Abstract
Insulin detemir is a soluble long-acting human insulin analogue at neutral pH with a unique mechanism of action. Following subcutaneous injection, insulin detemir binds to albumin via fatty acid chain, thereby providing slow absorption and a prolonged metabolic effect. Insulin detemir has a less variable pharmacokinetic profile than insulin suspension isophane or insulin ultralente. The use of insulin detemir can reduce the risk of hypoglycemia (especially nocturnal hypoglycemia) in type 1 and type 2 diabetic patients. However, overall glycemic control, as assessed by glycated hemoglobin, is only marginally and not significantly improved compared with usual insulin therapy. The weight gain commonly associated with insulin therapy is rather limited when insulin detemir is used. In our experience, this new insulin analogue is preferably administrated at bedtime but can be proposed twice a day (in the morning and either before the dinner or at bedtime). Detemir is a promising option for basal insulin therapy in type 1 or type 2 diabetic patients.
Keywords: diabetes mellitus, insulin detemir, hypoglycemia, insulin analogue, insulin therapy
Introduction
The history of treatment for diabetic patients can be resumed in several milestones. More than eighty years ago, insulin was discovered and it is probably one of the greatest medical advances of the 20th century (Rosenfeld 2002). Manufacturing techniques improved rapidly but the origin of the insulin proposed to diabetic patients was still inadequate (bovine and porcine sources). The first long-acting preparation, protamine zinc insulin, was developed in order to reduce the number of injections necessary for adequate insulin replacement (Joslin 1941). This preparation was often used once daily, without the addition of regular insulin. Later, insulin neutral protamine Hagedorn (NPH) and insulin zinc (Lente) were introduced. Then, a movement toward more complete coverage of insulin requirements resulted in the twice daily mix regimen of NPH and regular insulin (Jackson 1986). Nevertheless, the best definition for physiological insulin replacement consists of prandial (bolus) insulin, basal insulin, and a correction-dose insulin supplement when necessary (DeWitt and Hirsch 2003).
Another big step in insulin therapy was effective when the development of purified pork insulin and then recombinant human insulin virtually eliminated insulin allergy and immune-mediated lipoatrophy (McNally et al 1988).
Despite many improvements in the management of diabetes, the nonphysiological time-action profiles of conventional insulin formulations remain a significant obstacle. In 1993 and 1998, the reports of the Diabetes Control and Complications Trial (DCCT 1993) and the United Kingdom Prospective Diabetes Study (UKPDS 1998), respectively, confirmed the value of glycemic control in the delay or prevention of complications of diabetes. The limiting pharmacokinetic and pharmacodynamic features of standard insulins, which frequently lead to hypoglycemia as glycosylated hemoglobin (HbA1C) values approach the normal range, renewed interest in producing safer insulin formulations that more closely mimic the basal and mealtime components of endogenous insulin secretion. This interest has yielded insulin analogues that are characterized by action profiles that afford more flexible treatment regimens with a lower risk of hypoglycemia (Hirsch 2005).
The first insulin analogues used in insulin therapy were the rapid-acting insulin analogues (aspart and lispro): their pharmacodymamic properties are particularly interesting because their profile is closer to the physiologic profile of postprandial endogenous insulin (Mudaliar et al 1999). They are able to decrease glycemia more rapidly than usual rapid-acting insulin preparations (regular); the peak insulin action occurs approximately twice as fast with analogues as with regular insulin. The more rapid pharmacodynamic effects of insulin lispro and insulin aspart make postabsorptive hypoglycemia less of a problem with these analogues than with regular insulin (Hirsch 2005).
The first long-acting insulin analogue, insulin glargine, was introduced in the US in spring 2001. This analogue is produced by the substitution of the asparagine by a glycine at position A21 of the insulin molecule and by the addition of two arginine molecules at position B30 (Heinemann et al 2000). These changes lead to a shift in the isoelectric point toward a neutral pH, which results in an insulin molecule that is less soluble at the injection site and that precipitates in the subcutaneous tissue to form a deposit from which insulin is slowly released (Yki-Jarvinen et al 2000).
