Abstract
Clinicians involved in the care of patients with cardiovascular conditions have recently been confronted with an important body of literature linking inflammation and cardiovascular disease. Indeed, the level of systemic inflammation as measured by circulating levels of C-reactive protein (CRP) has been linked to prognosis in patients with atherosclerotic disease, congestive heart failure, atrial fibrillation, myocarditis, aortic valve disease and heart transplantation. In addition, a number of basic science reports suggest an active role for CRP in the pathophysiology of cardiovascular diseases. This article explores the potential role of CRP in disease initiation, progression, and clinical manifestations and reviews its role in the prediction of future events in clinical practice. Therapeutic interventions to decrease circulating levels of CRP are also reviewed.
Keywords: C-reactive protein, inflammation, atherosclerosis, risk prediction, cardiovascular disease, acute coronary syndrome
Introduction
Cardiovascular disease is one of the leading causes of death worldwide and accounts for 40% of all deaths in the US. Understanding the underlying basic biologic principles and prognostic risk factors is fundamental to identifying the most appropriate therapeutic strategies. Clinicians involved in the care of patients with cardiovascular conditions have recently been confronted with an important body of literature linking inflammation and cardiovascular disease. Indeed, the level of systemic inflammation as measured by circulating levels of high sensitivity C-Reactive protein (hs-CRP) has been linked to prognosis in patients with atherosclerotic disease, congestive heart failure (CHF), atrial fibrillation (AF), myocarditis, aortic valve disease, and heart transplantation. In addition, a number of basic science reports suggest an active role for CRP in the pathophysiology of cardiovascular diseases. This article reviews the underlying mechanisms by which CRP potentially participates in disease initiation, progression, and clinical manifestations, and reviews its role as a predictor of future clinical events. Therapeutic strategies to decrease CRP are reviewed.
C-reactive protein and atherosclerosis/atherothrombosis
Atherosclerosis initiation and progression
Atherogenesis begins with endothelial dysfunction in response to various injuries (L'Allier 2004). Central to this disease process are circulating low density lipoprotein (LDL) molecules which transmigrate across the endothelium and are oxidized by local reactive oxygen species (ROS). Oxidized LDL (Ox-LDL) molecules (and not native, unmodified LDLs) possess direct cytotoxicity and stimulate endothelial cells to express adhesion molecules that allow white blood cells to abnormally adhere to the endothelium and to differentiate into macrophages. Macrophages express scavenger receptors on their surface allowing unopposed phagocytosis of Ox-LDLs, leading to the well known cytopathological designation of “foam cells”. These foam cells are very active biologically and secrete a host of chemotactic factors and cytokines promoting smooth muscle cell activation/migration, cellular apoptosis, and vascular inflammation. The known “classical” risk factors associated with atherosclerosis – dyslipidemia, diabetes, smoking, and hypertension – create an environment of increased oxidative stress through formation of ROS (Tardif et al 2003). Elevated levels of ROS then activate redox-sensitive signaling pathways and transcriptional factors in the cell nucleus such as nuclear factor kappa B (NF-κB), peroxisome proliferator-activated receptors (PPARs), and activator protein-1 (AP-1). Once activated, transcriptional factors preferentially promote the transcription of “atherogenic genes” that subsequently express a host of proinflammatory factors, including cytokines, chemokines, and adhesion molecules that are responsible for endothelial activation, vascular dysfunction, and inflammation.
Key inflammatory mediators believed to be involved in atherosclerotic disease initiation and progression include vascular-cell adhesion molecule-1 (VCAM-1), monocyte chemotactic protein-1 (MCP-1), CD40 ligand, and CRP. CRP is particularly interesting to study in the clinical setting because of its biological properties that allow easy and reliable measurements. The preferred methods of CRP measurement today are high-sensitivity nephelometric assays that can be performed on fresh, stored, and frozen plasma (ex. Dade Behring BN II [Deerfield, IL, USA], Abbott IMx [Abbott Park, IL, USA], Diagnostic Products Corporation IMMULITE [Los Angeles, CA, USA], and Beckman Coulter IMMAGE [Fullerton, CA, USA]) (Roberts et al 2000). These assays allow discrimination within what was previously recognized as the normal range (levels of CRP as low as 0.15 mg/L can now be measured, corresponding to <2.5 percentile of the general population) (Ledue et al 1998; Kapyaho et al 1989). Indeed, this discrimination appears to be crucial in the realm of cardiovascular diseases since most patients fall within the “normal” range (<5.0 mg/L) of previous assays.
CRP was originally isolated as a protein that binds to the C-polysaccharide of the cell wall of pneumococci. It is a major acute phase reactant produced mainly by hepatocytes after stimulation by cytokines, of which interleukin-6 (IL-6) appears the major inducer. It is part of the so-called innate immunity system. CRP levels increase six hours after acute stimuli, reaching a peak within 48 hours (up to 100-fold) (Kushner 1990). With abrupt cessation of stimuli, values decrease exponentially at a rate close to the half-life of CRP (18–20 hours) (Ridker 2003). Early reports showed no diurnal variation and no age or gender dependence (Meier-Ewert et al 2001; Imhof et al 2003; Rifai and Ridker 2003). However, these reports were based on comparisons of CRP levels across dissimilar studies with heterogeneous populations. A recent large scale cohort study included 2749 white and black subjects aged 30 to 65 participating in the Dallas Heart Study compared levels of CRP between different race and gender groups. After adjustment for traditional risk factors, body mass index, estrogen, and statin use, a CRP level >3 mg/L was more common in white women (odds ratio [OR] 1.6; 95% confidence interval [CI] 1.1 to 2.5) and black women (OR 1.7; 95% CI 1.2 to 2.6), but not in black men (OR, 1.3; 95% CI, 0.8 to 1.9) when compared with white men (Khera et al 2005).
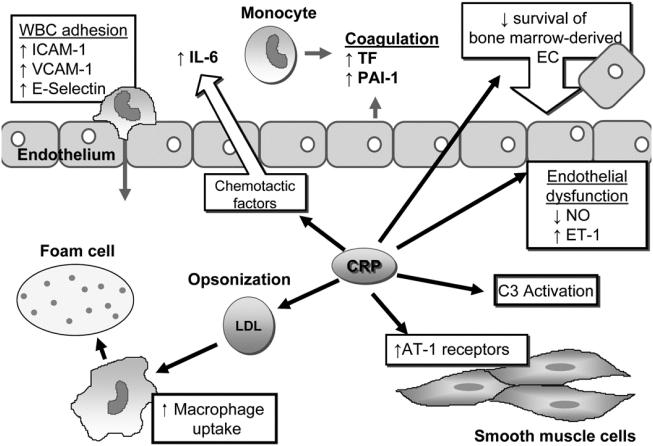
CRP appears to play an active role in endothelial dysfunction and atherosclerotic plaque formation and progression and has been found within atherosclerotic plaques (Figure 1). Indeed, CRP down-regulates endothelial nitric oxide (NO) synthase (eNOS) transcription in endothelial cells (EC) and destabilizes eNOS mRNA, resulting in decreased NO release (Verma, Wang, et al 2002). This inhibition of NO production facilitates endothelial cell apoptosis and blocks angiogenesis (Verma, Szmitko, et al 2004). Among other proatherogenic effects, CRP upregulates adhesion molecules (intercellular adhesion molecule-1 (ICAM-1), VCAM-1, and E-selectin through up-regulation of NF-κB involved in the nuclear transcription of several proatherosclerotic genes (Pasceri et al 2000; Verma et al 2003) and can facilitate leukocyte transmigration by stimulating the release of MCP-1 (Pasceri et al 2001). It also up-regulates angiotensin type-1 receptor (AT-1) in vascular smooth muscle cells and stimulates migration, proliferation, neointimal formation, and ROS production (Pasceri et al 2000). In addition, CRP inhibits bone marrow-derived endothelial progenitor cell survival and differentiation, impairing maintenance of vascular integrity (Verma, Kuliszewski, et al 2004; Verma, Szmitko, et al 2004).
Figure 1.
CRP in the pathogenesis of atherosclerosis and atherothrombosis.
Abbreviations: AT-1, angiotensin-1; CRP, C-reactive protein; EC, endothelial cells; ET-1, endothelin-1; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; LDL, low-density lipoprotein; NO: nitric oxide; PAI-1, plasminogen activator inhibitor-1; TF, tissue factor; VCAM-1, vascular cell adhesion molecule-1; WBC: white blood cells.
Another mechanism by which CRP actively participates in atheroma formation is facilitation of LDL uptake by macrophages by opsonization, leading to foam-cell formation (Zwaka et al 2001). Furthermore, CRP activates complement (particularly C3) (Wolbink et al 1996), which has been found within atherosclerotic lesions and is believed to be implicated in both the initiation and progression of atherosclerosis (Niculescu and Rus 1999). CRP may also induce the production of endothelin-1 (ET-1), a potent endogenous vasoconstrictors and mediator of endothelial dysfunction, leukocyte and platelet activation and cellular proliferation (Miyauchi and Masaki 1999). Finally, CRP stimulates the production of IL-6 in the vasculature (Yudkin et al 2000). This finding is particularly important since IL-6 is involved in a positive feedback loop to stimulate CRP production by the liver.
Interestingly, Verma and colleagues recently suggested that CRP may require dissociation from a pentameric to a monomeric form (mCRP) to exert its proatherosclerotic effects and that agents interfering with the conversion of pentameric CRP to the more active monomeric form may serve to limit CRP's proinflammatory effects (Verma, Kuliszewski, et al 2004). These findings were also confirmed by Khreiss et al (2004). mCRP was able to increase expression of adhesion molecules E-selectin, ICAM-1, and VCAM-1 by human coronary artery endothelial cells (HCAECs) as early as 4 hours after incubation. The increase of expression was associated with increased adhesion of neutrophils to HCAECs cultured with mCRP. Unlike mCRP, the induction with the native pentameric CRP didn't become evident until 6 to 12 hours of incubation with a maximal at 24 hours, coincident with its dissociation time. These findings support also the theory proposed by Verma and colleagues that CRP requires dissociation to exert its full proatherosclerotic effect (Verma, Szmitko, et al 2004). Currently, there is no assay to measure mCRP specifically.
