in the NPH insulin group (29.6% versus 16.8%, p value not reported) (Raskin et al 2000).
While similar rates of hypoglycemia were observed in the 16-week study, a 28-week trial of 534 patients found that compared with NPH insulin, insulin glargine was associated with a lower rate of symptomatic hypoglycemia (39% versus 49%, p = 0.0219) and nocturnal hypoglycemia (18.2% versus 27%, p = 0.0116) (Ratner et al 2000). As with the shorter-term trial, insulin glargine was more effective at reducing FPG levels (−1.76 mmol/L versus −0.33 mmol/L for insulin glargine and NPH insulin, respectively, p = 0.0145) (Ratner et al 2000). Consistent results were reported from another 28-week trial of 394 patients (Hershon and Blevins 2004). In this case, insulin glargine was compared with a twice-daily NPH insulin basal regimen. FPG levels were reduced by −1.17 mmol/L versus −0.56 mmol/L in the insulin glargine and NPH insulin groups, respectively (p = 0.015), and a greater percentage of patients treated with insulin glargine reached the target FPG (32.6% versus 21.3%; p = 0.015). As with the earlier trial, significantly fewer symptomatic hypoglycemic events occurred with insulin glargine than with NPH insulin (36.6% versus 46.2%; p = 0.033).
One study has compared insulin glargine with ultralente insulin and the results were consistent with those found for comparisons with NPH formulations. Although the ability to assess clinical significance is limited by its small size, this randomized, cross-over trial (n = 22) found that in addition to providing more stable nocturnal glucose control (continuous subcutaneous glucose monitoring [CSGM], nocturnal values: 49.06 ± 4.74 versus 62.36 ± 5.21, p = 0.036), being more effective at lowering FPG levels (between group difference −35.70 ± 15.97, p = 0.047) and lowering the number of hypoglycemic episodes (between group difference for day and night episodes of symptoms suggestive of hypoglycemia and simultaneous capillary blood glucose <3.3 mmol/L: −1.5, p = 0.002 and −3.0, p = 0.0015, respectively), insulin glargine significantly reduced HbA1c levels compared with ultralente insulin (6.82% versus 7.02%, p = 0.03) (Kudva et al 2005).
Insulin glargine was found to provide at least equivalent glycemic control as NPH insulin in children and adolescents with type 1 diabetes. This 6-month trial randomized 349 patients aged 5–16 years to receive insulin glargine once-daily at bedtime or NPH insulin either once- (at bedtime in 114 patients) or twice-daily (in the morning and at bedtime in 61 patients). Changes in HbA1c from baseline to endpoint were similar in the insulin glargine and NPH insulin groups (−0.28 ± 0.09% and −0.27 ± 0.09%, respectively), but the corresponding changes in FPG decreased more in the insulin glargine than NPH insulin groups (−1.29 mmol/L versus −0.68 mmol/L, p = 0.02). Overall rates of hypoglycemia were similar in both groups (78.9% and 79.3% for insulin glargine and NPH insulin, respectively) (Schober et al 2001).
Type 2 diabetes
The efficacy of insulin glargine has also been demonstrated in patients with type 2 diabetes with inadequate glycemic control on oral antidiabetic agents. Two trials compared NPH insulin with insulin glargine added to existing oral agents.
Riddle et al (2003) compared the addition of either insulin glargine or NPH insulin with existing regimens of one or two oral antidiabetic agents. This 24-week, randomized trial enrolled 756 patients. Although both basal insulins reduced FPG and HbA1c levels by similar amounts (FPG: 6.4 mmol/L versus 6.6 mmol/L; HbA1c: 6.96% versus 6.97% for insulin glargine and NPH insulin, respectively), 25% more patients attained a target HbA1c level of =7% without experiencing nocturnal hypoglycemia with insulin glargine than with NPH insulin (p < 0.05) (Riddle et al 2003). The overall rate of hypoglycemia, rate of symptomatic events, and rate of confirmed events in the insulin glargine group were reduced by 21%, 29%, and 41%, respectively (Riddle et al 2003). Yki-Jarvinen's group designed a similar trial, but the treatment period was one year. Again, insulin glargine and NPH insulin reduced HbA1c levels by a similar amount, but there was less nocturnal hypoglycemia (9.9% versus 24.0% of patients, p < 0.001) and insulin glargine was associated with better post-meal glucose control than NPH insulin (9.9 mmol/L versus 10.7 mmol/L, p < 0.002) (Yki-Jarvinen et al 2000).