Later on, insulin detemir was introduced and is now available as the last insulin analogue proposed for insulin therapy. The advantages and properties of this new basal human insulin analogue are discussed in this article.
Pharmacodynamic and pharmacokinetic properties
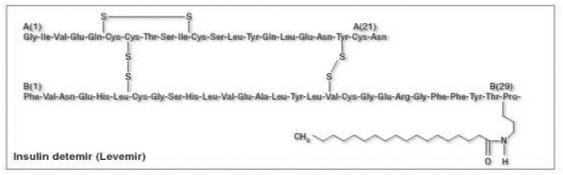
Insulin detemir is a neutral, soluble, long-acting insulin analogue in which threonine is omitted from position B30 of the insulin β-chain and replaced by myristic acid, a C14 fatty acid chain (Figure 1). This fatty acid modification allows insulin detemir to reversibly bind to the long-chain fatty acid binding sites (Havelund et al 2004). Insulin detemir is soluble at neutral pH, which enables it to remain in a liquid form following subcutaneous injection, unlike NPH insulin and glargine. The solubility of insulin detemir may be a factor contributing to the reduced variability in glycemic control observed in recipients of this agent compared with NPH or glargine because precipitation and dissociation of a precipitate are unpredictable processes. Anyway, comparison of intra-subject variability of glucose-lowering action between detemir and glargine use is still limited since the paper documenting this possible better stability of glucose levels with detemir is based on a comparison after four injections of each insulin analogue in a limited number of patients (Heise et al 2004). So far, there is no extended clinical study on such a comparison available. A trial designed to describe the 24 h pharmacodynamic profile (including duration of action and dose-response relationship) of insulin detemir in subjects with type 1 diabetes reported that insulin detemir provides a flat and protracted pharmacodynamic profile. This study used a 24 h isoglycemic clamp and showed a linear dose response over a range of clinically relevant doses (Plank et al 2005). These data were also confirmed in a crossover trial including children, adolescents and adults who received subcutaneous single doses of 0.5 units/kg insulin detemir or 0.5 IU/kg NPH insulin on two separate days. Less total variability in the pharmacokinetics of insulin detemir than NPH insulin was observed in all three age-groups (Danne et al 2003).
Figure 1.
Structure of insulin detemir.
Following subcutaneous injection, insulin detemir binds to albumin and, at steady state, the concentration of free, unbound insulin is then greatly reduced, resulting in more stable plasma insulin levels. The protracted action of insulin detemir, achieved by slow absorption from the subcutaneous depot, appears to be mediated via two mechanisms: initially, self association of the insulin detemir molecule at the site of injection and, subsequently, binding to albumin via fatty acid chain (Havelund et al 2004). Although few data are available in humans, there is no major relationship between pharmacodynamic and pharmacokinetic properties of detemir and the plasma level of albumin (perhaps except in severe hypoalbuminemia). A significant relationship between the injected dose of insulin detemir and its duration of hypoglycemic action has been recently reported (Plank et al 2005), and such observation may influence the use of insulin detemir in one or two injections per day to insure basal insulin levels over the 24 h period (Oiknine et al 2005).
Insulin detemir acts as a full agonist of the insulin receptor but dissociates from the insulin receptor twice as fast as human insulin in vitro. This finding explains the fact that insulin detemir demonstrated lower metabolic potency than human insulin. The drug has been shown to have molar potency approximately 25% lower than that of human insulin in patients with diabetes (Kurtzhals 2004). For this reason, insulin detemir is formulated at a molar concentration four times higher than that of human insulin.
Some data suggest that insulin detemir, when compared with NPH insulin, has a greater effect on the liver than on peripheral tissues (Hordern et al 2005). As discussed below, this particularity may explain some particular clinical observations.
When creating new insulin analogues, the risk of mitogenicity is an important and potentially dangerous adverse event to look after. The affinity of insulin detemir for the insulin-like growth factor-1 receptor is about 16% (100% for human insulin) and the mitogenic potency of the drug is low (Kurtzhals et al 2000).
Concerning metabolism and elimination, human insulin is internalized after binding to its receptor and the same is presumed for insulin detemir. Data suggest an elimination half-life of 6.8 minutes which is five to six times more slowly than human insulin.