Therefore, it is plausible that CRP plays an active role in disease initiation and progression by directly affecting pro-atherogenic gene expression, pro-inflammatory pathways and vascular homeostasis. Important results of a post hoc analysis of the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial (n=502) evaluating the impact of moderate versus intensive statin therapy on atherosclerosis progression assessed by intravascular ultrasound (IVUS) were recently reported (Nissen et al 2005). The authors applied statistical methods to examine the relationship between LDL-cholesterol (LDL-C) and CRP levels and between levels of both markers and the rate of disease progression (by IVUS). They found a significant relationship (although relatively weak) between the reduction in LDL-C and the reduction in CRP levels in the entire population (r=0.13, p=0.005). They also reported that the reduced rate of progression seen with intensive (vs moderate) treatment was significantly and independently related to both greater reductions in atherogenic lipoprotein and CRP levels. However, the correlation coefficients were also relatively weak (and of similar magnitude) for both (r values 0.11 and 0.14, respectively), suggesting that these biomarkers account for only a small fraction of the overall progression. Patients with reductions in both biomarkers greater than the median had significantly slower progression than those with smaller reductions (p=0.001). These findings support the hypothesis that CRP level may eventually become an interesting therapeutic target.
It is important to note that various other inflammatory biomarkers such as IL-6, tumor necrosis factor-α (TNF-α), ICAM-1, P-Selectin, E-Selectin, MCP-1, Il-1, fibrinogen, and soluble CD40 ligand, have also been shown to be independent predictors of cardiovascular risk (Hwang et al 1997; Ridker, Buring, et al 1998; Harris et al 1999; Ridker, Hennekens, et al 2000; Blake and Ridker 2001; Cipollone et al 2001; Heeschen et al 2003; Kervinen et al 2004; L'Allier et al 2005). However, their measurement is difficult in clinical practice because of their short half-lives, lack of standardized assays, and high cost.
Atherothrombosis
Numerous cohort studies link inflammation and thrombosis. A postulated mechanism through which inflammation shifts the hemostatic system in favor of thrombosis is the induction of peripheral blood monocytes to synthesize tissue factor by CRP (Cermak et al 1993). This finding appears particularly important because tissue factor is a well-known and potent stimulus for the extrinsic pathway of coagulation and for endothelial cell activation and expression of adhesion molecules in clinical atherothrombosis (Pasceri et al 2001). Further in vitro studies revealed that incubation of human aortic endothelial cells with CRP also resulted in a time and dose-dependent increase in secreted plasminogen activator inhibitor-1 (PAI-1), PAI-1 activity, intracellular PAI-1 protein, and PAI-1 mRNA. In addition, CRP was shown to stabilize PAI-1 mRNA (Devaraj et al 2003). Together with its role in endothelial dysfunction and impaired homeostasis, these data support CRP's active role in clinical atherothrombosis in addition to its role in atherosclerosis progression.
Prediction of future clinical events (primary prevention)
Beyond its basic functions as an important inflammatory biomarker and a proatherosclerotic/prothrombotic agent, CRP has also been show to predict incidence of myocardial infarction (MI), stroke, and sudden death. CRP emerged as a potent risk marker of recurrent events among patients with no or stable coronary artery disease (CAD), and those undergoing coronary revascularization (Table 1). Many large-scale epidemiological studies among apparently healthy men and women have found CRP to be an independent and strong predictor of future cardiovascular risk (see Table 1 for relative risks and corresponding cutoff values) (Kuller et al 1996; Ridker et al 1997; Tracy et al 1997; Ridker, Hennekens, et al 1998; Ridker, Cushman, et al 1998; Ridker, Buring, et al 1998, 2001; Harris et al 1999; Koenig et al 1999; Ridker, Rifai, et al 2000, 2001; Danesh et al 2000; Mendall et al 2000; Roivainen et al 2000). This finding was consistent across different subpopulations, including elderly patients (Tracy et al 1997), smokers (Kuller et al 1996), and post-menopausal woman (Ridker et al 1997; Tracy et al 1997; Ridker, Hennekens, et al 1998; Ridker, Rifai, et al 2000). Specifically, hs-CRP was the strongest predictor of future vascular events when compared with serum amyloid-A (SA-A), IL-6, ICAM-1, homocysteine, lipoprotein (a), and LDL-C in the Women's Health Study (Liuzzo et al 1996; Ridker, Rifai, et al 2000). Furthermore, an association between hs-CRP levels and all-cause mortality has been demonstrated (Hwang et al 1997; Ridker, Hennekens, et al 1998; Harris et al 1999). A practical approach in the evaluation of the predictive value of CRP has been to divide CRP levels into population-based percentiles and quintiles: absolute hs-CRP values of 0.3 mg/L, 0.6 mg/L, 1.5 mg/L, 3.5 mg/L, and 6.6 mg/L have been reported as estimates of the 10th, 25th, 50th, 75th, and 90th percentile cut-points for middle-aged Americans (Ridker et al 2002). The proposed quintile cut-points are <0.5 mg/L, 0.5–1.0 mg/L, 1.0–2.0 mg/L, 2.0–4.0 mg/L, and >4.0 mg/L, respectively (Ridker et al 1997, 2002; Ridker, Rifai, et al 2000; Ridker 2001b). The authors also proposed a simplified approach using levels of <1 mg/L, 1–3 mg/L, and >3 mg/L corresponding to low, moderate and high-risk groups, respectively. It is suggested to repeat CRP test when CRP levels are above 10 mg/L to exclude inflammatory diseases. The risk of future cardiovascular events associated with each quintile increment was 26% for men and 33% for women after adjustment for age, smoking status, family history of premature coronary disease, diabetes, hypertension, hyperlipidemia, exercise level, and body-mass index (Ridker, Buring, et al 2001). In addition and importantly, CRP was found to enhance global coronary risk as assessed by lipid-derived indices (such as LDL-C) and by the Framingham Risk Score (FRS) that integrates age, lipid profile, smoking status, blood pressure and diabetes (Ridker, Cushman, et al 1998; Ridker, Rifai, et al 2000; Ridker 2001b; Ridker, Buring, et al 2001; Ridker et al 2002). Indeed, hs-CRP was shown to add prognostic information at all levels of LDL-C and at all levels of FRS (Ridker et al 2002; Koeing et al 2004).
Table 1.
Prognostic value of CRP in primary prevention
| Study | n | Endpoint | Cutoff | Risk estimates |
|---|---|---|---|---|
| Ridker, Rifai, et al 2000 | 28 | Death/MI/stroke/revasc | >8.5 mg/L vs <0.6 mg/L | RR=1.5 |
| 263 | ||||
| Harris et al 1999 | 1293 | Death | ≥2.78 mg/L | RR=1.6 |
| Ridker et al 1997 | 543 | MI | ≥2.11 mg/L vs ≤0.55 mg/L | RR=2.9 |
| Ischemic stroke | ≥2.11 mg/L vs ≤0.55 mg/L | RR=1.9 | ||
| Tracy et al 1997 | 5201 | MI | >2.79 mg/L vs <0.97 mg/L | RR=2.67 |
| Ridker, Hennekens, et al 1998 | 122 | Death/MI/stroke/revasc | >7.3 mg/L vs <1.5 mg/L | RR=4.8 |
| MI/stroke | >7.3 mg/L vs <1.5 mg/L | RR=7.3 | ||
| Ridker, Glynn, et al 1998 | 144 | PAD | 2.1 mg/L vs 0.55 mg/L | RR=2.1 |
| Koeing et al 1999 | 936 | CV Death/MI | 6.6 mg/L vs 0.4 mg/L | RR=2.4 |
| Danesh et al 2000 | 1531 | CHD | >2.4 mg/L vs <0.9 mg/L | RR=2.13 |
| Mendall et al 2000 | 1395 | CHD | <0.83 mg/L vs >3.87 mg/L | p=ns |
| CV Death | p=ns | |||
| All cause mortality | p=0.0033 | |||
| Danesh et al 2004 | 6428 | CHD | >2.0 mg/L vs <0.78 mg/L | RR=1.45 |
Abbreviations: CHD, coronary artery disease; CRP, C-reactive protein; CV Death, fatal acute myocardial infarction or sudden death; MI, myocardial infarction; Revasc, revascularization; RR, relative risk; PAD, peripheral arterial disease.
Patients with elevated levels of both LDL-C and CRP were shown to have almost 8 times the cardiovascular risk of those with low levels of both markers. Moreover, it was suggested that in the primary prevention setting, CRP is an even stronger predictor of CV events than is LDL-C (reduction of LDL-C to the lowest quintile would reduce CV risk by 19% whereas CRP reduction to the lowest quintile would theoretically afford a 40% reduction in CV risk). Koenig et al (2004) confirmed that CRP enhances global coronary risk as assessed by the FRS in a large cohort of middle aged men from general population and the contribution of CRP to coronary event risk was independent of the FRS. The value of CRP levels in risk assessment might be particularly high in the “low-and intermediate-risk” subgroups identified by conventional methods of risk detection.
