Abstract
In patients with diabetes, dysregulation of multiple glucoregulatory hormones results in chronic hyperglycemia and an array of associated microvascular and macrovascular complications. Optimization of glycemic control, both overall (glycosylated hemoglobin [A1C]) and in the postprandial period, may reduce the risk of long-term vascular complications. However, despite significant recent therapeutic advances, most patients with diabetes are unable to attain and/or maintain normal or near-normal glycemia with insulin therapy alone. Pramlintide, an analog of amylin, is the first in a new class of pharmaceutical agents and is indicated as an adjunct to mealtime insulin for the treatment of patients with type 1 and type 2 diabetes. By mimicking the actions of the naturally occurring hormone amylin, pramlintide complements insulin by regulating the appearance of glucose into the circulation after meals via three primary mechanisms of action: slowing gastric emptying, suppressing inappropriate post-meal glucagon secretion, and increasing satiety. In long-term clinical trials, adjunctive pramlintide treatment resulted in improved postprandial glucose control and significantly reduced A1C and body weight compared with insulin alone. The combination of insulin and pramlintide may provide a more physiologically balanced approach to managing diabetes.
Keywords: diabetes, pramlintide, postprandial glucose, insulin, A1C, weight
Background
Diabetes results from the dysregulation of multiple glucoregulatory hormones that normally act to maintain glucose homeostasis. These hormonal imbalances lead to chronic hyperglycemia, which results in an array of microvascular complications including retinopathy, nephropathy, and neuropathy. The Diabetes Control and Complications Trial (DCCT 1993) and the UK Prospective Diabetes Study (UKPDS 1998) demonstrated that in patients with type 1 and type 2 diabetes, respectively, improved overall glycemic control, as measured by glycosylated hemoglobin (A1C), reduced the risk of development and progression of microvascular complications (DCCT 1993; UKPDS 1998). Importantly, there does not appear to be a discernable glycemic threshold, indicating that any reduction in A1C conveys clinical benefits (UKPDS 1995, 1998; DCCT 1996). In addition to microvascular complications, patients with diabetes also have at least a 2- to 10-fold greater risk for cardiovascular disease than individuals without diabetes (Manson et al 1991; Stamler et al 1993; ADA 2001). Cardiovascular disease is the leading cause of morbidity and mortality in this patient population (Gaba et al 1999; ADA 2001). Recent evidence indicates that improved overall glycemic control also reduces the risk for development of cardiovascular disease in patients with type 1 diabetes (DCCT/EDIC 2005).
Unfortunately, despite intensive insulin therapy, many insulin-treated patients are unable to achieve and maintain normal or near-normal glycemic control on a long-term basis (DCCT 1993; UKPDS 1998). This is due to a number of limitations of exogenously administered insulin, including weight gain and difficulty controlling postprandial glucose excursions. Intensification of insulin therapy almost inevitably results in weight gain (DCCT 1988; UKPDS 1998). This is not only a cosmetic deterrent to optimal therapy for many patients, but it has been associated with various components of the metabolic syndrome and increased cardiovascular risk (Purnell et al 1998; Purnell and Weyer 2003). A second important limitation of insulin therapy is its frequent inability to adequately control post-meal glucose excursions. Even when used optimally, insulin injected subcutaneously does not replicate normal postprandial insulin and amylin secretion into the portal vein, frequently resulting in persistent postprandial hyperglycemia (Hirsh 1999).
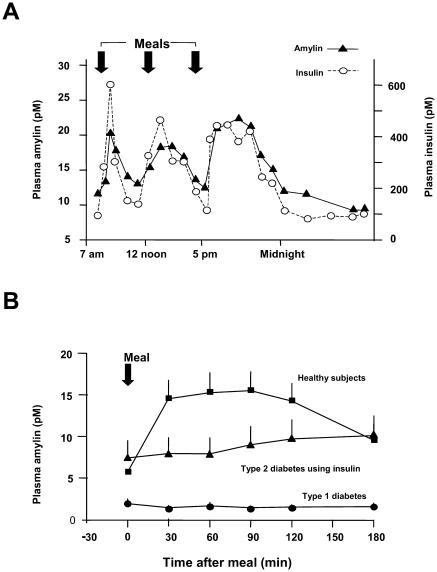
Investigation of additional therapies to optimize glycemic control in patients with diabetes has led to the development of pramlintide, an agent that mimics the actions of the neuroendocrine hormone amylin. Amylin is co-secreted with insulin from pancreatic beta cells in response to nutrient intake (Young 1997; Kruger et al 1999) (Figure 1A). Insulin and amylin have complementary effects on glucose metabolism. During the postprandial period, insulin facilitates glucose disappearance from the circulation by stimulating peripheral glucose uptake. Amylin regulates glucose appearance into the circulation by three principal mechanisms: regulation of gastric emptying (Young, Gedulin, et al 1996), suppression of postprandial glucagon secretion (Gedulin et al 1996), and regulation of food intake (Bhavsar et al 1996). Both insulin and amylin are absent in patients with type 1 diabetes and deficient in later stages of type 2 diabetes (Fineman et al 1996; Young 1997; Kruger and Gloster 2004) (Figure 1B). Due to several physiochemical characteristics, including insolubility and a propensity to aggregate, native amylin cannot be formulated as a pharmaceutical preparation.
