Abstract
Background
Angiotensin-converting enzyme inhibitors (ACEI) have a well-established role in the prevention of cardiovascular events in hypertension, left ventricular dysfunction, and heart failure. More recently, ACEI have been shown to prevent cardiovascular events in individuals with increased cardiovascular risk, where hypertension, left ventricular dysfunction, or heart failure was not the primary indication for ACEI therapy.
Objective
To review studies of the effects of the ACEI perindopril on cardiovascular events.
Method
The EUROPA (European Trial on Reduction of Cardiac Events with Perindopril in Patients with Stable Coronary Artery Disease Study), PROGRESS (Perindopril Protection Against Recurrent Stroke Study), and ASCOT-BPLA (Anglo-Scandinavian Cardiac Outcomes Trial – Blood Pressure Lowering Arm) trials are reviewed.
Results
Perindopril alone reduced cardiovascular events in subjects with stable coronary heart disease. Perindopril in combination with indapamide reduced cardiovascular events in subjects with cerebrovascular disease. Perindopril in combination with amlodipine reduced cardiovascular events in subjects with hypertension.
Conclusion
Perindopril reduced cardiovascular events. The reduction of cardiovascular events by perindopril was in large part associated with reduction of blood pressure, and greater reduction in cardiovascular events was associated with greater reduction of blood pressure. Perindopril may need to be combined with other antihypertensive agents to maximize reduction of cardiovascular events.
Keywords: Angiotensin-converting enzyme inhibitor, hypertension, coronary heart disease, stroke, myocardial infarction, heart failure
Introduction
Angiotensin-converting enzyme inhibitors (ACEI) have a well established role in prevention of cardiovascular events in hypertension (Chobanian et al 2003; BPLTTC 2005), left ventricular dysfunction (Flather et al 2000), and heart failure (Flather et al 2000; Remme and Swedberg 2001; Hunt et al 2005). More recently, ACEI have been shown to prevent cardiovascular events in individuals with increased cardiovascular risk, where hypertension, left ventricular dysfunction, or heart failure was not the primary indication for ACEI therapy (HOPE 2000; PROGRESS 2001, 2003; EUROPA 2003). This review will summarise and comment on three recent studies of the effects of the ACEI perindopril on cardiovascular events, the EUROPA (European Trial on Reduction of Cardiac Events with Perindopril in Patients with Stable Coronary Artery Disease Study) and PROGRESS (Perindopril Protection Against Recurrent Stroke Study) studies where the effects of perindopril were studied alone (PROGRESS 2001, 2003; EUROPA 2003), or in combination with the diuretic indapamide (PROGRESS 2001, 2003), and the ASCOT-BPLA (Anglo-Scandinavian Cardiac Outcomes Trial – Blood Pressure Lowering Arm) study where perindopril was added to therapy with the calcium-channel blocker amlodipine (Dahlof et al 2005).
ASCOT-BPLA provides no direct evidence about the effects of perindopril on cardiovascular events (Dahlof et al 2005). However, it does provide limited evidence for the effect of perindopril in combination with amlodipine on cardiovascular events. The vast majority of subjects allocated to amlodipine-based therapy in ASCOT-BPLA required one or more additional antihypertensive agents which, for 59% of subjects, included perindopril. Thus, perindopril played an essential role in blood pressure (BP) reduction for most subjects allocated to the amlodipine-based regimen.
EUROPA
EUROPA was a randomized placebo-controlled, double-blind study of the effects of perindopril therapy on cardiovascular events in 12 218 subjects with previous myocardial infarction (MI), angiographic evidence of coronary heart disease (CHD), coronary revascularization, or a positive stress test (Table 1) (EUROPA 2003). Past history of heart failure was recorded in 1.3% of subjects, but none had clinical signs of heart failure, with 10% in New York Heart Association class I and none in class II or higher. Subjects were randomized to either 8 mg perindopril or placebo.
Table 1.