Clinical efficacy in type 1 diabetes
The clinical efficacy, tolerability, and safety of insulin detemir has been studied in several large (number of subjects >250), randomized, parallel-group, multicenter trials of 16–26 weeks duration in adults with type 1 diabetes (Hermansen et al 2001, Hermansen, Fontaine, et al 2004; Home et al 2004). Some publications also reported results with 12-month data from two extension studies (Standl et al 2004; De Leeuw et al 2005). A recent large trial in children and adolescents with type 1 diabetes is also available (Robertson et al 2004). In all these trials, insulin detemir was compared with NPH insulin (Table 1).
Table 1.
Changes in glycosylated hemoglobin (HbA1C) levels, body weight and incidence of hypoglycemic episodes (HYPO) with insulin detemir versus NPH insulin in most important randomized studies performed in patients with type 1 diabetes (Type 1 DM) and with type 2 diabetes (Type 2 DM)
| DETEMIR | NPH | Detemir versus NPHp value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| References | HbA1c changes % | Body weight kg | Overall HYPO | Nocturnal HYPO % | HbA1c changes kg | Body weight | Overall HYPO | Nocturnal HYPO | HbA1c | Weight | Overall HYPO | Nocturnal HYPO |
| Events/subject/month | Events/subject/month | |||||||||||
| Type 1 DM | ||||||||||||
| Vague et al 2003 | 8.18 | 71.5 | 8.18 | 71.5 | ||||||||
| ↓ | ↓ | 5.18 | 0.64 | ↓ | ↓ | 6.7 | 0.96 | 0.61 | 0.001 | 0.029 | <0.005 | |
| 7.60 | 70.9 | 7.64 | 71.8 | |||||||||
| De Leeuw et al 2005 | 8.18 | 71.3 | 8.18 | 71.3 | ||||||||
| ↓ | ↓ | NA | 0.63 | ↓ | ↓ | NA | 0.89 | NS | <0.001 | NA | 0.016 | |
| 7.53 | 71.2 | 7.59 | 72.7 | |||||||||
| Pieber et al 2005 | 8.07 | 76.3 | 8.08 | 74.8 | ||||||||
| ↓ | ↓ | 2.31 | 0.40 | ↓ | ↓ | 2.18 | 0.43 | 0.63 | 0.050 | 0.52 | 0.58 | |
| 7.66 | 75.8 | 7.73 | 75.5 | |||||||||
| Type 2 DM | ||||||||||||
| Haak et al 2005 | 7.90 | 85.7 | 7.80 | 89.3 | ||||||||
| ↓ | ↓ | 0.64 | 0.09 | ↓ | ↓ | 0.78 | 0.09 | 0.16 | 0.017 | 0.48 | 0.95 | |
| 7.60 | 86.7 | 7.50 | 91.1 | |||||||||
| Raslova et al 2004 | 8.16! | 82.0 | 8.08 | 79.6 | ||||||||
| ↓ | ↓ | 0.34 | 0.06 | ↓ | ↓ | 0.40 | 0.10 | NS | 0.038 | 0.65 | 0.14 | |
| 7.51 | 82.5 | 7.50 | 80.7 | |||||||||
Abbreviations: HYPO, hypoglycemia; NA, not available; NPH, insulin neutral protamine Hagedorn; NS, not significant.
Glucose control, as assessed by HbA1C fasting plasma glucose (FPG) levels, or 9-point blood glucose profiles, was similar to, or better than, that with NPH insulin (Vague et al 2003; Hermansen et al 2001; Home et al 2004; Standl et al 2004; Pieber et al 2005). Use of insulin detemir, administered once or twice daily, in combination with bolus insulin aspart or regular human insulin, resulted in FPG levels similar to or lower than those achieved when treating patients with NPH plus insulin aspart or regular.
