Abstract
Atherosclerosis is a chronic inflammatory disease characterized by infiltration of blood vessels by lipids and leukocytes. There is a growing body of evidence that among risk factors that promote atherosclerosis, the metabolic syndrome is a powerful and prevalent predictor of cardiovascular events. The systemic inflammatory process associated with the metabolic syndrome has numerous deleterious effects that promote plaque activation, which is responsible for clinical events. Interactions between the innate immune system with lipid-derived products seem to play a major role in the pathophysiology of atherosclerosis in relation with the metabolic syndrome. The multiple links among adipose tissue, the vascular wall, and the immune system are the topics of this review, which examines the roles of oxidized low-density lipoprotein, inflammatory cytokines, and adipokines in triggering and perpetuating a danger signal response that promotes the development of atherosclerosis. Furthermore, therapeutic options that specifically target the metabolic syndrome components are reviewed in light of recent developments.
Keywords: atherosclerosis, inflammation, metabolic syndrome, innate immune system, danger signal theory
Introduction
Atherosclerosis is a chronic disease that develops over a lifetime, with clinical manifestations that occur after decades of silent progression. Despite recent progress in the treatment of cardiovascular disease associated with a significant reduction of death rates from 1990 to 2000, atherosclerotic disease is still the leading cause of mortality in developed countries (AHA 2006). Furthermore, atherosclerosis-related diseases represent an enormous socio-economic burden increasing healthcare expenditures and are an important source of human suffering (Hoffman et al 1996). Lifestyle modifications such as poor dietary patterns and physical inactivity are recent events in the human evolution and are responsible for the development of obesity, which has reached an epidemic proportion in North America. It is estimated that overweight individuals (as defined by a body mass index [BMI] of >25 kg/m2) represent ∼60% of the adult US population (Troiano and Flegal 1999). Obesity, is an important risk factor for cardiovascular disorders, it is often associated with hypertension and it increases the risk of metabolic perturbations including insulin resistance, hypertriglyceridemia, and low plasma high-density lipoprotein cholesterol (HDL-C) concentrations. In the 1980s, Reaven (1988) proposed that patients with these metabolic abnormalities had a plurimetabolic syndrome which he, at the time, described as syndrome X commonly referred to as the metabolic syndrome.
Although the concept of metabolic syndrome was accepted for years, it is only recently that an attempt to develop a recognized definition was established by different organizations (Table 1) (Alberti and Zimmet 1998; NCEP–ATP 2001). Although obesity is often found among individuals with the metabolic syndrome, it appears that a substantial amount of patients with a BMI less than 30 kg/m2 have metabolic abnormalities. Among those individuals that are not clinically obese, a high accumulation of visceral adipose tissue appears to predict the features of the metabolic syndrome (St-Pierre et al 2005).
Table 1.
The proposed criteria for the metabolic syndrome by the National Cholesterol Education Program–Adult Treatment Panel III (NCEP–ATP III) and by the World Health Organization (WHO)
| NCEP ATP III | WHO | ||
|---|---|---|---|
| 3 or more of the following | |||
| Visceral adiposity | Diabetes or insulin resistance | ||
| Waist circumference: | male >102 cm | (hyperinsulinaemic, euglycemic clamp-glucose uptake in lowest 25%) | |
| female >88 cm | |||
| Hypertriglyceridemia: | TG ≥1.7 mmol/L | Plus two of the following criteria: | |
| Low HDL-C: | male <1.0 mmol/L | Obesity: BMI >30 or waist-to-hip ratio | male >0.9 |
| female <1.3 mmol/L | female >0.85 | ||
| Hypertension ≥135/85 mm Hg or medication | Lipid abnormalities: TG ≥1.7 mmol/L or HDL-C | male <0.9 mmol/L | |
| Fasting plasma glucose ≥6.1 mmol/L | female <1.0 mmol/L | ||
| Microalbuminuria >20 μg/min | |||
| Hypertension >140/90 | |||
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein-cholesterol; TG, triglycerides.
Prospective observational studies have clearly established that classical risk factors such as age, diabetes, hypertension, smoking and high plasma low-density lipoprotein cholesterol (LDL-C) concentrations predicted coronary events (Wilson et al 1998). However, a substantial proportion of patients with coronary heart disease (CHD) do not have high level of LDL-C, suggesting that other factors beyond traditional risk factors influence evolution of atherosclerotic disease (Genest et al 1992; Despres et al 2000). In the prospective Québec Cardiovascular Study, men with features of the metabolic syndrome had a 20-fold increase in their risk of CHD compared with men without such abnormalities, suggesting that these metabolic perturbations could act synergistically with classical risk factors (Lamarche et al 1998). It is now well documented that metabolic syndrome is also characterized by an elevation of inflammatory markers that can predict cardiovascular events such as the risk of myocardial infarction, stroke, and peripheral arterial disease (Ridker et al 2002). There is evidence that the expanded visceral adipose depot is a source of cytokines and adipokines that influence interactions between the immune system and the vascular wall (Despres 2003). Indeed, atherosclerosis is an inflammatory disease characterized by vascular wall infiltration by macrophages and T cells associated with lipid infiltration (Hansson 2005). Implication of the immune system in atherosclerosis is still incompletely understood, but recent works have highlighted the role of the innate immune system in generating a response in the presence of tissue aggression, which would subsequently lead to the activation of inflammatory pathways (Matzinger 2002). Therefore, the metabolic perturbations that mediate important and complex interactions with the immune system and the vascular wall in relation with atherosclerosis are reviewed with an emphasis on the mechanisms that mediate immune activation.
The danger model of immunity and atherosclerosis
The immune system evolved to ensure a protection against ‘non-self’, allowing a defense line against the infectious threat. For years it was taught that the immune system functions as sensor that allows recognition of ‘self’ from ‘non-self’, and to mount a specific response against ‘non-self’ by the use of adaptive immune responses. However, this immunological paradigm has been challenged in the 1990s with the danger model of immunity in which the immune system responds to damaged cells rather than to foreign (Gallucci and Matzinger 2001). This model of immunity has allowed the expansion of the scope of immunological implication in diseases with an inflammatory component that might be detrimental to the host. The discovery at the end of the 20th century of the Toll-like receptors (TLRs) in mammalian innate immune cells such as macrophages and dendritic cells has reinforced the view that innate immune system plays a key role in inflammatory response (Medzhitov et al 1997). Furthermore, the discovery that, beside bacterial products, endogenous substances such as oxidized LDL (ox-LDL) and heat shock proteins (HSPs) mediate activation of TLRs has reinforced the view that innate immune system play a key role in the genesis of atherosclerosis (Michelsen et al 2004). Therefore, immune cells are the gate keepers that detect cellular damage and initiate a response allowing our body to defend against ‘offending’ insults.
Endogenous danger signals are from intracellular or secreted extracellular products. Some are constitutive whereas others are inducible and require either neosynthesis or modifications before they can activate the innate immune system. Atherosclerosis is characterized by a chronic inflammatory state in which interplay between metabolic factors and cytokines leads to stimulation of the innate immune system when these signals are detected as danger. Therefore, signals from different sources including: modified lipid products, endogenous inducible factors, and cytokines are implicated in a complex inflammatory response that relies on tissue damage as the primary stimulating event leading to immune activation.
Oxidized LDL-C as a danger signal
Macrophage and scavenger receptor
The hallmark of atherosclerosis is the fatty streak lesion, which consists of macrophages filled with lipid droplets forming the so called foam-cells. While initial interest with ox-LDL stemmed from its ability to induce foam cells formation, it is now viewed as a potent activator of the inflammatory pathway and as a key factor promoting atherosclerosis by different mechanisms (Holvoet 2004). The discovery that LDL undergoes modification within tissue, forming oxidized products from minimally modified ox-LDL (mm-LDL) to fully oxidized LDL (ox-LDL), lead many investigations to discover the receptors and pathways by which the immune cells are activated (Yla-Herttuala et al 1989; Shaw 2004). Ox-LDL recruits monocytes and T cells to the intima and promotes the transformation of monocytes into macrophages (Weber et al 1995). Recent investigations have shown that ox-LDL attracts monocytes to the artery wall through the induction of the monocyte chemotactic protein 1 (MCP-1) by the endothelial cells (Navab et al 1996). Furthermore, ox-LDL increase the expression of CC-chemokine receptor 2 (CCR2), the receptor for MCP-1, in monocytes, explaining the fact that monocytes of hypercholesterolemic patients exhibit an increase chemotactic activity to MCP-1 (Han et al 1998). The discovery of the scavenger receptors on monocytes/macrophages, which recognize ox-LDL, has shed light on how the foam cells are formed (de Villiers and Smart 1999).