Figure 1.
(A) Amylin secretion mirrors insulin secretion in healthy subjects (n=6) (adapted from Kruger et al 1999). (B) Amylin concentrations increase in response to meals in healthy individuals (n=27). In patients with type 2 diabetes (n=12), the amylin response is attenuated and in patients with type 1 diabetes (n=190), it is virtually absent (adapted from Kruger and Gloster 2004).
Pramlintide, the active ingredient in SYMLIN® (pramlintide acetate) injection, is a soluble, non-aggregating, synthetic analog of amylin. It mimics the glucoregulatory effects of amylin, which collectively limit postprandial glucose excursions (Young, Vine, et al 1996). Pramlintide is indicated for use as an adjunct to mealtime insulin in patients with type 1 and type 2 diabetes who are unable to achieve glycemic goals with insulin therapy alone. Together the complementary effects of insulin and pramlintide may provide a more balanced approach to managing hyperglycemia in patients with diabetes.
Pramlintide mechanisms of action
Clinical studies have demonstrated that pramlintide reduces postprandial glucose concentrations through at least three distinct mechanisms of action including modulation of gastric emptying, prevention of abnormal postprandial glucagon secretion, and increased satiety leading to a reduction in caloric intake.
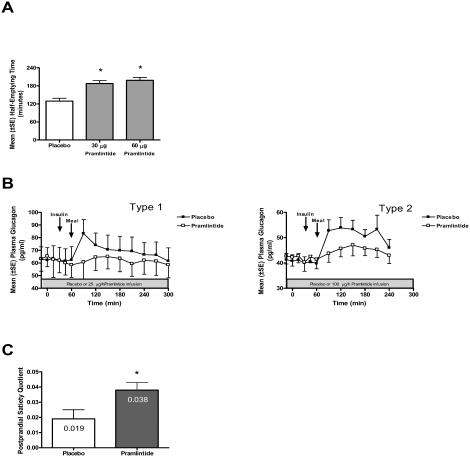
Gastric emptying in patients with diabetes is often accelerated compared with healthy individuals (Schvarcz et al 1997). This may, at least in part, be due to deficient amylin secretion in response to meals. Since gastric emptying is the rate-limiting step for glucose entering the circulation, accelerated gastric emptying may exacerbate postprandial glucose excursions in patients with diabetes. Pramlintide slows gastric emptying, limiting the rate at which nutrients are delivered from the stomach to the small intestine and subsequently, the rate at which glucose enters the circulation following meals. Pramlintide administered to patients with diabetes slows gastric emptying of both solid (Figure 2A) and liquid meals (Kong et al 1997, 1998). This effect results in a decreased postprandial glucose excursion, but does not affect the net absorption of nutrients.
Figure 2.
Pramlintide mechanisms of action. (A) Pramlintide (30 μg or 60 μg) or placebo were injected subcutaneously 15 minutes prior to a solid meal in patients with type 1 diabetes (n=11). Pramlintide significantly increased the gastric half-emptying compared to placebo (*indicates p<0.004) (adapted from Kong et al 1998). (B) Patients with type 1 (n=9) and type 2 (n=12) diabetes were infused with either pramlintide (type 1 at 25 μg/h, type 2 at 100 μg/h) or placebo for 5 hours during a SUSTACAL® meal challenge test. Pramlintide significantly reduced postprandial plasma glucagon concentrations in patients with both type 1 and type 2 diabetes compared with placebo control (p<0.05) (adapted from Fineman et al 2002). (C) Postprandial satiety quotients (5 h minus individual end of buffet VAS hunger ratings/total energy intake) were calculated for patients with type 2 diabetes (n=11) receiving a single subcutaneous injection of either placebo or 120 μg pramlintide prior to an ad libitum buffet meal. Pramlintide treatment significantly increased the postprandial satiety quotient (*indicates p=0.029) compared with placebo (adapted from Chapman et al 2005).
Abbreviations: SE, standard error of mean.
Pramlintide also suppresses the inappropriately elevated postprandial glucagon secretion that is often seen in patients with diabetes. Glucagon, secreted from pancreatic alpha cells, is a catabolic hormone that stimulates hepatic glucose production in the fasting state to protect against hypoglycemia. Normally, glucagon secretion is suppressed after meals. However, in patients with type 1 and type 2 diabetes, postprandial glucagon secretion is abnormally elevated. This inappropriate secretion of glucagon leads to excess hepatic glucose production and is an important contributor to postprandial hyperglycemia in patients with diabetes (Unger et al 1970; Unger and Orci 1977). In clinical studies of patients with type 1 and type 2 diabetes, pramlintide administered prior to a meal significantly reduced postprandial glucagon secretion compared with placebo (Fineman, Koda, et al 2002; Fineman, Weyer, et al 2002) (Figure 2B).
Finally, pramlintide affects satiety, which leads to a reduced caloric intake. A recent study in patients with type 2 diabetes and overweight subjects without diabetes demonstrated that a single, preprandial, subcutaneous injection of pramlintide reduced food intake by 16% to 23% without changing mean meal duration (Chapman et al 2005).
