Abstract
Low ethanol intake is known to have a beneficial effect on cardiovascular disease. In cardiovascular disease, insulin resistance leads to altered glucose and lipid metabolism resulting in an increased production of aldehydes, including methylglyoxal. Aldehydes react non-enzymatically with sulfhydryl and amino groups of proteins forming advanced glycation end products (AGEs), altering protein structure and function. These alterations cause endothelial dysfunction with increased cytosolic free calcium, peripheral vascular resistance, and blood pressure. AGEs produce atherogenic effects including oxidative stress, platelet adhesion, inflammation, smooth muscle cell proliferation and modification of lipoproteins. Low ethanol intake attenuates hypertension and atherosclerosis but the mechanism of this effect is not clear. Ethanol at low concentrations is metabolized by low Km alcohol dehydrogenase and aldehyde dehydrogenase, both reactions resulting in the production of reduced nicotinamide adenine dinucleotide (NADH). This creates a reductive environment, decreasing oxidative stress and secondary production of aldehydes through lipid peroxidation. NADH may also increase the tissue levels of the antioxidants cysteine and glutathione, which bind aldehydes and stimulate methylglyoxal catabolism. Low ethanol improves insulin resistance, increases high-density lipoprotein and stimulates activity of the antioxidant enzyme, paraoxonase. In conclusion, we suggest that chronic low ethanol intake confers its beneficial effect mainly through its ability to increase antioxidant capacity and lower AGEs.
Keywords: low ethanol, hypertension, cardiovascular disease, biochemical mechanisms, advanced glycation end products
Introduction
Ethanol intake is a common lifestyle factor found across many cultures and geographic regions. Since cardiovascular disease is also found ubiquitously and accounts for more than 16 million deaths per year worldwide, it is essential to understand how they relate to each other (WHO 2003a). Various epidemiological and controlled clinical studies have investigated the effects of varying levels and patterns of ethanol intake on cardiovascular health (Reynolds et al 2003; Corrao et al 2004). In contrast to high ethanol intake which is detrimental to cardiovascular health, chronic low ethanol has been shown to have a beneficial effect (Camargo et al 1997; Okubo et al 2001; Corrao et al 2004; Piano 2005). Understanding the biological mechanism of this beneficial effect will aid in identifying potential preventative or therapeutic agents. In this review, we discuss the factors involved in the development and progression of cardiovascular disease and the possible biochemical mechanisms by which low ethanol counters these factors to prevent or attenuate this disease.
Cardiovascular disease
Cardiovascular disease includes atherosclerosis and hypertension. Hypertension affects more than 600 million people worldwide and results in 13% of the total deaths globally (WHO 2003b). Approximately 90% of all hypertension is classified as “essential” meaning that the cause is not known. Essential hypertension involves endothelial dysfunction with alterations in nitric oxide (NO) bioavailability and calcium handling, smooth muscle cell proliferation, thickening of the vessel walls, and increased peripheral vascular resistance and blood pressure (Resnick 1993; Oshima and Young 1995; Taddei et al 2003; Portaluppi et al 2004). Risk factors for hypertension include family history, diabetes, obesity, smoking, excessive alcohol intake, and a diet high in salt and/or low in antioxidant nutrients. Most of these risk factors are modifiable through lifestyle changes such as participating in moderate physical activity and eating a well-balanced diet. Healthy lifestyle choices also include not smoking and limiting alcohol intake (WHO 2003b). Individuals with hypertension are at increased risk for atherosclerotic diseases such as stroke, and heart and kidney disease.
Atherosclerosis is a leading cause of death in the world. Heart disease and stroke are the two leading causes of death in adults in developed countries and are responsible for a third of all deaths in developing countries (WHO 2003a). Atherosclerosis is an inflammatory condition of the blood vessels (Ross 1999). Damage to, or activation of, the endothelium promotes entry of modified low-density lipoprotein (LDL) into the intima, a process enhanced by an elevation in circulating levels of LDL. Alteration in endothelium also increases the expression of adhesion molecules on the cell surface resulting in recruitment of monocytes and platelet adhesion. The monocytes transmigrate to the sub-endothelial space where they differentiate into macrophages. Modified LDL is scavenged by macrophages in the interstitial space transforming over time into foam cells. Accumulation of foam cells and other cellular debris evolve into atherosclerotic plaques (Chakarvarti et al 1991; Witztum and Steinberg 1991; Palinski et al 1995; Ross 1999; Sima and Stancu 2002; Hansson 2005). Although atherosclerotic lesions generally occur at junctions of large- and medium-size vessels, they may also arise throughout the vasculature (Tegos et al 2001; Hansson 2005). Through stenosis or embolytic occlusion, lesions within the coronary, cerebral, peripheral or renal vessels result in the clinical manifestations of angina, myocardial infarction, stroke, peripheral arterial disease or renal failure (Tegos et al 2001). Atherosclerosis also involves increased smooth muscle cell migration and proliferation. Stiffening of the vessel walls may hinder elasticity exacerbating hypertension. Hypertension is considered a risk factor for atherosclerosis, as increased blood pressure itself can contribute to vascular injury, making vessels more susceptible to inflammation (Chobanian and Alexander 1996). However, studies show that lowering blood pressure alone does not completely eliminate the risk of cardiovascular disease (Neutel 2000). Other risk factors include smoking, diabetes mellitus, obesity, dyslipidemias, and a diet high in saturated fat (WHO 2003a, 2003b). Hypertension and atherosclerosis share similar risk factors and both are characterized by modified vascular structure and function (Pepine and Handberg 2001). These cardiovascular conditions are also linked by another common feature, insulin resistance.
Etiological factors of cardiovascular disease
Insulin resistance
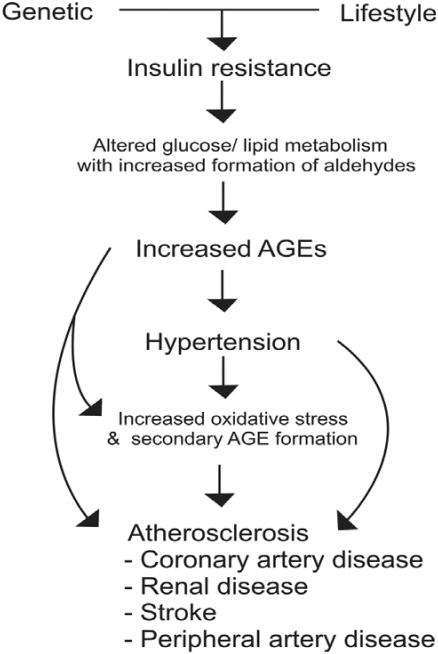
We suggest that the key etiological factor in cardiovascular disease is insulin resistance (Figure 1). Insulin resistance is characterized by an inadequate glucose uptake in peripheral tissues at a given concentration of plasma insulin. It involves an impairment of the nonoxidative (glycolytic) pathways of intracellular glucose metabolism (Ferrannini et al 1987). Insulin resistance has been well documented in hypertension and atherosclerosis. Humans with essential hypertension and normotensive offspring of essential hypertensives have insulin resistance (Ferrannini et al 1987; Saad et al 2004; Vlasakova et al 2004). Abnormalities in glucose metabolism exist in up to 80% of subjects with essential hypertension (Ferrannini et al 1987; Flack and Sowers 1991). Insulin resistance has also been documented in humans with atherosclerosis (DeFronzo and Ferrannini 1991; Howard et al 1996; Shinozaki et al 1996; Reaven 2003). It has been suggested that hypertensives who are insulin resistant are at increased risk for atherosclerotic disease (Reaven 2003; Liao et al 2004). In metabolic syndrome, also known as syndrome X or insulin resistance syndrome, primary insulin resistance is linked to a group of co-existing conditions including hypertension, dyslipidemias, diabetes, and atherosclerotic cardiovascular disease (DeFronzo and Ferrannini 1991).
Figure 1.
Mechanism of cardiovascular disease. In insulin resistant state, excess aldehydes formed due to altered glucose/lipid metabolism react with proteins to form advanced glycation end products (AGEs). AGEs alter the functions of cellular proteins including vascular ion channels, and metabolic and antioxidant enzymes, with oxidative stress leading to hypertension and atherosclerosis.