However, the absolute value of CRP in the prediction of CAD remains uncertain. Danesh et al (2004) published results from a large prospective study on CAD (Reykjavik Study) and a meta-analysis of 22 prospective studies to evaluate the relevance of CRP and other inflammatory markers in the prediction of CAD. After adjustment for baseline values of established risk factors, the OR for CAD was 1.45 (95% CI, 1.25–1.68) in a comparison of participants in the top third of the group with respect to baseline CRP values with those in the bottom third in the Reykjavik Study. Similar overall findings were observed in an updated meta-analysis involving a total of 7068 patients with coronary heart disease. By comparison, the ORs in the Reykjavik Study for coronary heart disease were generally stronger for established risk factors, such as an increased total cholesterol concentration (2.35) and cigarette smoking (1.87). The authors therefore concluded that CRP is a relatively modest predictor of coronary heart disease and that recommendations regarding its use in predicting the likelihood of coronary heart disease may need to be reviewed. Of note, the independent relative risk associated with increased CRP levels in this study appeared considerably lower than in some earlier reports (Danesh et al 2004). Furthermore, the investigators of the Dallas Heart Study (n=3373) recently reported that CRP was a poor predictor of atherosclerotic burden as assessed by coronary calcium score (electron-beam computed tomography) and aortic plaque (magnetic resonance imaging). In this study, subjects with higher CRP levels had slightly more subclinical atherosclerosis, but this association was not independent of traditional cardiovascular risk factors (Khera et al 2006).
Based on the available data and before the latter two studies, the Center for Disease Control and the American Heart Association suggested further clarification of the predictive value of CRP in CAD in general population before recommending its widespread use in clinical practice (Pearson et al 2003).
Prediction of future clinical events (Acute coronary syndrome)
Accumulating data suggest that markers of inflammation may be reliable markers of risk of CAD in the short- and medium-term. This finding may be particularly pertinent for acute coronary syndrome (ACS) (Table 2). Numerous small studies showed a correlation between increasing levels of CRP and risk of cardiac events in this clinical setting (cardiac death, MI, recurrent angina, urgent revascularization) (Liuzzo et al 1994; Morrow et al 1998; Rebuzzi et al 1998; Verheggen et al 1999). The cutoff value of CRP in those studies was variable and ranged between 3.0 mg/L and 15.5 mg/L. Overall, the results suggest that elevated levels of baseline CRP are associated with increased recurrent clinical events (such as death, MI, revascularization, and refractory angina) within 14 days. Bazzino et al (2001) showed that after a 90-day follow-up period, an elevated CRP level at discharge appeared to be a more sensitive (88% vs 47%) and specific (81% vs 70%) marker of increased risk of future events than a positive treadmill test. The negative predictive value was 98% for CRP versus 90% for stress test. While two sub-analyses of large multicenter trials involving ACS patients showed no association between CRP levels at entry and risk of death and MI during follow-up (Oltrona et al 1997; Montalescot et al 1998), this was not the case in a Global Use of Strategies To Open Occluded Coronary Arteries IV-Acute Coronary Syndrome Trial (GUSTO IV-ACS) sub-analysis. Patients participating in the GUSTO IV randomized trial not undergoing early revascularization (n=7, 108) were included in a dedicated post-hoc analysis to evaluate CRP and Troponin T (TnT) as predictors of individual endpoints (James et al 2003). Investigators reported that baseline levels of TnT and hs-CRP were both independently related to 30-day mortality and that the combination of both markers provided a better stratification than either marker alone. However, only TnT levels were related to 30-day MI. Along the same line, Sabatine et al (2002) have proposed a multimarker approach to stratification for ACS patients using TnT, CRP, and B-Type Natriuretic Peptide (BNP). Currently, CRP is recognized as a useful adjunct to standard risk stratification in patients with ACS. The bulk of evidence suggests that the predictive value of CRP is independent of and additive to the predictive value of troponin levels. However, there are currently no definitive data to support the concept of tailoring therapy according to CRP levels to decrease clinical events.
Table 2.
Prognostic value of CRP in acute coronary syndromes
| Study | n | Endpoint | Cutoff | Risk estimates |
|---|---|---|---|---|
| Short term | ||||
| Liuzzo et al 1994 | 31 | Death/MI/urg. revasc | 3.0 mg/L | RR=2.6 |
| Morrow et al 1998 | 437 | Death | 15.5 mg/L | RR=16.1 |
| Rebuzzi et al 1998 | 102 | MI | 3 mg/L | RR=6.0 |
| Verheggen et al 1999 | 211 | Refractory angina | 6 mg/L | OR=2.19 |
| Oltrona et al 1997 | 140 | Death/MI/revasc | 10 mg/L | p=ns |
| Montalescot et al 1998 | 68 | Death/MI/angina/revasc | 5 mg/L | p=ns |
| Mid term | ||||
| Ridker et al 2005 | 3,745 | Death/MI | 2 mg/L | RR=1.4 |
| Muller et al 2002 | 1,042 | Death | 10 mg/L | OR=4.1 |
| Heeschen et al 2000 | 447 | Death/MI | 10 mg/L | RR=2.0 |
| Lindhal et al 2000 | 917 | Death | 10 mg/L | RR=2.6 |
| Ferreiros et al 1999 | 194 | Death/MI/refractory angina | 15 mg/L | HR=3.16 |
| Biasucci et al 1999 | 53 | UA | 3 mg/L | OR=8.6 |
| Toss et al 1997 | 965 | Death/MI | >10 mg/L vs <2.0 mg/L | RR=3.5 |
| Haverkate et al 1997 | 2,121 | Sudden death/MI | 3.6 mg/L | RR=2.0 |
Abbreviations: CRP, C-reactive protein; HR, hazard ratio; MI, myocardial infarction; Mid-term, in-hospital, 3–37 months; OR, odds ratio; RR, relative risk; Short term, in-hospital, 3 months; UA, unstable angina; urg. revasc, urgent revascularization.
Beside its potential role in predicting short-term prognosis and recurrent clinical events in patients with ACS, CRP may also have a role in medium-term prognosis (5–37 months). The results of numerous large-scale studies are concordant (Haverkate et al 1997; Toss et al 1997; Biasucci et al 1999; Ferreiros et al 1999; Lindahl et al 2000; Heeschen et al 2000; Muller et al 2002). The relative risk of meaningful clinical events (such as death, MI, angina) in patients with highest levels of baseline CRP varied between 2.0 and 4.1 (cutoff CRP values of 3.0–10.0 mg/L) (Table 2). Persistently elevated CRP levels for >3 months after waning of symptoms are also associated with increased risk of cardiac events (Bazzino et al 2001). The Fragmin during Instability in Coronary Artery Disease (FRISC) investigators showed that the risk associated with elevated CRP levels at the time index event continues to increase for several years (Lindahl et al 2000). Despite these concordant results, it is worth mentioning that other well designed studies showed that high CRP level measured at 72 hours did not predict adverse outcome in patients hospitalized for unstable angina (Oltrona et al 1997). The best evidence to date comes from an analysis of the Pravastatin and Atorvastatin Evaluation and Infection Therapy — Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) study (n=3745) evaluating the risk of death from coronary causes and recurrent MI after an ACS (Ridker at al 2005). The authors evaluated relationships between LDL-C and CRP levels achieved on pravastatin 40 mg or atorvastatin 80 mg and clinical events during mean follow-up of 24 months. They found a significant relationship (although relatively weak) between the reduction in LDL-C and that in CRP levels in the entire population (r=0.16, p=0.001). Although the authors stated that there was a completely independent linear relationship between levels of LDL-C achieved after statin therapy and events and CRP levels and events, the respective correlation coefficients were not reported. While patients who achieved LDL-levels lower than the median had lower event-rate than those with higher levels (2.7 vs 4.0 events per 100 person years, p=0.008), an almost identical difference was observed in patients who achieved CRP levels lower than the median as compared with those with higher levels (2.8 vs 3.9 events per 100 person-years, p=0.006). This effect was present at all levels of LDL-C achieved. In addition, patients with both low LDL-C and low CRP levels had significantly less events (1.9 events per 100 person-years) than either patients with high LDL-C and low CRP, low LDL-C and high CRP levels, or high LDL-C and high CRP levels (respective hazard ratios 1.0, 1.3, 1.4, and 1.9, p<0.001). These observations led the authors to conclude that patients who have low CRP levels after statin therapy have better clinical outcomes than those with higher levels, regardless of LDL-C levels achieved. Strategies to reduce cardiovascular risk with statins might eventually include CRP monitoring in addition to cholesterol, since CRP levels were lower in the high-dose statin group. Such results are concordant with those of atherosclerosis progression studies (Nissen et al 2005) and fuel the hypothesis that reducing inflammation in general and perhaps CRP in particular may have a significant role in controlling atherothrombosis and atherosclerosis progression.
Prediction of future clinical events (following revascularization procedures)
CRP predicts future clinical events after percutaneous coronary interventions (PCI) (Table 3) and rises proportionally to the instability of the culprit coronary lesions (Tomoda and Aoki 2001). Furthermore, the pattern of increase was correlated with restenosis. In patients with stable CAD and subsequent restenosis, CRP increased up to 96 hours of stent implantation, whereas in patients without restenosis, CRP peaked at 48 hours then decreased. Patients with angiographic evidence of restenosis at follow-up also had significantly higher peak post-procedural CRP levels, supporting the role of inflammation in restenosis after stent implantation (Gottsauner-Wolf et al 2000). In addition, higher levels of CRP were evident during follow-up of patients with in-stent restenosis as compared with those without, suggesting that inflammatory processes play an important role in the occurrence of in-stent restenosis (Angioi et al 2001). Similarly, in patients with stable angina and one vessel disease who had stent placement, normalization of hs-CRP levels within 72 hours after coronary artery stenting identified a large subgroup of patients who did not develop cardiovascular events during a 12-month follow-up period (Gaspardone et al 1998). CRP can also be a risk factor of stent thrombosis and recurrent ischemic events after PCI. Chew et al (2001a) showed that elevated baseline levels of CRP (>10.1 mg/L) were associated with a 3.7-fold increase in the risk of death or MI at 30 days after PCI, and that CRP is an independent and additive factor to the increasing American College of Cardiology/American Heart Association (ACC/AHA) lesion score.