While different scavenger receptors for ox-LDL have been described such as scavenger receptor class A (SR-A), CD36, macrosialin (CLA-1, CD68), and others, their redundant roles in other cellular functions such as adhesion and elimination of apoptotic cells suggest that clearance of lipids play an important biological role in the elimination of modified lipids and lipoproteins (Greaves et al 1998). When the apolipoprotein E (ApoE−/−) mice (a genetic model of hypercholesterolemia and spontaneous formation of extensive atherosclerosis) is crossed with SR-A−/− animals, there is a reduction of atherosclerotic lesions, suggesting that scavenger receptors play a permissive role in the development of atherosclerosis (Suzuki et al 1997). Furthermore, deletion of CD36 in hypercholesterolemic mice has led to an impressive reduction of atherosclerosis (Febbraio et al 2000). Recently, it has been demonstrated that CD36 expression in macrophages is upregulated by ox-LDL products such as 4-hydroxy-2-nonenal (HNE), using the peroxisome proliferator-activated receptor-γ (PPAR-γ) and nuclear respiratory factor-2 (Nrf2) pathways (Nagy et al 1998; Chawla, Barak, et al 2001; Ishii et al 2004). Therefore, these studies have highlighted the fact that ox-LDL mediates the formation of foam cells through the binding to an array of scavenger receptors, which are regulated through different pathways. Whereas the expression of these scavenger receptors are well documented in macrophages and smooth muscle cells they are undetectable or expressed at very low level in the endothelium. In 1997, Sawamura et al (1997) described the presence of a lectin-like ox-LDL receptor-1 (LOX-1) in endothelial cells. LOX-1 binds to ox-LDL where it induces endothelial dysfunction and it is thought that this receptor plays a critical role in the development of atherosclerosis. LOX-1 expression is rapidly induced in endothelial cells by ox-LDL, angiotensin II (Ang II), tumor necrosis factor-α (TNF-α), and shear stress (Mehta et al 2006). In apoE−/− mice fed with a high-fat diet, increased expression of LOX-1 in transgenic animals is associated with augmented level of adhesion molecules and atheroma formation (Inoue et al 2005). Therefore, in addition to the formation of foam cells, it appears that ox-LDL through the LOX-1 receptor is an important molecule by which the endothelial cell is activated and become dysfunctional.
Peroxisome proliferators-activated receptor
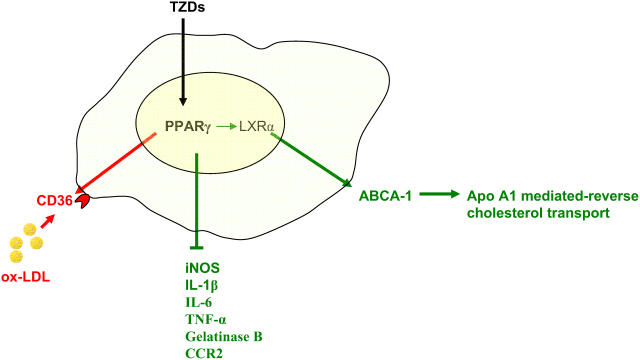
Peroxisome proliferator-activated receptors are lipid sensing ligand-activated transcription factors that are involved in glucose homeostasis, lipid metabolism, and inflammatory activity (Evans et al 2004). They are potent regulators that have an important implication in atherosclerosis development. PPAR-γ, which has been extensively studied, has an important role in glucose homeostasis and adipocyte function. Potent synthetic PPAR-γ agonists have been developed, including the thiazolidinediones (TZDs), which are in clinical use as insulin sensitizers (Willson et al 1996). Natural PPAR-γ agonists, although not completely elucidated, include several metabolites of the polyunsaturated fatty acids such as 13-hydroxyoctadecadienoic acid (HODE) and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2). Furthermore, ox-LDL-derived lipids such as 9-HODE and 13-HODE have been described as potent agonists of PPAR-γ, and to mediate by this pathway the uptake of ox-LDL and the formation of foam cells (Navab et al 1996). Indeed, PPAR-γ is an important regulator of the scavenger receptor, CD36, which is upregulated through this pathway (Tontonoz et al 1998; Matsumoto et al 2000). PPAR-γ expression has been documented in atherosclerotic lesions and in human monocytes/macrophages, suggesting that it mediates important function in the formation of foam cells (Ricote et al 1998). On the other hand, PPAR-γ is a potent antiinflammatory regulator, it has been found to negatively regulate inducible nitric oxide synthase, gelatinase B, interleukin-1β (IL-1β), IL-6, and TNF-α, revealing a potential pathway by which inflammation could be controlled (Jiang et al 1998). Furthermore, PPAR-γ agonists inhibit CCR2 expression by monocytes, which would decrease the recruitment of monocytes/macrophages to the artery wall (Han et al 2000). Although indirectly, PPAR-γ pleiotropic effects also include the regulation of cholesterol efflux in macrophages by controlling the expression of LXRα, a liver X nuclear receptor that control gene expression in response to oxysterols (Chinetti et al 2001). LXRα target genes include the adenosine triphopshate-binding cassette protein-1 (ABCA-1) which is involved in the control of apolipoprotein A1 (apo A1)-mediated cholesterol efflux, which returns to the liver as HDL-C (Chawla, Boisvert, et al 2001). Therefore, cholesterol efflux and antiinflammatory effects of the PPAR-γ pathway might counterbalance the upregulation of CD36, which would be detrimental if unopposed (Figure 1). The overall antiatherosclerotic effect of the PPAR-γ agonists is substantiated by animal studies that showed a reduction of lesions in mice treated with TZDs, and one animal study in which reconstitution of the bone marrow with PPAR-γ−/− cells increased significantly atherosclerosis in LDL-R−/− mice (Babaev et al 2005). However, in one study pharmacologic PPAR-γ agonist in LDL-R−/− mice failed to reduce lesions in females, whereas atherosclerotic lesions were significantly decreased in males, suggesting that hormonal status might influence the development of atherosclerosis in a manner that is not significantly influenced by the PPAR-γ pathway (Li et al 2000). In addition, a recent human randomized study, has demonstrated that pioglitazone reduced the composite endpoint of all-cause mortality, non-fatal myocardial infarction and stroke in type 2 diabetic patients, but it did not significantly impact a primary composite end-point which included in addition to the aforementioned points revascularisation procedures of coronary or leg arteries and leg amputation (Dormandy et al 2005). Therefore, animal and clinical studies suggest at this point that TZDs affect favourably the evolution of atherosclerotic diseases in function of hormonal status or the forms of atherosclerosis. However, it remains to be confirmed with other ongoing clinical studies.
Figure 1.
Peroxisome proliferators-activated receptor (PPAR-γ) is a member of nuclear receptor superfamily that regulates in macrophages numerous functions that have important impact on the development of atherosclerosis. It up-regulates the expression of the CD-36 scavenger receptor and has therefore a potential to increase atherosclerosis (in red). On the other hand, PPAR-γ reduces the expression of pro-inflammatory mediators and increases LXR expression, a nuclear receptor that control the level of adenosine triphosphate (ATP)-binding cassette protein-1 (ABCA-1), which mediates the apo-A1 mediated reverse cholesterol transport (green). Thus, the balance of PPAR-γ activity indicates that an antiatherosclerotic effect (green) might be superior to the pro-atherosclerotic effect (red), explaining the beneficial effect of the thiazolidinediones (TZDs).
Abbreviations: apo-A1, apolipoprotein A1; CCR2, CC-chemokine receptor 2; IL-1β, interleukin 1β; IL-6, interleukin 6; LXRα, liver X nuclear receptor; ox-LDL, oxidized low-density lipoprotein; TNF-α, tumor necrosis factor-α.
Besides PPAR-γ, PPAR-α, and PPAR-δ are nuclear receptors subtype that have recently received more attention and studies indicate that these transcriptional regulators are important metabolic regulators that might play important roles in the pathogenesis of atherosclerosis. PPAR-α is highly expressed in the liver, and to a lesser extent, in heart and the skeletal muscle. PPAR-α activation induces fatty acid oxidation and improves the lipid profile. PPAR-α activation results in a decrease of triglyceride through different mechanisms including increased lipoprotein lipolysis and inhibition of triglyceride synthesis (Puddu et al 2003). Moreover, PPAR-α activation has been shown to increase cholesterol efflux through the ABCA-1 pathway and an increased synthesis of apo A1 (Chinetti et al 2001). In smooth muscle cells, PPAR-α activation reduced inflammatory activity by interfering with the nuclear factor kappa B (NF-κB) (Staels et al 1998). The hypolipidemic fibrate class of drugs is a strong PPAR-α agonist that reduces the triglyceride level and increases the HDL-C level. Post-hoc analysis of clinical trials have suggested that fibrate therapy was more effective in patients with type 2 diabetes or the metabolic syndrome, giving the impetus for further randomized studies (Verges 2005). In respect to this hypothesis, the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was conducted in diabetic patients (Keech et al 2005). In this study, fenofibrate did not reduce the primary outcome of coronary events but it did reduce the nonfatal myocardial infarction and revascularizations. There was a nonsignificant excess rate of death in the fibrate-treated patients. Thus, in view of the mixed results of the FIELD study the ongoing Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial will attempt to provide more information of the combination therapy of statins and fibrate therapy (ACCORD 2006).
PPAR-δ, which is ubiquitously distributed, has recently received more attention with the development of specific agonists. It is involved in the control of lipid metabolism and energy homeostasis (Grimaldi 2005). Transgenic mice overexpressing PPAR-δ are protected against diet-induced obesity through an increased catabolism of fatty acid (Wang et al 2003). Furthermore, administration of PPAR-δ agonist to mice fed with a high-fat diet decreased insulin resistance through enhanced fatty acid oxidation and decreased skeletal muscle lipid content (Tanaka et al 2003). In macrophages from mice, both PPAR-δ−/− cells and transgenic cells overexpressing PPAR-δ have a proinflammatory profile (Lee et al 2003). On the other hand, treatment of cells from wild type mice with the PPAR-δ agonist, GW501516, inhibited the expression of MCP-1 and IL-1β (Wang et al 2003). It was then proposed that PPAR-δ regulates the expression of inflammatory cytokines through a switch, releasing a transcriptional repressor. When PPAR-δ is not activated by a ligand, it sequesters the transcriptional repressor, whereas when activated by a ligand it allows the liberation of the repressor leading to antiinflammatory response. Treatment of obese rhesus monkeys with the PPAR-δ agonist, GW501516, increased HDL-C considerably while it decreased fasting insulin level, triglyceride, and the proportion of small and dense LDL particles (Oliver et al 2001). Therefore, in view of the aforementioned points it has been hypothesized that PPAR-δ agonists could have a protective role on atherosclerosis development. In mice lacking the LDL receptor (LDLR−/−), one study has concluded that PPAR-δ agonist failed to reduced the formation of atherosclerosis whereas Graham et al (2005) demonstrated a dramatic reduction of atherosclerosis following administration of agonist (Li et al 2004). Thus, further studies with PPAR-δ agonists are needed in order to clarify the exact role of this pathway in the pathophysiology of atherosclerosis.