Aldehydes
Under normal physiological conditions, glucose is metabolized via the glycolytic pathway to glyceraldehyde-3-phosphate (G3P) which is converted to 1,3-diphosphoglycerate by the enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), with further metabolism to pyruvate. Any factor which affects GAPDH, whether through inhibition or upregulation, has an impact on the rate of glucose metabolism. It has been shown that GAPDH is upregulated by insulin (Alexander et al 1988). In insulin resistant states, altered insulin function may down regulate GAPDH, slowing glucose metabolism through the glycolytic pathway, thus increasing metabolism via the polyol pathway. This may result in a build-up of G3P leading to an increase in the highly reactive aldehyde, methylglyoxal (Mayes 1983; Thornalley 1993; Beisswenger et al 2003). Methylglyoxal itself has been shown to inhibit GAPDH, resulting in further abnormalities in glucose metabolism (Morgan et al 2002). Methylglyoxal also induces aldose reductase which is known to stimulate flux of glucose through the polyol pathway with further formation of methylglyoxal (Chang et al 2002). An excess of dietary sugar or fat, or both, typical of western diets, may overload these pathways and exacerbate this altered metabolism. Insulin resistance is also associated with dyslipidemia (Fuh et al 1987; Liao et al 2004); elevated LDL without the mitigating antioxidant effect of high high-density lipoprotein (HDL), may contribute to an increase in reactive aldehydes (Dargel 1992).
Advanced glycation end products
Advanced glycation end products (AGEs) are formed when aldehydes (eg, methylglyoxal, glyoxal, glucose) react nonenzymatically with free sulfhydryl (SH) and amino (NH2) groups of amino acids including cysteine, arginine or lysine, of proteins (Schauenstein et al 1977; Thorpe and Baynes 2003; Thornalley et al 2003). This direct modification of protein structure results in functional changes (Morgan et al 2002; Nagaraj et al 2002; Karachalias et al 2003). AGE-modified protein has also been shown to stimulate receptors of AGEs (RAGEs) and various scavenger receptors to influence protein function and expression (Schmidt et al 2001; Wautier et al 2001; Horiuchi et al 2003). Normally, methylglyoxal is formed but kept at a low level through catabolism via the glutathione-dependent glyoxalase enzyme system or by binding to cysteine and being excreted in bile and urine (Schauenstein et al 1977). It has been suggested that AGEs formed in low concentrations contribute to the regulation of normal tissue remodeling (Kirstein et al 1992) but when found in excess are pathogenic (Vasdev et al 2000a, 2000b; Mizutani et al 2002; Alderson et al 2003; Bidasee et al 2003; Karachalias et al 2003; Stitt et al 2004; Vasdev et al 2005). It has also been proposed that certain AGEs have normal biological functions whereas others referred to as toxic AGEs, play a pathophysiological role (Takeuchi and Yamagishi 2004). Whatever the case, several specific AGEs including carboxymethyl-lysine, carboxyethyl-lysine, argpyrimidine, and glycoaldehyde-pyridine, have been identified, and have been implicated in the pathology of hypertension and atherosclerosis (Anderson et al 1999; Oya et al 1999; Baynes and Thorpe 2000; Nagai et al 2002; Alderson et al 2003; Wang et al 2004).
Causative role of advanced glycation end products in cardiovascular disease
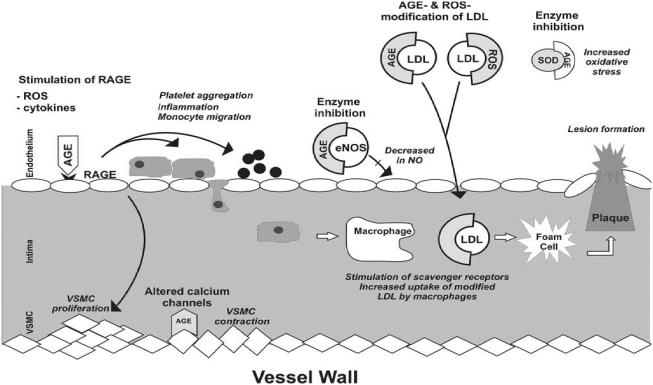
It is known that AGEs act directly or via receptors to alter the function of cellular proteins including calcium channels, metabolic and antioxidant enzymes, receptors and structural proteins leading to endothelial dysfunction, inflammatory responses, and increased oxidative stress (Figure 2). Thus, it is becoming increasingly clear that AGEs play a major causative role in hypertension and atherosclerosis. We have shown that methylglyoxal given in the diet to Wistar-Kyoto (WKY) rats increased tissue AGEs and caused hypertension (Vasdev et al 1998). Levels of tissue methylglyoxal and AGEs are higher in spontaneously hypertensive rats (SHR) and sugar-induced hypertensive rats (Vasdev et al 2000a, 2000b; Wu and Juurlink 2002; Midaoui et al 2003; Wang et al 2004). Although research on AGEs in human essential hypertension is scant, in preeclampsia, a hypertensive condition of pregnancy, RAGE expression was increased in vascular tissue (Cooke et al 2003). AGE-mediated crosslinks in collagen and elastin, also contribute to arterial stiffening, hindering vessel elasticity, and exacerbating hypertension (Aronson 2003). There is strong evidence in diabetes, another insulin resistant state, that AGEs are responsible for cellular protein modifications which contribute to diabetic atherosclerotic complications (Kislinger et al 2001; Degenhardt et al 2002; Nagaraj et al 2002; Babaei-Jadidi et al 2003; Karachalias et al 2003; Alt et al 2004; Stitt et al 2004). Evidence supporting the concept that AGEs are involved in the etiology of cardiovascular disease shows that compounds which lower AGEs also lower blood pressure and attenuate atherosclerotic conditions (Vasdev et al 2000a, 2000b, 2002; Kislinger et al 2001; Degenhardt et al 2002; Mizutani et al 2002; Alderson et al 2003; Babaei-Jadidi et al 2003; Midaoui et al 2003; Sakaguchi et al 2003; Smit and Lutgers 2004; Stitt et al 2004).
Figure 2.
Atherosclerotic and hypertensive effects of advanced glycation end products (AGEs) on blood vessels. AGEs act directly and via receptors of AGEs (RAGES) to alter the function of cellular proteins including calcium channels, endothelial nitric oxide synthase (eNOS), antioxidant enzyme superoxide dismutase (SOD) resulting in a decrease in NO and an increase of reactive oxygen species (ROS), cytokines, imflammation, platelet aggregation and vascular smooth muscle cell (VSMC) proliferation. AGEs and ROS also modify low density lipoprotein (LDL) increasing uptake by macrophages contributing to the formation of plaque. These alterations lead to hypertension and atherosclerosis.
Vascular dysfunction
Maintaining normal endothelial function is essential to blood pressure homeostasis and vessel integrity. One of the major factors involved in regulation of endothelial function is NO. Endothelium derived NO is not only a potent vasodilator but it inhibits platelet aggregation, vascular smooth muscle cell (VSMC) proliferation and intimal migration, and monocyte adhesion, thus regulating blood pressure and protecting vascular function (Taddei et al 2003). Abnormalities in NO have been demonstrated in both hypertension and atherosclerosis (Taddei et al 2003; Shinozaki et al 2004).
The ability of vascular tissue to form adequate amounts of NO depends on the availability of its substrate, arginine, and the endothelial enzyme, nitric oxide synthase (eNOS) (Taddei et al 2003). Methylglyoxal reacts with arginine residues to form several AGEs including argpyrimidine (Shamsi et al 1998; Oya et al 1999; Thornalley et al 2003), which may limit substrate availability (Degenhardt et al 2002). eNOS is an SH-dependent enzyme with a cysteine residue identified at its active site (Chen et al 1994). AGEs have been shown to inhibit eNOS activity and expression (Verbeke et al 2000; Rojas et al 2000). We have shown that methylglyoxal given in the diet to WKY rats increased tissue AGEs and decreased plasma NO (Vasdev et al 1998). NO production is also regulated by insulin acting on specific receptors on the cell surface (Trovati et al 1997; Kahn et al 2000). In insulin resistant states, aldehydes may alter receptor function decreasing NO formation (Sechi et al 1996; Vasdev et al 1998, 2005). NO has a very short half life. Under physiological conditions, it has been shown to readily react with SH groups of plasma proteins forming biologically stable adducts (S-nitrosothiols) which represent a pool of available NO. Low molecular weight thiols such as cysteine and glutathione act as transfer agents for NO from these pools to NO's site of action (Scharfstein et al 1994; Alencar et al 2003). Since aldehydes also readily react with SH groups, this suggests a competitive role limiting both protein binding sites and transfer agent availability resulting in an increase in NO degradation and possible endothelial dysfunction (Farkas and Menzel 1995; Thorpe and Baynes 2003).
Vascular calcium channels are dependent on SH groups for normal function (Schauenstein et al 1977; Zaidi et al 1989) and their alteration can lead to increased cytosolic free calcium, abnormal contractile activity and increased peripheral vascular resistance. AGEs impair type 2 ryanodine receptor calcium release channels (calcium receptors which regulate cardiac contractility) during chronic diabetes (Bidasee et al 2003) and AGE-modified protein increases intracellular calcium (Scivattaro et al 2000). We have shown increased vascular AGEs and platelet cytosolic free calcium in SHRs, a genetic model of hypertension and in methylglyoxal- and fructose-treated WKY rats, dietary models of hypertension (Vasdev et al 1998, 2000a, 2000b).