Table 3.
Prognostic value of CRP after percutaneous coronary intervention
| Study | n | Endpoint | Cutoff | Risk estimates |
|---|---|---|---|---|
| Heeschen et al 2000 | 447 | Restenosis | 10 mg/L | RR=3.0 |
| Muller et al 2002 | 1042 | Death | 10 mg/L | OR=4.1 |
| Chew et al 2001 | 727 | Death/MI (30 days) | 3.0 mg/L | OR=3.68 |
Abbreviations: CRP, C-Reactive protein; MI, myocardial infarction; OR, odds ratio; RR, risk ratio.
Table 4.
Reported independent prognostic value of CRP in other clinical situations
| Clinical situation | Event(s) |
|---|---|
| Atrial fibrillation | AF development, LA thrombus, success of ECV |
| Myocarditis | NYHA functional class, overall prognosis |
| Heart transplant vasculopathy | Survival |
| Aortic stenosis | Disease progression |
Abbreviations: AF, atrial fibrillation; CRP, C-reactive protein; LA, left atrium; ECV, electrical cardioversion; NYHA, New York Heart Association.
The association between systemic inflammation and short-term adverse clinical outcome was not seen in patients undergoing coronary bypass grafting (CABG). However there is a significant increase in recurrent angina, MI, and new revascularization procedures up to 6 years in patients who had a high baseline CRP. No difference in cardiac and total mortality was noted (Milazzo et al 1999). Further studies are needed to assess more completely the impact of higher levels of CRP on adverse clinical outcomes in patients undergoing CABG.
Therefore, there is strong epidemiological and ex vivo evidence that CRP plays an active role in atherosclerosis initiation/progression and atherothrombosis. A definitive causal relationship between CRP and clinical events is still lacking in humans.
C-reactive protein and other cardiovascular conditions
Congestive heart failure
Patients with either ischemic or non-ischemic heart failure show activation of proinflammatory cytokines possibly by the activation of renin–angiotensin–aldosterone system and the sympathetic nervous system (Ferrari et al 1995; MacGowan et al 1997; Samsonov et al 1998). Elster et al (1956) demonstrated that CRP was present in the serum of thirty of forty patients manifesting CHF, independently of age, sex or race. Higher CRP appeared to predict severity of disease (Elster et al 1956) and CRP was more elevated in patients with severe acute than those with severe chronic heart failure. This finding, in conjunction with the tendency of CRP level to drop with treatment, suggests that the concentration of CRP is influenced by the severity of disease (Pye et al 1990). Higher CRP levels were again observed in patients with higher New York Heart Association (NYHA) functional class and were related to higher rates of readmission and mortality (Alonso-Martinez et al 2002). To evaluate amino terminal-pro-BNP (NT-proBNP) and CRP, separately and together, for assessment of risk of CHF, Campbell et al (2005) performed a nested case-control study of the 6105 participants of the Perindopril pROtection aGainst REcurrent Stroke Study (PROGRESS), 258 subjects who developed CHF resulting in death, hospitalization, or withdrawal of randomized therapy during a mean followup of 3.9 years were matched to one to three control subjects. They found that NT-proBNP and CRP were independent predictors of CHF risk after stroke or transient ischemic attack (TIA). Moreover, NT-proBNP and CRP may be markers of mechanisms of CHF pathogenesis distinct from those responsive to angiotensin-converting enzyme inhibitor-based therapy. While there appears to be a correlation between CRP levels and prognosis, CRP's usefulness in predicting adverse outcomes in patients with CHF beyond known predictors of adverse outcomes in this patient population (such as left ventricular ejection fraction [LVEF], functional class, markers of neurohumoral activation, etc) remains to be proven.
Atrial fibrillation
A link between inflammation and AF has been proposed based on post-operative histologic and genetic studies, and the clinical association between AF and pericarditis. Pathology studies support such a relationship in light of inflammatory infiltrates, myocyte necrosis, and fibrosis on atrial biopsies of patients with refractory arrhythmias. This potential link was further established in a small case-control study by Chung et al (2001) of patients with AF outside the post-operative setting (n=202). They found elevated levels of CRP in patients with AF as compared with those without (21 mg/L vs 0.96 mg/L; p<0.001). The same group published the results of a larger population-based cohort (n=5806; median follow-up 7.8 years) assessing CRP as a predictor of baseline and future development of AF (Aviles et al 2003). In this study, baseline CRP predicted higher risk for developing future AF (fourth vs first quartile adjusted hazard ratio 1.31, 95% CI 1.08–1.58; p=0.005). Similar findings were reported from an independent cohort study (n=2796; OR of developing AF 1.19 per quartile of CRP) (Anderson et al 2004). It remains uncertain whether AF triggers an inflammatory response or baseline systemic inflammation promotes AF. In addition, higher IL-6 and CRP levels were found to be associated with higher fibrinogen and plasma viscosity in patients with chronic AF, suggesting a link between inflammation and prothombotic state in AF (Conway et al 2004b, 2004c) as well as the surrogates of thromboembolism as documented by transesophageal echocardiography (left atrial thrombus, spontaneous echo contrast, aortic atheroma, and low left atrial appendage shear rate) (Conway et al 2004a; Thambidorai et al 2004). Moreover, lower CRP levels have been shown to predict higher conversion to sinus rhythm in patients with AF who undergo direct current (DC) cardioversion, but failed to predict mediumterm outcome following cardioversion (Conway et al 2004a). Although these studies showed a correlation between higher CRP levels and the occurrence of AF and its thrombotic state, further studies are needed to confirm a strong and direct causal relationship between inflammation and subsequent development of AF and its complications.
Aortic valve disease
Small pilot studies have tested the hypothesis of an association between elevated CRP levels and aortic valve disease progression. Galante et al (2001) were among the first investigators to demonstrate the association between rate of progression of degenerative aortic valve disease and elevated CRP levels, independent of the presence of subclinical infection (H. pylori, C. pneumonia). Retrospective analyses showed that statin drugs exert a beneficial effect on calcific aortic stenosis progression, possibly through their antiinflammatory effect rather than through their lipid lowering effects (Novaro et al 2001; Bellamy et al 2002). In a small study, CRP levels decreased after aortic valve replacement for non-rheumatic valve stenosis (degenerative and bicuspid). However the decrease in CRP was not substantial in all patients and all patients did not have high CRP levels at baseline (Gerber et al 2003). A recent randomized trial assigned 77 patients with calcific aortic stenosis to treatment with high dose statins (a treatment known to decrease CRP) and 78 to placebo. At 24 months follow-up, there was no significant difference in the rate of progression of aortic stenosis. This finding was similar regardless of the degree of the stenosis as assessed by echocardiography and helical CT. Moreover there was no relationship between LDL levels and the progression of the disease (Cowell et al 2005).
Thus, large scale clinical trials with long-term followup are needed to establish the role of CRP in aortic valve stenosis progression.
Myocarditis
In lymphocytic myocarditis (LM) – the most common type of myocarditis – an increase in CRP level correlates positively with the NYHA functional class and inversely with LVEF. Serum CRP levels were found to be significantly higher in patients who die from LM within a 28-day period of follow-up than in those who survive suggesting a prognostic value for CRP in patients presenting with this condition implying that the intensity of the inflammatory reaction is of prognostic value (Kaneko et al 2000). Of note, CRP levels measured in this setting are well above the ranges assessed by hs-CRP assays.
Heart transplantation
CRP was shown to be higher in patients with advanced stages of cardiac allograft vasculopathy disease (CAVD), the major cause of death after transplantation. Eisenberg et al (2000) demonstrated that hs-CRP might indeed be a useful marker for survival after heart transplantation. In a prospective study of 99 heart transplant recipients, doubling of CRP baseline levels was independently associated with an OR of 1.32 (p=0.025) of graft failure during the 5.0±2.7-year follow-up. Furthermore, Hognestad et al (2003) have reported a relationship between CRP levels and development of transplant allograft vasculopathy. Indeed, CRP increase during follow-up was the only independent predictor for transplant vasculopathy in their study. Prospective treatment with pravastatin was associated with a 25% reduction in CRP levels. In parallel, the use of statin drugs were shown to decrease the incidence of severe cardiac rejection accompanied by hemodynamic compromise, improve the one-year survival, and reduce the incidence of transplant vasculopathy (Kobashigawa et al 1995). Therefore, these preliminary results are encouraging for a role of CRP in the prediction of graft failure in heart transplant recipients and in immunosuppression monitoring.
Interventions to reduce circulating levels of CRP
Pharmacologic interventions
Antiplatelet agents
Early evidence of a link between systemic inflammation and cardiovascular events came from the Physician Health Study. In this study, Ridker at al (1997) identified 543 apparently healthy men in whom acute MI, stroke, or venous thrombosis subsequently developed and 543 participants who did not develop such complications and measured baseline hs-CRP. Patients were randomly assigned to receive aspirin or placebo. Baseline hs-CRP was indeed higher in patients who went on to develop MI or stroke. Interestingly, men with the highest initial level of CRP (4th quartile) had the greatest benefit of aspirin, with a relative MI risk reduction of 55.7% (risk ratio [RR] 4.16 vs 1.80) as compared with 13.9% reduction for those in the lowest quartile (RR 1.16 vs 1.00) (Ridker et al 1997). These results fuelled the hypothesis that antiinflammatory agents (such as aspirin) may have clinical benefits in preventing cardiovascular disease. In a small case-control study of patients with chronic stable angina (n=40), 300 mg of aspirin daily was associated with a 29% decrease in CRP levels after six weeks (Ikonomidis et al 1999). Furthermore, in a study of consecutive patients (n=304) admitted for non-ST elevation ACS, those pretreated with aspirin tended to have lower CRP and lower troponin I levels (Kennon et al 2001). In this study, pretreatment with aspirin neutralized the incremental risk of death/MI at 12 months associated with high CRP levels, suggesting a role for aspirin to decrease subclinical inflammation and an additional mechanism through which aspirin can exert its protective effect. However, subsequent dedicated trials did not show convincing evidence that low-dose (75 mg–325 mg) could lower circulating levels of hs-CRP (and hence systemic inflammation) (Feng et al 2000; Feldman et al 2001; Azar et al 2003) (Table 5). Therefore, lowdose aspirin is not currently recognized as a potent antiinflammatory agent and is believed to exert its beneficial effect mainly through its anti-platelet function.