Summary of EUROPA trial
| Inclusion criteria: Men and women, aged >18 years, with CHD (previous MI, PCI, CABG, or angiographic evidence*), and without clinical evidence of heart failure. Men were also recruited if they had a history of chest pain and a positive ECG, echocardiograph, or nuclear stress test. |
| Exclusion criteria: Clinical evidence of heart failure, planned revascularization, hypotension (SBP <110 mm Hg), uncontrolled hypertension (SBP >180 mm Hg, DBP >100 mm Hg, or both), recent use of ACEI or ARB, creatinine >0.15 mmol/L, serum potassium >5.5 mmol/L. |
| Primary outcome: composite of cardiovascular death, non-fatal MI, and cardiac arrest with successful resuscitation. |
| Secondary outcomes: composite of total mortality, non-fatal MI, hospital admission for unstable angina, and cardiac arrest with successful resuscitation; cardiovascular mortality and non-fatal MI, as well as individual components of these secondary outcomes and revascularization, stroke, and admission for heart failure. |
| Data derived from EUROPA 2003. |
| Baseline clinical characteristics | Perindopril (n=6110) | Placebo (n=6108) |
|---|---|---|
| Age, years (SD) | 60 (9) | 60 (9) |
| Female sex | 14.5% | 14.7% |
| History of CHD | ||
| MI | 64.9% | 64.7% |
| PCI | 29.0% | 29.5% |
| CABG | 29.3% | 29.4% |
| Documented CHD | ||
| Angiographic evidence* | 60.4% | 60.5% |
| Positive stress test† | 22.6% | 23.3% |
| Previous stroke or TIA | 3.4% | 3.3% |
| Peripheral vascular disease | 7.1% | 7.4% |
| Hypertension‡ | 27.0% | 27.2% |
| Diabetes mellitus | 11.8% | 12.8% |
| Hypercholesterolemia§ | 63.3% | 63.3% |
| Medication | ||
| Platelet inhibitors | 91.9% | 92.7% |
| Lipid-lowering therapy | 57.8% | 57.3% |
| β blockers | 62.0% | 61.3% |
| Calcium-channel blockers | 31.7% | 31.0% |
| Nitrates | 42.8% | 43.0% |
| Diuretics | 9.1% | 9.4% |
| SBP (SD) | 137 (16) | 137 (15) |
Angiographic evidence of CHD: stenosis > 70%.
Positive stress test: only in men.
Hypertension: BP > 160/95mm Hg or receiving antihypertensive treatment.
Hypercholesterolemia: cholesterol > 6.5 mmol/L or receiving lipidlowering treatment.
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin type 1 receptor blocker; CABG, coronary artery bypass graft; CHD, coronary heart disease; DBP, diastolic blood pressure; ECG, electrocardiograph; PCI, percutaneous coronary intervention, MI, myocardial infarction; SBP, systolic blood pressure; SD, standard deviation; TIA, transient ischemic attack.
It is notable that although only 27% of subjects had a diagnosis of hypertension (BP > 160/95 mm Hg or receiving antihypertensive treatment), the mean baseline systolic BP (SBP) was 137 mm Hg, with a standard deviation of 16 mm Hg, indicating that a sizable proportion were hypertensive (Chobanian et al 2003). In comparison with placebo, perindopril reduced BP by 5/2 mm Hg.
During a mean follow-up of 4.2 years, perindopril reduced the primary outcome (composite of cardiovascular death, non-fatal MI, cardiac arrest with successful resuscitation) from 9.9% to 8.0% (20% decrease, 95% confidence interval (CI) 9% to 29%; number needed to treat (NNT)=53). The main contributor to this reduction in the primary outcome was the reduction in non-fatal MI from 6.2% to 4.8% (22% decrease, 95% CI 10% to 33%; NNT=74); the reduction in cardiovascular mortality from 4.1% to 3.5% (14% decrease, 95% CI −3% to 28%) did not achieve statistical significance. The incidence of cardiac arrest was only 0.2% with placebo and 0.1% with perindopril (46% decrease, 95% CI −47% to 80%). Perindopril also reduced the incidence of heart failure requiring hospitalization from 1.7% to 1.0% (39% decrease, 95% CI 17% to 56%; NNT=153), but produced only a slight reduction in stroke from 1.7% to 1.6%. Perindopril produced similar benefits in both hypertensive (BP >160/95 mm Hg or receiving antihypertensive treatment on enrollment) and nonhypertensive subjects.
Perindopril produced similar effects in EUROPA participants with diabetes, although this substudy had insufficient power to show statistical significance (Daly et al 2005).
PROGRESS
The PROGRESS study was a randomized placebocontrolled double-blind study of the effects of perindoprilbased therapy on stroke and other cardiovascular outcomes in 6105 subjects with a history of ischemic or hemorrhagic stroke, or transient ischemic attack within the previous 5 years (Table 2) (PROGRESS 2001, 2003). Active therapy was 4 mg perindopril, with the addition of 2.5 mg indapamide (2 mg in Japan) if the physician felt there was no contraindication to a diuretic. Those for whom their physician recommended single drug therapy were randomized to receive either perindopril or single placebo. Those for whom their physician recommended combination drug therapy were randomized to receive either perindopril plus indapamide or double placebo.
Table 2.
