Abstract
Several publications have attested to the ability of herpes simplex viruses to protect cells against apoptosis. We investigated the ability of the virus to protect cells in continuous cultivation from apoptosis induced by the virus itself, and by other known inducers such as exposure to the tumor necrosis factor α (TNFα), antibody to Fas, C2-ceramide, osmotic shock (sorbitol), and thermal shock. The salient features of the results were that the virus was able to protect cells against apoptosis by all of the agents tested, and that apoptosis induced by the virus was a very early event that did not require de novo expression of viral genes. However, these events were cell-type specific. Thus: (i) The cell lines tested exhibited fragmented chromosomal DNA following infection with a virus lacking functional α4 and US3 genes encoding the major regulatory protein and a viral protein kinase, respectively, but not by wild-type virus. (ii) Wild-type virus protected subconfluent SK-N-SH but not HeLa cells against induction of apoptosis by anti-Fas antibody, TNFα, C2-ceramide, and thermal shock. Confluent SK-N-SH cells were not protected from osmotic shock-induced apoptosis by wild-type infection. (iii) Wild-type virus protected SK-N-SH but not HeLa cells against induction of apoptosis by sorbitol, anti-Fas antibody, or TNFα and C2-ceramide. (iv) Mutant HSV-1(HFEM)tsB7 at the nonpermissive temperature infects cells but the DNA is not released from capsids, and therefore viral gene expression is restricted to the function of viral proteins introduced into the cell along with the capsid containing the viral DNA. HSV-1(HFEM)tsB7 induced apoptosis in Vero cells but not in SK-N-SH cells infected and maintained at 39.5°C. (v) Tests of two caspase inhibitors showed that they blocked apoptosis induced by C2-ceramide and sorbitol, but were not able to block apoptosis induced by the virus lacking functional α4 and US3 genes. We conclude that HSV-1 triggers apoptosis at multiple metabolic checkpoints and in turn has evolved mechanisms to block apoptosis at each point and that some of the pathways of induction are shared with exogenous inducers tested in this study whereas others are not.
Keywords: anti-Fas antibody, tumor necrosis factor α, thermal shock, sorbitol, caspase inhibitors, uncoating mutants
Cellular apoptosis serves two functions: to clear the body of unwanted cells and to dispose of cells whose cascades of sequentially ordered events are irreversibly damaged by factors internal or external to the cell. Once apoptosis is triggered, a series of programmed events leads to the death of the cell manifested by morphological changes, activation of specific enzymes, and especially by the degradation of cellular DNA (1). Viruses are major external factors that disrupt this orderly sequence of programmed cellular events, and as a result programmed cell death is triggered in the infected cells either intrinsically or by mediators of the host immune response. In situations in which endogenously or exogenously induced apoptosis threatens the capacity of the cell to produce the required number and quality of infectious virus progeny, viruses have evolved mechanism to block apoptosis. There is considerable evidence that herpesviruses and particularly herpes simplex viruses 1 and 2 (HSV-1 and HSV-2) block apoptosis (2–9). This laboratory reported that mutants lacking the HSV-1 US3 gene induce apoptosis, and, by extension, that the protein kinase expressed by the US3 gene blocks it (10). The studies described in this report were designed initially to determine whether HSV-1 blocks apoptosis induced by other agents and thereby begin to define the pathways by which HSV-1 both induces and blocks apoptosis. The fundamental hypothesis driving these studies was that HSV triggers apoptosis as a consequence of the function of one viral gene and that identification of the gene product may elucidate both the cellular metabolic event disrupted by the virus and the mechanism by which the apoptotic response is blocked.
The evidence we present in this report suggests that the likely scenario is more complex than predicted by the hypothesis underlying these studies. The evidence, basically, is that the ability of HSV-1 mutants to induce apoptosis is cell-type dependent, as is the ability of wild-type virus to protect cells from apoptosis induced by exogenously applied agents. Furthermore, whereas caspase inhibitors block apoptosis induced by nonviral agents at least in some cell lines tested, they were ineffective against virus-induced apoptosis even though in these cells wild-type HSV-1 blocked apoptosis induced by these agents. These results lead us to conclude that (i) HSV-1 induces apoptosis by disruption of a plethora of metabolic checkpoints and in turn has evolved mechanisms to block apoptosis at each step and that (ii) some steps in the pathways of induction by HSV-1 are shared with the inducers tested in this study, whereas others are not.