Table 5.
Treatment effect on hs-CRP levels
| Study | n | Treatment | Effect | Clinical setting | p value |
|---|---|---|---|---|---|
| Ridker, Hennekens, et al 1999 | 472 | Pravastatin 40 mg | ↓ 17.4% | Secondary prevention | 0.007 |
| Placebo | ↑ 4.2% | ||||
| Kinlay et al 2003 | 2402 | Atorvastatin 80 mg | ↓ 83% | Unstable | <0.0001 |
| Placebo | ↓ 74% | ||||
| Ridker et al 2005 | 3745 | Atorvastatin 80 mg | ↓ 89% | Unstable | <0.001 |
| Pravastatin 40 mg | ↓ 82% | ||||
| Nissen et al 2005 | 502 | Atorvastatin 80 mg | ↓ 36% | Stable and unstable | <0.001 |
| Pravastatin 40 mg | ↓ 3% | ||||
| Bogaty 2004 | 35 | Rofecoxib 25 mg | ↓ 59% | Stable | 0.03 |
| Placebo | ↑ 35% | ||||
| Monakier 2004 | 34 | Rofecoxib 25 mg | ↓ 98% | Unstable | <0.02 |
| Placebo | ↑ 100% | ||||
| Fliser 2004 | 199 | Olmesartan 20 mg | ↓ 15.1% | Hypertension | <0.05 |
| Després et al 2005 | 1036 | Rimonabant 20 mg | ↓18% | Overweight | 0.020 |
| Placebo | ↓7.5% | ||||
| Wannamethee et al 2002 | 3628 | Physical activity | ↓ 18% | Stable (elderly men) | 0.0005 |
| Mattusch et al 1999 | 14 | Endurance training | ↓ 31% | Healthy subjects | <0.05 |
| Chrysohoou et al 2004 | 3042 | Mediterranean diet | ↓ 20% | Stable | 0.015 |
Abbreviations: hs-CRP, high sensitivity C-reactive protein.
Investigators from the Cleveland Clinic Foundation showed that pre-treatment with inhibitors of the P2Y12 adenosine receptor, such as clopidogrel, is associated with a substantial reduction in 30-day death or MI in patients with baseline CRP greater than 1.1 mg/dL undergoing PCI and stenting (Chew et al 2001b). Furthermore, periprocedural treatment with clopidogrel was associated with an attenuation of the increase in CRP by up to 65% (Vivekananthan et al 2004).
Statins
The primary mechanism of action of this class of drugs is certainly inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase with consequent decrease in LDL-C. However, a non-lipid effect was first noticed in the Cholesterol and Recurrent Events (CARE) trial. In a post hoc study of 472 randomly selected participants who remained free of recurrent coronary events during five years follow-up (Ridker, Rifai, et al 1999), those allocated to placebo experienced an increase in median CRP level while those allocated to pravastatin experienced a decrease in median CRP level (difference between groups, −21.6%; p=0.007). Attempts to relate the magnitude of change in CRP to the magnitude of change in lipids in both the pravastatin and placebo groups did not reveal any significant relationship. The authors concluded that these data supported the potential for non-lipid effects of pravastatin. Similar results were reported in the Myocardial Ischemia Reduction with Acute Cholesterol Lowering (MIRACL) study involving high-risk ACS patients (Kinlay et al 2003). Patients randomized to high-dose atorvastatin (vs placebo) experienced greater reductions in CRP (−83% vs −74%, p<0.001). Reductions in CRP were observed in patients with baseline LDL-C <3.2 mmol/L and >3.2 mmol/L and among all other important subgroups. By 16 weeks, CRP was 34% lower with atorvastatin than with placebo. In a small 22 patients study, treatment with simvastatin, pravastatin, and atorvastatin produced similar significant reduction of hs-CRP at six weeks (Jilal et al 2001). The JUPITER trial is currently recruiting patients (objective n=15000) with low LDL and high CRP to specifically test the hypothesis that decreasing CRP with a statin drug translates into clinical benefits (Ridker 2003a). Statins therapy in patient undergoing PCI significantly decreased the one year mortality and MI without affecting the need for revascularization in patients with high baseline CRP levels (Chan et al 2003).
Statins currently represent the most promising cardiovascular drugs to reduce CRP in clinical practice. Two recent large scale clinical trials have reported larger reductions of hs-CRP levels with atorvastatin 80 mg as compared with pravastatin 40 mg (Nissen et al 2005; Ridker et al 2005).
Angiotensin-converting enzyme inhibitors and receptor blockers
The impact of an angiotensin-converting enzyme (ACE) inhibition on systemic inflammation has not been completely documented. Early studies have suggested a favorable effect. Data from a prospective study (n=507) suggested that treatment with ACE inhibitors was associated with lower (2.6-fold; p<0.0001) median CRP levels in patients following stroke (Di Napoli and Papa 2003). However, treatment of high-risk patients with the ACE inhibitor ramipril did not result in CRP reduction in the Heart Outcomes Prevention Evaluation (HOPE) study (Yusuf et al 2000). Investigators from other largescale clinical trials evaluating ACE inhibition in patients with coronary atherosclerosis have not yet reported significant benefits on CRP levels (Fox 2003; Braunwald et al 2004). Definitive data in CAD patients are not available and no conclusion can be drawn at this time, although ACE inhibition is expected to have a modest impact at best on CRP levels.
The EUropean Trial on Olmesartan and Pravastatin in Inflammation and Atherosclerosis (EUTOPIA) investigators found significant reduction (15.1%) in measured CRP levels in hypertensive patients with microvascular inflammation treated with the angiotensin receptor blocker olmesartan. The reduction was present at six weeks (as compared with baseline levels) while there was no significant change with placebo (Fliser et al 2004).
Cannabinoid-1 receptor blockers
A recent randomized controlled trial (n=1036) showed that the specific cannabinoid-1 receptor blocker rimonabant reduces body weight and waist circumference and improves the profile of several metabolic risk factors in overweight or obese individual who have hyperlipidemia. Moreover, plasma CRP levels decreased by 0.9 mg/L in the rimonabant 20 mg daily group as compared with 0.4 mg/L in the placebo group (p=0.020) (Després et al 2005).
Hormone replacement therapy
Recent studies demonstrated that oral hormone replacement therapy (HRT) causes a sustained increase in CRP. Indeed, as compared with nonusers of HRT, median CRP levels were higher among women using HRT preparations (Ridker, Rifai, et al 1999). Furthermore, in a large-scale randomized trial of HRT, post-menopausal hormones were found to rapidly increase the concentration of CRP (approximately 2-fold), which may explain the early adverse effects of estrogen therapy seen in this trial (Cushman et al 1999). The addition of progesterone is associated with an insignificant increase in CRP levels (Yildirir et al 2002). Interestingly, droloxifene, a selective estrogen receptor modulator (SERM), was found to have little or no proinflammatory effects on CRP or IL-6 and had mixed effects on endothelial adhesion molecules as compared with estrogen therapy. These observations provide rationale for continuing to evaluate the potential cardiovascular benefits of SERM's (Herrington et al 2002) and to limit the use of HRT.
Cyclooxygenase-2 inhibitors
The case of cyclooxygenase-2 (COX-2) inhibitors is of particular interest. Indeed, this class of drugs represents potent antiinflammatory agents capable of rapidly and decisively decreasing circulating CRP levels and systemic inflammation through selective inhibition of COX-2. This enzyme, along with cyclooxygenase-1 (COX-1), catalyzes the transformation of the substrate arachidonic acid into molecular entities that include thromboxane A2 (prothrombotic) and prostacyclin (antithrombotic). The possibility of selectively inhibiting COX-2 generated the hypothesis that inflammation could be effectively controlled without the major adverse effect of COX-1 inhibition (gastrointestinal bleeding). This therapeutic potential combined with the more widely accepted concept that atherosclerosis is modulated by inflammation was enough to generate enthusiasm for a new strategy to limit its progression and clinical manifestations through chronic COX-2 inhibition. Early randomized trials demonstrated that COX-2 inhibitors could effectively reduce hs-CRP levels in patients with ACS (Bogaty et al 2004; Monakier et al 2004). However, an unexpected problem was first noted in the Vioxx Gastrointestinal Outcomes Research (VIGOR) study (Mukherjee et al 2001). Patients treated with rofecoxib (a COX-2 inhibitor) were found to have more MIs than patients treated with naproxen (COX-1 inhibitor) in the control group. More recent placebo controlled trials clearly confirmed this toxicity (Bresalier et al 2005; Nussmeier et al 2005; Solomon et al 2005). The most likely reason for the increased atherothrombotic events is that COX-2 inhibitors, while reducing CRP levels, also reduce the production of the antithrombotic substance prostacyclin without changing the production of the prothrombotic thromboxane A2 (McAdam et al 1999). These findings illustrate that although vascular inflammation is a key factor in atherosclerosis progression and clinical manifestations and decreasing vascular inflammation to reduce events is an appealing therapeutic strategy, the mechanisms of action of antiinflammatory agents are extremely important and caution should be exercised.