Summary of PROGRESS trial
| Inclusion criteria: Men and women, aged >18 years, with history of stroke or TIA within the previous 5 years. Individuals with hypertension treated with agents other than ACEI. |
| Exclusion criteria: Definite indication for treatment with ACEI (such as heart failure); definite contraindication to treatment with ACEI (such as previous intolerance). |
| Primary outcome: total stroke (fatal and non-fatal). |
| Secondary outcomes: fatal or disabling stroke; total major vascular events comprising the composite of non-fatal stroke, non-fatal MI, or death due to any vascular cause (including unexplained sudden death); total and cause-specific deaths; and hospital admissions. Other outcomes analyzed included major coronary events (defined as non-fatal MI or death ascribed to CHD); total coronary events (non-fatal MI, death ascribed to CHD, coronary revascularization (PCI, CABG), or hospitalization due to unstable angina; congestive heart failure resulting in death, hospitalization, or requiring withdrawal of randomized therapy. Two subgroup analyses were prespecified: separate estimates of treatment effects for combination (perindopril and indapamide) therapy and single (perindopril alone) therapy; and separate estimates of treatment effects for participants classified as hypertensive (SBP >160 mm Hg or DBP >90 mm Hg) and those classified as nonhypertensive at enrollment. |
| Data derived from PROGRESS 2001, 2003. |
| Baseline clinical characteristics | Perindopril (n=3051) | Placebo (n=3054) |
|---|---|---|
| Age, years (SD) | 64 (10) | 64 (10) |
| Female sex | 30% | 30% |
| Asian* | 39% | 39% |
| Cerebro-vascular disease history | ||
| Ischemic stroke | 71% | 71% |
| Cerebral hemorrhage | 11% | 11% |
| Unknown stroke | 4% | 5% |
| TIA or amaurosis fugax | 22% | 22% |
| CHD† | 16% | 16% |
| Peripheral vascular disease | 4% | 4% |
| Hypertension‡ | 48% | 48% |
| Atrial fibrillation | 8% | 8% |
| LVH on ECG | 7% | 7% |
| Diabetes mellitus | 13% | 12% |
| Current smoker | 20% | 20% |
| Medication | ||
| Platelet inhibitors | 73% | 72% |
| Statins | 8% | 8% |
| β blockers | 17% | 18% |
| Calcium-channel blockers | 40% | 40% |
| Diuretics | 11% | 12% |
| Other antihypertensives | 11% | 12% |
| Oral anticoagulants | 9% | 10% |
| SBP (SD) | 147 (19) | 147 (19) |
Asian: participants recruited from People's Republic of China or Japan;
history of MI or coronary revascularization, or angina (supported by documented ECG or angiographic evidence of coronary disease);
Hypertension: SBP ≥160mm Hg or DBP ≥90 mm Hg.
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; CABG, coronary artery bypass graft; CHD, coronary heart disease; DBP, diastolic blood pressure; ECG, electrocardiograph; LVH, left ventricular hypertrophy; PCI, percutaneous coronary intervention, MI, myocardial infarction; SBP, systolic blood pressure; SD, standard deviation; TIA, transient ischemic attack.
Whereas all PROGRESS participants had cerebro-vascular disease, only 16% had CHD (history of MI or coronary revascularization, or angina). While there were no BP entry criteria, it was recommended that subjects enrolled in PROGRESS have their hypertension controlled by medication other than ACEI and angiotensin type 1 receptor blocker (ARB) before enrolment. Despite this recommendation, the mean SBP on enrolment was 147 mm Hg, and 48% had SBP >160 mm Hg or diastolic BP (DBP) >90 mm Hg (PROGRESS 2001, 2003). Heart failure was an exclusion criterion. In comparison with placebo, combination of perindopril and indapamide during the PROGRESS trial reduced BP by 12/5 mm Hg, whereas perindopril alone reduced BP by 5/3 mm Hg.
During a mean follow-up of 3.9 years, the combination of perindopril and indapamide reduced stroke incidence from 14.4% to 8.4% (43% decrease, 95% CI 30% to 54%; NNT=17), reduced major coronary events (non-fatal MI or death ascribed to CHD) from 5.7% to 3.9% (35% decrease, 95% CI 12% to 52%; NNT=51), and reduced heart failure incidence (hospitalization or requiring withdrawal of randomized therapy) from 4.6% to 3.1% (34% decrease, 95% CI 7% to 53%; NNT=66). Perindopril alone produced non-statistically significant reductions in stroke incidence from 12.9% to 12.3% (5% decrease, 95% CI −19% to 23%), major coronary events from 4.1% to 3.7% (7% decrease, 95% CI −37% to 38%) and heart failure incidence from 5.4% to 4.5% (16% decrease, 95% CI −19% to 41%). The effects of perindopril-based therapy were similar for both hypertensive (SBP ≥160 mm Hg or DBP ≥90 mm Hg on enrollment) and nonhypertensive subjects.