Relevant to this report are the following: (i) Virus-specific T lymphocytes kill infected cells by expressing Fas/Apo-1 ligand (CD95L), which triggers apoptosis upon binding to CD95, or by granule exocytosis through the granzyme B-perforin pathway (reviewed in ref. 11). Tumor necrosis factor α (TNFα) is a potent antiviral cytokine; it effects the apoptosis-dependent cytolysis of infected cells by binding to the TNF receptor that is present in most cell types. The TNFα and Fas-activated pathways share many mediator molecules including members of a cysteine protease enzymes known as caspases (12, 13). Proteolytically activated caspase-3 has been found to be necessary and sufficient to trigger apoptosis by cleavage of several substrates, among which only DNA fragmentation factor has been demonstrated to play a role in apoptosis (14). The specific inhibitor Ac-DEVD-CHO, an acetylated tetrapeptide aldehyde, inhibits caspase-3 both in vitro and in vivo by binding irreversibly to the active site of the enzyme (15). z-VAD-fmk is a general inhibitor of cysteine proteases extensively used to block caspase activity (16).
(ii) TNFα and Fas have been suggested to induce apoptosis by hydrolysis of sphyngomyelin and subsequent generation of ceramide that acts as a second messenger transducing the signal through the stress-activated protein kinase (SAPK/JNK) cascade (17). It has been suggested that constitutively produced ceramide is necessary but not sufficient in TNFα-induced apoptosis (18) and that induction of apoptosis by Fas in T cells does not involve the generation of sphingosine-based ceramides (19). In studies reported here, the cell-permeant analog C2-ceramide induced apoptosis in most cell types tested.
(iii) Osmotic shock by exposure of cells to high concentrations of sorbitol has been demonstrated to induce apoptosis by altering the structure of the plasma membrane such that membrane-embedded proteins cluster and activate their signaling pathways (20, 21). An extreme shift in temperature is also thought to alter the structure of the plasma membrane in a manner similar to that resulting from osmotic shock.
MATERIALS AND METHODS
Cells.
Vero, SK-N-SH, and HeLa S3 cells were obtained from American Type Culture Collection (Rockville, MD) and were grown in DMEM containing 5% fetal bovine serum (Vero and HeLa) or 10% fetal bovine serum (SK-N-SH).
Viruses.
HSV-1(F), is the prototype HSV-1 strain used in this laboratory, like most wild-type virus isolates with a history of limited cell culture passages, carries a temperature-sensitive mutation in the α4 gene (22). At the nonpermissive temperature (39.5°C), HSV-1(F) expresses predominantly α genes. The HSV-1 mutant d120 (a kind gift of N. DeLuca) carries a deletion in both copies of the α4 gene and was grown in a Vero cell line (E5) expressing α4 (23). It also carries a defective US3 gene (24). The temperature-sensitive (ts) mutation in HSV-1(HFEM)tsB7syn+ (tsB7) was mapped to the UL36 ORF. At 39.5°C the virus infects cells, the capsids are transported to the nuclear pore but viral DNA is not released from the capsids, and consequently it is not transcribed (25, 26).
Induction of Apoptosis.
Thermal shock was induced by shifting cells maintained in DMEM containing 1% newborn calf serum at 4°C for 2 hr to a 39.5°C water bath for 36 hr. Osmotic shock was induced by exposing SK-N-SH and HeLa cells to sorbitol (1.0 and 0.5 M, respectively) for 1 hr, followed by 5 hr of incubation in 5% fetal bovine serum. The optimal concentrations for induction of apoptosis by C2-ceramide (Biomol, Plymouth Meeting, PA), recombinant TNFα (Calbiochem) or CH-11 anti-Fas mAb (IgM) (Calbiochem) were determined by assessing the viability of the cells by the trypan blue exclusion method. The cells were exposed to the drugs or antibody in DMEM containing 1% newborn calf serum. The effect of cycloheximide (CHX) on induction of apoptosis by anti-Fas IgM or TNFα was also evaluated by addition of 1 μg of CHX per ml of medium to each concentration of inducer tested. Cells treated with C2-ceramide were collected at 5 hr after treatment. Cells treated with anti-Fas IgM or TNFα both in the presence or absence of CHX were collected at 16 hr after treatment. In all cases, cells were trypsinized, pelleted, and resuspended in 0.2% trypan blue and viable and nonviable cells were counted in a hematocytometer.
z-VAD-fmk was purchased from Enzyme Systems Products (Livermore, CA) and used at a concentration of 50 μM. Ac-DEVD-CHO (Ki = 12 μM) was purchased from Calbiochem and used at a concentration of 100 μM.