Clinical efficacy
Glycemic control
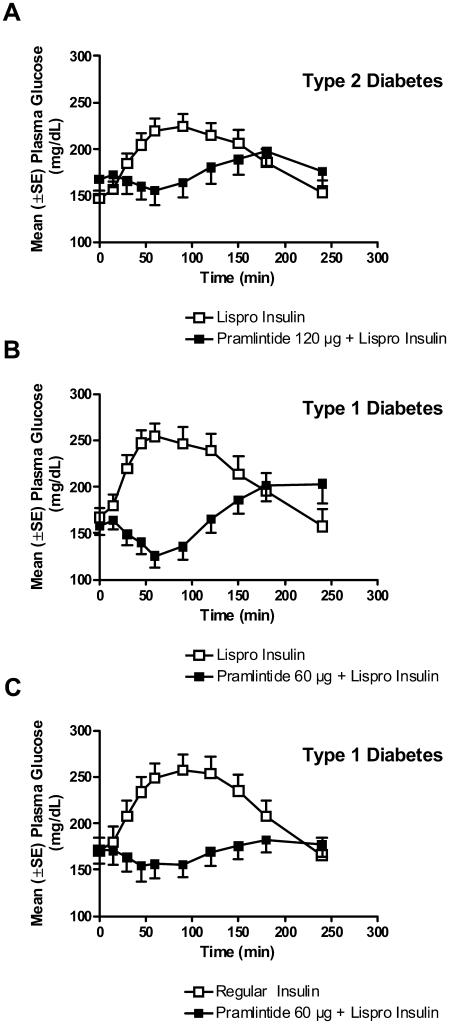
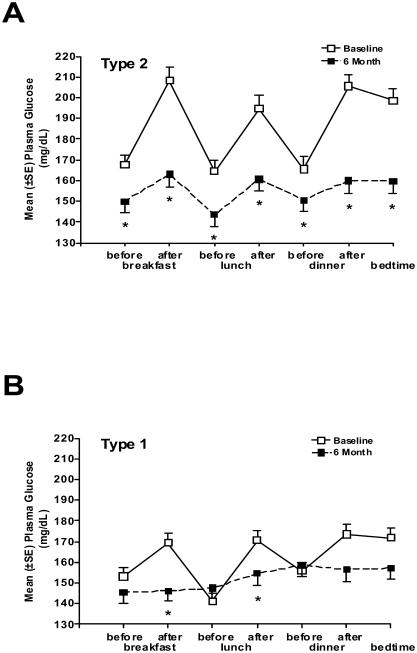
Pramlintide's mechanisms of action collectively reduce postprandial glucose concentrations. The effects of pramlintide on the appearance of glucose in the circulation during the postprandial period have been demonstrated in several clinical studies. In patients with type 2 diabetes using insulin lispro and type 1 subjects using either insulin lispro or regular human insulin, pramlintide administered immediately prior to a standardized meal significantly reduced postprandial glucose excursions compared with insulin therapy alone (Weyer et al 2003; Maggs et al 2004) (Figure 3). Another study in patients with type 1 diabetes using a continuous glucose monitoring system (CGMS) demonstrated that following four weeks of pramlintide treatment, glucose excursions were attenuated, resulting in a reduction of ∼2.5 hours per day spent in the hyperglycemic range (>140 mg/dL) compared with baseline (Levetan et al 2003). Finally, in an open-label clinical practice study, glucose fluctuations were examined in patients with type 1 and type 2 diabetes using self-monitored blood glucose (SMBG) 7-point glucose profiles. Patients recorded blood glucose values before and after the three major meals and before bedtime on a single day at baseline while treated with insulin alone, and again following 6 months of pramlintide therapy as an adjunct to insulin. Six months of pramlintide treatment significantly reduced postprandial blood glucose excursions in both patients with type 1 and type 2 diabetes (Guthrie et al 2005; Karl et al 2005) (Figure 4A, 4B). In each of these studies, improved postprandial glucose control was observed in the context of significant reductions in mealtime insulin (Levetan et al 2003; Weyer et al 2003; Maggs et al 2004; Guthrie et al 2005; Karl et al 2005).
Figure 3.
Pramlintide reduces postprandial glucose concentrations. (A) Patients with type 2 diabetes (n=19) were administered placebo or 120 μg pramlintide and rapid-acting insulin lispro immediately prior to a meal. Patients with type 1 diabetes were administered placebo or 60 μg pramlintide with either (B) rapid acting insulin lispro (n=21) or (C) regular human insulin (n=19) immediately prior to a meal. In all cases, pramlintide significantly reduced postprandial glucose concentrations in the postprandial period compared with placebo controls (p<0.05) (adapted from Weyer et al 2003; Maggs et al 2004; and SYMLIN 2005).
Abbreviations: SE, standard error of mean.
Figure 4.
Pramlintide reduces diurnal glucose fluctuations. Patients with type 1 or type 2 diabetes in an open-label study self-monitored blood glucose for a 7 point glucose profile at baseline and following 6-months of pramlintide treatment. Readings were recorded before and after the 3 major meals of the day and before bedtime. (A) In patients with type 2 diabetes (n=166), pramlintide 120 μg three times daily (TID) and twice daily (BID) significantly reduced blood glucose concentrations following all three major meals (*indicates p<0.05). (B) In patients with type 1 diabetes (n=265), pramlintide 30–60 μg TID and four times daily (QID) significantly reduced blood glucose concentrations following breakfast and lunch (*indicates p<0.05) (Guthrie et al 2005; Karl et al 2005).