Optimizing treatment algorithms
As insulin glargine is still a relatively new form of therapy, the definition of the optimal initiation and maintenance regimen is ongoing. Recently published data from the AT. LANTUS (A Trial comparing Lantus® Algorithms to achieve Normal blood glucose Targets in patients with Uncontrolled blood Sugar) study suggest that a greater degree of self-management can improve glycemic control. The study compared two algorithms for the initiation of therapy with insulin glargine in 4961 patients with type 2 diabetes (Davies et al 2005). Patients were randomized to a physician-managed algorithm, where insulin doses were titrated at each physician visit, or to a self-management algorithm where patients adjusted their own dose every 3 days. After 24 weeks, HbA1c levels were lowered significantly more in the self-versus physician-managed algorithm (−1.22% versus −1.08% mmol/L, p < 0.001), as were FPG levels (−3.4% versus 3.1% mmol/L, p < 0.001). Importantly, this improvement in glycemic control was achieved without an increase in the incidence of severe hypoglycemia (Davies et al 2005).
Safety profile of insulin glargine
Various definitions and methods have been used to measure hypoglycemia in trials of insulin glargine, which limits the ability to generalize the findings across all patients with diabetes. While some studies have shown similar rates of hypoglycemia when compared with NPH insulin, there is also evidence insulin glargine can maintain effective glucose control and reduce the risk of hypoglycemia. Rosenstock et al (2001) randomized 518 patients with type 2 diabetes, who were already being treated with basal NPH insulin and regular insulin, to receive either insulin glargine or NPH insulin once- or twice daily. While improvements in HbA1c were comparable, the group who switched to insulin glargine showed a 25% decrease in the rate of nocturnal hypoglycemia (26.5% versus 35.5%, p = 0.0136). A recent metaanalysis of four open-label, randomized trials of insulin glargine versus NPH insulin adds further weight to this assertion. In total, 2304 patients were randomized and while glycemic control was similar between groups, there was a significant and consistent reduction in the risk of hypoglycemia (Table 1) (Rosenstock et al 2005).
Table 1.
Meta-analysis of episodes of hypoglycemia with insulin glargine versus NPH insulin (Derived from Table 3 of Rosenstock et al 2005)
| Type of documented symptomatic hypoglycemia | Insulin glargine (% of patients) | NPH insulin (% of patients) | p | Insulin glargine significant % risk reduction |
|---|---|---|---|---|
| Overall | 54.2 | 61.2 | 0.0006 | 11 |
| Nocturnal | 28.4 | 38.2 | <0.0001 | 26 |
| Non-nocturnal | 49.6 | 51.7 | 0.2553 | – |
| Severe | 1.4 | 2.6 | 0.0422 | 46 |
| Severe nocturnal | 0.7 | 1.7 | 0.0231 | 59 |
| Severe non-nocturnal | 0.8 | 0.9 | 0.7296 | – |
Abbreviations: NPH, neutral protamine Hagedorn.
The overall safety profile of insulin glargine is similar in patients with type 1 or type 2 diabetes. Insulin glargine is generally well tolerated and appears to have a very similar safety profile to NPH insulin, the most relevant comparator. The only difference is a greater frequency of injection-site pain with insulin glargine reported in some, but not all, trials (Raskin et al 2000; Ratner et al 2000; Rosenstock et al 2001). This increase in pain may be related to the product's acid pH, but this rarely leads to discontinuation.