ASCOT-BPLA
ASCOT-BPLA was a prospective randomized comparison of amlodipine–perindopril and atenolol–bendro-flumethiazide antihypertensive regimens on non-fatal MI (including silent MI) and fatal CHD in 19 257 hypertensive subjects with at least three other cardiovascular risk factors (Table 3) (Dahlof et al 2005). The amlodipine-based regimen was 5–10 mg amlodipine with addition of 4–8 mg perindopril as required. The atenolol-based regimen was 50–100 mg atenolol with addition of 1.25–2.5 mg bendro-flumethiazide and potassium as required. By the end of the study, most subjects were taking at least two antihypertensive agents, and only 15% and 9% were taking amlodipine and atenolol monotherapy, respectively. On average, of total time, 83% were taking amlodipine as allocated, 79% were taking atenolol, 59% were taking perindopril, and 66% were taking bendroflumethiazide. Overall, throughout the trial, a mean of 50% were taking the combination of amlodipine with perindopril as allocated, with or without other antihypertensive drugs. Compared with those allocated to the atenolol-based regimen, BP values were lower throughout the trial in those allocated to the amlodipine-based regimen. These differences were largest (5.9/2.4 mm Hg) at 3 months, and the average difference throughout the trial was 2.7/1.9 mm Hg.
Table 3.
Summary of ASCOT-BLPA trial
| Inclusion criteria: Men and women, aged 40–70 years, with either untreated hypertension (SBP ≥160 mm Hg, DBP ≥100 mm Hg, or both) or treated hypertension with SBP ≥140 mm Hg or DBP ≥90 mm Hg or both. In addition, subjects had to have at least three of the following cardiovascular risk factors: LVH, specified abnormalities on ECG, type 2 diabetes, peripheral arterial disease, previous stroke or TIA, male sex, age ≥55 years, microalbuminuria or proteinuria, smoking, ratio of total/HDL cholesterol ≥6, or family history of premature CHD. |
| Exclusion criteria: Previous MI, currently treated angina, a cerebro-vascular event within the previous 3 months, fasting triglycerides >4.5 mmol/L, heart failure, uncontrolled arrhythmias, or clinically important hematological or biochemical abnormality. |
| Primary outcome: composite of non-fatal MI (including silent MI) and fatal CHD. |
| Secondary outcomes: all-cause mortality, total stroke, primary outcome minus silent MI, all coronary events, total cardiovascular events and procedures, cardiovascular mortality, and non-fatal and fatal heart failure. |
| Data derived from Dahlof et al 2005. |
| Baseline clinical characteristics | Amlodipine/Perindopril (n=9639) | Atenolol/Bendroflumethiazide (n=9618) |
|---|---|---|
| Age, years (SD) | 63 (9) | 63 (9) |
| Female sex | 23% | 23% |
| Previous stroke or TIA | 11% | 11% |
| Peripheral vascular disease | 6% | 6% |
| Diabetes mellitus | 27% | 27% |
| LVH | 22% | 22% |
| Atrial fibrillation | 1% | 1% |
| ECG abnormality other than LVH | 23% | 23% |
| Other relevant cardiovascular disease | 6% | 5% |
| Current smoker | 33% | 32% |
| Total cholesterol, mmol/L (SD) | 5.9 (1.1) | 5.9 (1.1) |
| LDL cholesterol, mmol/L (SD) | 3.8 (1.0) | 3.8 (1.0) |
| HDL cholesterol, mmol/L (SD) | 1.3 (0.4) | 1.3 (0.4) |
| Triglycerides mmol/L (SD) | 1.8 (1.0) | 1.9 (1.0) |
| Medication | ||
| Previous antihypertensive medications | ||
| None | 19% | 19% |
| 1 | 44% | 45% |
| ≥2 | 36% | 36% |
| Lipid-lowering therapy | 11% | 10% |
| Aspirin use | 19% | 19% |
| SBP, mm Hg (SD) | 164 (18) | 164 (18) |
Abbreviations: CHD, coronary heart disease; DBP, diastolic blood pressure; ECG, electrocardiograph; HDL, high density lipoprotein; LDL, low density lipoprotein; LVH, left ventricular hypertrophy; PCI, percutaneous coronary intervention, MI, myocardial infarction; SBP, systolic blood pressure; SD, standard deviation; TIA, transient ischemic attack.