Since calcium plays a key role in platelet activation, elevated intracellular free calcium may contribute to the enhanced platelet aggregation seen in hypertension and atherosclerosis (Ding 1996). AGEs may also contribute to platelet aggregation and thrombus formation. AGEs increased superoxide production and aggregation in human platelets in vitro (Hangaishi et al 1998). It has been suggested that AGEs stimulate externalization of phosphatidylserine which activates clotting factors leading to platelet adhesion (Wang et al 2005).
Oxidative stress
Under normal physiological conditions, reactive oxygen species (ROS) are produced in low concentrations and act as signaling molecules to regulate VSMC contraction-relaxation, and VSMC growth (Touyz and Schiffrin 2004). Oxidative stress occurs when ROS outweigh the body's antioxidant capacity. Oxidative stress is controlled in part by antioxidant enzymes, including glutathione peroxidase and glutathione reductase (Kaul et al 1993). These enzymes have SH and NH2 groups at their active sites and AGEs inhibit their activity increasing oxidative stress (Morgan et al 2002; Wu and Juurlink 2002; Park et al 2003). Excess ROS can lead to the secondary production of aldehydes such as malondialdehyde and hydroxynonenal, through lipid peroxidation (Brooks and Klamerth 1968; Schauenstein et al 1977; Benedetti et al 1980; Thornalley et al 1984; Dargel 1992; Touyz and Schiffrin 2004). These lipid-derived aldehydes have been shown to react with cysteine or lysine residues of proteins to form a type of AGE known as advanced lipoxidation end products (ALEs) (Baynes and Thorpe 2000; Thorpe and Baynes 2003; Stitt et al 2004).
There is strong evidence that oxidative stress contributes to the progression of essential hypertension and the development of atherosclerosis (Chakravarti et al 1991; Kumar and Das 1993; Chobanian and Alexander 1996; Stocker and Keaney 2004; Touyz and Schiffrin 2004). Oxidative modification of LDL increases uptake by macrophages via scavenger receptors (further discussed in dislipidemia section). This results in an increased production and accumulation of foam cells in the vascular intima leading to the formation of atherosclerotic lesions. Oxidized LDL also stimulates atherogenic processes including inflammation, platelet aggregation, and VSMC proliferation (Ross 1999; Stocker and Keaney 2004; Hansson 2005).
Oxidative stress impairs endothelial function. ROS promote uncoupling of eNOS resulting in a decreased production of NO (Alp and Channon 2004; Shinozaki et al 2004). ROS also react directly with NO, forming peroxynitrite, also known as reactive nitrogen species. This not only limits NO bioavailability, but peroxynitrite and peroxynitrous acid are powerful and cyto-toxic oxidants which may cause damage to vascular tissue (Beckman and Koppenol 1996). Methylglyoxal and AGEs have been shown to increase oxidative stress (Anderson et al 1999; Scivittaro et al 2000; Wautier et al 2001; Mizutani et al 2002; Wu and Jurrlink 2002; Midaoui et al 2003; Wong et al 2003) and in VSMC in vitro induced significant generation of superoxide radical and peroxynitrite (Chang et al 2005).
Dyslipidemia
Dyslipidemia has long been associated with atherosclerosis, and controlling elevated cholesterol and abnormal lipoprotein ratios is a recognized preventative cardiovascular health strategy (Fodor et al 2000). It is well known that high levels of HDL offer protective cardiovascular effect, and that elevated LDL is associated with higher risk of cardiovascular disease.
However, the roles of these lipoproteins are complex and have not yet been fully elucidated. It has been proposed that in its native state LDL is not atherogenic but becomes so under conditions where its structure is modified (Figure 2). Alterations to lysine residues of apolipoprotein B of LDL result in a decreased binding by LDL receptors and an increased stimulation of macrophage scavenger receptors (Witztum and Steinberg 1991; Haberland et al 1992; Horiuchi et al 2003). Oxidatively modified LDL initiates atherogenic processes including inflammation, platelet aggregation and smooth muscle cell proliferation (Stocker and Keaney 2004). Oxidized LDL has been identified as a main component in atherosclerotic lesions, and hypertensive subjects exhibit an enhanced susceptibility to LDL oxidation (Witztum and Steinberg 1991; Maggi et al 1993).
AGEs can form on lipoproteins themselves and AGE-LDL has been shown to have similar atherogenic properties as oxidized LDL (Figure 2). AGE-LDL and AGE-modified protein are ligands for class AI/AII scavenger receptors (Horiuchi et al 2003). Binding to these receptors leads to endocytic uptake of LDL and accumulation in human monocytes-macrophages (Haberland et al 1992; Horiuchi et al 2003). AGE-LDL has been identified in the cytoplasm of foam cells and extracellularly in the core of atherosclerotic lesions in humans and animals (Palinski et al 1995; Nagai et al 2002; Sima and Stancu 2002).
HDL's function in reverse cholesterol transport plays a key role in its cardioprotective effect. HDL removes cholesterol from vascular tissue and either transfers it to very low density lipoprotein (VLDL) or LDL for transport to the liver, or it carries cholesterol directly to the liver where it may be recycled or excreted. In dyslipidemia, where HDL is low, cholesterol may be left to accumulate in vascular tissue. It has been suggested that AGEs interfere with reverse cholesterol transport by suppressing scavenger receptor B1 (SR-B1)-mediated uptake of cholesterol ester from HDL by liver and SR-B1-mediated cholesterol efflux from peripheral cells (Horiuchi et al 2003). AGEs have been shown to cause cholesterol and cholesterol ester accumulation in macrophages in vitro (Brown et al 2005).
HDL also has antioxidant ability. It is closely associated with the antioxidant enzyme paraoxonase. Low HDL and paraoxonase levels are associated with oxidative stress, hypertension and coronary heart disease (Suh et al 1992; Mackness et al 2001; Uzun et al 2004; Mancia et al 2005). Paraoxonase protects both HDL and LDL from oxidation and lipid peroxidation (Aviram, Rosenblat, et al 1998; Aviram, Billecke, et al 1998; Rao et al 2003). In vitro studies show that paraoxonase lowers levels of oxidized LDL by converting them to biologically inactive products (Rao et al 2003). Blockage of free SH groups (cysteine residues) of paraoxonase reduced its ability to protect LDL from oxidation (Aviram, Billecke, et al 1998) suggesting a possible inhibitory role for AGEs (Hedrick et al 2000; Ferretti et al 2006).
HDL has also been shown to have antiinflammatory properties. It inhibited cytokine-induced expression of adhesion proteins and reduced neutrophil infiltration into the wall of injured arteries (Barter et al 2004). AGE-modification of HDL resulted in a loss of these anti-inflammatory properties (Hedrick et al 2000; Ferretti et al 2006).
Low ethanol intake
Effect on hypertension and atherosclerosis
It has been well documented that excessive alcohol intake can be detrimental to cardiovascular health (Thun et al 1997; Reynolds et al 2003; Corrao et al 2004). Although some studies of ethanol intake of greater than 30 g/day have shown improvement in cardiovascular risk, this level of intake increases risk of other diseases and death by other means (Camargo et al 1997; Fagrell et al 1999). Therefore, we have focused our attention in this review on the effects of low ethanol intake (Table 1). Since there is no standard definition of “low” ethanol intake, we have chosen a level of up to, and including, 24 g per day (equivalent to 2 drinks), for the purposes of this discussion. A drink has been defined as a 12 oz bottle or can of beer (4%–5% ethanol), a 4 oz glass of table wine (10%–12% ethanol), or a 1–1.5 oz shot of liquor or spirits (40% ethanol) (Ellison et al 2004).
Table 1.