Abbreviations: SE, standard error of mean.
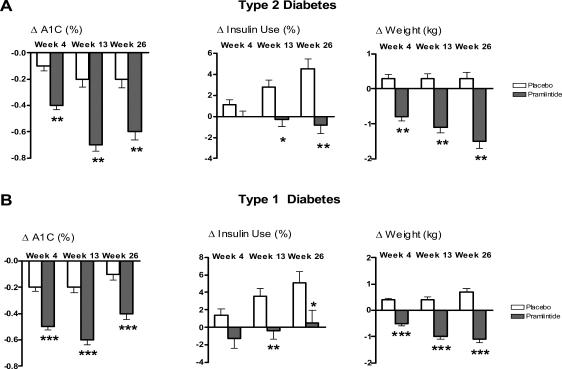
In placebo-controlled long-term clinical studies in insulin-using patients with type 1 and type 2 diabetes, pramlintide treatment also improved overall glycemic control as measured by A1C (Ratner et al 2002, 2004; Whitehouse et al 2002; Hollander et al 2003). Pramlintide significantly reduced A1C over 26 weeks with mean reductions of approximately 0.6% in patients with type 2 diabetes at the 120 μg dose (p<0.0001) and 0.4% in patients with type 1 diabetes at 30 μg to 60 μg doses (p<0.0001) (Figure 5A and 5B). Reductions in placebo-treated patients were approximately 0.1% and 0.2% in type 1 and type 2 patients, respectively. In these studies, improved glycemic control was achieved over 6 months with significant reductions compared with placebo controls in total daily insulin dose in both patients with type 1 (p<0.05) and type 2 (p<0.0001) diabetes (Figure 5), consistent with insulin and pramlintide having complementary roles in postprandial glucose homeostasis. A1C reductions were sustained up to 52 weeks in a placebo-controlled setting in both type 1 and type 2 patients, as well as for an additional 52 weeks during an open-label extension (104 weeks total) in patients with type 1 diabetes.
Figure 5.
Results for combined pivotal studies with pramlintide in patients with type 1 and type 2 diabetes. The change in A1C, total daily insulin use and weight at 4, 13, and 26 weeks are expressed as mean ± SE, ITT observed. (A) Patients with type 2 diabetes at 120 μg pramlintide BID (n=292) versus placebo (n=284), *p<0.01, **p<0.0001. (B) Patients with type 1 diabetes, 30–60μg doses TID and QID (n=716) versus placebo (n=538), *p<0.05, **p<0.01, ***p<0.0001 (combined data adapted from Ratner et al 2002, 2004; Whitehouse et al 2002; Hollander et al 2003).
Abbreviations: A1C, glycosylated hemoglobin; ITT, intention to treat; QID, four times daily; SE, standard error of mean; TID, three times daily.
As mentioned previously, long-term, prospective clinical trials have demonstrated that improvement of overall glucose control (A1C) results in reduced risk of development and progression of microvascular complications (DCCT 1993; UKPDS 1998). Long-term follow-up data from patients with type 1 diabetes (DCCT/EDIC 2005) have demonstrated that intensive treatment leading to improved glycemic control also reduces long-term cardiovascular complications. Clinical trials that address this relationship in patients with type 2 diabetes are currently ongoing (Action to Control Cardiovascular Risk in Diabetes [ACCORD] and Veterans Affairs Diabetes Trial [VADT]) (Abraira et al 2003; ACCORD 2006).
Recently, increasing emphasis has been placed on the importance of controlling postprandial glucose excursions. Postprandial glucose control is potentially important for at least two reasons. First, it is an important component of overall glycemic exposure (A1C). Studies have demonstrated that at A1C levels approaching the American Diabetes Association target of <7.0% (ADA 2005), the relative contributions of postprandial glucose and fasting glucose to overall glycemic control is approximately 70% and 30%, respectively (Monnier et al 2003). Second, independent of its effect on A1C, postprandial hyperglycemia has been implicated in the development of vascular diabetes complications. Brownlee and Hirsch (2005) have postulated that A1C may be an incomplete measure of glucose control and that glucose fluctuations should also be taken into account when assessing a patient's risk of potential complications. Their hypothesis is based on data from the DCCT which demonstrated that at similar A1C levels, conventionally treated patients had an increased risk of retinopathy compared with intensively treated patients. This may have been due to reduced glucose fluctuations in the intensively treated patients who were treated with prandial insulin at each meal. A large body of epidemiological data also supports the potential deleterious effects of abnormal postprandial glucose excursions in persons with impaired glucose tolerance and patients with diabetes (Donahue et al 1987; Lowe et al 1997; DECODE 1999; de Vegt et al 1999; Rodriguez et al 1999; Tominaga et al 1999). The mechanisms by which postprandial hyperglycemia may increase the risk of diabetic complications are emerging and the leading hypothesis appears to be glucose-induced generation of oxidative stress in the postprandial period (Brownlee 2005; Ceriello 2005; Hirsch and Brownlee 2005).