Importantly, insulin detemir was generally associated with more predictable glycemic control and less intra-patient variability than NPH insulin (Vague et al 2003; Hermansen et al 2001; Home et al 2004; Pieber et al 2005). Intra-patient variation in self-measured plasma or blood glucose was lower with insulin detemir than with NPH insulin in most trials. Glucose fluctuations (defined as the area between the individual glucose curve and its mean level) based on continuous glucose monitoring over 24 h and during the night were reported in a subgroup of patients after 5 months treatment, these glucose fluctuations over 24 h or at night were significantly lower with insulin detemir than with NPH insulin (Russell-Jones et al 2004).
Clinical evidence from different trials has shown a similar or lower risk of hypoglycemia (particularly nocturnal hypoglycemia) with insulin detemir compared with NPH insulin. Most of the trials report a significant (p<0.05) reduction in the risk of nocturnal hypoglycemia in recipients of insulin detemir compared with NPH insulin (Hermansen et al 2001; Chapman and Perry 2005). For example, a trial aimed to compare insulin analogues (insulin detemir, insulin aspart) versus traditional human insulins (insulin NPH, insulin regular) in type 1 diabetic patients with basal bolus therapy showed interesting results in term of hypoglycemic events, numbers of overall hypoglycemia episodes per person-year were 37.1 and 48.2 for the insulin detemir and insulin NPH, respectively, while corresponding numbers of nocturnal hypoglycemia episodes per person-year were 4.0 and 9.2, respectively (Hermansen, Fontaine, et al 2004).
Weight gain with insulin detemir use is rather limited when compared to insulin NPH. After 16–52 weeks of treatment, patients with type 1 diabetes receiving insulin detemir had a significantly (p<0.001) lower mean bodyweight or a significantly (p=0.002) lower bodyweight gain adjusted for change in HbA1C than NPH insulin recipients (Vague et al 2003; Standl et al 2004). The explanation for this interesting observation is still unclear and discussed below.
Clinical efficacy in type 2 diabetes
Two trials had reported data about insulin detemir in combination with mealtime insulin aspart (like the usual insulin therapy in type 1 diabetes) (Haak et al 2003; Raslova et al 2004). This association produced glycemic control similar to that of NPH insulin plus either insulin aspart or regular human insulin. In terms of HbA1C levels, mean FPG levels, and self-measured blood glucose profiles, there were no difference at the end of the treatment in the two trials. Intra-individual variation in FPG levels was, however, significantly lower in patients receiving insulin detemir. The risk of hypoglycemia (during the day and/or the night) was generally reduced when using insulin detemir as compared with NPH insulin although differences between the two groups were not always statistically significant (Raslova et al 2003; Haak et al 2004; Hermansen, Derezinski, et al 2004). While the frequency of hypoglycemia (especially severe events) is an important issue in some patients, it is less important in type 2 diabetic compared with type 1 diabetic patients.
An important point, especially in the context of type 2 diabetes, is that insulin detemir was consistently associated with significantly less bodyweight gain than NPH. This effect may be considered as a relevant clinical benefit concerning the optimal treatment of patients with type 2 diabetes. Insulin therapy is often delayed because of the risk of weight gain when stopping oral antidiabetic drugs for insulin injections. A recent trial showed that, in type 2 diabetic patients with impaired metabolic control with oral agents, the use of insulin detemir versus insulin NPH plus oral agents was associated with less bodyweight gain and hypoglycemic events (Hermansen, Derezinski, et al 2004). There is no clear explanation for the lesser weight gain with insulin detemir as compared with NPH insulin. This may be a consequence of the increased predictability and smoother and more consistent pharmacodynamic profile of insulin detemir, resulting in reduced risk of hypoglycemia. Patients may be able to reduce defensive eating against hypoglycemia while maintaining more optimal blood glucose levels (Fritsche and Haring 2004). However, the reduction of hypoglycemic events does not seem to fully explain this effect because no prevention of weight gain has been reported with the use of glargine despite the fact that this insulin also reduces the number of hypoglycemic episodes in a similar extent to that reported with insulin detemir. Because of its pharmacokinetic properties, insulin detemir exerts greater effects on the liver than the periphery. It has been suggested that systemic hyperinsulinemia increases peripheral glucose uptake and lipogenesis and decreases lipolysis, contributing to weight gain associated with insulin therapy (Andreani 1999). Anyway, all these mechanisms that could explain the favorable effects of insulin detemir on body weight remain hypothetical.