Effect of low ethanol on hypertension and atherosclerosis
| Subject | Description | Result | Reference |
|---|---|---|---|
| Animal | |||
| New Zealand white rabbits | 0.5% ethanol given in drinking water for 10 weeks |
|
Merritt et al 1997 |
| Spontaneously hypertensive rats | 0.5% ethanol given in drinking water for 14 weeks |
|
Vasdev et al 1999 |
| Human | |||
| New Zealand males and females, 35–64 yrs | Epidemiologic study of 1429 subjects on the effect of ethanol consumption on BP |
|
Jackson et al 1985 |
| US males and females | Study of 129 170 individuals undergoing health examinations in a prepaid health plan |
|
Klatsky et al 1990 |
| US males and females, 30–104 yrs | 9-year prospective study of 490 000 subjects on the effect of ethanol consumption on CV and total mortality |
|
Thun et al 1997 |
| US male physicians, 40–84 yrs | 10.7-year prospective study of 22 071 subjects on the effect of ethanol consumption on mortality |
|
Camargo et al 1997 |
| Bulgarian males & females, 45–69 yrs | Study of 155 individuals hospitalized with ischemic heart disease (IHD) versus 154 normal control patients |
|
Genchev et al 2001 |
| Japanese normotensive males, 40–54 yrs | A 5-year observational study of 2143 subjects on the effect of ethanol consumption on BP |
|
Okubo et al 2001 |
| Males and females from various countries | Meta-analysis of 35 observational studies of varying duration examining the risk of stroke at various levels of alcohol consumption |
|
Reynolds et al 2003 |
| Diabetic males and females, 45–75 yrs | Study of 216 subjects hospitalized with first event of ACS versus 196 diabetic controls |
|
Pitsavos et al 2004 |
| Males and females from various countries | Meta-analysis of 116 702 subjects from 156 epidemiologic studies examining the risk of alcohol related diseases and injuries at various levels of alcohol consumption |
|
Corrao et al 2004 |
Abbreviations: ACS, acute coronary syndrome; AGEs, advanced glycation end products; CV, cardiovascular; BP, blood pressure; DBP, dystolic blood pressure; SBP, systolic blood pressure.
We have shown in rat models of hypertension that chronic low ethanol is effective in decreasing blood pressure (Vasdev et al 1999). Two studies of low ethanol intake in humans, one using up to 9 g/day and the other up to 18 g/day, showed antihypertensive effects (Jackson et al 1985; Okubo et al 2001). Most animal research that looked specifically at atherosclerotic vascular changes, used moderate to high levels of ethanol. However, one study which fed low ethanol to an atherosclerotic model of rabbit showed that it attenuated atherosclerosis with a decrease in the extent of neointimal proliferation, lipid oxidation, and number of foam cells (Merritt et al 1997). In humans, low ethanol consumption reduced risk for both cardiovascular and non-cardiovascular mortality (Klatsky et al 1990; Camargo et al 1997; Thun et al 1997) and was associated with a significantly reduced risk of stroke (Reynolds et al 2003) and ischemic heart disease (Genchev et al 2001). In diabetic patients, low ethanol intake was associated with a 47% reduction in the prevalence of acute coronary syndrome (Pitsavos et al 2005) and in individuals known to be at risk for cardiovascular disease, a 20% reduction in risk of coronary artery disease (Corrao et al 2004).
Mechanism of beneficial effect
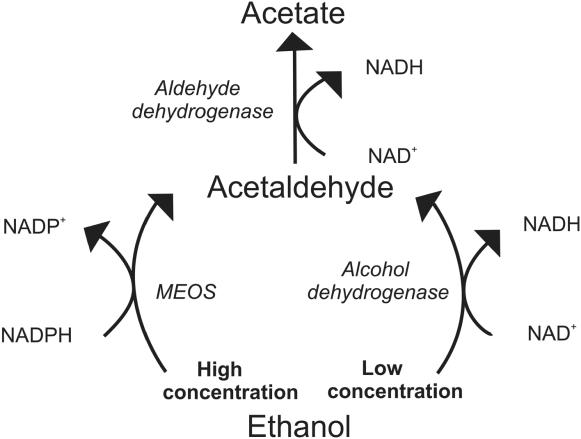
Low ethanol has been shown to improve the clinical manifestations of cardiovascular disease (Table 1), but what is the biochemical mechanism of this beneficial effect? We suggest that low ethanol intake has the ability to increase antioxidant capacity, improve insulin resistance and decrease AGEs, thus preventing subsequent hypertensive and atherosclerotic consequences. To begin, we need to understand the metabolism of ethanol (Figure 3). Ethanol is metabolized differently at high and low concentrations. With chronic high ethanol intake, the microsomal ethanol oxidizing system (MEOS) is induced. MEOS has a relatively high Km for ethanol, and at high concentrations ethanol is metabolized to acetaldehyde without producing reduced nicotinamide adenine dinucleotide (NADH). Instead, this pathway utilizes reduced nicotinamide adenine dinucleotide phosphate (NADPH), another reducing equivalent, thus producing an oxidative environment (Lieber 1990). Additionally, chronic high ethanol consumption inhibits aldehyde dehydrogenase resulting in a significant decrease in the ability of rat mitochondria to oxidize acetaldehyde. This high ethanol intake thus associated with an enhanced rate of metabolism by the MEOS pathway resulting in a decrease in reducing equivalents, elevated acetaldehyde, and increased oxidative stress. These factors may account for the detrimental effects of high ethanol intake. However, at low ethanol blood levels, ethanol is metabolized very efficiently by low Km alcohol dehydrogenase to acetaldehyde and then by aldehyde dehydrogenase to acetate, producing NADH in both reactions (Lieber 1990; Bello et al 1994). NADH has a major influence on total antioxidant capacity of the body. NADH is a key component of the electron transport chain and, due to its high reducing potential functions to promote regeneration of endogenous vitamin radicals back to their reduced form (Figure 4). Although ethanol is primarily metabolized in the liver, it is also metabolized in other tissues, including vascular tissue. This ability of ethanol at low concentrations to create a strong reducing environment, possibly at the site of atherosclerotic activity, may enhance its cardiovascular protective effect.
Figure 3.
Metabolism of high versus low concentrations of ethanol. In high concentrations, ethanol is metabolized by the microsomal ethanol oxidizing system (MEOS) system. In this reaction, reduced nicotinamide adenine dinucleotide phosphate (NADPH) is converted to oxidized nicotinamide adenine dinucleotide phosphate (NADP+) creating an oxidative environment. In low concentrations, ethanol is metabolized by the enzymes alcohol and aldehyde dehydrogenase producing reduced nicotinamide adenine dinucleotide (NADH) from oxidized nicotinamide adenine dinucleotide (NAD+) by both reactions, increasing antioxidant capacity. At the low levels produced, acetate, which is a normal metabolite of glucose and fatty acid metabolism, is further metabolized in the citric acid cycle.
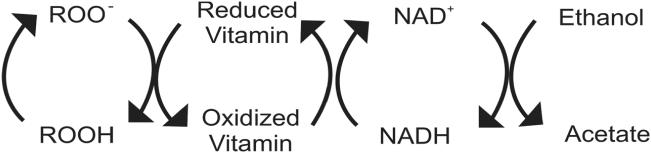
Figure 4.
Antioxidant activity of low ethanol. Free radicals (ROO−) are reduced (ROOH) by vitamins which become oxidized in the process. These vitamin radicals are reduced by nicotinamide adenine dinucleotide (NADH) which forms oxidized nicotinamide adenine dinucleotide (NAD+). Ethanol in low concentrations converts NAD+ back into NADH, via its metabolism to acetate. At this low level, acetate, which is a normal metabolite of glucose and fatty acid metabolism, is further metabolized in the citric acid cycle.
An increase in antioxidant capacity would offer protection against oxidative stress (Figures 4 and 5) and secondary production of aldehydes through lipid peroxidation (Bello et al 1994). In vitro, ethanol has been shown to act as an antioxidant by decreasing the rate of consumption of LDL antioxidants and reducing the formation of lipid peroxides (Bonnefont-Rousselot et al 2001). In humans, it decreased urinary 8-hydroxydeoxyguanosine, a measure of oxidative stress (Yoshida et al 2001). NADH treatment has been shown to reduce blood pressure and lipid peroxidation in SHRs (Bushehri et al 1998). NADH may also increase overall antioxidant capacity, increasing tissue levels of cysteine by converting cystine to cysteine, via the NADH dependent enzyme, cystine reductase (Rodwell 1983). Cysteine is a precursor of glutathione, a major endogenous antioxidant. Additionally, glutathione is a cofactor in methylglyoxal catabolism and cysteine has the ability to bind aldehydes to foster excretion and reduce AGE formation (Schauenstein et al 1977; Vasdev et al 1998).
Figure 5.
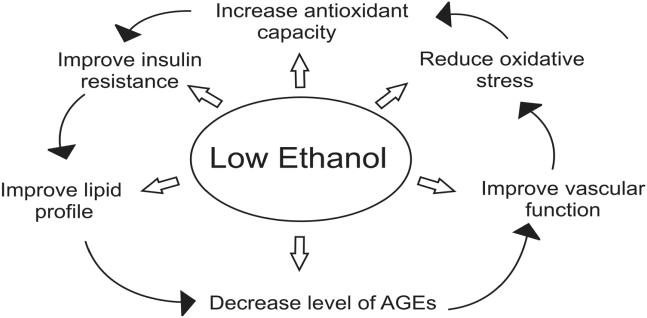
Mechanism of action of low ethanol.