RESULTS
Determination of Conditions for Induction of Apoptosis by Temperature Shift and Osmotic Shock in Different Cell Lines.
In preliminary studies we established the effects of the physiological state of the cells, serum concentration, and temperature of incubation on induction of apoptosis. In all of these studies the criterion for induction of apoptosis was the fragmentation of chromosomal DNA resulting from random cleavage at internucleosomal intervals. The results (data not shown), were as follows:
(i) Significant DNA fragmentation was seen in subconfluent (80% of surface covered by cells) SK-N-SH and HeLa cells subject to temperature shift from 4°C to 39.5°C irrespective of the concentration of serum present in the medium. No significant DNA fragmentation was observed in control cultures maintained at 37°C. SK-N-SH and HeLa cells therefore respond similarly to apoptosis induced by a shift in temperature from 4°C to 39.5°C.
(ii) Confluent cultures of HeLa but not of SK-N-SH cells showed high amounts of DNA fragmentation, even in samples incubated at 37°C.
(iii) Titrations of sorbitol in the range of 0 to 2.5 M indicated that concentrations of 0.5 and 1.0 M were sufficient to induce apoptosis in HeLa and SK-N-SH cells, respectively.
The Effects of TNFα, Anti-Fas IgM, or C2-Ceramide on HeLa and SK-N-SH Cells.
We determined the sensitivity of HeLa and SK-N-SH cells to undergo apoptosis following exposure to increasing concentrations of TNFα or anti-Fas IgM in the presence or absence of 1 μg of CHX per ml. Simultaneous addition of inhibitors of protein or RNA synthesis have been shown to dramatically increase the sensitivity of many cells to TNFα (27) or anti-Fas IgM (28). Apoptosis was assayed as a function of cell viability and measured by the trypan blue exclusion method. The results were as follows:
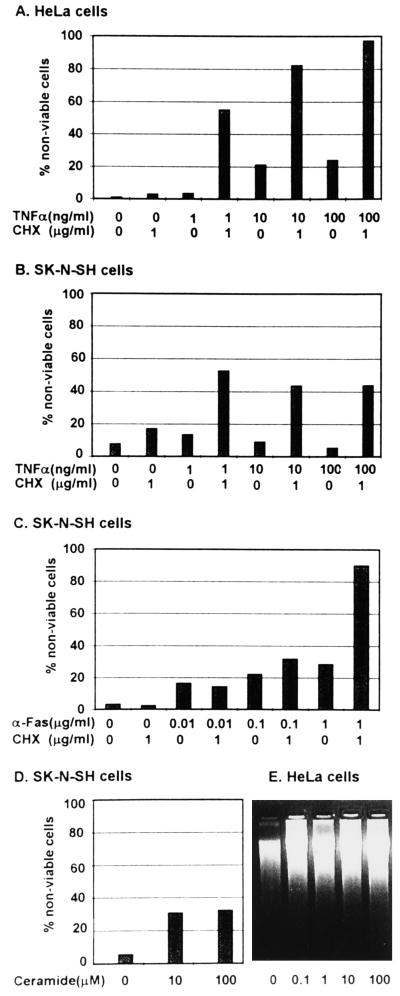
(i) CHX alone had little or no effect and could not be differentiated from medium lacking drugs with respect to cell death in any of the cell lines tested.
(ii) Nearly 100% of HeLa cells were nonviable after 16 hr of incubation in medium containing 100 ng of TNFα per ml in the presence of CHX (Fig. 1A), compared with 44% nonviable SK-N-SH cells treated in a similar fashion (Fig. 1B).
Figure 1.