Effects on weight
Weight gain is associated with the majority of antihyperglycemic therapies and is often cited as one of the primary reasons for patient non-compliance with insulin therapy (Edelman and Weyer 2002). Excess body weight is not only a cosmetic problem for patients, but also has deleterious effects on glucose homeostasis due to its effects on insulin resistance (Edelman and Weyer 2002). Furthermore, obesity increases cardiovascular risk due to effects on blood pressure and lipids (Alexander 2001; Vidal 2002) and also appears to have effects independent of these established risk factors (Yan et al 2006). Weight control is recognized as an important component of managing diabetes and moderate reductions in weight (5% to 10%) often result in improvements in glycemic control and cardiovascular health, particularly in patients with type 2 diabetes (Goldstein 1992; Williamson et al 2000; Vidal 2002).
Clinical studies have shown that improved glycemic control in pramlintide-treated patients is not accompanied by weight gain, and instead is associated with a sustained and significant reduction in body weight (Ratner et al 2002, 2004; Whitehouse et al 2002; Hollander et al 2003). Combined data from pramlintide clinical trials showed significant (p<0.0001) reductions in body weight at 26 weeks of approximately 1.5 kg in patients with type 2 diabetes (Figure 5A) and 1.2 kg in patients with type 1 diabetes (Figure 5B) compared with an average gain of 0.2 kg to 0.5 kg, respectively, in placebo-treated patients. In long-term trials, weight reductions were sustained up to 52 weeks in patients with both type 1 and type 2 diabetes. Stratification of patients with type 2 diabetes based on baseline body mass index (BMI) demonstrated that body weight reductions were greatest in patients who were overweight or obese, and that pramlintide was weight-neutral in lean patients (Hollander et al 2004). Furthermore, weight reduction in pramlintide treated patients occurred without prescribed modification of diet or exercise routines (Ratner et al 2002, 2004; Whitehouse et al 2002; Hollander et al 2003).
One potential contributor to pramlintide-dependent reductions in body weight may be pramlintide-induced satiety. Chapman and colleagues (2005) observed that single dose of pramlintide prior to a buffet meal reduced food intake without affecting mean meal duration or macronutrient preferences. Similar hunger and fullness ratings were reported following pramlintide and placebo treatment, despite significantly reduced caloric intake in pramlintide-treated patients during the buffet meal. These data suggest that pramlintide exerts a satiety effect resulting in reduced food intake.
Safety and tolerability
The primary adverse events associated with pramlintide therapy apart from hypoglycemia were nausea, vomiting, and anorexia (Ratner et al 2002, 2004; Whitehouse et al 2002; Hollander et al 2003). Gastrointestinal side effects were more common in patients with type 1 diabetes, were generally mild to moderate, and diminished over time. Gradual dose-titration of pramlintide during the initiation period (initial 4 weeks of treatment) effectively reduced the incidence and severity of nausea in patients with type 1 diabetes (Edelman et al 2006). Importantly, the weight reductions associated with pramlintide treatment appear to be independent of pramlintide-induced nausea since stratification of patients into groups that either did or did not experience nausea during pramlintide treatment did not reveal significant differences in body weight reductions (Hollander et al 2003).
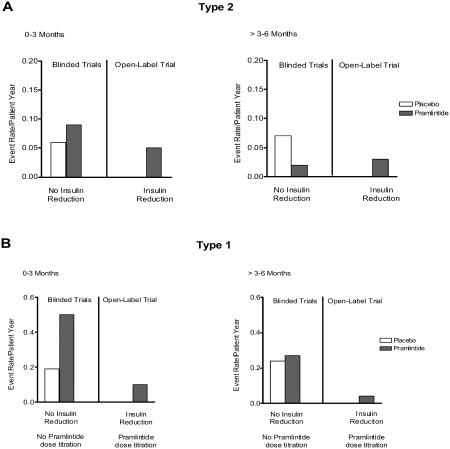
Although pramlintide itself does not cause hypoglycemia, the addition of any antihyperglycemic agent to an insulin regimen can increase the risk of severe insulin-induced hypoglycemia. In the clinical development program, pramlintide was studied in insulin-using patients with type 2 diabetes and patients with type 1 diabetes. To isolate the effect of pramlintide during the initial clinical trials, insulin dosages were not reduced when pramlintide was introduced and an increase in severe hypoglycemia was often observed. In subsequent clinical trials, mealtime insulin was proactively reduced by 30% to 50% during initiation of pramlintide treatment and, in patients with type 1 diabetes, pramlintide dose was also progressively escalated based on tolerability to nausea. Together these measures reduced the incidence of severe hypoglycemia in patients with both type 1 and type 2 diabetes (Edelman et al 2006; SYMLIN 2005). Therefore, the risk of hypoglycemia in pramlintide-treated patients is manageable with careful titration of insulin and pramlintide during initiation (Figure 6).
Figure 6.