ASCOT-BPLA was stopped prematurely after 5.5 years' median follow-up. Although the primary endpoint did not achieve statistical significance, compared with the atenolol–bendroflumethiazide regimen, individuals on the amlodipine–perindopril regimen had 10% (95% CI −2% to 21%) lower incidence of fatal CHD or non-fatal MI, 23% (95% CI 11% to 34%; NNT=101) lower incidence of fatal or non-fatal stroke, 16% (95% CI 10% to 22%; NNT=40) lower incidence of total cardiovascular events and procedures, and 11% (95% CI 1% to 19%; NNT=117) lower all-cause mortality. However, the reduction in fatal and non-fatal heart failure from 3.0% to 2.5% (16% decrease, 95% Perindopril and vcardiovascular events CI −5% to 34%) with the amlodipine–perindopril regimen did not achieve statistical significance.
Why does perindopril reduce cardiovascular events?
There is continuing debate about the role of BP reduction in the prevention of cardiovascular events by BP-lowering therapy. This debate is fuelled by studies showing decreases in cardiovascular event rate greater than can be accounted for by BP reduction (Lewis et al 1993; HOPE 2000; EUROPA 2003). This debate is based in part on the assumption that BP has a causal role in cardiovascular disease pathogenesis. This assumption may not, however, be correct, except in extreme situations such as flash pulmonary edema due to acute severe elevation of BP.
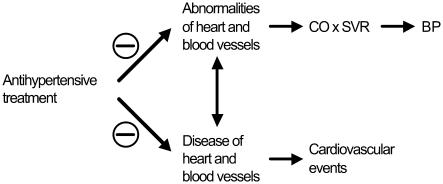
Rather than cause cardiovascular events, elevated BP may be the consequence of changes in the heart and vasculature that overlap those that lead to cardiovascular events (Figure 1). BP is the product of cardiac output (CO) and systemic peripheral resistance (SVR). Thus, change in BP is necessarily dependent upon change in cardiac and vascular function that produces change in CO and/or SVR. Many candidate mechanisms for the pathogenesis of hypertension have been proposed (Guyton et al 1995; Beevers et al 2001; Johnson et al 2002; Zandi-Nejad et al 2006), including the possible role of structural and functional changes in the vasculature (Mark 1984; Noon et al 1997; Simon 2004; Skov and Mulvany 2004; Wong et al 2004; Safar and Boudier 2005; Ikram et al 2006). Moreover, hypertension is associated with endothelial dysfunction and other risk factors for cardiovascular disease (Lip et al 1995, 1999; Landray et al 2002; King et al 2004). Of particular interest is the possibility that angiotensin II may participate in the pathogenesis of both hypertension and cardiovascular disease (Simon 2004).
Figure 1.
Possible relationship between heart and blood vessel abnormalities that influence cardiac output (CO) and systemic vascular resistance (SVR), thereby influencing blood pressure (BP), and heart and blood vessel diseases that cause cardiovascular events.
BP may therefore have little if any causal role in the pathogenesis cardiovascular events, and may show variable change in response to cardiovascular disease-reducing therapy. However, when this therapy does lower BP, even within the normal range, it can serve as a valuable marker of reduced cardiovascular risk. Most cardiovascular disease is associated with BP levels below the current definition of hypertension (WHO 2002), and there is a continuous association between usual BP and cardiovascular event risk down to 115 mm Hg SBP and 75 mm Hg DBP (PSC 2000).
There is ample evidence that perindopril has actions on the heart and blood vessels that may reduce cardiovascular disease. In addition to many studies in animals, studies in humans show perindopril corrected endothelial dysfunction (Bijlstra et al 1995; Watanabe et al 2005), and reduced ventricular remodelling following MI (Ferrari et al 2006). In addition, perindopril–indapamide improved arterial stiffness and wave reflection, and reduced left ventricular mass in hypertensive subjects (Asmar et al 2001; de Luca et al 2004), and reduced albuminuria in diabetic subjects (Mogensen et al 2003). Amlodipine–perindopril reduced central pulse pressure more than atenolol in a substudy of the ASCOT study, indicative of increased aortic compliance (CAFE 2006).
The EUROPA investigators proposed the reduction in cardiovascular events by perindopril in the EUROPA study was greater than might be expected for the observed reduction in BP (mean 5/2 mm Hg), and may have been due to antiatherosclerotic effects of ACE inhibition. In contrast to the EUROPA study, BP reduction was a key indicator of reduced cardiovascular event rates in the PROGRESS study, given the greater reductions in BP and cardiovascular events seen with 4 mg perindopril in combination with indapamide, than with 4 mg perindopril alone. The EUROPA study suggests that 8 mg perindopril may have produced greater reduction in cardiovascular events than produced by 4 mg perindopril alone in the PROGRESS study. Conversely, the PROGRESS data raise the possibility that a greater reduction in cardiovascular events may have been seen in the EUROPA study if perindopril had been combined with a diuretic.