An increase in the low molecular weight thiols, cysteine and glutathione, may also enhance the transfer of NO from protein-bound reserves improving endothelial dysfunction (Scharfstein et al 1994). Decreasing levels of AGEs may also preserve eNOS and prevent breakdown of NO. Low ethanol increased the expression of eNOS (Venkov et al 1999) and stimulated calcium-activated potassium channels increasing production of NO in vascular endothelial cells in culture (Kuhlmann et al 2004). Humans consuming low amounts of ethanol showed significant dilatation of brachial artery at rest and at reactive hyperaemic conditions (Vlachopoulos et al 2003). Low ethanol decreases cytosolic free calcium, an initiator of smooth muscle cell contraction. We have shown in rat models of essential hypertension that low ethanol reduced AGEs and platelet cytosolic free calcium (Vasdev et al 1999). Low ethanol activated sarco/endoplasmic reticulum uptake of calcium in platelets (Mitidieri and de Meis 1995) which would lower cytosolic free calcium concentration. This effect of ethanol on vascular dilation/contraction regulators may contribute to its beneficial cardiovascular effect (Figure 5).
Low alcohol intake increased insulin sensitivity in humans (Facchini et al 1994; Flanagan et al 2000) and in rats (Furuya et al 2003). Improving insulin resistance, the source of excess aldehydes, would limit formation of AGES and their subsequent hypertensive and atherosclerotic complications (Figure 5). Since aldehydes and AGEs have also been shown to inhibit GAPDH (Morgan et al 2002; Beisswenger et al 2003), likely further exacerbating insulin resistance, reducing AGE formation may limit the effect of this cycle. Low ethanol may also work to improve insulin resistance by lowering plasma free fatty acids and improving glucose uptake and metabolism (Avogaro et al 2002).
Low ethanol intake has a beneficial effect on lipoprotein profiles (Figure 5). Low ethanol has been shown to increase HDL (Facchini et al 1994; De Oliviera e Silva et al 2000; Ellison et al 2004). This increase may be a result of mediating the AGE-induced inhibitory effect on reverse cholesterol transport. In hypertensive patients low ethanol decreased lipoprotein a (Lp[a]), an independent predictor for atherosclerosis (Catena et al 2003). It has been suggested that this may be due to an increase in the transportation rate of apolipoprotein AI and AII (De Oliviera e Silva et al 2000) or to an ethanol-induced redistribution of cholesteryl ester transport proteins between HDL and VLDL (Hannuksela et al 1996). Chronic low ethanol intake stimulated paraoxonase activity by upregulating liver paraoxonase mRNA in rats and humans (Rao et al 2003). Ethanol may also prevent inhibition of paraoxonase by lowering AGEs.
Implication for treatment
We have suggested that low ethanol intake affords beneficial cardiovascular effects due to its ability to improve insulin resistance and its subsequent detrimental effects. However, due to the addictive nature of alcohol, recommending ethanol intake at any level may not be prudent. It may be more appropriate to suggest other agents which act in a similar fashion and may provide the same beneficial effects without the complication of addiction. Supplementation with antioxidants including vitamin C, E, or B6, N-acetylcysteine, lipoic acid and coenzyme Q10 has been shown to lower blood pressure in animal models and humans with essential hypertension (Duffy et al 1999; Singh et al 1999; Vasdev et al 2000a, 2000b, 2002; Morcos et al 2001; Barrios et al 2002; Boshtam et al 2002). In humans, antioxidants have also shown a beneficial effect on atherosclerotic endpoints in several studies (Stampfer et al 1993; Losonczy et al 1996; Stephens et al 1996). As well, various anti-AGE therapies, which either prevent or reverse AGE formation, such as pyridoxamine, thiamine, metformin, alagebrium, or soluble RAGE have been shown to attenuate hypertension and atherosclerotic disease (Kislinger et al 2001; Degenhardt et al 2002; Mizutani et al 2002; Alderson et al 2003; Babaei-Jadidi et al 2003; Midaoui et al 2003; Sakaguchi et al 2003; Smit and Lutgers 2004; Stitt et al 2004).
Conclusion
In summary, insulin resistance leads to altered glucose and lipid metabolism. A subsequent increase in AGEs, oxidative stress, and endothelial dysfunction leads to the development of hypertension and atherosclerosis. Low ethanol intake provides cardiovascular beneficial effect primarily through its ability to increase antioxidant capacity, improving insulin resistance and decreasing AGEs. Considering the potential for addiction with ethanol consumption, agents such as antioxidants which have similar antihypertensive and anti-atherosclerotic mechanisms, may provide an appropriate alternative.
Acknowledgments
We would like to thank the Canadian Institutes of Health Research for their financial support.
References
- Alderson NL, Chachich ME, Youssef NN, et al. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int. 2003;63:2123–33. doi: 10.1046/j.1523-1755.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- Alencar JL, Lobysheva I, Geffard M, et al. Role of s-nitosation of cysteine residues in long-lasting inhibitory effect of nitric oxide on arterial tone. Mol Pharmac. 2003;63:1148–58. doi: 10.1124/mol.63.5.1148. [DOI] [PubMed] [Google Scholar]
- Alexander MC, Lomanto M, Nasrin N, et al. Insulin stimulates glyceraldehyde-3-phosphate dehydrogenase gene expression through CIS-acting DNA sequences. Proc Natl Acad Sci U S A. 1988;85:5092–6. doi: 10.1073/pnas.85.14.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–20. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- Alt N, Carson JA, Alderson NL, et al. Chemical modification of muscle protein in diabetes. Arch Biochem Biophys. 2004;425:200–6. doi: 10.1016/j.abb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Anderson MM, Requena JR, Crowley JR, et al. The myeloperoxidase system in human phagocytes generates Nɛ-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest. 1999;104:103–13. doi: 10.1172/JCI3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M, Bisgaier CL, et al. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its function. J Clin Invest. 1998;101:1581–90. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram M, Billecke S, Sorenson R, et al. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities. Selective action of human paraoxonase allozymes Q nd R. Arterioscler Thromb Vasc Biol. 1998;18:1617–24. doi: 10.1161/01.atv.18.10.1617. [DOI] [PubMed] [Google Scholar]
- Avogaro A, Watanabe RM, Gottardo L, et al. Glucose tolerance during moderate alcohol intake: Insights on insulin action from glucose/lactate dynamics. J Clin Endocrinol Metab. 2002;87:1233–8. doi: 10.1210/jcem.87.3.8347. [DOI] [PubMed] [Google Scholar]
- Babaei-Jadidi R, Karachalias N, Ahmed N, et al. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52:2110–20. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- Barrios V, Calderon A, Navarro-Cid J, et al. N-Acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Pressure. 2002;11:235–39. doi: 10.1080/08037050213760. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Rad Biol Med. 2000;28:1708–16. doi: 10.1016/s0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide and peroxynitrite: the good, the bad and the ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Beisswenger PJ, Howell SK, Smith K, et al. Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochim Biophys Acta. 2003;1637:98–106. doi: 10.1016/s09254439(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Bello AT, Bora NS, Lange LG, et al. Cardioprotective effects of alcohol: mediation by human vascular alcohol dehydrogenase. Biochem Biophys Res Commun. 1994;203:1858–64. doi: 10.1006/bbrc.1994.2404. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980;620:281–96. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- Bidasee KR, Nallani K, Yu Y, et al. Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes. 2003;52:1825–36. doi: 10.2337/diabetes.52.7.1825. [DOI] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D, Rouscilles A, Bizard C, et al. Antioxidant effect of ethanol toward in vitro peroxidation of human low-density lipoproteins initiated by oxygen free radicals. Radiat Res. 2001;155:279–87. doi: 10.1667/0033-7587(2001)155[0279:aeoeti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Boshtam M, Rafiei M, Sadeghi K, et al. Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitam Nutr Res. 2002;72:309–14. doi: 10.1024/0300-9831.72.5.309. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Klamerth OJ. Interaction of DNA with bifunctional aldehydes. Euro J. Biochem. 1968;5:178–82. doi: 10.1111/j.1432-1033.1968.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Brown BE, Dean RT, Davies MJ. Glycation of low-density lipoproteins by methylglyoxal and glycoaldehyde gives rise to the in vitro formation of lipid-laden cells. Diabetologia. 2005;48:361–9. doi: 10.1007/s00125-004-1648-4. [DOI] [PubMed] [Google Scholar]