Non-pharmacologic interventions
Cardiac rehabilitation and exercise training
Cardiac rehabilitation programs improve numerous cardiac risk factors. The relationship between physical activity and CRP levels was examined in many studies. Men enrolled in the British Regional Heart Study (n=3810) were evaluated by questionnaire to assess physical activity and provided a fasting blood sample between 1998 and 2000 (approximately 20 years after enrolment). Physical activity showed a significant and inverse dose-relationship with CRP (increasing quartiles of physical activity: 1.73 mg/L, 1.73 mg/L, 1.57 mg/L, and 1.42 mg/L, p<0.0005) (Wannamethee et al 2002). Similar findings were reported from the Aerobics Center Longitudinal Study (Dallas, TX, US) (Church et al 2002), and by Aronson et al (2004). Interestingly, Mattusch et al (2000) reported that subjects preparing for a marathon experienced a 31% decrease in circulating hs-CRP levels (1.19 mg/L vs 0.82 mg/L, p<0.05). Therefore, aerobic exercise training and improved cardiorespiratory endurance are associated with reduced CRP levels, suggesting that exercise training has antiinflammatory effects. Regular physical exercise appears important in a global therapeutic strategy designed to decrease CRP in the high-risk patient population identified by high level of circulating CRP.
Weight loss and diet
CRP levels are higher in obese subjects, particularly in those with the metabolic syndrome. Indeed, CRP levels are related to abdominal obesity and insulin-resistance (Visser et al 1999; Yudkin et al 1999). Adipocytes are known to secrete IL-6 which increases the liver production of CRP. Thus, one mechanism of reduction of cardiovascular risk by weight loss may through a decrease in the major stimulus (IL-6) for hepatic CRP production and consequent decrease in CRP's proatherogenic effects. Caloric restriction and weight loss lowers IL-6 and CRP levels and may beneficially suppress a chronic and systemic inflammatory response (Heilbronn and Clifton 2002). Weight loss and diet are intrinsically linked. The Mediterranean diet has been one of the most thoroughly studied regimens with regard to its impact on cardiovascular diseases (CVD) and it has been shown to have a beneficial role by reducing BP, body mass index, platelet aggregation, and other hematological factors. Specifically, Chrysohoou et al (2004) recently demonstrate that the Mediterranean diet was independently associated with a reduction in various inflammatory and coagulation markers related to CVD. Participants who were closer to this diet had lower CRP, IL6, homocysteine, fibrinogen levels, and white blood counts compared with those who were away from this pattern diet. The authors concluded a correlation between the beneficial actions of this diet on the cardiovascular system. Reductions of CRP levels (40% reduction at two years) were confirmed in other major trials and adherence to this diet was also associated with a significant reduction in metabolic syndrome (Esposito et al 2003). Furthermore, this diet has also been associated with a reduction in all-cause mortality (Trichopoulou et al 2003; Knoops et al 2004). Moderate alcohol consumption is also associated with a decrease in CRP. The relationship between alcohol consumption and CRP was specifically evaluated in a cross-sectional survey and over time among 1732 men and 1101 women participating in the Pravastatin Inflammation/CRP Evaluation Study. CRP levels were lower in those with moderate alcohol intake versus light or occasional intake. In five categories of alcohol intake (no alcohol or < one drink monthly, one to three drinks monthly, one to four drinks weekly, five to seven drinks weekly, and ≥ or = two drinks daily), median CRP levels were 2.60 mg/L, 2.20 mg/L, 1.70 mg/L, 1.60 mg/L, and 1.80 mg/L, respectively (Albert et al 2003). In a cohort of wellfunctioning men and women aged 70 to 79 years, Volpato et al (2004) found that compared with subjects who consumed one to seven drinks per week, those who never drank had an increased likelihood of having high levels of both IL-6 and CRP, as did those who drank eight or more drinks per week. In this study and after adjustment for age, race, smoking status, history of diabetes, history of cardiovascular disease, physical activity, high-density lipoprotein cholesterol, antiinflammatory medications, statins, and total fat mass, alcohol intake showed a J-shaped relationship with mean IL-6 (p for quadratic term <0.001) and CRP (p=0.014) levels. Alcohol may therefore attenuate cardiovascular mortality in part through an antiinflammatory mechanism. These results might suggest an additional biological explanation for the attenuation of cardiovascular morbidity/mortality associated with moderate alcohol consumption.
The inflammation hypothesis and C-Reactive protein-guided therapy
In light of the intensive basic and clinical research in the pathophysiology in CVD, arterial inflammation has emerged as a new risk factor for the progression of atherosclerosis and its associated clinical manifestations. CRP was extensively investigated as a marker and prognostic factor in epidemiologic studies and elevated CRP levels predict worse outcomes even in apparently healthy individuals as well as in patients with unstable coronary syndromes. In a recent review, Bhatt and Topol (2002) proposed a clinical trial design to evaluate the practical utilization of CRP to allocate medical therapy. This trial would test the “inflammation hypothesis” by randomizing patients with a history of CVD to either standard therapy or CRP-guided strategy. All patients would receive aspirin. Statins would be prescribed per protocol if LDLs were >100 mg/dL and ACE-I if LVEF was decreased, respectively. Additional therapies would be initiated and medications would be titrated according to CRP levels in the CRP-guided arm (with the goal of achieving optimal CRP levels in this group). This “inflammation-guided” strategy would also include deoxyribonucleic acid (DNA) analysis to ascertain the relationship between polymorphisms of inflammatory markers and drug interactions. Positive results would validate this strategy, which could be easily implemented by clinicians.
Conclusion
Atherosclerosis is a complex and systemic disease in which vascular inflammation likely plays a key role. Indeed, there is evidence that inflammation is closely linked to atherosclerotic disease at all stages, from silent progression to clinical manifestations. Experimental studies suggest that CRP is an active player in the pathophysiology of atherosclerosis. Moreover, there is strong epidemiologic evidence that mediators of inflammation, especially hs-CRP, predict cardiovascular risk independently of “classical” risk factors. This finding is particularly pertinent when considering that a significant proportion of CAD cases are not associated with any prominent “classical” risk. Although an elevated hs-CRP level is now clearly established as an independent risk factor for CAD, the magnitude of the effect remains the subject of heated debate and strong recommendations for its use in clinical practice have not been issued. Its impact may be particularly important in the intermediate risk patients as assessed by conventional methods. CRP levels also have important prognostic implications in other cardiovascular diseases, such as CHF, AF, aortic valve disease, myocarditis and heart transplantation. A number of pharmacological and non-pharmacological interventions have been shown to decrease hs-CRP levels. Because most of these interventions have other known beneficial effects, it is currently difficult to independently assess the impact of hs-CRP reduction on cardiovascular diseases. However, dedicated clinical trials are ongoing and should provide important insight into the “inflammatory-hypothesis”.