Toll-like receptors
TLR signaling has recently emerged as one of the most important pathways leading to innate immune system activation in atherosclerosis (de Kleijn and Pasterkamp 2003). First discovered in Drosophila, mammalian homologues of the Toll receptors were soon discovered, leading to extensive research to understand the function of these receptors. Among the mammalian there are at least eleven TLRs that have been described, from which TLR1–9 are conserved between human and mouse (Takeda and Akira 2005). TLRs have evolved to recognize highly conserved pathogen motifs as a first line of defense; however the discovery of endogenous ligands has expanded the scope of their function. Upon binding of their ligands, TLRs initiate a cellular response leading to activation of NF-κB, and regulation of immune responsive genes. Several reports have documented the expression of TLRs in atherosclerosis: TLR1, TLR2, TLR4, and to a lesser extent TLR5 have been documented in human plaques and mouse model of atherosclerosis (Xu et al 2001; Edfeldt et al 2002). TLR1 is ubiquitously expressed and is present on monocytes/macrophages, neutrophils, B-cells, and natural killer cells. TLR5 and TLR2 seems to be restricted to monocytes/macrophages, neutrophils and dendritic cells, whereas TLR4 is present on endothelial cells, adventitial fibroblast, and monocytes/macrophages (Hopkins and Sriskandan 2005). TLR4 is the well known bacterial lipopolysaccharide (LPS) receptor, which also binds to endogenous ligands that include mm-LDL and HSP60. HSPs are highly conserved proteins that are synthesized upon exposition to various stressors including ox-LDL; they promote inflammation and autoimmunity, and are viewed as important mediators of atherosclerosis (Xu 2002). Both HSP60 and HSP70 are considered as endogenous ligands of TLR2 and signaling through this receptor in mice has been shown to induce atherosclerotic lesion development associated with the production of IL-6, IL-8, and MCP-1 (Schoneveld et al 2005). Interestingly, the discovery of TLRs brings back the idea that infectious agents could contribute to atherosclerosis. At the end of 1980s, Saikku and colleagues reported a serologic evidence that Chlamydia pneumoniae and coronary atherosclerosis were linked (Saikku et al 1988). Thereafter, histopathologic studies have confirmed the presence of C. pneumoniae in atherosclerotic plaques (Kuo et al 1993). Furthermore, a number of infectious agents have now been regarded as potential candidates implicated in the pathogenesis of atherosclerosis (Yamashiroya et al 1988; Horvath et al 2000). Recently, an immunologic cross reaction between human and bacterial HSP60 has been documented, suggesting that chronic infections might contribute or exacerbate atherosclerosis (Bulut et al 2002). Chronic infections were associated with an increased risk for the development of atherosclerosis and injection of LPS in animals increased atherosclerotic lesions (Lehr et al 2001). Therefore, the total infectious burden, rather than specific microorganisms, is now considered as potential exacerbating or initiating events in atherosclerosis. Thus, metabolic factors such as ox-LDL are among the most important mediators of inflammation in atherosclerosis, whereas infections might have a contributory role that exacerbates the overall immune activation amplifying the danger signal to innate cells. In relation to their ubiquitous distribution within the innate immune system, TLRs represent an important pathway by which the danger signals are detected, and this will lead to the immune responses implicated in atherosclerosis.
Autoantibodies to neoepitopes are present in patients and in experimental models of atherosclerosis. Transformation of native LDL to ox-LDL creates autoantigens that are recognized by the B cells to generate autoantibodies. Antibodies to ox-LDL are found in atherosclerotic lesions and bind to circulating LDL. In ApoE−/− mouse, B cell clones derived from the spleen secrete immunoglobin M (IgM) autoantibodies to ox-LDL, indicating a T cell independent production of antibodies by B cells, which would rely on the innate signaling pathway (Palinski et al 1996). Indeed, some primordial B cells, such as B-1 cells are a part of the innate immune system expressing TLRs and are known to secrete antibodies against self antigens (Pasare and Medzhitov 2004; Carroll and Holers 2005). Immunization against ox-LDL and apoB-100, an important protein found in LDL particles, has been shown to decrease atherosclerotic lesions in mouse models (Fredrikson et al 2003, 2005). Thus, it is speculated that autoantibodies block the uptake of ox-LDL by macrophages and enhance plasma clearance of modified lipids. Therefore, although incompletely understood, autoantibodies generated by the innate system might have anti-atherogenic properties to overcome the increase burden of ox-LDL.
Metabolic syndrome and ox-LDL
In light of the numerous pro-inflammatory activities of ox-LDL, clinical studies have highlighted its role as a pro-atherogenic particle (Carmena et al 2004). Indeed, ox-LDL plasma level is increased in patients with CHD, and several components of the metabolic syndrome independently predicted high levels of ox-LDL (Holvoet 2004). It is often observed that patients with the metabolic syndrome have a LDL-C within normal range (Despres 2001). In fact, there is a poor correlation between visceral obesity and plasma LDL-C, suggesting that other factors might influence LDL phenotype toward a more atherogenic particle. Elevated triglycerides (TGs) and increased release of free fatty acids (FFAs) in patients with the metabolic syndrome lead to assembly and secretion of very LDL (VLDL). Through exchange of cholesteryl esters and TGs between VLDL and LDL, production of TGs-enriched LDL particles occurs. TGs-enriched LDL particles then undergo lipolysis and become smaller and denser (Nicholls and Lundman 2004). Therefore, patients with the metabolic syndrome have a higher proportion of small and dense LDL particles, which are highly atherogenic. Small LDL particles penetrate the arterial wall with more facility than their buoyant counterpart, and are more susceptible to oxidative transformation, explaining at least in part their pro-atherogenic activities (Tribble et al 1992; Bjornheden et al 1996). In the Québec Cardiovascular Study, small LDL size independently predicted the rate of CHD. Furthermore, when associated with hyperinsulinemia and elevated apo B concentration, small LDL size was found to increase CHD risk remarkably (Lamarche et al 1999). Accordingly, although LDL-C level is an accepted predictor of CHD, other factors that influence LDL phenotype such as particle size and oxidative transformation, have a profound effect on their atherogenicity.
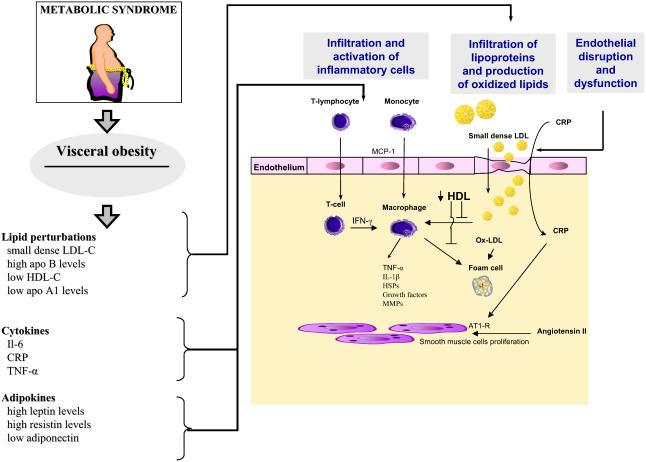
While metabolic syndrome is associated with LDL particle modifications, it also affects HDL-C level and phenotype. HDLs are a group of heterogeneous particles with anti-atherosclerotic properties. Indeed, the association between low levels of HDL-C and CHD is well established trough clinical and epidemiological studies (Gordon and Rifkind 1989). The protective role of HDL in atherosclerosis has been attributed to its reverse cholesterol effect, in which cholesterol from the peripheral tissues is returned to the liver for excretion in the bile (von Eckardstein et al 2005). Regulation of cholesterol efflux from macrophages through HDL-mediated transport relies on a broad spectrum of pathways including: the scavenger receptor type BI (SR-BI) and ABCA-1. The predominant HDL protein, apo A1, interacts with ABCA-1 to mediate cholesterol efflux from macrophages, while expression of SR-BI on hepatocytes mediates selective uptake of cholesteryl esters (Assman and Gotto 2004). While most investigators considered that the beneficial effect of HDL was mostly related to the reverse cholesterol transport, several studies have recently highlighted its antioxidant and antiinflammatory activities. It has been demonstrated that HDL protects LDL from oxidative transformation through the paraoxonase-mediated breakdown of oxidized phospholipids, which have a proinflammatory activity. Indeed, mice fed with cholesterol rich diet and deficient for paraoxonase developed more atherosclerotic lesions, reinforcing the view that HDL mediated antioxidative actions play a key role in the genesis of atherosclerosis (Getz et al 2004). Furthermore, recent studies have stressed the role of apo A1 as an antiinflammatory component of HDL which was independent of HDL-C level; atherosclerosis susceptible mice deficient for apo A1 exhibited more atherosclerotic lesions and had an increase production of MCP-1, suggesting that in addition to its ABCA-1-mediated reverse cholesterol transport effect, apo A1 has an important antiinflammatory activity (Moore et al 2005). Moreover, HDLs decrease TNF-α and IL-1β release of monocytes when activated by T cells (Hyka et al 2001; Calabresi et al 2003). Atherosclerotic calcification are commonly observed in patients and, it has been demonstrated in vitro that TNF-α is one of the mediators with oxidized lipids that strongly influence the phenotypic switch of vascular cells toward bone forming cells. Interestingly, calcification process and transformation of vascular cells to osteoblast-like cells is prevented by HDL, reinforcing the view that HDL has a broader scope of action which goes beyond the reverse cholesterol transport (Figure 2) (Parhami et al 2002).