Weight gain has long been an issue with insulin therapy. Insulin glargine has been associated with a mean weight gain of up to 2.02 kg in a 39-month study of 239 patients being treated in combination with oral antidiabetes agents, but many studies have reported no significant weight gain despite significant improvements in HbA1c (Dunn et al 2003). Some evidence suggests that insulin glargine may be associated with less weight gain than NPH insulin. In three studies, NPH insulin was associated with significantly more weight gain than insulin glargine (Raskin et al 2000; Rosenstock et al 2001; Garg et al 2004). In one 16-week trial in type 1 diabetes, weight gain was greater with NPH insulin than with insulin glargine (−0.12 kg with insulin glargine versus 0.54 kg with NPH insulin, p = 0.034) (Raskin et al 2000) In a further study of 196 patients with type 1 diabetes, mean weight gain from baseline was significantly higher with NPH insulin (1.4 kg ± 1.8 kg, p = 0.004) compared with insulin glargine (no significant weight gain, p = 0.4). In a 16-week trial in patients with type 2 diabetes, weight gain was 0.4 kg with insulin glargine versus 1.4 kg with NPH insulin (p < 0.0007) (Rosenstock et al 2001). In contrast, one study reported similar gains in mean body weight following 1 year of treatment of patients with type 2 diabetes with insulin glargine (+2.6 kg, n = 214) and NPH insulin (+2.3 kg, n = 208) (Yki-Jarvinen et al 2000).
Insulin glargine has up to 6-fold greater potency at the insulin-like growth factor-1 (IGF-1) receptor than regular human insulin and this has raised the concern of mitogenic effects in cells that express a high number of IGF-1 receptors (Kurtzhals et al 2000). IGF-1 has also been implicated in the progression of retinopathy. Reports that insulin glargine was associated with an increase in three-step progression of retinopathy in the Early Treatment Diabetic Retinopathy study were refuted when an independent panel convened by Aventis reviewed the data (Bolli and Owens 2000).
Treatment of rats and mice for up to 2 years at doses up to 10-times the normal starting dose of insulin glargine have not suggested an increase in carcinogenicity (Bolli and Owens 2000; Lantus 2004). The mitogenic activity of insulin glargine was not increased compared with regular human insulin in in vitro tests using cell lines that mainly express insulin receptors (Berti et al 1998). Furthermore, the uptake of thymidine into DNA in human cultured skeletal muscle cells from patients with type 2 diabetes was equivalently stimulated by regular human and insulin glargine, but was significantly more potently stimulated by IGF-1 (EC50s: 51 ± 14 nM, 63 ± 18 nM and 0.57 ± 0.20 nM, respectively) (Ciaraldi et al 2001).
While there is no evidence that insulin glargine is carcinogenic in routine clinical use, the theoretical risk of increased mitogenesis reinforces the need for appropriate pharmacovigilance.
The concerns on mitogenesis mean insulin glargine is not recommended for use during pregnancy, despite the fact this treatment may enhance the ability to reach the tight glycemic control recommended for diabetic pregnancies. Di Cianni et al (2005) reported the use of insulin glargine in five women with unplanned pregnancy from the pre-conception period to 6–12 weeks post-conception, when they were switched to NPH insulin or CSII. Insulin glargine did not seem to affect embryo–fetal development during this critical period of embryo-genesis as all deliveries were viable, had no minor or major congenital malformations, or any complications during the post-partum period. This evidence adds to the small number of reported case studies where the use of insulin glargine did not affect embryo-genesis in pregnant women with diabetes (Devlin et al 2002; Holstein et al 2003).
Case reports of nausea and vomiting in patients receiving insulin glargine have been published, although this adverse event appears to be rare (Dixon and Bain 2005).
Insulin glargine – patient satisfaction
The clinical benefits of insulin glargine over traditional basal insulins have been demonstrated in clinical trials. Combined with the convenience of once-daily injection and the absence of suspension problems, this agent may help to take a step closer to achieving tight glycemic control in many patients with diabetes. Do these changes lead to an improvement in treatment satisfaction? A prospective audit of the introduction of insulin glargine in 83 type 1 diabetes patients transferred from NPH insulin found that the switch reduced morning blood glucose and HbA1c levels and improved patient satisfaction and subjective well-being (Gallen and Carter 2003). This appears to confirm another study that showed insulin glargine was associated with consistent and significant improvements in treatment satisfaction, as measured by the Diabetes Treatment Satisfaction Questionnaire (Witthaus et al 2001). The study gathered questionnaires from 517 patients with type 1 diabetes enrolled in a 28-week randomized, controlled clinical trial of insulin glargine versus NPH insulin. While general well being was not significantly different between groups at any point during the trial, treatment satisfaction improved over the trial period with insulin glargine and slightly deteriorated in the NPH insulin group (p = 0.0001). Furthermore, scores relating to perceived frequency of hyperglycemia and hypoglycemia were significantly better for insulin glargine and NPH insulin (Witthaus et al 2001). One weakness of this trial is the open-label design, which might have allowed the bias of the subjective measures being tested through knowledge of the treatment being allocated.