References
- Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–7. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- Alonso-Martinez JL, Llorente-Diez B, Echegaray-Agara M, et al. C-reactive protein as a predictor of improvement and readmission in heart failure. Eur J Heart Fail. 2002;4:331–6. doi: 10.1016/s1388-9842(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Allen Maycock CA, Lappe DL, et al. Frequency of elevation of C-reactive protein in atrial fibrillation. Am J Cardiol. 2004;94:1255–9. doi: 10.1016/j.amjcard.2004.07.108. [DOI] [PubMed] [Google Scholar]
- Angioi M, Abdelmouttaleb I, Rodriguez RM, et al. Increased C-reactive protein levels in patients with in-stent. Am J Cardiol. 2001;87:1189–93. doi: 10.1016/s0002-9149(01)01492-8. [DOI] [PubMed] [Google Scholar]
- Aronson D, Sella R, Sheikh-Ahmad M, et al. The association between cardiorespiratory fitness and C-reactive protein in subjects with the metabolic syndrome. J Am Coll Cardiol. 2004;44:2003–7. doi: 10.1016/j.jacc.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- Azar RR, Klayme S, Germanos M, et al. Effects of aspirin (325 mg/day) on serum high-sensitivity C-reactive protein, cytokines, and adhesion molecules in healthy volunteers. Am J Cardiol. 2003;92:236–9. doi: 10.1016/s0002-9149(03)00549-6. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Topol EJ. Need to test the arterial inflammation hypothesis. Circulation. 2002;106:136–40. doi: 10.1161/01.cir.0000021112.29409.a2. [DOI] [PubMed] [Google Scholar]
- Bazzino O, Ferreiros ER, Pizarro R, et al. C-reactive protein and the stress tests for the risk stratification of patients recovering from unstable angina pectoris. Am J Cardiol. 2001;87:1235–9. doi: 10.1016/s0002-9149(01)01511-9. [DOI] [PubMed] [Google Scholar]
- Bellamy MF, Pellikka PA, Klarich KW, et al. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723–30. doi: 10.1016/s0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- Biasucci LM, Liuzzo G, Grillo RL, et al. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation. 1999;99:855–60. doi: 10.1161/01.cir.99.7.855. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–71. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- Bogaty P, Brophy JM, Noel M, et al. Impact of prolonged cyclooxygenase-2 inhibition on inflammatory markers and endothelial function in patients with ischemic heart disease and raised C-reactive protein: a randomized placebo-controlled study. Circulation. 2004;110:934–9. doi: 10.1161/01.CIR.0000139338.12464.5F. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Domanski MJ, Fowler SE, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–68. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Woodward M, Chalmers JP, et al. Prediction of heart failure by amino terminal-pro-B-type natriuretic peptide and C-reactive protein in subjects with cerebrovascular disease. Hypertension. 2005;45:69–74. doi: 10.1161/01.HYP.0000151103.02424.c3. [DOI] [PubMed] [Google Scholar]
- Cermak J, Key NS, Bach RR, et al. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–20. [PubMed] [Google Scholar]
- Chan AW, Bahtt DL, Chew DP, et al. Relation of inflammation and benefit of statins after percutaneous interventions. Circulation. 2003;107:1750–6. doi: 10.1161/01.CIR.0000060541.18923.E9. [DOI] [PubMed] [Google Scholar]
- Chew DP, Bhatt DL, Robbins MA, et al. Incremental prognostic value of elevated baseline C-reactive protein among established markers of risk in percutaneous coronary intervention. Circulation. 2001a;104:992–7. doi: 10.1161/hc3401.095074. [DOI] [PubMed] [Google Scholar]
- Chew DP, Bhatt DL, Robbins MA, et al. Effect of clopidogrel added to aspirin before percutaneous coronary intervention on the risk associated with C-reactive protein. Am J Cardiol. 2001b;88:672–74. doi: 10.1016/s0002-9149(01)01813-6. [DOI] [PubMed] [Google Scholar]
- Chrysohoou C, Panagiotakos DB, Pitsavos C, et al. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004;44:152–8. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- Church TS, Barlow CE, Earnest CP, Kampert JB, et al. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–76. doi: 10.1161/01.atv.0000036611.77940.f8. [DOI] [PubMed] [Google Scholar]
- Cipollone F, Marini M, Fazia M, et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2001;21:327–34. doi: 10.1161/01.atv.21.3.327. [DOI] [PubMed] [Google Scholar]
- Conway DS, Buggins P, Hughes E, et al. Predictive value of indexes of inflammation and hypercoagulability on success of cardioversion of persistent atrial fibrillation. Am J Cardiol. 2004a;94:508–10. doi: 10.1016/j.amjcard.2004.04.070. [DOI] [PubMed] [Google Scholar]
- Conway DS, Buggins P, Hughes E, et al. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004b;43:2075–82. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- Conway DS, Buggins P, Hughes E, et al. Relation of interleukin-6, C-reactive protein, and the prothrombotic state to transesophageal echocardiographic findings in atrial fibrillation. Am J Cardiol. 2004c;93:1368–73. doi: 10.1016/j.amjcard.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–97. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- Cushman M, Legault C, Barrett-Connor E. Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation. 1999;100:717–22. doi: 10.1161/01.cir.100.7.717. [DOI] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. Br Med J. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Després JP, Golay A, Sjostrom L, et al. Effects of Rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Papa F. Angiotensin-converting enzyme inhibitor use is associated with reduced plasma concentration of C-reactive protein in patients with first-ever ischemic stroke. Stroke. 2003;34:2922–9. doi: 10.1161/01.STR.0000099124.84425.BB. [DOI] [PubMed] [Google Scholar]
- Eisenberg MS, Chen HJ, Warshofsky MK, et al. Elevated levels of plasma C-reactive protein are associated with decreased graft survival in cardiac transplant recipients. Circulation. 2000;102:2100–4. doi: 10.1161/01.cir.102.17.2100. [DOI] [PubMed] [Google Scholar]
- Elster SK, Braunwald E, Wood HF. A study of C-reactive protein in the serum of patients with congestive heart failure. Am Heart J. 1956;51:533–41. doi: 10.1016/0002-8703(56)90099-0. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- Feldman M, Jialal I, Devaraj S, et al. Effects of low-dose aspirin on serum C-reactive protein and thromboxane B2 concentrations: a placebo-controlled study using a highly sensitive C-reactive protein assay. J Am Coll Cardiol. 2001;37:2036–41. doi: 10.1016/s0735-1097(01)01289-x. [DOI] [PubMed] [Google Scholar]
- Feng D, Tracy RP, Lipinska I, et al. Effect of short-term aspirin use on C-reactive protein. J Thromb Thrombolysis. 2000;1:37–41. doi: 10.1023/a:1018644212794. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Bachetti T, Confortini R, et al. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92:1479–86. doi: 10.1161/01.cir.92.6.1479. [DOI] [PubMed] [Google Scholar]
- Ferreiros ER, Boissonnet CP, Pizarro R, et al. Independent prognostic value of elevated C-reactive protein in unstable angina. Circulation. 1999;100:1958–63. doi: 10.1161/01.cir.100.19.1958. [DOI] [PubMed] [Google Scholar]
- Fliser D, Buchholz K, Haller H, et al. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–7. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- Fox KM. EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–8. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- Galante A, Pietroiusti A, Vellini M, et al. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol. 2001;38:1078–82. doi: 10.1016/s0735-1097(01)01484-x. [DOI] [PubMed] [Google Scholar]
- Gaspardone A, Crea F, Versaci F, et al. Predictive value of C-reactive protein after successful coronary-artery stenting in patients with stable angina. Am J Cardiol. 1998;82:515–18. doi: 10.1016/s0002-9149(98)00370-1. [DOI] [PubMed] [Google Scholar]
- Gerber IL, Stewart RA, Hammett CJ, et al. Effect of aortic valve replacement on C-reactive protein in nonrheumatic aortic stenosis. Am J Cardiol. 2003;92:1129–32. doi: 10.1016/j.amjcard.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Gottsauner-Wolf M, Zasmeta G, Hornykewycz S, et al. Plasma levels of C-reactive protein after coronary stent implantation. Eur Heart J. 2000;21:1152–8. doi: 10.1053/euhj.1999.1987. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Haverkate F, Thompson SG, Pyke SD, et al. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349:462–6. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Hamm CW, Bruemmer J, et al. Predictive value of C-reactive protein and troponin T in patients with unstable angina: a comparative analysis. CAPTURE Investigators. Chimeric c7E3 AntiPlatelet Therapy in Unstable angina REfractory to standard treatment trial. J Am Coll Cardiol. 2000;35:1535–42. doi: 10.1016/s0735-1097(00)00581-7. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Dimmeler S, Hamm CW, et al. Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation. 2003;107:2109–14. doi: 10.1161/01.CIR.0000065232.57371.25. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Clifton PM. C-reactive protein and coronary artery disease: influence of obesity, caloric restriction and weight loss. J Nutr Biochem. 2002;13:316–21. doi: 10.1016/s0955-2863(02)00187-0. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Howard TD, Brosnihan KB, et al. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation. 2002;105:1879–82. doi: 10.1161/01.cir.0000016173.98826.88. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Brosnihan KB, Pusser BE, et al. Differential effects of E and droloxifene on C-reactive protein and other markers of inflammation in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86:4216–22. doi: 10.1210/jcem.86.9.7799. [DOI] [PubMed] [Google Scholar]
- Hognestad A, Endresen K, Wergeland R, et al. Plasma C-reactive protein as a marker of cardiac allograft vasculopathy in heart transplant recipients. J Am Coll Cardiol. 2003;42:477–82. doi: 10.1016/s0735-1097(03)00645-4. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–25. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- Ikonomidis I, Andreotti F, Economou E, et al. Increased proinflammatory cytokines in patients with chronic stable. Circulation. 1999;100:793–8. doi: 10.1161/01.cir.100.8.793. [DOI] [PubMed] [Google Scholar]
- Imhof A, Frohlich M, Loewel H, et al. Distributions of C-reactive protein measured by high-sensitivity assays in apparently healthy men and women from different populations in Europe. Clin Chem. 2003;49:669–72. doi: 10.1373/49.4.669. [DOI] [PubMed] [Google Scholar]
- James SK, Armstrong P, Barnathan E, et al. Troponin and C-reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome: a GUSTO-IV substudy. J Am Coll Cardiol. 2003;41:916–24. doi: 10.1016/s0735-1097(02)02969-8. [DOI] [PubMed] [Google Scholar]
- Jialal I, Stein D, Balis D, et al. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor. Circulation. 2001;103:1933–35. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kanda T, Hasegawa A, et al. C-reactive protein as a prognostic marker in lymphocytic myocarditis. Jpn Heart J. 2000;41:41–7. doi: 10.1536/jhj.41.41. [DOI] [PubMed] [Google Scholar]
- Kapyaho K, Welin MG, Tanner P, et al. Rapid determination of C-reactive protein by enzyme immunoassay using two monoclonal antibodies. Scand J Clin Lab Invest. 1989;49:389–93. doi: 10.3109/00365518909089112. [DOI] [PubMed] [Google Scholar]
- Kennon S, Price CP, Mills PG, et al. The effect of aspirin on C-reactive protein as a marker of risk in unstable angina. J Am Coll Cardiol. 2001;37:1266–70. doi: 10.1016/s0735-1097(01)01130-5. [DOI] [PubMed] [Google Scholar]