- Bushehri N, Jarrell ST, Lieberman S, et al. Oral reduced B-nicotinamide adenine dinucleotide (NADH) affects blood pressure, lipid peroxidation, and lipid profile in hypertensive rats (SHR) Geriat Nephrol Urol. 1998;8:95–100. doi: 10.1023/a:1008242900153. [DOI] [PubMed] [Google Scholar]
- Camargo CA, Hennekens CH, Gaziano JM, et al. Prospective study of moderate alcohol consumption and mortality in US male physicians. Arch Intern Med. 1997;157:79–85. [PubMed] [Google Scholar]
- Catena C, Novello M, Dotto L, et al. Serum lipoprotein(a) concentrations and alcohol consumption in hypertension: possible relevance for cardiovascular damage. J Hypertens. 2003;21:281–8. doi: 10.1097/00004872-200302000-00018. [DOI] [PubMed] [Google Scholar]
- Chakravarti RN, Kirshenbaum LA, Singal PK. Atherosclerosis: Its pathophysiology with special reference to lipid peroxidation. J Appl Cardiol. 1991;6:91–112. [Google Scholar]
- Chang KC, Paek KS, Kim HJ, et al. Substrate-induced up-regulation of aldose reductase by methylglyoxal, a reactive oxoaldehyde elevated in diabetes. Mol Pharmacol. 2002;61:1184–91. doi: 10.1124/mol.61.5.1184. [DOI] [PubMed] [Google Scholar]
- Chang T, Wang R, Wu L. Methylglyoxal-induced nitric oxide and peroxynitrite production in vascular smooth muscle cells. Free Radic Biol Med. 2005;38:286–93. doi: 10.1016/j.freeradbiomed.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Chen P, Tsai A, Wu KK. Cysteine 184 of endothelial nitric oxide synthase is involved in heme coordination and catalytic activity. J Biol Chem. 1994;269:25062–6. [PubMed] [Google Scholar]
- Chobanian AV, Alexander RW. Exacerbation of atherosclerosis by hypertension. Arch Intern Med. 1996;156:1952–6. [PubMed] [Google Scholar]
- Cooke CLM, Brockelsby JC, Baker PN, et al. The receptor for advanced glycation end products (RAGE) is elevated in women with preeclampsia. Hypertens Preg. 2003;22:173–84. doi: 10.1081/PRG-120021068. [DOI] [PubMed] [Google Scholar]
- Corrao G, Bagnardi V, Zambon A, et al. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prevent Med. 2004;38:613–19. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Dargel R. Lipid peroxidation - a common pathogenetic mechanism? Exp Toxic Pathol. 1992;44:169–81. doi: 10.1016/S0940-2993(11)80202-2. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E. Insulin resistance: A multifaceted syndrome responsible for NIDDM, obesity, hypertension and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Degenhardt TP, Alderson NL, Arrington DD, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in streptozotocin-diabetic rat. Kidney Int. 2002;61:939–50. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- De Oliveira e Silva ER, Foster D, McGee Harper M, et al. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102:2347–52. doi: 10.1161/01.cir.102.19.2347. [DOI] [PubMed] [Google Scholar]
- Ding YA. Thrombogenic and lipid risk factors in hypertension and coronary artery disease. Jpn Circ J. 1996;60:75–84. doi: 10.1253/jcj.60.75. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Gokce N, Holbrook M, et al. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048–9. doi: 10.1016/s0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- Ellison RC, Zhang Y, Qureshi MM, et al. Lifestyle determinants of high-density lipoprotein cholesterol: The National Heart, Lung, and Blood Institute Family Heart Study. Am Heart J. 2004;147:529–35. doi: 10.1016/j.ahj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Facchini F, Chen YDI, Reaven GM. Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care. 1994;17:115–19. doi: 10.2337/diacare.17.2.115. [DOI] [PubMed] [Google Scholar]
- Fagrell B, De Faire U, Bondy S, et al. The effects of light to moderate drinking on cardiovascular diseases. J Intern Med. 1999;246:331–40. doi: 10.1046/j.1365-2796.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Farkas J, Menzel EJ. Proteins lose their nitric oxide stabilizing function after advanced glycosylation. Biochim Biophys Acta. 1995;1245:305–10. doi: 10.1016/0304-4165(95)00113-1. [DOI] [PubMed] [Google Scholar]
- Ferretti G, Bacchetti T, Negre-Salvayre A, et al. Structural modifications of HDL and functional consequences. Atheroscler. 2006;184:1–7. doi: 10.1016/j.atherosclerosis.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–7. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- Flack JM, Sowers JR. Epidemiologic and clinical aspects of insulin resistance and hyperinsulinemia. Am J Med. 1991;91:11S–21S. doi: 10.1016/0002-9343(91)90058-6. [DOI] [PubMed] [Google Scholar]
- Flanagan DEH, Moore VM, Godsland IF, et al. Alcohol consumption and insulin resistance in young adults. Euro J Clin Invest. 2000;30:297–301. doi: 10.1046/j.1365-2362.2000.00624.x. [DOI] [PubMed] [Google Scholar]
- Fodor JG, Frohlich JJ, Genest JJG, et al. Recommendations for the management and treatment of dyslipidemia. Report of the Working Group on Hypercholesterolemia and Other Dyslipidemias. CMAJ. 2000;162:1441–7. [PMC free article] [PubMed] [Google Scholar]
- Fuh MM, Sheih SM, Wu DA, et al. Abnormalities of carbohydrate and lipid metabolism in patients with hypertension. Arch Intern Med. 1987;147:1035–8. [PubMed] [Google Scholar]
- Furuya DT, Binsack R, Machado UF. Low ethanol consumption increases insulin sensitivity in Wistar rats. Braz J Med Biol Res. 2003;36:125–30. doi: 10.1590/s0100-879x2003000100017. [DOI] [PubMed] [Google Scholar]
- Genchev GD, Georgieva LM, Weijenberg MP, et al. Does alcohol protect against ischemic heart disease in Bulgaria? A case-control study of non-fatal myocardial infarction in Sofia. Centr Eur J Public Health. 2001;9:83–6. [PubMed] [Google Scholar]
- Haberland ME, Fless GM, Scanu AM, et al. Malondialdehyde modification of lipoprotein(a) produces avid uptake by human monocyte-macrophages. J Biol Chem. 1992;267:4143–51. [PubMed] [Google Scholar]
- Hangaishi M, Taguchi J, Miyata T, et al. Increased aggregation of human platelets produced by advanced glycation end products in vitro. Biochem Biophys Res Comm. 1998;248:285–92. doi: 10.1006/bbrc.1998.8945. [DOI] [PubMed] [Google Scholar]
- Hannuksela ML, Rantala M, Kesaniemi YA, et al. Ethanol-induced redistribution of cholesteryl ester transfer protein (CEPT) between lipoproteins. Atheroscler Thromb Vasc Biol. 1996;16:213–21. doi: 10.1161/01.atv.16.2.213. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hedrick CC, Thorpe SR, Fu MX, et al. Glycation impairs high-density lipoprotein function. Diabetalogia. 2000;43:312–20. doi: 10.1007/s001250050049. [DOI] [PubMed] [Google Scholar]
- Horiuchi S, Sakamoto Y, Sakai M. Scavenger receptors for oxidized and glycated proteins. Amino Acids. 2003;25:283–92. doi: 10.1007/s00726-003-0029-5. [DOI] [PubMed] [Google Scholar]
- Howard G, O'Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. Circulation. 1996;93:1809–17. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- Jackson R, Stewart A, Beaglehole R, et al. Alcohol consumption and blood pressure. Am J Epidemiol. 1985;122:1037–44. doi: 10.1093/oxfordjournals.aje.a114185. [DOI] [PubMed] [Google Scholar]
- Kahn NN, Acharya K, Bhattacharya S, et al. Nitric oxide: The “second messenger” of insulin. IUBMB Life. 2000;49:441–50. doi: 10.1080/152165400410308. [DOI] [PubMed] [Google Scholar]
- Karachalias N, Babaei-Jadidi R, Ahmed N, et al. Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats. Biochem Soc Trans. 2003;31:1423–5. doi: 10.1042/bst0311423. [DOI] [PubMed] [Google Scholar]
- Kaul M, Siveski-Iiskovic M, Hill M, et al. Free radicals and the heart. J Pharmacol Toxicol Methods. 1993;30:55–67. doi: 10.1016/1056-8719(93)90008-3. [DOI] [PubMed] [Google Scholar]
- Kirstein M, Aston C, Hintz R, et al. Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product–modified proteins. J Clin Invest. 1992;90:439–46. doi: 10.1172/JCI115879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislinger T, Tanji N, Wendt T, et al. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2001;21:905–10. doi: 10.1161/01.atv.21.6.905. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Armstrong MA, Friedman GD. Risk of cardiovascular mortality in alcohol drinkers, ex-drinkers and nondrinkers. Am J Cardiol. 1990;66:1237–42. doi: 10.1016/0002-9149(90)91107-h. [DOI] [PubMed] [Google Scholar]