Whereas analyses by the Blood Pressure Treatment Trialists' Collaboration suggest the size of the absolute BP reduction is a more important indicator of the relative cardiovascular disease-prevention by antihypertensive drugs than is the antihypertensive drug choice, there are exceptions emerging (BPLTT 2005). Thus, calcium channel blockers appear to be inferior to other antihypertensive agents for the prevention of heart failure (BPLTT 2005). Moreover, data from the Losartan Intervention For Endpoint Reduction (LIFE) study suggest losartan may provide better protection from stroke than atenolol, although losartan- and atenolol-based therapies had similar effects on cardiac outcomes (Dahlof et al 2002). By contrast, the lower MI event rate with amlodipine than valsartan therapy in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) study was associated with a greater BP reduction by amlodipine (Julius et al 2004; Weber et al 2004).
Extensive analyses of the relationship between the benefits of therapy and BP lowering in the ASCOT-BPLA study provide indirect support for non-BP mechanisms in mediating the superior prevention of cardiovascular events by the amlodipine–perindopril regimen. Reduction in cardiovascular events by the amlodipine–perindopril regimen was associated not only with a greater reduction of BP, but also with a faster pulse, and beneficial alterations in body weight, serum high density lipoprotein (HDL) cholesterol, triglycerides, creatinine, and potassium, and fasting glucose levels (Poulter et al 2005). All of these variables are independent risk factors for cardiovascular events. When each of these variables was analyzed separately for its effect on the difference between the cardiovascular event rates for the two treatment regimens, differences in HDL cholesterol had the biggest association with differences in coronary event rates, whereas only differences in BP were materially associated with the stroke event rate. The effects of the amlodipine–perindopril regimen, relative to the atenolol–bendrofluthiazide regimen, on cardiovascular events were attenuated, but remained statistically significant, when analyses were adjusted for BP alone. However, multivariate adjustment for all risk factors accounted for half of the difference in coronary events and for about 40% of the difference in stroke events between the amlodipine–perindopril and atenolol–bendrofluthiazide regimens, and the residual differences in event rates were no longer statistically significant (Poulter et al 2005).
The EUROPA investigators argued that some of the benefits of lipophilic ACEI such as perindopril and ramipril may be due to increased tissue penetration (EUROPA 2003). However, the effects of ACEI are dose-related (Campbell et al 1994), and studies in man showed that although perindoprilat is more lipophilic, with more rapid and efficient myocardial uptake than enalaprilat, the two ACEI produced similar changes in angiotensin and bradykinin peptide levels in arterial and coronary sinus blood of humans with CHD (Zeitz et al 2003).
Should ACEI be given to all patients with CHD?
Discussion of the EUROPA study has emphasized that the benefits of perindopril were in addition to other preventive measures, including the relatively high use of platelet inhibitors (92%), β blockers (62%) and lipid lowering therapy (58%) (EUROPA 2003). Nevertheless, the Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) trial suggests ACEI may have less benefit in CHD if other preventive measures are more effectively implemented (PEACE 2004). The PEACE study was a randomized placebo-controlled double-blind study of the effects of 4 mg trandolapril on cardiovascular events in 8290 subjects with stable CHD and preserved left ventricular function (PEACE 2004). During a median follow-up of 4.8 years, trandolapril produced non-statistically significant reductions in the primary endpoint (composite of cardiovascular death, MI, and coronary revascularization) from 22.5% to 21.9% (4% decrease, 95% CI −6% to 12%), and in cardiovascular death and non-fatal MI from 8.5% to 8.3% (3% decrease, 95% CI −12% to 17%), although trandolapril reduced hospitalization or death due to heart failure from 3.7% to 2.8% (25% decrease, 95% CI 5% to 41%; NNT=112). However, BP (an indicator of cardiovascular disease risk) at study baseline in PEACE participants (133/78 mm Hg) was lower than the baseline BP in the Heart Outcomes Prevention Evaluation (HOPE) and EUROPA studies, and was similar to the level achieved with ACEI therapy in HOPE and EUROPA. In addition, PEACE participants received more intensive management of risk factors than did those in the HOPE and EUROPA studies, with 70% of PEACE participants receiving lipid lowering therapy (29% in HOPE, 56% in EUROPA), and 72% had undergone coronary revascularization before enrollment (40% in HOPE, 54% in EUROPA). Thus, PEACE participants had an event rate similar to that of the general population (1.6% annualized rate of death), and the more aggressive management of their risk factors may have negated any potential benefit from ACEI therapy (PEACE 2004). Pitt (2004) suggested that the lack of benefit from trandolapril in the PEACE study may have been due to lower low density lipoprotein (LDL) cholesterol levels in PEACE participants. The Ischemia Management with Accupril post bypass Graft via Inhibition of angiotensin converting enzyme (IMAGINE) study similarly showed a lack of benefit from 40 mg quinapril in optimally treated low-risk patients after coronary artery bypass grafting (Keuper and Verheugt 2005). The PEACE and IMAGINE studies emphasize the importance of addressing modifiable risk factors and incorporating an assessment of individual risks and benefits in making decisions about ACEI therapy in low risk patients with CHD and preserved left ventricular function.