Figure 2.
The metabolic syndrome is characterized by visceral obesity and its attendant metabolic perturbations that have numerous pro-atherosclerotic effects on the arterial wall. Production of small dense LDL particles and decrease HDL levels increase vascular infiltration by lipids and the production of oxidized LDL (ox-LDL). Ox-LDL delivers a danger signal to the macrophages and the production of foam cells producing cytokines and growth factors that will promote the development of atherosclerosis. In addition, elevated blood levels of cytokines and of adipokines contribute to increase the inflammatory reaction.
Abbreviations: apo A1, apolipoprotein A1; apo B, apolipoprotein B; AT1-R, angiotensin 1 receptor; CRP, C-reactive protein; HDL-C, high-density lipoproteincholesterol; HSPs, heat shock proteins; IL-1β, interleukin 1β; IL-6, interleukin 6; INF-γ, interferon-γ LDL-C, low-density lipoprotein-cholesterol; MCP-1, monocyte chemotactic protein 1; MMPs, matrix metalloproteinases; TNF-α, tumor necrosis factor-α.
While the HDL beneficial effects are well documented, recent evidence supports that HDL might also behave as pro-inflammatory particles (Navab et al 2005). During an acute phase response, HDL became pro-inflammatory enhancing LDL-induced monocyte migration, indicating that some modifications of HDL occurred and modified its activity. Modification of HDL phenotype includes a decreased level of apo A1, whereas proteins such as apo E, apo A1V, apo A-V are increased (Khovidhunkit et al 2004). Furthermore, oxidative changes of apo A1 might explain the reduced ability of modified apo A1 to promote reverse cholesterol transport and their accumulation within atherosclerotic lesions (Mackness et al 1997; Zheng et al 2004). Patients with CHD have an increased amount of lipid hydroperoxides content in HDLs (Ansell et al 2003). Furthermore, HDLs in the metabolic syndrome have an altered composition with a core enrichment of TG and a depletion of cholesteryl ester and are associated with a decrease in antioxidative properties and an increase systemic oxidative stress (Hansel et al 2004). Therefore, phenotype of HDLs may vary in accordance with the systemic inflammatory activity and are susceptible to oxidativemediated changes toward pro-inflammatory particles, which might deliver or amplify the danger signal to the immune system. On the other hand, ‘healthy’ HDL, which is dependent on its chemical constituent, is a powerful antiinflammatory particle that has anti-atherosclerotic properties. Therefore, future therapeutic development aim to modulate HDL levels should also target the HDL phenotype to ensure a protective action.
Cytokines, adipokines and acute phase proteins as danger signals
C-reactive protein
Clinical and epidemiological studies have stressed the role of C-reactive protein (CRP) as a marker of CHD (Ridker 2003). Obesity and particularly the visceral adipose depot correlate with CRP level, suggesting that metabolic activity of abdominal adipocytes with their production of IL-6 influence CRP production by the liver (Yudkin et al 1999; Lemieux et al 2001). Whereas CRP is clearly associated with clinical manifestations of atherosclerosis, a question remains as to whether CRP is a bystander or an active player. Recent works suggest the second hypothesis, CRP can induce the release of cytokines, chemokines and tissue factor from monocytes, and therefore could explain the link between CRP level and atherothrombotic events (Du Clos and Mold 2004). Furthermore, enzymatically remodeled LDL (E-LDL), which is present in atherosclerotic lesion, has the capacity to trigger complement activation through a CRP dependent pathway (Bhakdi et al 2004). Whereas at low E-LDL levels, CRP initiates complement activation but halts before the terminal sequence, high concentrations of E-LDL drives complement activation through completion in a CRP-independent manner. Indeed, it has been demonstrated that E-LDL but not CRP colocalizes with C5b-9 in atherosclerotic lesions (Torzewski et al 1998). It has been suggested that CRP might have a physiological role by eliminating self generated E-LDL in attracting innate immune cells and preventing or limiting inflammatory reaction. Proteases such as cathepsin H and plasmin, which are present in atherosclerotic lesions, generate E-LDL and expose phosphocholines groups, which are then available for binding to CRP and triggers complement activation. When the removal machinery for E-LDL is overwhelmed, deesterified cholesterol, which is present in large amount in atherosclerotic plaques, and E-LDL by the action of cholesteryl esterase activate the complement through terminal sequence. Recently, van Tits et al (2005) have demonstrated that CRP binds to the phosphatidylcholine group of ox-LDL and, by a complement-independent pathway, enhances binding to monocytes/macrophages through Fc receptor, suggesting that CRP might contributes to rapid clearance of ox-LDL at moderate concentrations, whereas at high levels this mechanism might promote foam cell formation.
While CRP seems to be involved in LDL homeostasis, recent findings suggest that it modulates vascular function and, therefore, represents a potential pathway by which it might influence inflammation and atherogenesis. In cultured endothelial cells, CRP induces the expression of vascular cell adhesion molecules (VCAM-1), intercellular adhesion molecules (ICAM-1), and IL-6, whereas in smooth muscle cells it upregulates angiotensin type 1 receptor (AT1-R) (Pasceri et al 2000; Nickenig and Harrison 2002). The stimulation of receptor AT1-R by ANG II induces cell proliferation and matrix synthesis, which is responsible for vascular remodeling. Furthermore, in endothelial cells, CRP increases the production of plasminogen activator inhibitor-1 (PAI-1), a potent inhibitor of fibrinolysis and a pro-thrombotic protein (Devaraj et al 2003). Besides endothelial cells, adipocytes also produce PAI-1, and elevated concentrations are observed in obese subjects and among individuals with the metabolic syndrome and this might account for the high rate of thrombotic events in these populations (Lau et al 2005). Therefore, recent studies suggest that CRP is not simply a clinical marker but a protein that has a major role in the physiology and pathophysiology of LDL-C homeostasis and vascular endothelial function.
Cytokines
Inflammation is associated with the production of numerous cytokines that influence the development of atherosclerosis. Interferon-γ (IFN-γ), which is produced by activated T cells, plays an important role in the development of atherosclerosis. Indeed, recent studies have demonstrated that the development of atherosclerotic lesions is decreased in apoE−/− mouse lacking IFN-γ, whereas administration of IFN-γ enhanced vascular lesions (Whitman et al 2000). IFN-γ activates macrophages to produce TNF-α and IL-1β, which act synergistically to instigate the production of metalloproteinases, oxygen radicals, and growth factors (Hansson et al 1989). Furthermore, IFN-γ has been shown to negatively influence the reverse cholesterol transport by inhibiting the ABCA-1 protein (Panousis and Zuckerman 2000). Thus, IFN-γ-mediated activities on the macrophages tend to increase inflammatory activity and to promote atherosclerosis. Whereas production of IFN-γ has been classically restricted to T cells, recent investigations have indicated that macrophages, dendritic cells, and vascular smooth muscle cells had the ability to produce this cytokine (Tenger et al 2005). Furthermore, γδ T cells and natural killer (NK) T cells, which are part of both the innate and adaptive immune systems, are activated by modified lipids (Vanderlaan and Reardon 2005). Indeed, NK T cells, which are activated when an antigen is presented on Cd1 molecules by either macrophages or dendritic cells, have been shown to produce IFN-γ when activated by glycosphingolipids or gangliosides, which are present in the atherosclerotic plaque (Garner et al 2002). Thus, it has been suggested that NK T cells may be activated by modified lipid neoantigen either present or induced by ox-LDL. Therefore, IFN-γ along with TNF-α and IL-1β are part of a dual system in which those cytokines can act as an immunostimulant signal when produced by activated T cells or as a primal alarm signal when produced by innate immune cells and cells from the vascular wall.
Emerging data have highlighted the fact that besides their interactions with the vascular cells and the immune cells, cytokines are also implicated through complex interactions with the metabolism. TNF-α is a true pleiotropic factor that is produced by the activated macrophages, NK cells, vascular smooth muscle cells, fibroblasts and adipocytes (Goetz et al 2004). Recent studies in rodents have linked TNF-α systemic levels with obesity and insulin resistance. In humans TNF-α plasma concentrations are very low and were only found to correlate weakly with obesity and insulin resistance (Staiger and Haring 2005). Furthermore, administration of neutralizing antibodies against TNF-α in humans did not improve insulin resistance, suggesting that adipocyte-derived circulating TNF-α does not account for a significant role in glucose metabolism in vivo (Ofei et al 1996). Nevertheless, it is possible that local production of TNF-αby a paracrine or an autocrine effect has a significant impact on adipocyte metabolism and on the induction of insulin resistance at the tissue level (Xu et al 2002). Along with TNF-α, IL-6 is another cytokine with pleiotropic properties that has a profound metabolic effect. Circulating IL-6 levels are increased in patients with atherosclerosis, obesity, type 2 diabetes, and metabolic syndrome (Yudkin et al 2000). This cytokine is produced and released by numerous cells including among others: monocytes/macrophages, T cells, endothelial cells, and adipocytes (Van Snick 1990). Interestingly, it has been recently demonstrated that omental fat cells produce 3 times more IL-6 than their subcutaneous counterparts (Fried et al 1998). IL-6 production by omental adipocytes drains into the portal vein up to the liver, where this cytokine has been demonstrated to induce the production of acute phase proteins and to affect glucose metabolism. Indeed, it was recently demonstrated that IL-6 impaired insulin signaling in hepatocytes (Senn et al 2002). Moreover, in adipocytes, IL-6 was reported to enhance lipolysis, which would in turn increase the FFAs' delivery to the liver with the consequence of an increased hepatic TG synthesis (Lyngso et al 2002). In summary, cytokines are involved in many steps of immune activation and, in addition to their role as a danger signal to immune cells, they are also committed as powerful regulators of the glucose and lipid metabolism.