- Kervinen H, Manttari M, Kaartinen M, et al. Prognostic usefulness of plasma monocyte/macrophage and T-lymphocyte activation markers in patients with acute coronary syndromes. Am J Cardiol. 2004;94:993–6. doi: 10.1016/j.amjcard.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, et al. Race and Gender Differences in C-Reactive Protein Levels. J Am Coll Cardiol. 2005;46:464–9. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Khera A, de Lemos JA, Peshock RM, et al. Relationship between C-reactive protein and subclinical atherosclerosis. The Dallas Heart Study. Circulation. 2006;113:38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- Khreiss T, Jozsef L, Potempa L, et al. Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation. 2004;109:2016–22. doi: 10.1161/01.CIR.0000125527.41598.68. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Schwartz GG, Olsson AG, et al. High dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation. 2003;108:1560–6. doi: 10.1161/01.CIR.0000091404.09558.AF. [DOI] [PubMed] [Google Scholar]
- Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–9. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- Koenig W, Sund M, Frohlich M, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- Koenig W, Lowel H, Baumert J, et al. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004;109:1349–53. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Tracy RP, Shaten J, et al. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;144:537–47. doi: 10.1093/oxfordjournals.aje.a008963. [DOI] [PubMed] [Google Scholar]
- Kushner I. C-reactive protein and the acute-phase response. Hosp Pract 30. 1990;25:21–8. [PubMed] [Google Scholar]
- L'Allier PL, Tardif JC, Grégoire J, et al. Sustained elevation of serum CD40 ligand levels one month after coronary angioplasty predicts angiographic restenosis. Can J Cardiol. 2005;21:495–500. [PubMed] [Google Scholar]
- L'Allier PL. Inflammation in atherothrombotic disease. J Inv Cardiol. 2004:41S–44S. [PubMed] [Google Scholar]
- Ledue TB, Weiner DL, Sipe JD, et al. Analytical evaluation of particle-enhanced immunonephelometric assays. Ann Clin Biochem. 1998;35:745–53. doi: 10.1177/000456329803500607. [DOI] [PubMed] [Google Scholar]
- Lindahl B, Toss H, Siegbahn A, et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000;343:1139–47. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–24. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Biasucci LM, Rebuzzi AG, et al. Plasma protein acute-phase response in unstable angina is not induced. Circulation. 1996;94:2373–80. doi: 10.1161/01.cir.94.10.2373. [DOI] [PubMed] [Google Scholar]
- MacGowan GA, Mann DL, Kormos RL, et al. Circulating interleukin-6 in severe heart failure. Am J Cardiol. 1997;79:1128–31. doi: 10.1016/s0002-9149(96)00063-x. [DOI] [PubMed] [Google Scholar]
- Mattusch F, Dufaux B, Heine O, et al. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int J Sports Med. 2000;21:21–4. doi: 10.1055/s-2000-8852. [DOI] [PubMed] [Google Scholar]
- McAdam BF, Catella-Lawson F, Mardini IA, et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999;96:272–7. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, et al. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem. 2001;47:426–30. [PubMed] [Google Scholar]
- Mendall MA, Strachan DP, Butland BK, et al. C-reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J. 2000;21:1584–90. doi: 10.1053/euhj.1999.1982. [DOI] [PubMed] [Google Scholar]
- Milazzo D, Biasucci LM, Luciani N, et al. Elevated levels of C-reactive protein before coronary artery bypass grafting predict recurrence of ischemic events. Am J Cardiol. 1999;84:459–61. doi: 10.1016/s0002-9149(99)00333-1. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol. 1999;61:391–415. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- Monakier D, Mates M, Klutstein MW, et al. Rofecoxib, a COX-2 inhibitor, lowers C-reactive protein and interleukin-6 levels in patients with acute coronary syndromes. Chest. 2004;125:1610–15. doi: 10.1378/chest.125.5.1610. [DOI] [PubMed] [Google Scholar]
- Montalescot G, Philippe F, Ankri A, et al. Early increase of von Willebrand factor predicts adverse outcome in unstable coronary artery disease: beneficial effects of enoxaparin. French Investigators of the ESSENCE Trial. Circulation. 1998;98:294–9. doi: 10.1161/01.cir.98.4.294. [DOI] [PubMed] [Google Scholar]
- Morrow DA, Rifai N, Antman EM, et al. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11A substudy. Thrombolysis in myocardial infarction. J Am Coll Cardiol. 1998;31:1460–5. doi: 10.1016/s0735-1097(98)00136-3. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–9. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- Muller C, Buetter HJ, Hoggson JM, et al. Inflammation and long-term mortality after non ST elevation acute coronary syndromes treated with a very early invasive strategy in 1042 consecutive patients. Circulation. 2002;105:1412–15. doi: 10.1161/01.cir.0000012625.02748.62. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus H. Complement activation and atherosclerosis. Mol Immunol. 1999;36:949–55. doi: 10.1016/s0161-5890(99)00117-0. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu M, Shoenhagen P, et al. Statin Therapy, LDL Cholesterol, C-Reactive Protein, and Coronary Artery Disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- Novaro GM, Tiong IY, Pearce GL, et al. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–9. doi: 10.1161/hc4301.098249. [DOI] [PubMed] [Google Scholar]
- Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–91. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- Oltrona L, Ardissino D, Merlini PA, et al. C-reactive protein elevation and early outcome in patients with unstable angina pectoris. Am J Cardiol. 1997;80:1002–6. doi: 10.1016/s0002-9149(97)00593-6. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–8. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Cheng JS, Willerson JT, et al. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–4. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, et al. Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pye M, Rae AP, Cobbe SM. Study of serum C-reactive protein concentration in cardiac failure. Br Heart J. 1990;63:228–30. doi: 10.1136/hrt.63.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuzzi AG, Quaranta G, Liuzzo G, et al. Incremental prognostic value of serum levels of troponin T and C-reactive protein on admission in patients with unstable angina pectoris. Am J Cardiol. 1998;82:715–19. doi: 10.1016/s0002-9149(98)00458-5. [DOI] [PubMed] [Google Scholar]
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001a;103:1813–18. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Role of inflammatory biomarkers in prediction of coronary heart disease. Lancet. 2001b;358:946–8. doi: 10.1016/S0140-6736(01)06112-8. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003b;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491–5. doi: 10.1161/01.cir.103.4.491. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Shih J, et al. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Eng J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, et al. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Rifai N, et al. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999;100:713–16. doi: 10.1161/01.cir.100.7.713. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Roitman-Johnson B, et al. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- Ridker PM, JUPITER Study Group Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003a;108:2292–7. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Clearfield M, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study Investigators. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Pfeffer MA, et al. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–5. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rifai N, Ridker PM. Population distributions of C-reactive protein in apparently healthy men and women in the United States implication for clinical interpretation. Clin Chem. 2003;49:666–9. doi: 10.1373/49.4.666. [DOI] [PubMed] [Google Scholar]
- Roberts WL, Sedrick R, Moulton L, et al. Evaluation of four automated high-sensitivity C-reactive protein. Clin Chem. 2000;46:461–8. [PubMed] [Google Scholar]
- Roivainen M, Viik-Kajander M, Palosuo T, et al. Infections, inflammation, and the risk of coronary heart disease. Circulation. 2000;101:252–7. doi: 10.1161/01.cir.101.3.252. [DOI] [PubMed] [Google Scholar]
- Sabatine MS, Morrow DA, de Lemos JA. Multi-marker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–3. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- Samsonov M, Lopatin J, Tilz GP, et al. The activated immune system and the renin-angiotensin-aldosterone system in congestive heart failure. J Intern Med. 1998;243:93–8. doi: 10.1046/j.1365-2796.1998.00240.x. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Grégoire J, Lavoie MA, et al. Vascular protectants for the treatment of atherosclerosis. Expert Rev Cardiovasc Ther. 2003;1:385–92. doi: 10.1586/14779072.1.3.385. [DOI] [PubMed] [Google Scholar]
- Thambidorai SK, Parakh K, Martin DO, et al. Relation of C-reactive protein correlates with risk of thromboembolism in patients with atrial fibrillation. Am J Cardiol. 2004;94:805–7. doi: 10.1016/j.amjcard.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Tomoda H, Aoki N. Instability of coronary lesions in unstable angina assessed by. Am J Cardiol. 2001;87:221–3. doi: 10.1016/s0002-9149(00)01323-0. [DOI] [PubMed] [Google Scholar]
- Toss H, Lindahl B, Siegbahn A, et al. Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. Circulation. 1997;96:4204–10. doi: 10.1161/01.cir.96.12.4204. [DOI] [PubMed] [Google Scholar]
- Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17:1121–7. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- Trichopoulou A, Costacou T, Bamia C, et al. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- Verheggen PW, de Maat MP, Cats VM, et al. Inflammatory status as a main determinant of outcome in patients with unstable angina, independent of coagulation activation and endothelial cell function. Eur Heart J. 1999;20:567–74. doi: 10.1053/euhj.1998.1312. [DOI] [PubMed] [Google Scholar]
- Verma S, Li SH, Badiwala MV, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105:1890–6. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–19. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- Verma S, Badiwala MV, Weisel RD, et al. C-reactive protein activates the nuclear factor-kappaB signal transduction pathway in saphenous vein endothelial cells: implications for atherosclerosis and restenosis. J Thorac Cardiovasc Surg. 2003;126:1886–91. doi: 10.1016/j.jtcvs.2003.07.026. [DOI] [PubMed] [Google Scholar]
- Verma S, Kuliszewski MA, Li SH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–67. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- Verma S, Szmitko PE, Yeh ET. C-reactive protein: structure affects function. Circulation. 2004;109:1914–17. doi: 10.1161/01.CIR.0000127085.32999.64. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Vivekananthan DP, Bhatt DL, Chew DP, et al. Effect of clopidogrel pretreatment on periprocedural rise in C-reactive protein after percutaneous coronary intervention. Am J Cardiol. 2004;94:358–60. doi: 10.1016/j.amjcard.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Volpato S, Pahor M, Ferrucci L, et al. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: the Health, Aging, and Body Composition study. Circulation. 2004;109:607–12. doi: 10.1161/01.CIR.0000109503.13955.00. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Lowe GD, Whincup PH, et al. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–90. doi: 10.1161/hc1502.107117. [DOI] [PubMed] [Google Scholar]
- Wolbink GJ, Brouwer MC, Buysmann S, et al. CRP-mediated activation of complement in vivo: assessment by measuring circulating complement-C-reactive protein complexes. J Immunol. 1996;157:473–9. [PubMed] [Google Scholar]
- Yildirir A, Aybar F, Tokgozoglu L, et al. Effects of hormone replacement therapy on plasma homocysteine and C-reactive protein levels. Gynecol Obstet Invest. 2002;53:54–8. doi: 10.1159/000049412. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–7. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]