Tolerability
All the data from randomized trials in type 1 and type 2 diabetes show that insulin detemir is well tolerated (Vague et al 2003; Hermansen, Derezinski, et al 2004; Hermansen, Fontaine, et al 2004; Home et al 2004; Robertson et al 2004; Standl et al 2004; Chapman and Perry 2005). Hypoglycemia was of course registered as an endpoint in the different trials and it is reasonable to consider these hypoglycemic episodes as a “usual” iatrogenic effect. As already mentioned, the incidence of hypoglycemic episodes was lower in diabetic patients treated with insulin detemir as compared with NPH insulin, especially at night. Otherwise, the nature and incidence of adverse events observed with insulin detemir are quite similar to those experienced with other human insulin preparations (including insulin analogues) (Chapman and Perry 2004). The majority of adverse events was mild and considered unrelated to the study drug (Chapman and Perry 2004).
Insulin detemir has not been studied in pregnant diabetic women and should therefore not be proposed to this special population.
Recently, the first case of type III allergy to the new long-acting insulin analogue detemir was reported (Darmon et al 2005). A severe injection site reaction to insulin detemir has also been recently reported (Blumer 2006).
Administration and posology
Insulin detemir can be used as basal therapy in conjunction with short-acting bolus insulin in both patients with type 1 or type 2 diabetes (Chapman and Perry 2005). It can be injected in the subcutaneous tissue one or two times a day. Initially, it is recommended to inject insulin detemir in the evening (at dinner or bedtime). Nevertheless, it was shown that morning plus evening administration of insulin detemir (plus insulin aspart at mealtime) provided also less variable glucose levels with no, or less, weight gain than NPH insulin administered in a similar way. Insulin detemir can be administered either at dinner or bedtime, with similar glycemic control (Pieber et al 2005). Adding short-acting insulin analogue or rapid-acting insulin at mealtimes to mimic as best as possible the normal insulin secretion is the basis of the basal-bolus therapy. Such insulin regimen particularly applies to insulin detemir administration. The dose of insulin detemir has to be appreciated and adjusted until the desired fasting plasma level has been attained. For patients in whom the desired pre-dinner target blood glucose level cannot be reached, it seems reasonable to split the total daily dose of insulin detemir. Two separate injections (morning and evening) may therefore be administrated. Mixing insulin detemir with a rapid-acting insulin should be avoided because the action profile of insulin detemir can be modified with a lower and delayed maximum effect compared with that provided by separate injections.
It appears that it is not necessary to make special adjustments to the dosage of insulin detemir in children or adolescents. It also seems safe to propose insulin detemir to patients with renal or hepatic impairment. Indeed, despite the fact that insulin detemir binds to albumin, no particular adaptation in insulin detemir doses, as compared with NPH insulin, is necessary for this kind of patient. Comparisons between continuous subcutaneous insulin infusion and multiple daily injections using insulin detemir (or insulin glargine) have been limited until now and no relevant longterm data are available yet.
Conclusions
Insulin detemir has been developed to ameliorate the profile of usual human basal insulin. The advantage to avoid an insulin peak and to keep a significant action for several hours gives to insulin detemir appreciated properties for diabetic patients. Despite the fact that rather few data are available so far, insulin detemir may be responsible for less intra-patient variability in glycemic control among patients with type 1 diabetes compared with NPH insulin or insulin glargine. Compared with NPH, use of insulin detemir may be associated with a lower risk of hypoglycemia, especially nocturnal hypoglycemia, in patients with type 1 or type 2 diabetes. This new insulin also provides the added clinical benefit of no appreciable bodyweight gain in patients with type 1 diabetes and less weight gain than NPH insulin in patients with type 2 diabetes. The need to administer insulin detemir twice a day to obtain a better basal insulin profile compared with the unique injection of insulin glargine may be a brake for detemir use in some patients.
In terms of overall glycemic control, there is no proof of significant improvement in HbA1C levels when using insulin detemir as compared with NPH insulin. Anyway, the new properties of insulin analogues (basal and rapid analogs) often give to diabetic patients some advantages (more flexibility and lower rates of hypoglycemic episodes for example). Therefore, insulin detemir can be considered as a valuable new option for basal therapy in patients with type 1 or type 2 diabetes.