Adipokines
In the past while viewed as a passive lipid storage depot, adipose tissue is now considered as an endocrine and a paracrine organ that produces a large number of mediators that affect metabolism, inflammation and coagulation (Figure 3). Leptin, which was discovered in 1994, is an adipocyte specific hormone that play a central role in the regulation of the body weight in exerting an anorexigenic function (Zhang et al 1994). Leptin is predominantly produced by subcutaneous adipocytes and, increased circulating levels have been documented in obese subjects (Hube et al 1996). While leptin decreases food intake through a central action, it also increases peripheral tissue fatty acid oxidation. It is thus believed that leptin-mediated actions are physiologically oriented in a response to decrease lipotoxicity in lean non-adipose tissue (Unger 2005). It has been suggested that some of the manifestations of the metabolic syndrome such as ectopic lipid deposition in skeletal muscle, liver, and heart are related to an impaired antilipotoxic protection of leptin. In such a model, the hyperleptinemia observed in obese would be low relative to the caloric excess and may contribute to lipotoxicity. Furthermore, impaired leptin sensitivity related to a leptin resistance factor, suppressor of cytokine signaling 3 (SOCS-3), has been described in aged rodents and might explain the age-related lipid metabolism abnormalities (Wang et al 2001).
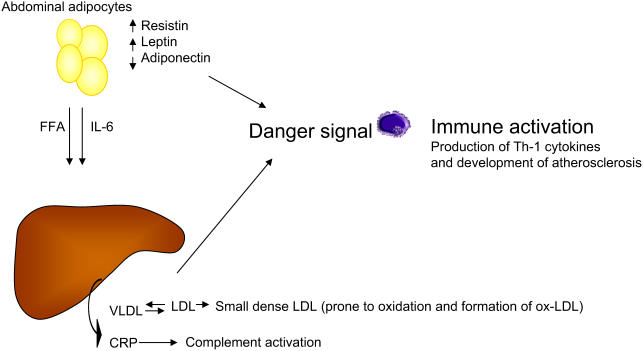
Figure 3.
Visceral obesity is a key component of the metabolic syndrome. Free fatty acids (FFA) and interleukin 6 (IL-6) produced by abdominal fat cells are drained up to the liver via the portal vein and contribute to the production of very low-density lipoprotein (VLDL) and C-reactive protein (CRP). VLDL through exchange of cholesteryl esters and triglycerides (TGs) contribute to the generation of highly atherogenic small and dense low-density lipoprotein (LDL) particles that are easily transformed to oxidized products that deliver a danger signal. In addition, abdominal fat cells produce resistin and leptins that deliver a danger signal, whereas lower levels of adiponectin contribute to amplify the pro-atherogenic signal through the loss of its protective effects.
Abbreviations: ox, oxidized; Th-1, T helper 1.
In addition to its metabolic activities, leptin has been shown to play an important role in the development of atherosclerosis. Indeed, leptin deficient mice (ob/ob) who exhibit an early onset morbid obesity, are markedly resistant against diet-induced atherosclerosis, whereas exogenous administration of leptin promotes arterial neointimal proliferation (Schafer et al 2004). It is now well established that leptin deficiency is associated in mice and humans with an immunodeficiency state characterized by a low T cell count, thymus atrophy, and an impairment of delayed-type hypersensitivity (DTH) (Chandra 1980). Recently, new insights into the immunologic actions of leptin have emerged from studies demonstrating that leptin receptors were present in immune cells such as monocytes/macrophages, T cells, and NK cells (Peelman et al 2004). It has been demonstrated that leptin induced the expression of IFN-γ, IL-6, and TNF-α in monocytes/macrophages, whereas it induced in T cells a switch toward Th-1 cytokines (IL-2, IFN-γ, TNF-α, IL-18), indicating that leptin is clearly an important immune regulator (Lord et al 1998; Zarkesh-Esfahani et al 2001).
Resistin is a recently identified adipocyte-derived protein which is involved in glucose metabolism and inflammation. In mice, resistin expression is increased in diet–induced obesity as well in genetic models of obesity (Steppan et al 2001). The association between obesity, resistin and insulin resistance is not a consistent finding in the literature (Way et al 2001). However, a recent investigation in mice has shown that animals treated with an adenovirus expressing resistin had an hyperinsulinemia associated with lipid abnormalities including high total cholesterol, hypertriglyceridemia, and low HDL-C (Sato et al 2005). Resistin blood levels are elevated in patients with atherosclerosis and are associated with coronary artery calcification, which is a strong predictor of cardiovascular events (Reilly et al 2005). Whereas resistin is exclusively produced by adipocytes in mice, it is secreted predominantly by macrophages in humans, suggesting that it might be involved in the regulation of immune cells function. It was recently demonstrated that resistin induced an inflammatory activation with the production of TNF-α and IL-12 by macrophages through a NF-κB pathway (Silswal et al 2005). Furthermore, when stimulated by resistin, endothelial cells expressed VCAM, indicating that resistin is involved in leukocytes recruitment and cell activation (Verma et al 2003). Interestingly, resistin expression is repressed by PPAR-γ activation through a direct binding of the transcription factor to the resistin promoter (Patel et al 2003).
Adiponectin is a recently discovered peptide with a broad range of actions including regulation of plasma glucose and lipid levels, endothelial dysfunction and inflammatory pathways, all of which are strongly implicated in atherosclerosis development (Chandran et al 2003). Adiponectin blood levels are inversely correlated with visceral obesity, insulin resistance, and hyperlipidemia (Cote et al 2005). It has been demonstrated that adiponectin production by adipocytes is negatively influenced by TNF-α and glucocorticoid whereas it is upregulated by insulin (Kobayashi 2005). Furthermore, in a recent study, low plasma adiponectin levels were associated with an increased risk of myocardial infarction in men, suggesting that, as opposed to other adipokines, adiponectin has a protective effect against the development of atherosclerosis and its manifestations (Pischon et al 2004). In vitro studies have corroborated the anti-atherosclerotic effect of adiponectin, showing that it prevented the formation of foam cells by inhibiting the expression of class A scavenger receptor in human macrophages (Ouchi et al 2001). In addition, animal studies have highlighted the role of adiponectin as a vascular protector. Adiponectin-deficient mice are resistant to insulin action and develop important neointimal proliferation in response to vascular injury (Kubota et al 2002). Whereas adiponectin has major effect on the metabolism, it also influences the inflammatory response. Indeed, recent reports have stressed the role of adiponectin in modulating immune cytokines production toward a Th2 profile type of response, which has a protective effect against the development of atherosclerosis (Wolf et al 2004). In isolated macrophages, adiponectin decreased IFN-γ production whereas it increased IL-10 and tissue inhibitor of metalloproteinase-1 (TIMP-1) (Kumada et al 2004). Moreover, in endothelial cells, adiponectin decreases VCAM-1 expression and NF-κB, a major transcription factor regulating the expression of cytokines (Ouchi et al 2000). Therefore, adiponectin is a fat produced multifaceted protein with important regulatory functions reducing inflammation and protecting blood vessels against injury and the development of atherosclerosis.
In recent years our growing understanding of adipocyte biology has revealed that fat cells are actively involved in the regulation of the inflammatory pathway. Whereas the production of adipokines in response to metabolic stimuli has a physiologic role to prevent tissue injury, it seems that western hypercaloric diet has the ability to overwhelm this system and eventually lead to immune activation with the development of pathologies such as atherosclerosis. Keeping in mind the danger model of immune response, adipocyte can now be considered as an active player which may deliver an alarm signal to the immune cells. Whereas an alarm signal can be delivered by adipokines such as resistin and leptin, other proteins such as adiponectin seems to have a protective effect, and its absence may trigger an activating signal.