Annual event rate of medically assisted severe hypoglycemia in patients with type 1 and type 2 diabetes from the combined pivotal trials and an open-label trial. (A) In blinded pivotal trials where there was no insulin reduction, the event rate for hypoglycemia in patients with type 2 diabetes treated with pramlintide was higher in the first three months (n=292) compared with those treated with placebo (n=284). In the second three months of treatment, the event rate for patients treated with pramlintide was less than placebo controls. In a subsequent open-label trial, in patients with type 2 diabetes (n=166) reduction of mealtime insulin and titration of pramlintide during initiation further decreased the incidence of severe hypoglycemia. (B) In blinded pivotal trials of patients with type 1 diabetes where there was no insulin reduction and no titration of pramlintide during initiation, the event rate of severe hypoglycemia in patients treated with pramlintide (n=716) was increased in the first three months compared with placebo (n=538), but was reduced to similar levels as placebo in months 3 to 6. In a subsequent open label study, titration of pramlintide during initiation combined with reduction of mealtime insulin further reduced the event rate of severe hypoglycemia in patients with type 1 diabetes (n=265) (combined data adapted from Ratner et al 2002, 2004; Whitehouse et al 2002; Hollander et al 2003; Karl et al 2005; Guthrie et al 2005; SYMLIN 2005).
Pramlintide indications and dosing
The clinical data presented above culminated in the current approved clinical indication for pramlintide as an adjunct to mealtime insulin in patients with type 1 or type 2 diabetes who have failed to reach desired glucose control despite optimal insulin therapy. For patients with type 2 diabetes, pramlintide can be used as an adjunct to mealtime insulin with or without concurrent oral sulfonylurea agents or metformin. Dosing of pramlintide differs depending on whether the patient has type 1 or type 2 diabetes. In patients with type 2 diabetes, pramlintide should be initiated at a dose of 60 μg, and subsequently increased to a maintenance dose of 120 μg based on tolerability of nausea. In patients with type 1 diabetes, pramlintide should be initiated at a dose of 15 μg and titrated upward in 15 μg increments to a maintenance dose of 60 μg based on tolerability to nausea. If doses of 45 μg and 60 μg are not well-tolerated, a maintenance dose of 30 μg can be used in patients with type 1 diabetes. Pramlintide should always be administered in a separate syringe and at a distinct injection site more than two inches away from concomitant insulin injections (SYMLIN 2005).
Pramlintide is used as an adjunct to insulin and has been associated with an increased risk of insulin-induced severe hypoglycemia, particularly in patients with type 1 diabetes. To minimize the risk of insulin-induced hypoglycemia, all patients should reduce premeal insulin by 50% upon initiation of pramlintide therapy, monitor blood glucose frequently, and contact their healthcare provider if symptoms of nausea and/or hypoglycemia are persistent. Careful patient selection, patient instruction, and insulin dose adjustments are critical for reducing the risk of hypoglycemia (SYMLIN 2005).
Patients considering pramlintide therapy should have an A1C <9%. Postprandial hyperglycemia is the major contributor to overall hyperglycemia as patients approach a more optimum A1C range. Pramlintide targets postprandial glucose concentrations and should be considered as a second-line therapy for those patients (type 2 or type 1 diabetes) who have an A1C <9%, but are unable to reach desired A1C targets. Due to its effects on gastric emptying, pramlintide should not be used in patients with diagnosed gastroparesis or in patients that require drugs that stimulate gastrointestinal motility. In addition, pramlintide should not be used in patients with hypoglycemia unawareness (SYMLIN 2005).
Conclusions
Pramlintide is a first-in-class amylinomimetic therapy for insulin-using patients with diabetes that reduces postprandial hyperglycemic excursions and improves overall glycemic control with concomitant reductions in weight and insulin use. There is accumulating evidence that postprandial glucose fluctuations are a key component to the management of overall glycemia and an important predictor of cardiovascular risks associated with diabetes. The actions of pramlintide to slow gastric emptying, suppress inappropriately elevated postprandial glucagon secretion, and reduce food intake act collectively to limit postprandial glucose fluctuations and improve overall glycemic control. The detrimental effects of excess weight gain associated with many current treatments for hyperglycemia and the cardiovascular risks associated with obesity make weight control another important aspect of therapeutic intervention in the treatment of diabetes. Improvements in glycemic control with pramlintide occur in the context of reductions in weight, making it an additional therapeutic choice for many insulin-using patients with type 1 or type 2 diabetes. Together, the benefits offered by pramlintide may address an important deficit in the treatment of diabetes. New therapies may initiate a new phase of diabetes management where multiple glucoregulatory agonists will be used to more closely approximate normal glucose homeostasis in patients with diabetes and may contribute to prevention of the associated vascular complications of the disease.