- Kuhlmann CRW, Li F, Ludders DW, et al. Dose-dependent activation of Ca2+-activated K+ channels by ethanol contributes to improved endothelial cell functions. Alcohol Clin Exp Res. 2004;28:1005–11. doi: 10.1097/01.alc.0000130811.92457.0d. [DOI] [PubMed] [Google Scholar]
- Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension? Free Rad Res Comm. 1993;19:59–66. doi: 10.3109/10715769309056499. [DOI] [PubMed] [Google Scholar]
- Liao Y, Kwan S, Shaughnessy S, et al. Critical evaluation of adult treatment panel III criteria in identifying insulin resistance with dyslipidemia. Diabet Care. 2004;27:978–83. doi: 10.2337/diacare.27.4.978. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Mechanism of ethanol induced hepatic injury. Pharmac Ther. 1990;46:1–41. doi: 10.1016/0163-7258(90)90032-w. [DOI] [PubMed] [Google Scholar]
- Losonczy KG, Harris TB, Havlik RJ. Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: the Established Populations for Epidemiologic Studies of the Elderly. Am J Clin Nutr. 1996;64:190–6. doi: 10.1093/ajcn/64.2.190. [DOI] [PubMed] [Google Scholar]
- Mackness B, Davies GK, Turkie W, et al. Paraoxonase status in coronary heart disease. Are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol. 2001;21:1451–7. doi: 10.1161/hq0901.094247. [DOI] [PubMed] [Google Scholar]
- Maggi E, Marchesi E, Ravetta V, et al. Low-density lipoprotein oxidation in essential hypertension. J Hypertens. 1993;11:1103–11. doi: 10.1097/00004872-199310000-00015. [DOI] [PubMed] [Google Scholar]
- Mancia G, Facchetti R, Bombelli M, et al. Relationship of office, home, and ambulatory blood pressure to blood glucose and lipid variables in the PAMELA population. Hypertens. 2005;45:1072–7. doi: 10.1161/01.HYP.0000165672.69176.ed. [DOI] [PubMed] [Google Scholar]
- Mayes PA. Carbohydrate metabolism. In: Martin DW, Rodwell VW, Mayes PA, editors. Harper's Review of Biochemistry 19th ed. Los Altos, CA: Lange Medical Pub; 1983. pp. 161–87. [Google Scholar]
- Merritt R, Guruge BL, Miller DD, et al. Moderate alcohol feeding attenuates postinjury vascular cell proliferation in rabbit angioplasty model. J Cardiovasc Pharmacol. 1997;30:19–25. doi: 10.1097/00005344-199707000-00004. [DOI] [PubMed] [Google Scholar]
- Midaoui AE, Elimadi A, Wu L, et al. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am J Hypertens. 2003;16:173–9. doi: 10.1016/s0895-7061(02)03253-3. [DOI] [PubMed] [Google Scholar]
- Mitidieri F, de Meis L. Ethanol has different effects on Ca (2+)–transport ATPases of muscle, brain and blood platelets. Biochem J. 1995;312:733–7. doi: 10.1042/bj3120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani K, Ikeda K, Tsuda K, et al. Inhibitor for advanced glycation end products formation attenuates hypertension and oxidative damage in genetic hypertensive rats. J Hypertens. 2002;20:1607–14. doi: 10.1097/00004872-200208000-00024. [DOI] [PubMed] [Google Scholar]
- Morcos M, Borcea V, Isermann B, et al. Effect of α-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: an exploratory study. Diab Res Clin Pract. 2001;52:175–83. doi: 10.1016/s0168-8227(01)00223-6. [DOI] [PubMed] [Google Scholar]
- Morgan PE, Dean RT, Davies MJ. Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Arch Biochem Biophys. 2002;403:259–69. doi: 10.1016/s0003-9861(02)00222-9. [DOI] [PubMed] [Google Scholar]
- Nagai R, Hayashi CM, Xia L, et al. Identification in human atherosclerotic lesions of GA-pyridine, a novel structure derived from glycoaldehyde-modified proteins. J Biol Chem. 2002;277:48905–12. doi: 10.1074/jbc.M205688200. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Sarkar P, Mally A, et al. Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch Biochem Biophys. 2002;402:110–19. doi: 10.1016/S0003-9861(02)00067-X. [DOI] [PubMed] [Google Scholar]
- Neutel JM. Why lowering blood pressure is not enough: the hypertension syndrome and the clinical context of cardiovascular risk reduction. Heart Dis. 2000;2:370–4. [PubMed] [Google Scholar]
- Okubo Y, Suwazono Y, Kobayashi E, et al. Alcohol consumption and blood pressure change: 5 year follow-up study of the association in normotensive workers. J Hum Hypertens. 2001;15:367–72. doi: 10.1038/sj.jhh.1001191. [DOI] [PubMed] [Google Scholar]
- Oshima T, Young EW. Systemic and cellular calcium metabolism and hypertension. Semin Nephrol. 1995;15:496–503. [PubMed] [Google Scholar]
- Oya T, Hattori N, Mizuno Y, et al. Methylglyoxal modification of protein. J Biol Chem. 1999;274:18492–502. doi: 10.1074/jbc.274.26.18492. [DOI] [PubMed] [Google Scholar]
- Palinski W, Koschinsky T, Butler SW, et al. Immunological evidence for the presence of advanced glycosylation end products in atherosclerotic lesions of euglycemic rabbits. Arterioscler Thromb Vasc Biol. 1995;15:571–82. doi: 10.1161/01.atv.15.5.571. [DOI] [PubMed] [Google Scholar]
- Park YS, Koh YH, Takahashi M, et al. Identification of the binding site of methylglyoxal on glutathione peroxidase: Methylglyoxal inhibits glutathione peroxidase activity via binding to glutathione binding sites Arg 184 and 185. Free Rad Res. 2003;37:205–11. doi: 10.1080/1071576021000041005. [DOI] [PubMed] [Google Scholar]
- Pepine CJ, Handberg EM. The vascular biology of hypertension and atherosclerosis and intervention with calcium antagonists and angiotensin-converting enzyme inhibitors. Clin Cardiol. 2001;24:V1–5. doi: 10.1002/clc.4960241702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano MR. The cardiovascular effects of alcohol: the good and the bad. How low-risk drinking differs from high-risk drinking. AJN. 2005;105:87–91. doi: 10.1097/00000446-200507000-00041. [DOI] [PubMed] [Google Scholar]
- Pitsavos C, Makrilakis K, Panagiotakos DB, et al. The J-shape effect of alcohol intake on the risk of developing acute coronary syndromes in diabetic subjects: the CARDIO 2000 II Study. Diabet Med. 2005;22:243–8. doi: 10.1111/j.1464-5491.2004.01384.x. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Boari B, Manfredini R. Oxidative stress in essential hypertension. Curr Pharmaceut Design. 2004;10:1695–8. doi: 10.2174/1381612043384619. [DOI] [PubMed] [Google Scholar]
- Rao MN, Marmillot P, Gong M, et al. Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabol. 2003;52:1287–94. doi: 10.1016/s0026-0495(03)00191-4. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J Clin Endocrin Metab. 2003;88:2399–403. doi: 10.1210/jc.2003-030087. [DOI] [PubMed] [Google Scholar]
- Resnick LM. Ionic basis of hypertension, insulin resistance, vascular disease, and related disorders. The mechanism of “syndrome X”. Am J Hyperten. 1993;6:123S–134S. doi: 10.1093/ajh/6.4s.123s. [DOI] [PubMed] [Google Scholar]
- Reynolds K, Lewis LB, Nolen JDL, et al. Alcohol consumption and risk of stroke. JAMA. 2003;289:579–88. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- Rodwell VW. Catabolism of the carbon skeletons of amino acids. In: Martin DW, Rodwell VW, Mayes PA, editors. Harper's Review of Biochemistry 19th ed. Stamford, CO: Appleton & Lange; 1983. pp. 283–306. [Google Scholar]
- Rojas A, Romay S, Gonzalez D, et al. Regulation of endothelial nitric oxide synthase expression by albumin-derived advanced glycosylation end products. Circ Res. 2000;86:e50–e54. doi: 10.1161/01.res.86.3.e50. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – An inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Saad MF, Rewers M, Selby J, et al. Insulin resistance and hypertension. The insulin resistance atherosclerosis study. Hypertens. 2004;43:1324–31. doi: 10.1161/01.HYP.0000128019.19363.f9. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Yan SF, Yan SD, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–72. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfstein JS, Keaney JF, Slivka A, et al. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest. 1994;94:1432–9. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauenstein E, Esterbauer H, Zollner H. Aldehydes in biological systems. In: Lagnado JR, editor. Aldehydes in biological systems, their natural occurrence and biological activities. London: Pion Limited; 1977. pp. 1–7. [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, et al. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–55. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scivittaro V, Ganz MB, Weiss MF. AGEs induce oxidative stress and activate protein kinase C-βII in neonatal mesangial cells. Am J Physiol Renal Physiol. 2000;278:F676–83. doi: 10.1152/ajprenal.2000.278.4.F676. [DOI] [PubMed] [Google Scholar]