Disclosure
The authors declare that there is no conflict of interest.
References
- Andreani D. Lights and shadows of insulin treatment seen by a senior diabetologist. Exp Clin Endocrinol Diabetes. 1999;107:S1–5. [Google Scholar]
- Blumer I. Severe injection site reaction to insulin detemir. Diabetes Care. 2006;29:946. doi: 10.2337/diacare.29.04.06.dc05-2503. [DOI] [PubMed] [Google Scholar]
- Chapman T, Perry C. Insulin detemir: a review of its use in the management of type 1 and 2 diabetes mellitus. Drugs. 2004;64:2577–95. doi: 10.2165/00003495-200464220-00008. [DOI] [PubMed] [Google Scholar]
- Chapman T, Perry C. Spotlight on insulin detemir in type 1 and 2 diabetes mellitus. BioDrugs. 2005;19:67–9. doi: 10.2165/00063030-200519010-00008. [DOI] [PubMed] [Google Scholar]
- Danne T, Lupke K, Walte K, et al. Insulin detemir is characterized by a consistent pharmacokinetic profile across age-groups in children, adolescents, and adults with type 1 diabetes. Diabetes Care. 2003;26:3087–92. doi: 10.2337/diacare.26.11.3087. [DOI] [PubMed] [Google Scholar]
- Darmon P, Castera V, Koeppel MC, et al. Type III allergy to insulin detemir. Diabetes Care. 2005;28:2980. doi: 10.2337/diacare.28.12.2980. [DOI] [PubMed] [Google Scholar]
- [DCCT] Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- De Leeuw I, Vague P, Selam J, et al. Insulin detemir used in basalbolus therapy in people with type 1 diabetes is associated with a lower risk of nocturnal hypoglycemia and less weight gain over 12 months in comparison to NPH insulin. Diabetes Obes Metab. 2005;7:73–82. doi: 10.1111/j.1463-1326.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- DeWitt D, Hirsch B. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254–64. doi: 10.1001/jama.289.17.2254. [DOI] [PubMed] [Google Scholar]
- Fritsche A, Haring H. At last, a weight neutral insulin? Int J Obesity Relat Metab Disord. 2004;28(Suppl 2):S41–6. doi: 10.1038/sj.ijo.0802749. [DOI] [PubMed] [Google Scholar]
- Haak T, Tiengo A, Waldhausl W, et al. Treatment with insulin detemir is associated with predictable fasting glucose levels and favourable weight development in subjects with type 2 diabetes [abstract] Diabetologia. 2003;46(Suppl 2):A120. [Google Scholar]
- Havelund S, Plum A, Ribel U, et al. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res. 2004;8:1498–504. doi: 10.1023/b:pham.0000036926.54824.37. [DOI] [PubMed] [Google Scholar]
- Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23:644–9. doi: 10.2337/diacare.23.5.644. [DOI] [PubMed] [Google Scholar]
- Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–20. doi: 10.2337/diabetes.53.6.1614. [DOI] [PubMed] [Google Scholar]
- Hermansen K, Madsbad T, Perilld H, et al. Comparison of soluble basal insulin analog detemir with NPH insulin: a randomized open crossover trial in type 1 diabetic subjects on basal-bolus therapy. Diabetes Care. 2001;24:296–301. doi: 10.2337/diacare.24.2.296. [DOI] [PubMed] [Google Scholar]
- Hermansen K, Derezinski T, Kim H, et al. Treatment with insulin detemir in combination with oral agents is associated with less risk of hypoglycemia and less weight gain than NPH insulin at comparable levels of glycaemic improvement in people with type 2 diabetes [abstract] Diabetologia. 2004a;47(Suppl 1):A273–4. [Google Scholar]
- Hermansen K, Fontaine P, Kukolja K, et al. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia. 2004b;47:622–9. doi: 10.1007/s00125-004-1365-z. [DOI] [PubMed] [Google Scholar]
- Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174–83. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- Home P, Bartley P, Russell-Jones D, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial. Diabetes Care. 2004;27:1081–7. doi: 10.2337/diacare.27.5.1081. [DOI] [PubMed] [Google Scholar]