References
- Abraira C, Duckworth W, McCarren M, et al. VA Cooperative Study of Glycemic Control and Complications in Diabetes Mellitus Type 2 Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003;17:314–22. doi: 10.1016/s1056-8727(02)00277-5. [DOI] [PubMed] [Google Scholar]
- ACCORD. ACCORD trial [online] 2006 Accessed 10 January 2006. URL: http://www.accordtrial.org/
- Alexander JK. Obesity and coronary heart disease. Am J Med Sci. 2001;321:215–24. doi: 10.1097/00000441-200104000-00002. [DOI] [PubMed] [Google Scholar]
- [ADA] American Diabetes Association. Diabetes vital statistics. Alexandria, VA: ADA; 2001. [Google Scholar]
- [ADA] American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(S1):4–36. [Google Scholar]
- Bhavsar S, Watkins J, Rink T. Synergistic effect of amylin and cholecystokinine octapeptide on food intake in mice. Diabetes. 1996;45(Suppl2):333A. [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- Chapman I, Parker B, Doran S, et al. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia. 2005;48:838–48. doi: 10.1007/s00125-005-1732-4. [DOI] [PubMed] [Google Scholar]
- [DCCT] Diabetes Control and Complications Trial Research Group. Weight gain associated with intensive therapy in the diabetes control and complications trial. Diabetes Care. 1988;11:567–73. doi: 10.2337/diacare.11.7.567. [DOI] [PubMed] [Google Scholar]
- [DCCT] Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- [DCCT] Diabetes Control and Complications Trial Research Group. The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45:1289–98. [PubMed] [Google Scholar]
- [DCCT/EDIC] Diabetes Control and Complications Trial Research Group, Epidemiology of Diabetes Interventions and Complications Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [DECODE] DECODE Study Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:617–21. [PubMed] [Google Scholar]
- de Vegt F, Dekker JM, Ruhe HG, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999;42:926–31. doi: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- Donahue RP, Abbott RD, Reed DM, et al. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987;36:689–92. doi: 10.2337/diab.36.6.689. [DOI] [PubMed] [Google Scholar]
- Edelman SV, Weyer C. Unresolved challenges with insulin therapy in type 1 and type 2 diabetes: potential benefit of replacing amylin, a second beta-cell hormone. Diabetes Technol Ther. 2002;4:175–89. doi: 10.1089/15209150260007390. [DOI] [PubMed] [Google Scholar]
- Edelman SV, Garg SK, Frias JP, et al. A double-blind, placebo-controlled trial asessing pramlintide teatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care. 2006 doi: 10.2337/dc06-0042. in press. [DOI] [PubMed] [Google Scholar]
- Fineman MS, Giotta MP, Thompson RG, et al. Amylin response following Sustacal ingestion is diminished in type II diabetic patients treated with insulin. Diabetologia. 1996;39(Suppl 1):A149. [Google Scholar]
- Fineman MS, Koda JE, Shen LZ, et al. The human amylin analog, pramlintide, corrcts postprandial hyperglucagonemia in patients with type 1 diabetes. Metabolism. 2002;51:636–41. doi: 10.1053/meta.2002.32022. [DOI] [PubMed] [Google Scholar]
- Fineman M, Weyer C, Maggs DG, et al. The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res. 2002;34:504–8. doi: 10.1055/s-2002-34790. [DOI] [PubMed] [Google Scholar]
- Gaba MK, Gaba S, Clark LT. Cardiovascular disease in patients with diabetes: clinical considerations. J Assoc Acad Minor Phys. 1999;10:15–22. [PubMed] [Google Scholar]
- Gedulin B, Jodka D, Green D, et al. Amylin inhibition of arginine-induced glucagon secretion: comparison with glucagon like peptide-1 (7-36)-amide (GLP-1) Diabetologia. 1996;39(Suppl 1):A154. [Google Scholar]
- Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- Guthrie R, Karl DM, Wang Y, et al. In an open-label clinical study pramlintide lowered A1c, body weight, and insulin use in patients with type 1 diabetes failing to achieve glycemic targets with insulin therapy. Diabetes. 2005;54(Suppl 1A):118. [Google Scholar]
- Hirsch IB. Type 1 diabetes mellitus and the use of flexible insulin regimens. Am Fam Physician. 1999;60:2343–52. 2355–6. [PubMed] [Google Scholar]
- Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178–81. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784–90. doi: 10.2337/diacare.26.3.784. [DOI] [PubMed] [Google Scholar]
- Hollander P, Maggs DG, Ruggles JA, et al. Effect of pramlintide on weight in overweight and obese insulin-treated type 2 diabetes patients. Obes Res. 2004;12:661–8. doi: 10.1038/oby.2004.76. [DOI] [PubMed] [Google Scholar]
- Karl DM, Wang Y, Lorenzi G, et al. In an open-label clinical study pramlintide lowered A1c, body weight, and insulin use in patients with type 2 diabetes failing to achieve glycemic targets with insulin therapy. Diabetes. 2005;54(Suppl 1):A12. [Google Scholar]
- Kong MF, King P, Macdonald IA, et al. Infusion of pramlintide, a human amylin analogue, delays gastric emptying in men with IDDM. Diabetologia. 1997;40:82–8. doi: 10.1007/s001250050646. [DOI] [PubMed] [Google Scholar]
- Kong MF, Stubbs TA, King P, et al. The effect of single doses of pramlintide on gastric emptying of two meals in men with IDDM. Diabetologia. 1998;41:577–83. doi: 10.1007/s001250050949. [DOI] [PubMed] [Google Scholar]