Emerging uses of insulin glargine
The long duration of action and lack of peak activity has raised the possibility of insulin glargine being useful in a number of special clinical settings. Frail elderly patients with diabetes, in whom hypoglycemia is a particular risk, may benefit from a reduced number of injections and from a reduced risk of hypoglycemia. As insulin glargine is a clear solution, concerns of adequate mixing of isophane insulins are alleviated for those with problems with dexterity. Furthermore, for those who cannot self-inject, the once-a-day regimen can facilitate the administration of insulin glargine at a time convenient for a community nurse.
Insulin glargine may also be useful in those who require continuous enteric tube feeding as the peakless nature and long duration of action may circumvent the need for continuous intravenous or subcutaneous insulin infusion. In fact, a case study reported by Putz and Kabadi (2002) outlines the successful use of insulin glargine in a 60-year old man who required enteral tube feeding following radiotherapy for squamous cell carcinoma.
Conclusion
Managing diabetes with insulin is primarily based on the balance between the necessity of tight glycemic control and the risks associated with hypoglycemia. Insulin glargine appears to improve this balance such that at least equivalent glycemic control can be achieved with a lower risk of hypoglycemia than traditional basal insulins. In patients with type 2 diabetes, the reduced risk of hypoglycemia with insulin glargine, combined with the flexibility of once-daily dosing at any time of the day, is likely to make insulin a more acceptable option, which may mean that patients are more open to start insulin earlier and to intensify their insulin sooner. In the long-term this may lead to improvements in HbA1c and thereby a reduction in the long-term complications of diabetes. In type 1 diabetes the reduced risk of hypoglycemia with insulin glargine, combined with improved treatment satisfaction, means that patients should be able to intensify their treatment and improve their ability to reach HbA1c targets, which, in the long term, may also lead to a reduced risk of complications. These hypotheses need to be tested with long-term comparator studies of insulin glargine and other insulins.
Disclosure
Professor Barnett has provided advice and given lectures for remuneration and has received research grants from all the major companies involved in producing insulin and oral diabetic medication.
References
- Bahr M, Kolter T, Seipke G, et al. Growth promoting and metabolic activity of the human insulin analogue [GlyA21, ArgB31, ArgB32] insulin (HOE 901) in muscle cells. Eur J Pharmacol. 1997;320:259–65. doi: 10.1016/s0014-2999(96)00903-x. [DOI] [PubMed] [Google Scholar]
- Berti L, Kellerer M, Bossenmaier B, et al. The long acting human insulin analog HOE 901: characteristics of insulin signalling in comparison to Asp(B10) and regular insulin. Horm Metab Res. 1998;30:123–9. doi: 10.1055/s-2007-978849. [DOI] [PubMed] [Google Scholar]
- Bohannon NJV. Optimizing insulin regimens in type 1 diabetes. How to help patients get control of their life. Postgraduate Medicine. Postgrad Med. 2003;113:39–42. 45–8, 54. doi: 10.3810/pgm.2003.06.1430. [DOI] [PubMed] [Google Scholar]
- Bolli GB, Owens DR. Insulin glargine. Lancet. 2000;356:443–5. doi: 10.1016/S0140-6736(00)02546-0. [DOI] [PubMed] [Google Scholar]
- Campbell RK, White JR., Jr Insulin therapy in type 2 diabetes. J Am Pharm Assoc (Wash) 2002;42:602–11. doi: 10.1331/108658002763029580. [DOI] [PubMed] [Google Scholar]
- Ciaraldi TP, Carter L, Seipke G, et al. Effects of the long-acting insulin analog insulin glargine on cultured human skeletal muscle cells: comparisons to insulin and IGF-I. J Clin Endocrinol Metab. 2001;86:5838–47. doi: 10.1210/jcem.86.12.8110. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev. 1999;15:42–6. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Davies M, Storms F, Shutler S, et al. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:128–8. doi: 10.2337/diacare.28.6.1282. [DOI] [PubMed] [Google Scholar]