- Hordern S, Wright J, Umpleby A, et al. Comparison of the effects on glucose metabolism of equipotent doses of insulin detemir and NPH insulin with a 16-h euglycaemic clamp. Diabetologia. 2005;48:420–6. doi: 10.1007/s00125-005-1670-1. [DOI] [PubMed] [Google Scholar]
- Jackson R. Historical background. In: Jackson RL, Guthrie RA, editors. The physiological management of diabetes in children. New York: Medical Examination Publishing; 1986. pp. 6–16. [Google Scholar]
- Joslin EP. A diabetic manual for the mutual use of doctor and patient. 7th ed. Philadelphia: Lea & Febiger; 1941. [Google Scholar]
- Kurtzhals P, Schaffer L, Sorensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49:999–1005. doi: 10.2337/diabetes.49.6.999. [DOI] [PubMed] [Google Scholar]
- Kurtzhals P. Engineering predictability and protraction in a basal insulin analogue: the pharmacology of insulin detemir. Int J Obes Relat Metab Disord. 2004;28(Suppl 2):S23–8. doi: 10.1038/sj.ijo.0802746. [DOI] [PubMed] [Google Scholar]
- McNally PG, Jowett NI, Kurinczuk JJ, et al. Lipohypertrophy and lipoatrophy complicating treatment with highly purified bovine and porcine insulins. Postgrad Med J. 1988;64:850–3. doi: 10.1136/pgmj.64.757.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudaliar SR, Lindberg FA, Joyce M, et al. Insulin aspart (B28 aspinsulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22:1501–6. doi: 10.2337/diacare.22.9.1501. [DOI] [PubMed] [Google Scholar]
- Oiknine R, Bernbaum M, Mooradian AD. A critical appraisal of the role of insulin analogues in the management of diabetes mellitus. Drugs. 2005;65:325–40. doi: 10.2165/00003495-200565030-00003. [DOI] [PubMed] [Google Scholar]
- Pieber T, Draeger E, Kristensen A, et al. Comparison of three multiple injection regimens for type 1 diabetes: morning plus dinner or bedtime administration of insulin detemir vs. morning plus bedtime NPH insulin. Diabetic Med. 2005;22:850–7. doi: 10.1111/j.1464-5491.2005.01545.x. [DOI] [PubMed] [Google Scholar]
- Plank J, Bodenlenz M, Sinner F, et al. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long acting insulin analog detemir. Diabetes Care. 2005;28:1107–12. doi: 10.2337/diacare.28.5.1107. [DOI] [PubMed] [Google Scholar]
- Raslova K, Bogoev M, Raz I, et al. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract. 2004;66:193–201. doi: 10.1016/j.diabres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Robertson K, Schonle E, Gucev Z, et al. Benefits of insulin detemir in children and adolescents with type 1 diabetes: lower and more predictable fasting glucose plasma and lower risk of nocturnal hypoglycemia (abstract) Diabetes. 2004;53(Suppl 2):A130–1. [Google Scholar]
- Rosenfeld L. Insulin: discovery and controversy. Clin Chem. 2002;48:2270–88. [PubMed] [Google Scholar]
- Russell-Jones D, Simpson R, Hylleberg B, et al. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal-bolus regimen. Clin Ther. 2004;26:724–36. doi: 10.1016/s0149-2918(04)90072-0. [DOI] [PubMed] [Google Scholar]
- Standl E, Lang H, Roberts A. The 12-month efficacy and safety of insulin detemir and NPH insulin in basal-bolus therapy for the treatment of type 1 diabetes. Diabetes Technol Ther. 2004;6:579–88. doi: 10.1089/dia.2004.6.579. [DOI] [PubMed] [Google Scholar]
- [UKPDS] UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- Vague P, Selam JL, Skeie S, et al. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart. Diabetes Care. 2003;26:590–6. doi: 10.2337/diacare.26.3.590. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen, Dressler A, Ziemen M, HOE 901/300s Study Group Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care. 2000;23:1130–6. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]