- Kruger DF, Gatcomb PM, Owen SK. Clinical implications of amylin and amylin deficiency. Diabetes Educ. 1999;25:389–97. doi: 10.1177/014572179902500310. [DOI] [PubMed] [Google Scholar]
- Kruger DF, Gloster MA. Pramlintide for the treatment of insulinrequiring diabetes mellitus: rationale and review of clinical data. Drugs. 2004;64:1419–32. doi: 10.2165/00003495-200464130-00003. [DOI] [PubMed] [Google Scholar]
- Levetan C, Want LL, Weyer C, et al. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care. 2003;26:1–8. doi: 10.2337/diacare.26.1.1. [DOI] [PubMed] [Google Scholar]
- Lowe LP, Liu K, Greenland P, et al. Diabetes, asymptomatic hyperglycemia, and 22-year mortality in black and white men. The Chicago Heart Association Detection Project in Industry Study. Diabetes Care. 1997;20:163–9. doi: 10.2337/diacare.20.2.163. [DOI] [PubMed] [Google Scholar]
- Maggs DG, Fineman M, Kornstein J, et al. Pramlintide reduces postprandial glucose excursions when added to insulin lispro in subjects with type 2 diabetes: a dose-timing study. Diabetes Metab Res Rev. 2004;20:55–60. doi: 10.1002/dmrr.419. [DOI] [PubMed] [Google Scholar]
- Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med. 1991;151:1141–7. [PubMed] [Google Scholar]
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c) Diabetes Care. 2003;26:881–5. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Hokanson JE, Marcovina SM, et al. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280:140–6. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus; from promotion to alleviation of obesity. Treat Endocrinology. 2003;2:33–47. doi: 10.2165/00024677-200302010-00004. [DOI] [PubMed] [Google Scholar]
- Ratner RE, Want LL, Fineman MS, et al. Adjunctive therapy with the amylin analogue pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with type 2 diabetes. Diabetes Technol Ther. 2002;4:51–61. doi: 10.1089/15209150252924094. [DOI] [PubMed] [Google Scholar]
- Ratner RE, Dickey R, Fineman M, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabetic Medicine. 2004;21:1204–12. doi: 10.1111/j.1464-5491.2004.01319.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez BL, Lau N, Burchfiel CM, et al. Glucose intolerance and 23-year risk of coronary heart disease and total mortality: the Honolulu Heart Program. Diabetes Care. 1999;22:1262–5. doi: 10.2337/diacare.22.8.1262. [DOI] [PubMed] [Google Scholar]
- Schvarcz E, Palmer M, Aman J, et al. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulindependent diabetes mellitus. Gastroenterology. 1997;113:60–6. doi: 10.1016/s0016-5085(97)70080-5. [DOI] [PubMed] [Google Scholar]
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- [SYMLIN] Amylin Pharmaceuticals, Inc. SYMLIN prescribing information [online] 2005 Accessed on 10 January 2006. URL: http://symlin.com/pdf/SYMLIN-pi-combined.pdf.
- Tominaga M, Eguchi H, Manaka H, et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–4. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- [UKPDSG] UK Prospective Diabetes Study Group. Overview of 6 years' therapy of type II diabetes: a progressive disease (UKPDS 16) Diabetes. 1995;44:1249–58. [PubMed] [Google Scholar]
- [UKPDSG] UK Prospective Diabetes Study Group. Intensive bloodglucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in subjects with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- Unger RH, Aguilar-Parada E, Muller WA, et al. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49:837–48. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Orci L. The role of glucagon in the endogenous hyperglycemia of diabetes mellitus. Ann Rev Med. 1977;28:119–30. doi: 10.1146/annurev.me.28.020177.001003. [DOI] [PubMed] [Google Scholar]
- Vidal J. Updated review on the benefits of weight loss. Int J Obes Relat Metab Disord. 2002;26(Suppl 4):S25–8. doi: 10.1038/sj.ijo.0802215. [DOI] [PubMed] [Google Scholar]
- Weyer C, Gottlieb A, Kim DD, et al. Pramlintide reduces postprandial glucose excursions when added to regular insulin or insulin lispro in subjects with type 1 diabetes: a dose-timing study. Diabetes Care. 2003;26:3074–9. doi: 10.2337/diacare.26.11.3074. [DOI] [PubMed] [Google Scholar]
- Whitehouse F, Kruger DF, Fineman M, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002;25:724–30. doi: 10.2337/diacare.25.4.724. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Thompson TJ, Thun M, et al. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care. 2000;23:1499–504. doi: 10.2337/diacare.23.10.1499. [DOI] [PubMed] [Google Scholar]
- Yan LL, Daviglus ML, Liu K, et al. Midlife body mass index and hospitalization and mortality in older age. JAMA. 2006;295:190–8. doi: 10.1001/jama.295.2.190. [DOI] [PubMed] [Google Scholar]
- Young AA, Gedulin BR, Rink TJ. Dose-responses for the slowing of gastric emptying in a rodent model by glucagon-like peptide (7-36) NH2, amylin, cholecystokinin, and other possible regulators of nutrient uptake. Metabolism. 1996;45:1–3. doi: 10.1016/s0026-0495(96)90192-4. [DOI] [PubMed] [Google Scholar]
- Young AA, Vine W, Gedulin BR, et al. Preclinical pharmacology of pramlintide in the rat: comparisons with human and rat amylin. Drug Dev Res. 1996;37:231–48. [Google Scholar]
- Young AA. Amylin's physiology and its role in diabetes. Curr Opin Endocrinol Diabetes. 1997;4:282–90. [Google Scholar]