- Sechi LA, Griffin CA, Giacchetti G, et al. Abnormalities of insulin receptors in spontaneously hypertensive rats. Hypertens. 1996;27:955–61. doi: 10.1161/01.hyp.27.4.955. [DOI] [PubMed] [Google Scholar]
- Shamsi FA, Partal A, Sady C, et al. Immunological evidence for methylglyoxal-derived modifications in vivo. J Biol Chem. 1998;273:6928–36. doi: 10.1074/jbc.273.12.6928. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Kashiwagi A, Masada M, et al. Molecular mechanism of impaired endothelial function associated with insulin resistance. Curr Drug Targets Cardiov Haemat Dis. 2004;4:1–11. doi: 10.2174/1568006043481248. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Suzuki M, Ikebuchi M, et al. Demonstration of insulin resistance in coronary artery disease documented with angiography. Diabetes Care. 1996;19:1–7. doi: 10.2337/diacare.19.1.1. [DOI] [PubMed] [Google Scholar]
- Sima A, Stancu C. Modified lipoproteins accumulate in human coronary atheroma. J Cell Mol Med. 2002;6:110–11. doi: 10.1111/j.1582-4934.2002.tb00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RB, Niaz MA, Rastogi SS, et al. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J Human Hypertens. 1999;13:203–8. doi: 10.1038/sj.jhh.1000778. [DOI] [PubMed] [Google Scholar]
- Smit AJ, Lutgers HL. The clinical relevance of advanced glycation endproducts (AGE) and recent developments in pharmaceutics to reduce AGE accumulation. Curr Med Chem. 2004;11:2767–84. doi: 10.2174/0929867043364342. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Hennekens CH, Manson JE, et al. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328:1444–9. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- Stephens NG, Parsons A, Schofield PM, et al. Randomized controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–6. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Frizzell N, Thorpe SR. Advanced glycation and advanced lipoxidation: possible role in initiation and progression of diabetic retinopathy. Curr Pharm Design. 2004;10:3349–60. doi: 10.2174/1381612043383124. [DOI] [PubMed] [Google Scholar]
- Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- Suh I, Shaten BJ, Cutler JA, et al. Alcohol use and mortality from coronary heart disease: The role of high-density lipoprotein cholesterol. Ann Intern Med. 1992;116:881–7. doi: 10.7326/0003-4819-116-11-881. [DOI] [PubMed] [Google Scholar]
- Taddei S, Ghiadoni L, Virdis A, et al. Mechanisms of endothelial dysfunction: Clinical significance and preventative non-pharmacological therapeutic strategies. Curr Pharmaceut Design. 2003;9:2385–402. doi: 10.2174/1381612033453866. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Yamagishi S. TAGE (toxic AGEs) hypothesis in various diseases. Med Hypoth. 2004;63:449–52. doi: 10.1016/j.mehy.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Tegos TJ, Kalodiki E, Sabetai MM, et al. The genesis of atherosclerosis and risk factors: A review. Angiology. 2001;52:89–98. doi: 10.1177/000331970105200201. [DOI] [PubMed] [Google Scholar]
- Thornalley P, Wolff S, Crabbe J, et al. The autoxidation of glyceraldehyde and other simple monosaccharides under physiological conditions catalysed by buffer ions. Biochim Biophys Acta. 1984;797:276–87. doi: 10.1016/0304-4165(84)90131-4. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Battah S, Ahmed N, et al. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J. 2003;375:581–92. doi: 10.1042/BJ20030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ. The glyoxalase system in health and disease. Mol Aspects Med. 1993;14:287–371. doi: 10.1016/0098-2997(93)90002-u. [DOI] [PubMed] [Google Scholar]
- Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids. 2003;25:275–81. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Peto R, Lopez AD, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–14. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications for hypertension. Histochem Cell Biol. 2004;122:339–52. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- Trovati M, Anfossi G, Massucco P, et al. Insulin stimulates nitric oxide synthesis in human platelets and, through nitric oxide, increases platelet concentrations of both guanosine-3′, 5′-cyclic monophosphate and adenosine-3′, 5′-cyclic monophosphate. Diabetes. 1997;46:742–9. doi: 10.2337/diab.46.5.742. [DOI] [PubMed] [Google Scholar]
- Uzun H, Karter Y, Aydin S, et al. Oxidative stress in white coat hypertension; role of paraoxonase. J Hum Hypertens. 2004;18:523–8. doi: 10.1038/sj.jhh.1001697. [DOI] [PubMed] [Google Scholar]
- Vasdev S, Ford CA, Longerich L, et al. Aldehyde induced hypertension in rats: Prevention by N-acetylcysteine. Artery. 1998;23:10–36. [PubMed] [Google Scholar]
- Vasdev S, Ford CA, Longerich L, et al. Antihypertensive effect of low ethanol intake in spontaneously hypertensive rats. Mol Cell Biochem. 1999;200:85–92. doi: 10.1023/a:1006950414560. [DOI] [PubMed] [Google Scholar]
- Vasdev S, Ford CA, Parai S, et al. Dietary alpha-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. J Hypertens. 2000a;18:567–73. doi: 10.1097/00004872-200018050-00009. [DOI] [PubMed] [Google Scholar]
- Vasdev S, Ford CA, Parai S, et al. Dietary lipoic acid supplementation prevents fructose-induced hypertension in rats. Nutr Metab Cardiovasc Dis. 2000b;10:339–46. [PubMed] [Google Scholar]
- Vasdev S, Gill V, Longerich L. Role of methylglyoxal in essential hypertension. In: Gupta SK, Singal PK, Agrawal, editors. New Delhi, India: Anamaya Pub; 2005. pp. 72–88. [Google Scholar]
- Vasdev S, Longerich L, Singal P. Nutrition and hypertension. Nutr Res. 2002;22:111–23. [Google Scholar]
- Venkov CD, Myers PR, Tanner MA, et al. Ethanol increases endothelial nitric oxide production through modulation of nitric oxide synthase expression. Thromb Haemost. 1999;81:638–42. [PubMed] [Google Scholar]
- Verbeke P, Perichon M, Friguet B, et al. Inhibition of nitric oxide synthase activity by early and advanced glycation end products in cultured rabbit proximal tubular epithelial cells. Biochim Biophys Acta. 2000;1502:481–94. doi: 10.1016/s0925-4439(00)00071-5. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Tsekoura D, Tsiamis E, et al. Effect of alcohol on endothelial function in health subjects. Vasc Med. 2003;8:263–5. doi: 10.1191/1358863x03vm505oa. [DOI] [PubMed] [Google Scholar]
- Vlasakova Z, Pelikanova T, Karasova L, et al. Insulin secretion, sensitivity, and metabolic profile of young healthy offspring of hypertensive patients. Metab. 2004;53:469–75. doi: 10.1016/j.metabol.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Wang X, Desai K, Clausen JT, et al. Increased methylglyoxal and advanced glycation end products in kidney from spontaneously hypertensive rats. Kid Internat. 2004;66:2315–21. doi: 10.1111/j.1523-1755.2004.66034.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Marshall SM, Thompson MG, et al. Cardiovascular risk in patients with end-stage renal disease: a potential role for advanced glycation end products. Contrib Nephrol. 2005;149:168–74. doi: 10.1159/000085483. [DOI] [PubMed] [Google Scholar]
- Wautier MP, Chappey O, Corda S, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–92. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RKM, Pettit AI, Quinn PA, et al. Advanced glycation end products stimulate an enhanced neutrophil respiratory burst mediated through the activation of cytosolic phospholipase A2 and generation of arachidonic acid. Circ. 2003;108:1858–64. doi: 10.1161/01.CIR.0000089372.64585.3B. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Health Report. Chapter 6: Neglected global epidemics: three growing threats [online] 2003a:1–15. Accessed on 8 February 2006. URL: http://www.who.int/entity/whr/2003/en/Chapter6-en.pdf.
- World Health Organization. Global strategy on diet, physical activity and health: chronic disease risk factors. 2003b Accessed on 8 February 2006. URL: http://www.who.int/dietphysicalactivity/publications/facts/riskfactors/en/
- Wu L, Juurlink BHJ. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertens. 2002;39:809–14. doi: 10.1161/hy0302.105207. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Shioji I, Kishida A, et al. Moderate alcohol consumption reduces urinary 8-hydroxydeoxyguanosine by inducing of uric acid. Indust Health. 2001;39:322–9. doi: 10.2486/indhealth.39.322. [DOI] [PubMed] [Google Scholar]
- Zaidi NF, Lagenaur CF, Abramson JJ, et al. Reactive disulfides trigger Ca2+ release from sarcoplasmic reticulum via an oxidation reaction. J Biol Chem. 1989;264:21725–36. [PubMed] [Google Scholar]