Therapeutic implications
From the above discussion it appears that metabolic syndrome-associated perturbations of the lipid metabolism affect many pathways by which inflammation is activated with its attendant negative impact on atherosclerotic disease. Thus, detection of patients with metabolic syndrome is crucial in order to initiate appropriate therapeutic interventions. The use of simple criteria proposed in the World Health Organisation (WHO) and National Cholesterol Education Program–Adult Treatment Panel III (NCEP–ATP III) guidelines have indicated that prevalence of metabolic perturbations is extremely high in westernized societies (Table 1). According to NCEP–ATP III criteria (abdominal obesity, low HDL, hypertriglyceridemia, hypertension and dysglycemia), up to 22% of the US population older than 20 years is affected by this syndrome (Roberts and Barnard 2005). Although a genetic predisposition might be involved in the etiology of the metabolic syndrome, acquired risk factors are readily modifiable by appropriate therapy and are, of course, the mainstay of clinical management (Table 2). Weight loss, even modest, has been shown to reduce insulin resistance and also to decrease circulating levels of CRP and IL-6 (Knowler et al 2002). Thus, dietary interventions reducing body weight by negative energy balance are effective means by which metabolic benefits are encountered. Although many diets have been proposed in order to treat obesity, it is suggested that obese patients consume a diet with an energy deficit of 500–1000 kcal/d (Klein et al 2004). The macronutrient composition of a diet has been studied in several randomized studies. Whereas, weight loss at 6 months is increased in low-carbohydrate diet, there is no difference at one year with low-fat diet (Foster et al 2003; Stern et al 2004). The low-carbohydrate diet is more effective at reducing triglyceride and increasing HDL-C, whereas low-fat diet is more beneficial at reducing LDL-C. However, whether one strategy is superior to another in reducing cardiovascular disease (CVD) risk is currently unknown (Bonow and Eckel 2003). In addition to diet interventions, exercise program combined with diet results in a greater weight loss and a sustained effect preventing weight regain (Jakicic et al 2001; Saris et al 2003). Moreover, in individuals who exercised for 2.5 h/wk, the levels of antiinflammatory cytokines such as IL-4 and IL-10 are increased significantly (Das 2004). In one study involving individuals with metabolic syndrome enrolled in a supervised exercise program for 20 weeks, it was found that 30% were no longer classified as having the syndrome after the completion of the exercise training program (Katzmarzyk et al 2003). In addition, endothelial function is improved by exercise training, independently of weight reduction, or changes in blood pressure (Lavrencic et al 2000). Although incompletely elucidated at the molecular level, it is speculated that modification of the insulin resistance associated with the reduction of the abdominal adipose depot tissue plays a major role in the improved lipid profile with its attendant benefit in reducing inflammation.
Table 2.
Therapeutic interventions for patients with the metabolic syndrome that have a regulatory role on the inflammation
| Therapeutics Interventions | |
|---|---|
| Lifestyle modifications | |
| Dietary interventions | A low fat diet is considered the standard approach to obesity. An energy deficit of 500–1000 kcal/d is usually suggested and a target reduction is 10% weight for the first year. |
| Physical activity | Exercise and particularly when combined with diet intervention help to maintain a healthy weight. These lifestyle modifications increase insulin sensitivity and and ameliorate lipid profile while decreasing the inflammatory state as measured with the CRP level. |
| Pharmacological interventions | |
| Lipid-lowering drugs | Statins are the mainstay of drug-lowering therapy. Their pleiotropic activity might have additional therapeutic benefits in reducing inflammatory activity. |
| PPAR-α agonists | Fibrate class of drugs are effective at reducing triglyceride level and increasing HDL-C level. Furthermore, fibrates have shown some antiinflammatory activity. Their clinical benefit might be in patients with metabolic syndrome/diabetes, however, their efficacy at reducing mortality remains to be established. |
| PPAR-γ agonists | TZDs are insulin sensitizers drugs that have a powerful antiinflammatory activity. Their ability to prevent cardiovascular events or mortality is not clearly established for the moment. |
| CB1 antagonists | Rimonabant, a CB1 antagonist, has been shown induce a weight reduction with a concomitant HDL-C and adiponectin rise. This is a new and promising avenue for the treatment of the metabolic syndrome. It remains to be seen if such approach will have a beneficial effect on the prevention of cardiovascular events. |
Abbreviations: CB1, cannabinoid-1 receptor; CRP, C-reactive protein; HDL-C, high density lipoprotein-cholesterol; PPAR, peroxisome proliferator-activated receptor; TZDs, thiazolidinediones.
The evolving role of inflammatory mediators in atherosclerosis, and particularly in patients with the metabolic syndrome, prompted the development of new class of drugs and also the rediscovery of new mechanisms by which older drugs such as statins interfere with atherosclerosis progression. Extensive clinical trial data have confirmed the beneficial effects of statin therapy in reducing cardiovascular events (Rosenson 2005). In patients with the metabolic syndrome, rosuvastatin treatment over 12 weeks decreased LDL-C by 48% whereas HDL-C was increased by 10%, indicating that statins and particularly rosuvastatin is an effective lipid-modifying agent in this high risk population (Stalenhoef et al 2005). Although, statins are notably known to reduce LDL-C levels, recent studies have emphasized their pleiotropic role including an antiinflammatory activity. Among the antiinflammatory activities, statins were shown to reduce the production of IFN-γ by activated T cells (Kwak et al 2000). Two recent clinical studies have demonstrated that statins reduced the cardiovascular events independently of the reduction in serum cholesterol levels (Nissen et al 2005; Ridker et al 2005). Indeed, in these studies, the observed reduction of CRP was only weakly correlated with the changes in serum lipids. The question of whether or not statins reduce cardiovascular events via an LDL-lowering-independent mechanism remains controversial, however, and has been recently questioned (Robinson et al 2005). Despite statin use, CVD remains a major public health problem in westernized societies. Thus, new therapeutic interventions are now developed to counterbalance the negative effect of inflammation in atherosclerosis. The PPAR-γ agonists, TZDs, have been developed as antidiabetic insulin-sensitizing agents, but it is now clear that this class of agents also has antiinflammatory activities. For example, TZDs downregulate the expression of TNF-α and upregulate adiponectin expression in obese and diabetics subjects (Yu et al 2002). Therefore, the TZDs are actually under investigation and it will be of great interest to see whether or not this new class of antidiabetic agent will reduce atherosclerotic-related events. Results of the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROACTIVE) trial suggest that TZDs may indeed confer CVD protection in diabetic patients (Dormandy et al 2005). Other trials are currently underway to clarify this issue.
Whereas currently used lipid-modifying agents have been of great help in managing one of the major risk factor namely LDL-C, it is considered that the major pathways by which metabolic syndrome influences atherosclerosis are not directly targeted by these pharmacological interventions. Over the recent years, the development of a new class of drug, the cannabinoid-1 (CB1) receptor antagonist, rimonabant, has generated evidence which suggest that this new pharmacological approach may be relevant for the management of key causal factors observed in the metabolic syndrome. The CB1 receptors are located in different areas of the brain and in a variety of peripheral tissues including the adipose tissue, the immune system and the heart (Howlett 2002). CB1 receptors are also located in the liver and play a major role in regulating hepatic lipid metabolism (Osei-Hyiaman et al 2005). The endocannabinoid system is overactivated in animal models of obesity and rimonabant has been shown to induce weight loss and to correct metabolic perturbations in experimental studies (Cota et al 2003). Recently, two clinical investigations have highlighted the beneficial effects of rimonabant in obese patients (Despres et al 2005; Van Gaal et al 2005). In the Rimonabant in Obesity Europe (RIO-Europe) study, a one year followup demonstrated that rimonabant induced a significant weight reduction compared with placebo, with 50.9% of the patients achieving a weight reduction greater or equal to 5% in the rimonabant group treated with 20mg per day. Plasma LDL-C levels were not affected by rimonabant but, of most interest, HDL-C levels were increased by 22.3% whereas TG levels fell by 6.8% in the treated group. Furthermore, the proportion of patients that fulfilled the NCEP–ATP III criteria for the metabolic syndrome was reduced by 64.8% in individuals who completed the treatment. Després and colleagues (2005) have recently demonstrated that rimonabant induced a significant rise of adiponectin blood levels that could not be explained only by the sole weight reduction. In addition, a positive correlation was found between the changes in adiponectin levels produced by rimonabant and changes in HDL-C concentrations, thus, suggesting that the loss of abdominal adiposity and a specific effect of rimonabant on adiponectin levels are among the important factors explaining the metabolic effects of CB-1 receptor blocker. Therefore, rimonabant appears to be a promising drug in the treatment of patients with abdominal obesity and related conditions, and it remains to be determined whether or not this pharmacological strategy will be the long-sought silver bullet to fight the plague of atherosclerotic disease.
Conclusion
The burden of atherosclerotic disease is a major public health problem in developed countries that is closely associated with the modern lifestyle, namely the hypercaloric diet and physical inactivity. Obesity, particularly visceral adiposity, is associated with the clustering of metabolic perturbations, which is termed the metabolic syndrome, and is associated with a substantially increased CHD risk. Perturbations of the metabolic syndrome including the LDL-C phenotype, low HDL-C, insulin resistance, and adipokines production are all potential pathways by which the immune system is activated. The discovery in the recent years, of the presence and function of Toll-like receptors, has reinforced the view that many danger signals can be detected and are involved in the genesis of atherosclerotic disease. The interactions between the adipose tissue, the vascular wall, and the immune system are complex, and these are the key players from which we have to understand the mechanisms implicated in the development of atherosclerosis. In light of recent developments, interventions to decrease inflammation associated with metabolic perturbations are actually developed and hopefully might in a near future be of a great benefit to decrease the ‘danger’ associated with our modern lifestyle.
Acknowledgments
Our research activities are supported by the Québec Heart Institute Foundation and the Canadian Institute of Health Research (CIHR), Ottawa, Canada, grant number MOP 79342.
Dr. Pibarot holds the Canada Research Chair in Valvular Heart Diseases, Canadian Institutes of Health Research, Ottawa, Ontario, Canada. Dr Després is the scientific director of the International Chair on Cardiometabolic Risk at University Laval, which is supported by two unrestricted grant from Sanofi-Aventis. Dr Mathieu is a research scholar from the Fonds de Recherche en Santé du Québec, Montreal, Canada.