- [DCCT] DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- [DCCT/EDIC] DCCT/EDIC Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Hothersall L, Wilkis JL. Use of insulin glargine during pregnancy in a type 1 diabetic woman. Diabetes Care. 2002;25:1095–6. doi: 10.2337/diacare.25.6.1095-a. [DOI] [PubMed] [Google Scholar]
- Di Cianni G, Volpe L, Lencioni C, et al. Use of insulin glargine during the first weeks of pregnancy in five type 1 diabetic women. Diabetes Care. 2005;28:982–3. doi: 10.2337/diacare.28.4.982. [DOI] [PubMed] [Google Scholar]
- Dixon AN, Bain SC. Nausea and vomiting due to insulin glargine in patient with type 1 diabetes mellitus. BMJ. 2005;330:455. doi: 10.1136/bmj.330.7489.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CJ, Plosker GL, Keating GM, et al. Insulin glargine: an updated review of its use in the management of diabetes mellitus. Drugs. 2003;63:1743–78. doi: 10.2165/00003495-200363160-00007. [DOI] [PubMed] [Google Scholar]
- Feher M, Bailey C. Reclassifying insulins. Br J Diabetes Vasc Dis. 2004;1:39–42. [Google Scholar]
- Gallen IW, Carter C. Prospective audit of the introduction of insulin glargine (lantus) into clinical practice in type 1 diabetic patients. Diabetes Care. 2003;26:3352–3. doi: 10.2337/diacare.26.12.3352. [DOI] [PubMed] [Google Scholar]
- Garg SK, Paul JM, Karsten JI, et al. Reduced severe hypoglycemia with insulin glargine in intensively treated adults with type 1 diabetes. Diabetes Technol Ther. 2004;6:589–95. doi: 10.1089/dia.2004.6.589. [DOI] [PubMed] [Google Scholar]
- Gillies PS, Figgitt DP, Lamb HM. Insulin glargine. Drugs. 2000;59:253–60. doi: 10.2165/00003495-200059020-00009. [DOI] [PubMed] [Google Scholar]
- Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23:644–9. doi: 10.2337/diacare.23.5.644. [DOI] [PubMed] [Google Scholar]
- Heinemann L, Richter B. Clinical pharmacology of human insulin. Diabetes Care. 1993;16(Suppl 3):90–100. doi: 10.2337/diacare.16.3.90. [DOI] [PubMed] [Google Scholar]
- Hershon KS, Blevins TC. Once-daily insulin glargine compared with twice-daily NPH insulin in patients with type 1 diabetes. Endocr Pract. 2004;10:10–17. doi: 10.4158/EP.10.1.10. [DOI] [PubMed] [Google Scholar]
- Hirsch IB. Intensive treatment of type 1 diabetes. Med Clin North Am. 1998;82:689–719. doi: 10.1016/s0025-7125(05)70020-1. [DOI] [PubMed] [Google Scholar]
- Holstein A, Plaschke A, Egberts EH. Use of insulin glargine during embryo-genesis in a pregnant woman with Type 1 diabetes. Diabet Med. 2003;20:779–80. doi: 10.1046/j.1464-5491.2003.00992.x. [DOI] [PubMed] [Google Scholar]
- Home PD, Boulton AJM, Jimenez J, et al. on behalf of the Worldwide Initiative for Diabetes Education (WorldWIDE) Issues relating to the early or earlier use of insulin in type 2 diabetes. Pract Diab Int. 2003;20:63–71. [Google Scholar]
- Jehle PM, Micheler C, Jehle DR, et al. Inadequate suspension of neutral protamine Hagendorn (NPH) insulin in pens. Lancet. 1999;354:1604–7. doi: 10.1016/S0140-6736(98)12459-5. [DOI] [PubMed] [Google Scholar]
- Kudva YC, Basu A, Jenkins GD, et al. Randomized controlled clinical trial of glargine versus ultralente insulin in the treatment of type 1 diabetes. Diabetes Care. 2005;28:10–14. doi: 10.2337/diacare.28.1.10. [DOI] [PubMed] [Google Scholar]
- Kurtzhals P, Schaffer L, Sorensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49:999–1005. doi: 10.2337/diabetes.49.6.999. [DOI] [PubMed] [Google Scholar]
- LANTUS®. Insulin glargine (rDNA origin) injection. Prescribing Information. Kansas City, MO 64137, USA: Aventis Pharmaceuticals, Inc; 2004. [Google Scholar]
- Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142–8. doi: 10.2337/diabetes.49.12.2142. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Lachin J, Cleary P, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE. Appraisal consultation document: The clinical effectiveness and cost effectiveness of long-acting insulin analogues for diabetes [online] 2002 Accessed October 2005. URL: http://www.nice.org.uk/article.asp?a=35497.