Another important benefit of ACEI and ARB therapy is reduction of diabetes incidence (Hansson et al 1999; HOPE 2000; Dahlof et al 2002; PEACE 2004). Whether perindopril reduces diabetes incidence is unknown, and combination with indapamide may compromise any antidiabetic action of perindopril. Current trials are of too short duration to reveal the cardiovascular impact of diabetes prevention by ACEI and ARB therapy. Given the increasing incidence of diabetes and its contribution to cardiovascular disease, prevention of diabetes may become one of the main contributors to long-term cardiovascular benefit from ACEI and ARB therapy in the future.
Conclusions
Perindopril reduces cardiovascular events. The reduction of cardiovascular events by perindopril was in large part associated with reduction of BP, and greater reduction of BP was associated with greater reduction in cardiovascular events. Perindopril may need to be combined with other antihypertensive agents to maximize reduction of cardiovascular events.
Disclosures
Duncan J Campbell is recipient of a Career Development Fellowship (Award CR 02M 0829) from the National Heart Foundation of Australia. Duncan J Campbell has had research contracts with Solvay Pharmaceutical Company and Novartis in the last 5 years, and has been a member of an advisory board for Novartis.
References
- Asmar RG, London GM, O'Rourke ME, et al. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922–6. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- Beevers G, Lip GY, O'Brien E. ABC of hypertension: The pathophysiology of hypertension. Br Med J. 2001;322:912–16. doi: 10.1136/bmj.322.7291.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlstra PJ, Smits P, Lutterman JA, et al. Effect of long-term angiotensin-converting enzyme inhibition on endothelial function in patients with the insulin-resistance syndrome. J Cardiovasc Pharmacol. 1995;25:658–64. doi: 10.1097/00005344-199504000-00021. [DOI] [PubMed] [Google Scholar]
- [BPLTT] Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165:1410–19. doi: 10.1001/archinte.165.12.1410. [DOI] [PubMed] [Google Scholar]
- [CAFE] The CAFE Investigators; for the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) investigators. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Kladis A, Duncan AM. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension. 1994;23:439–49. doi: 10.1161/01.hyp.23.4.439. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- Daly CA, Fox KM, Remme WJ, et al. The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: results from the PERSUADE substudy. Eur Heart J. 2005;26:1369–78. doi: 10.1093/eurheartj/ehi225. [DOI] [PubMed] [Google Scholar]
- de Luca N, Mallion JM, O'Rourke MF, et al. Regression of left ventricular mass in hypertensive patients treated with perindopril/indapamide as a first-line combination: the REASON echocardiography study. Am J Hypertens. 2004;17:660–7. doi: 10.1016/j.amjhyper.2004.03.681. [DOI] [PubMed] [Google Scholar]
- [EUROPA] European Trial on Reduction of Cardiac Events with Perindopril in Stable Coronary Artery Disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–8. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Perindopril and Remodeling in Elderly with Acute Myocardial Infarction Investigators Effects of angiotensin-converting enzyme inhibition with perindopril on left vascular remodeling and clinical outcome: results of the randomized Perindopril and Remodelling in Elderly with Acute Myocardial Infarction (PREAMI) study. Arch Intern Med. 2006;166:659–66. doi: 10.1001/archinte.166.6.659. [DOI] [PubMed] [Google Scholar]
- Flather MD, Yusuf S, Kober L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet. 2000;355:1575–81. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE, Coleman TG, et al. The dominant role of the kidneys in long term arterial pressure regulation in normal and hypertensive states. In: Laragh JH, Brenner BM, editors. Hypertension: pathophysiology, diagnosis, and management. New York: Raven Pr Ltd; 1995. pp. 1311–26. [Google Scholar]
- Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensinconverting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353:611–16. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- [HOPE] Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline update for the diagnosis and management of chronic heart failure in the adult – summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005;112:1825–52. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Ikram MK, Witteman JCM, Vingerling JR, et al. Retinal vessel diameters and risk of hypertension: the Rotterdam study. Hypertension. 2006;47:189–94. doi: 10.1161/01.HYP.0000199104.61945.33. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Herrera-Acosta J, Schreiner GF, et al. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–23. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–31. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- Keuper W, Verheugt FW. Hotline sessions of the 27th European congress of cardiology. Eur Heart J. 2005;26:2596–9. doi: 10.1093/eurheartj/ehi615. [DOI] [PubMed] [Google Scholar]