References
- ACCORD trial. Accord trial [online] 2006 Accessed 22 January 2006. URL: http://www.accordtrial.org/
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [AHA] American Heart Association. Heart disease and stroke statistics – 2006 update [online] 2006 doi: 10.1161/CIRCULATIONAHA.105.171600. Accessed 12 June 2006. URL: http://www.americanheart.org/presenter.jhtml. [DOI] [PubMed]
- Ansell BJ, Navab M, Hama S, et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–6. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- Assmann G, Gotto AM., Jr HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- Babaev VR, Yancey PG, Ryzhov SV, et al. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1647–53. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Torzewski M, Paprotka K, et al. Possible protective role for C-reactive protein in atherogenesis: complement activation by modified lipoproteins halts before detrimental terminal sequence. Circulation. 2004;109:1870–6. doi: 10.1161/01.CIR.0000124228.08972.26. [DOI] [PubMed] [Google Scholar]
- Bjornheden T, Babyi A, Bondjers G, et al. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system. Atherosclerosis. 1996;123:43–56. doi: 10.1016/0021-9150(95)05770-6. [DOI] [PubMed] [Google Scholar]
- Bonow RO, Eckel RH. Diet, obesity, and cardiovascular risk. N Engl J Med. 2003;348:2057–8. doi: 10.1056/NEJMp030053. [DOI] [PubMed] [Google Scholar]
- Bulut Y, Faure E, Thomas L, et al. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol. 2002;168:1435–40. doi: 10.4049/jimmunol.168.3.1435. [DOI] [PubMed] [Google Scholar]
- Calabresi L, Rossoni G, Gomaraschi M, et al. High-density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-alpha content and enhancing prostaglandin release. Circ Res. 2003;92:330–7. doi: 10.1161/01.res.0000054201.60308.1a. [DOI] [PubMed] [Google Scholar]
- Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation. 2004;109:III2–I7. doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
- Carroll MC, Holers VM. Innate autoimmunity. Adv Immunol. 2005;86:137–57. doi: 10.1016/S0065-2776(04)86004-8. [DOI] [PubMed] [Google Scholar]
- Chandra RK. Cell-mediated immunity in genetically obese C57BL/6J ob/ob) mice. Am J Clin Nutr. 1980;33:13–16. doi: 10.1093/ajcn/33.1.13. [DOI] [PubMed] [Google Scholar]
- Chandran M, Phillips SA, Ciaraldi T, et al. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- Chawla A, Barak Y, Nagy L, et al. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- Chawla A, Boisvert WA, Lee CH, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–71. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Lestavel S, Bocher V, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–8. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Lutz B, et al. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord. 2003;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- Cote M, Mauriege P, Bergeron J, et al. Adiponectinemia in visceral obesity: impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J Clin Endocrinol Metab. 2005;90:1434–9. doi: 10.1210/jc.2004-1711. [DOI] [PubMed] [Google Scholar]
- Das UN. Anti-inflammatory nature of exercise. Nutrition. 2004;20:323–6. doi: 10.1016/j.nut.2003.11.017. [DOI] [PubMed] [Google Scholar]
- deVilliers WJ, Smart EJ. Macrophage scavenger receptors and foam cell formation. J Leukoc Biol. 1999;66:740–6. doi: 10.1002/jlb.66.5.740. [DOI] [PubMed] [Google Scholar]
- deKleijn D, Pasterkamp G. Toll-like receptors in cardiovascular diseases. Cardiovasc Res. 2003;60:58–67. doi: 10.1016/s0008-6363(03)00348-1. [DOI] [PubMed] [Google Scholar]
- Despres JP. Health consequences of visceral obesity. Ann Med. 2001;33:534–41. doi: 10.3109/07853890108995963. [DOI] [PubMed] [Google Scholar]
- Despres JP. Inflammation and cardiovascular disease: is abdominal obesity the missing link? Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S22–4. doi: 10.1038/sj.ijo.0802495. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I, Dagenais GR, et al. HDL-cholesterol as a marker of coronary heart disease risk: the Québec cardiovascular study. Atherosclerosis. 2000;153:263–72. doi: 10.1016/s0021-9150(00)00603-1. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30:261–77. doi: 10.1385/IR:30:3:261. [DOI] [PubMed] [Google Scholar]
- Edfeldt K, Swedenborg J, Hansson GK, et al. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–61. [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–61. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–56. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- Fredrikson GN, Andersson L, Soderberg I, et al. Atheroprotective immunization with MDA-modified apo B-100 peptide sequences is associated with activation of Th2 specific antibody expression. Autoimmunity. 2005;38:171–9. doi: 10.1080/08916930500050525. [DOI] [PubMed] [Google Scholar]
- Fredrikson GN, Soderberg I, Lindholm M, et al. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–84. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–19. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Garner B, Priestman DA, Stocker R, et al. Increased glycosphingolipid levels in serum and aortae of apolipoprotein E gene knockout mice. J Lipid Res. 2002;43:205–14. [PubMed] [Google Scholar]
- Genest J, Jr, McNamara JR, Ordovas JM, et al. Lipoprotein cholesterol, apolipoprotein A-I and B and lipoprotein (a) abnormalities in men with premature coronary artery disease. J Am Coll Cardiol. 1992;19:792–802. doi: 10.1016/0735-1097(92)90520-w. [DOI] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Paraoxonase, a cardioprotective enzyme: continuing issues. Curr Opin Lipidol. 2004;15:261–7. doi: 10.1097/00041433-200406000-00005. [DOI] [PubMed] [Google Scholar]
- Goetz FW, Planas JV, MacKenzie S. Tumor necrosis factors. Dev Comp Immunol. 2004;28:487–97. doi: 10.1016/j.dci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Gordon DJ, Rifkind BM. High-density lipoprotein—the clinical implications of recent studies. N Engl J Med. 1989;321:1311–16. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- Graham TL, Mookherjee C, Suckling KE, et al. The PPARdelta agonist GW0742X reduces atherosclerosis in LDLR (−/−) mice. Atherosclerosis. 2005;181:29–37. doi: 10.1016/j.atherosclerosis.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Greaves DR, Gough PJ, Gordon S. Recent progress in defining the role of scavenger receptors in lipid transport, atherosclerosis and host defence. Curr Opin Lipidol. 1998;9:425–32. doi: 10.1097/00041433-199810000-00006. [DOI] [PubMed] [Google Scholar]
- Grimaldi PA. Regulatory role of peroxisome proliferator-activated receptor delta (PPAR delta) in muscle metabolism. A new target for metabolic syndrome treatment? Biochimie. 2005;87:5–8. doi: 10.1016/j.biochi.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Han KH, Chang MK, Boullier A, et al. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor gamma. J Clin Invest. 2000;106:793–802. doi: 10.1172/JCI10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KH, Tangirala RK, Green SR, et al. Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler Thromb Vasc Biol. 1998;18:1983–91. doi: 10.1161/01.atv.18.12.1983. [DOI] [PubMed] [Google Scholar]
- Hansel B, Giral P, Nobecourt E, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense highdensity lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89:4963–71. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–75. [PMC free article] [PubMed] [Google Scholar]
- Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. JAMA. 1996;276:1473–9. [PubMed] [Google Scholar]
- Holvoet P. Oxidized LDL and coronary heart disease. Acta Cardiol. 2004;59:479–84. doi: 10.2143/AC.59.5.2005219. [DOI] [PubMed] [Google Scholar]
- Hopkins PA, Sriskandan S. Mammalian Toll-like receptors: to immunity and beyond. Clin Exp Immunol. 2005;140:395–407. doi: 10.1111/j.1365-2249.2005.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R, Cerny J, Benedik J, Jr, et al. The possible role of human cytomegalovirus (HCMV) in the origin of atherosclerosis. J Clin Virol. 2000;16:17–24. doi: 10.1016/s1386-6532(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68-69:619–31. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Hube F, Lietz U, Igel M, et al. Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm Metab Res. 1996;28:690–3. doi: 10.1055/s-2007-979879. [DOI] [PubMed] [Google Scholar]
- Hyka N, Dayer JM, Modoux C, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–9. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- Inoue K, Arai Y, Kurihara H, et al. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circ Res. 2005;97:176–84. doi: 10.1161/01.RES.0000174286.73200.d4. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Ruiz E, et al. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 2004;94:609–16. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Clark K, Coleman E, et al. American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33:2145–56. doi: 10.1097/00005768-200112000-00026. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35:1703–9. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W, Duchateau PN, Medzihradszky KF, et al. Apolipoproteins A-IV and A-V are acute-phase proteins in mouse HDL. Atherosclerosis. 2004;176:37–44. doi: 10.1016/j.atherosclerosis.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Klein S, Burke LE, Bray GA, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K. Adipokines: therapeutic targets for metabolic syndrome. Curr Drug Targets. 2005;6:525–9. doi: 10.2174/1389450054021972. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Ouchi N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Shor A, Campbell LA, et al. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–9. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- Kwak B, Mulhaupt F, Myit S, et al. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- Lamarche B, Lemieux I, Despres JP. The small, dense LDL phenotype and the risk of coronary heart disease: epidemiology, pathophysiology and therapeutic aspects. Diabetes Metab. 1999;25:199–211. [PubMed] [Google Scholar]
- Lamarche B, Tchernof A, Mauriege P, et al. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279:1955–61. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- Lau DC, Dhillon B, Yan H, et al. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–41. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler Thromb Vasc Biol. 2000;20:551–5. doi: 10.1161/01.atv.20.2.551. [DOI] [PubMed] [Google Scholar]
- Lee CH, Chawla A, Urbiztondo N, et al. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003;302:453–7. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- Lehr HA, Sagban TA, Ihling C, et al. Immunopathogenesis of atherosclerosis: endotoxin accelerates atherosclerosis in rabbits on hypercholesterolemic diet. Circulation. 2001;104:914–20. doi: 10.1161/hc3401.093153. [DOI] [PubMed] [Google Scholar]