- Owens DR, Coates PA, Luzio SD, et al. Pharmacokinetics of 125Ilabeled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care. 2000;23:813–19. doi: 10.2337/diacare.23.6.813. [DOI] [PubMed] [Google Scholar]
- Pieber TR, Eugene-Jolchine I, Derobert E. Efficacy and safety of HOE 901 versus NPH insulin in patients with type 1 diabetes. The European Study Group of HOE 901 in type 1 diabetes. Diabetes Care. 2000;23:157–62. doi: 10.2337/diacare.23.2.157. [DOI] [PubMed] [Google Scholar]
- Putz D, Kabadi UM. Insulin glargine in continuous enteric tube feeding. Diabetes Care. 2002;25:1889–90. doi: 10.2337/diacare.25.10.1889. [DOI] [PubMed] [Google Scholar]
- Raskin P, Klaff L, Bergenstal R, et al. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care. 2000;23:1666–71. doi: 10.2337/diacare.23.11.1666. [DOI] [PubMed] [Google Scholar]
- Ratner RE, Hirsch IB, Neifing JL, et al. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes U.S Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23:639–43. doi: 10.2337/diacare.23.5.639. [DOI] [PubMed] [Google Scholar]
- Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Dailey G, Massi-Benedetti M, et al. Reduced hypoglycemia risk with insulin glargine: a meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care. 2005;28:950–5. doi: 10.2337/diacare.28.4.950. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Park G, Zimmerman J. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens. US Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Diabetes Care. 2000;23:1137–42. doi: 10.2337/diacare.23.8.1137. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Schwartz SL, Clark CM, Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631–6. doi: 10.2337/diacare.24.4.631. [DOI] [PubMed] [Google Scholar]
- Rosskamp RH, Park G. Long-acting insulin analogs. Diabetes Care. 1992;22(Suppl 2):B109–13. [PubMed] [Google Scholar]
- Schober E, Schoenle E, Van Dyk J, et al. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes. Diabetes Care. 2001;24:2005–6. doi: 10.2337/diacare.24.11.2005. [DOI] [PubMed] [Google Scholar]
- [UKPDS] UKPDS Study Group. United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995a;310:83–8. [PMC free article] [PubMed] [Google Scholar]
- [UKPDS] UKPDS Study Group. UK prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study (UKPDS) Group. Diabetes. 1995b;44:1249–58. [PubMed] [Google Scholar]
- [UKPDS] UKPDS Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998a;352:837–53. [PubMed] [Google Scholar]
- [UKPDS] UKPDS Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998b;352:854–65. [PubMed] [Google Scholar]
- Wallace TM, Matthews DR. Poor glycemic control in type 2 diabetes: a conspiracy of disease, suboptimal therapy and attitude. QJM. 2000;93:369–74. doi: 10.1093/qjmed/93.6.369. [DOI] [PubMed] [Google Scholar]
- Witthaus E, Stewart J, Bradley C. Treatment satisfaction and psychological well-being with insulin glargine compared with NPH in patients with Type 1 diabetes. Diabet Med. 2001;18:619–25. doi: 10.1046/j.1464-5491.2001.00529.x. [DOI] [PubMed] [Google Scholar]
- Wright A, Burden AC, Paisey R, et al. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57) Diabetes Care. 2002;25:330–6. doi: 10.2337/diacare.25.2.330. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130–6. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- Zinman B, Ross S, Campos RV, et al. Effectiveness of human ultralente versus NPH insulin in providing basal insulin replacement for an insulin lispro multiple daily injection regimen. A double-blind randomized prospective trial. The Canadian Lispro Study Group. Diabetes Care. 1999;22:603–8. doi: 10.2337/diacare.22.4.603. [DOI] [PubMed] [Google Scholar]