- King DE, Egan BM, Mainous AG, 3rd, et al. Elevation of C-reactive protein in people with prehypertension. J Clin Hypertens (Greenwich) 2004;6:562–8. doi: 10.1111/j.1524-6175.2004.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landray MJ, Edmunds E, Li-Saw-Hee FL, et al. Abnormal lowdensity lipoprotein subfraction profile in patients with untreated hypertension. Qjm. 2002;95:165–71. doi: 10.1093/qjmed/95.3.165. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensinconverting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- Lip GY, Blann AD, Beevers DG. Prothrombotic factors, endothelial function and left ventricular hypertrophy in isolated systolic hypertension compared with systolic-diastolic hypertension. J Hypertens. 1999;17:1203–7. doi: 10.1097/00004872-199917080-00021. [DOI] [PubMed] [Google Scholar]
- Lip GY, Blann AD, Zarifis J, et al. Soluble adhesion molecule Pselectin and endothelial dysfunction in essential hypertension: implications for atherogenesis? A preliminary report. J Hypertens. 1995;13:1674–8. [PubMed] [Google Scholar]
- Mark AL. Structural changes in resistance and capacitance vessels in borderline hypertension. Hypertension. 1984;6(Suppl III):III-69–III-73. doi: 10.1161/01.hyp.6.6_pt_2.iii69. [DOI] [PubMed] [Google Scholar]
- Mogensen CE, Viberti G, Halimi S, et al. Effect of low-dose perindopril/indapamide on albuminuria in diabetes: preterax in albuminuria regression: PREMIER. Hypertension. 2003;41:1063–71. doi: 10.1161/01.HYP.0000064943.51878.58. [DOI] [PubMed] [Google Scholar]
- Noon JP, Walker BR, Webb DJ, et al. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997;99:1873–9. doi: 10.1172/JCI119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [PEACE] PEACE Trial Investigators. Angiotensin-convertingenzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–68. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt B. ACE inhibitors for patients with vascular disease without left ventricular dysfunction—may they rest in PEACE? N Engl J Med. 2004;351:2115–17. doi: 10.1056/NEJMe048255. [DOI] [PubMed] [Google Scholar]
- Poulter NR, Wedel H, Dahlof B, et al. Role of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA) Lancet. 2005;366:907–13. doi: 10.1016/S0140-6736(05)67186-3. [DOI] [PubMed] [Google Scholar]
- [PROGRESS] PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- [PROGRESS] PROGRESS Collaborative Group. Effects of a perindopril-based blood pressure lowering regimen on cardiac outcomes among patients with cerebro-vascular disease. Eur Heart J. 2003;24:475–84. doi: 10.1016/s0195-668x(02)00804-7. [DOI] [PubMed] [Google Scholar]
- [PSC] Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–60. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- Safar ME, Boudier HS. Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension. 2005;46:205–9. doi: 10.1161/01.HYP.0000167992.80876.26. [DOI] [PubMed] [Google Scholar]
- Simon G. Pathogenesis of structural vascular changes in hypertension. J Hypertens. 2004;22:3–10. doi: 10.1097/00004872-200401000-00002. [DOI] [PubMed] [Google Scholar]
- Skov K, Mulvany MJ. Structure of renal afferent arterioles in the pathogenesis of hypertension. Acta Physiol Scand. 2004;181:397–405. doi: 10.1111/j.1365-201X.2004.01311.x. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Tagawa T, Yamakawa K, et al. Inhibition of the reninangiotensin system prevents free fatty acid-induced acute endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2005;25:2376–80. doi: 10.1161/01.ATV.0000187465.55507.85. [DOI] [PubMed] [Google Scholar]
- Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–51. doi: 10.1016/S0140-6736(04)16456-8. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization. World Health Report 2002: reducing risks, promoting healthy life. Geneva: WHO; 2002. [DOI] [PubMed] [Google Scholar]
- Wong TY, Shankar A, Klein R, et al. Prospective cohort study of retinal vessel diameters and risk of hypertension. Br Med J. 2004;329:79. doi: 10.1136/bmj.38124.682523.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease. The role of fetal programming. Hypertension. 2006;47:502–8. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- Zeitz CJ, Campbell DJ, Horowitz JD. Myocardial uptake and biochemical and hemodynamic effects of ACE inhibitors in humans. Hypertension. 2003;41:482–7. doi: 10.1161/01.HYP.0000054976.67487.08. [DOI] [PubMed] [Google Scholar]