- Lemieux I, Pascot A, Prud'homme D, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–7. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- Li AC, Binder CJ, Gutierrez A, et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–76. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AC, Brown KK, Silvestre MJ, et al. Peroxisome proliferator activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–31. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Lyngso D, Simonsen L, Bulow J. Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. J Physiol. 2002;543:379–86. doi: 10.1113/jphysiol.2002.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackness B, Hunt R, Durrington PN, et al. Increased immunolocalization of paraoxonase, clusterin, and apolipoprotein AI in the human artery wall with the progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:1233–8. doi: 10.1161/01.atv.17.7.1233. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Hirano K, Nozaki S, et al. Expression of macrophage (Mphi) scavenger receptor, CD36, in cultured human aortic smooth muscle cells in association with expression of peroxisome proliferator activated receptor-gamma, which regulates gain of Mphi-like phenotype in vitro, and its implication in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:1027–32. doi: 10.1161/01.atv.20.4.1027. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Chen J, Hermonat PL, et al. Lectin-like, oxidized lowdensity lipoprotein receptor-1 (LOX-1): A critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Michelsen KS, Doherty TM, Shah PK, et al. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol. 2004;173:5901–7. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- Moore RE, Navab M, Millar JS, et al. Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circ Res. 2005;97:763–71. doi: 10.1161/01.RES.0000185320.82962.F7. [DOI] [PubMed] [Google Scholar]
- Nagy L, Tontonoz P, Alvarez JG, et al. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–40. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- Navab M, Anantharamaiah GM, Fogelman AM. The role of high-density lipoprotein in inflammation. Trends Cardiovasc Med. 2005;15:158–61. doi: 10.1016/j.tcm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Navab M, Berliner JA, Watson AD, et al. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol. 1996;16:831–42. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- NCEP–ATP III. Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Nicholls S, Lundman P. The emerging role of lipoproteins in atherogenesis: beyond LDL cholesterol. Semin Vasc Med. 2004;4:187–95. doi: 10.1055/s-2004-835377. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Harrison DG. The AT (1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation. 2002;105:393–6. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- Ofei F, Hurel S, Newkirk J, et al. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–5. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- Oliver WR, Jr, Shenk JL, Snaith MR, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306–11. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–63. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- Palinski W, Horkko S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–14. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panousis CG, Zuckerman SH. Interferon-gamma induces downregulation of Tangier disease gene (ATP-binding-cassette transporter 1) in macrophage-derived foam cells. Arterioscler Thromb Vasc Biol. 2000;20:1565–71. doi: 10.1161/01.atv.20.6.1565. [DOI] [PubMed] [Google Scholar]
- Parhami F, Basseri B, Hwang J, et al. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002;91:570–6. doi: 10.1161/01.res.0000036607.05037.da. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–7. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–8. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–6. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- Peelman F, Waelput W, Iserentant H, et al. Leptin: linking adipocyte metabolism with cardiovascular and autoimmune diseases. Prog Lipid Res. 2004;43:283–301. doi: 10.1016/j.plipres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- Puddu P, Puddu GM, Muscari A. Peroxisome proliferator-activated receptors: are they involved in atherosclerosis progression? Int J Cardiol. 2003;90:133–40. doi: 10.1016/s0167-5273(02)00565-x. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988 Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Lehrke M, Wolfe ML, et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–9. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- Ricote M, Huang J, Fajas L, et al. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1998;95:7614–19. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM. Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation. 2003;108:e81–5. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Appl Physiol. 2005;98:3–30. doi: 10.1152/japplphysiol.00852.2004. [DOI] [PubMed] [Google Scholar]
- Robinson JG, Smith B, Maheshwari N, et al. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 2005;46:1855–62. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- Rosenson RS. New approaches in the intensive management of cardiovascular risk in the metabolic syndrome. Curr Probl Cardiol. 2005;30:241–79. doi: 10.1016/j.cpcardiol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Saikku P, Leinonen M, Mattila K, et al. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2:983–6. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- Saris WH, Blair SN, van Baak MA, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003;4:101–14. doi: 10.1046/j.1467-789x.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- Sato N, Kobayashi K, Inoguchi T, et al. Adenovirus-mediated high expression of resistin causes dyslipidemia in mice. Endocrinology. 2005;146:273–9. doi: 10.1210/en.2004-0985. [DOI] [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–7. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- Schafer K, Halle M, Goeschen C, et al. Leptin promotes vascular remodeling and neointimal growth in mice. Arterioscler Thromb Vasc Biol. 2004;24:112–17. doi: 10.1161/01.ATV.0000105904.02142.e7. [DOI] [PubMed] [Google Scholar]
- Schoneveld AH, Oude Nijhuis MM, van MB, et al. Toll-like receptor 2 stimulation induces intimal hyperplasia and atherosclerotic lesion development. Cardiovasc Res. 2005;66:162–9. doi: 10.1016/j.cardiores.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Senn JJ, Klover PJ, Nowak IA, et al. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–9. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- Shaw PX. Rethinking oxidized low-density lipoprotein, its role in atherogenesis and the immune responses associated with it. Arch Immunol Ther Exp (Warsz) 2004;52:225–39. [PubMed] [Google Scholar]
- Silswal N, Singh AK, Aruna B, et al. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- St-Pierre AC, Cantin B, Mauriege P, et al. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ. 2005;172:1301–5. doi: 10.1503/cmaj.1040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B, Koenig W, Habib A, et al. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature. 1998;393:790–3. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- Staiger H, Haring HU. Adipocytokines: fat-derived humoral mediators of metabolic homeostasis. Exp Clin Endocrinol Diabetes. 2005;113:67–79. doi: 10.1055/s-2004-830555. [DOI] [PubMed] [Google Scholar]
- Stalenhoef AF, Ballantyne CM, Sarti C, et al. A comparative study with rosuvastatin in subjects with metabolic syndrome: results of the COMETS study. Eur Heart J. 2005;26:2664–72. doi: 10.1093/eurheartj/ehi482. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: oneyear follow-up of a randomized trial. Ann Intern Med. 2004;140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kurihara Y, Takeya M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–6. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100:15924–9. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenger C, Sundborger A, Jawien J, et al. IL-18 accelerates atherosclerosis accompanied by elevation of IFN-gamma and CXCL16 expression independently of T cells. Arterioscler Thromb Vasc Biol. 2005;25:791–6. doi: 10.1161/01.ATV.0000153516.02782.65. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Nagy L, Alvarez JG, et al. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–52. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- Torzewski M, Klouche M, Hock J, et al. Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler Thromb Vasc Biol. 1998;18:369–78. doi: 10.1161/01.atv.18.3.369. [DOI] [PubMed] [Google Scholar]
- Tribble DL, Holl LG, Wood PD, et al. Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size. Atherosclerosis. 1992;93:189–99. doi: 10.1016/0021-9150(92)90255-f. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Flegal KM. Overweight prevalence among youth in the United States: why so many different numbers? Int J Obes Relat Metab Disord. 1999;23(Suppl 2):S22–7. doi: 10.1038/sj.ijo.0800855. [DOI] [PubMed] [Google Scholar]
- Unger RH. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie. 2005;87:57–64. doi: 10.1016/j.biochi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, et al. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–78. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- van Tits L, de Graaf J, Toenhake H, et al. C-reactive protein and annexin A5 bind to distinct sites of negatively charged phospholipids present in oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2005;25:717–22. doi: 10.1161/01.ATV.0000157979.51673.2c. [DOI] [PubMed] [Google Scholar]
- Vanderlaan PA, Reardon CA. Thematic review series: the immune system and atherogenesis. The unusual suspects:an overview of the minor leukocyte populations in atherosclerosis. J Lipid Res. 2005;46:829–38. doi: 10.1194/jlr.R500003-JLR200. [DOI] [PubMed] [Google Scholar]
- Verges B. Role for fibrate therapy in diabetes: evidence before FIELD. Curr Opin Lipidol. 2005;16:648–51. [PubMed] [Google Scholar]
- Verma S, Li SH, Wang CH, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–40. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- vonEckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005;8:147–52. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, et al. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–70. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- Wang ZW, Pan WT, Lee Y, et al. The role of leptin resistance in the lipid abnormalities of aging. FASEB J. 2001;15:108–14. doi: 10.1096/fj.00-0310com. [DOI] [PubMed] [Google Scholar]
- Way JM, Gorgun CZ, Tong Q, et al. Adipose tissue resistin expression is severely suppressed in obesity and stimulated by peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2001;276:25651–3. doi: 10.1074/jbc.C100189200. [DOI] [PubMed] [Google Scholar]
- Weber C, Erl W, Weber PC. Enhancement of monocyte adhesion to endothelial cells by oxidatively modified low-density lipoprotein is mediated by activation of CD11b. Biochem Biophys Res Commun. 1995;206:621–8. doi: 10.1006/bbrc.1995.1088. [DOI] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Elam H, et al. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–24. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson TM, Cobb JE, Cowan DJ, et al. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem. 1996;39:665–8. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Rumpold H, et al. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- Xu H, Hirosumi J, Uysal KT, et al. Exclusive action of transmembrane TNF alpha in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology. 2002;143:1502–11. doi: 10.1210/endo.143.4.8715. [DOI] [PubMed] [Google Scholar]
- Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–59. doi: 10.1161/01.atv.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–8. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- Yamashiroya HM, Ghosh L, Yang R, et al. Herpesviridae in the coronary arteries and aorta of young trauma victims. Am J Pathol. 1988;130:71–9. [PMC free article] [PubMed] [Google Scholar]
- Yla-Herttuala S, Palinski W, Rosenfeld ME, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–95. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JG, Javorschi S, Hevener AL, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–74. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- Zarkesh-Esfahani H, Pockley G, Metcalfe RA, et al. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593–9. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zheng L, Nukuna B, Brennan ML, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–41. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]