Induction of apoptosis by anti-Fas antibody, TNFα and C2-ceramide. (A–C) Cells were exposed to TNFα, or anti-Fas antibody alone or in the presence of CHX and assayed for viability. (D) SK-N-SH cells were exposed to C2-ceramide and tested for viability. (E) HeLa cells exposed to C2-ceramide and tested for degradation of DNA. The procedures were as described in Materials and Methods.
(iii) Anti-Fas (Fig. 1C) IgM treatment alone or in the presence of CHX induced relatively low levels of cell death in SK-N-SH cells at concentrations lower than 1 μg/ml, but cell death induced by incubation in 1 μg per ml of anti-Fas IgM was greatly enhanced by the concomitant addition of CHX (1 μg/ml of medium), consistent with the report that induction of apoptosis in nonlymphoid cells by Fas activation requires inhibition of RNA or protein synthesis (29). Although there have been contradictory reports regarding the susceptibility of HeLa cells to Fas engagement (30, 27), in our hands these cells showed high levels of apoptosis even after incubation in medium containing 1 μg per ml of anti-Fas IgM without concomitant addition of CHX (data not shown). (iv) Exposure to 10 μM C2-ceramide for 5 hr induced significant levels of cell death in SK-N-SH cells (Fig. 1D) whereas concentrations as low as 0.1 μM C2-ceramide were sufficient to induce apoptosis in HeLa cells (Fig. 1E). In general, we found HeLa cells to be extremely sensitive to all forms of induction of apoptosis tested.
HSV-1 Protection from Apoptosis Induced by Thermal Shock or Sorbitol Is Dependent on the Cell Type and Physiologic State of the Cell.
The effectiveness of HSV-1 infection in blocking apoptosis induced by temperature shift or by exposure to sorbitol is shown in Fig. 2. In the experiments whose results are shown in Fig. 2A, we tested subconfluent cultures of HeLa cells; in Fig. 2 B and C, we compared the effects of infection with HSV-1(F) on both subconfluent and confluent cultures of SK-N-SH cells. The results were as follows:
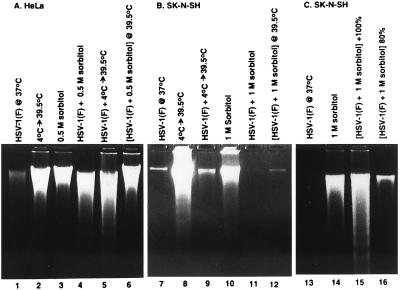
Figure 2.
Photograph of agarose gels containing electrophoretically separated low molecular weight DNA fractions from mock-infected or HSV-1(F)-infected cells and stained with ethidium bromide. Subconfluent HeLa (A) or SK-N-SH cells (B) or subconfluent and confluent SK-N-SH cells (C) were mock-infected (lanes 2, 3, 8, 10 and 14) or infected with HSV-1(F) (lanes 1, 4–7, 9, 11–13, 15 and 16), treated by temperature shift (4°C → 39.5°C) or osmotic shock (0.5 M or 1 M sorbitol) and incubated for 5 hr (sorbitol-treated cells) or for 36 hr (other cultures) at 37°C unless otherwise indicated. 2 × 106 cells per sample were collected, rinsed in PBS and lysed in a solution containing 10 mM Tris⋅HCl (pH 8.0), 10 mM EDTA and 0.5% Triton X-100 and centrifuged at 14,000 rpm for 30 min in an Eppendorf microcentrifuge to pellet chromosomal DNA. The supernatant fluids were digested with 0.1 mg/ml RNase A at 37°C for 1 hr and then with 1 mg/ml proteinase K at 50°C in the presence of 1% SDS, extracted with phenol and chloroform, precipitated in cold ethanol and subjected to electrophoresis in 2% agarose gels containing 5 μg/ml ethidium bromide. DNA was visualized by UV light transillumination and photographed with the Eagle Eye II (Stratagene).
(i) HeLa chromosomal DNA was not fragmented in cells infected with HSV-1(F) and maintained at 37°C (Fig. 2A, lane 1). Both mock-infected and HSV-1(F)-infected HeLa cells shifted from 4°C to 39.5°C exhibited fragmented chromosomal DNA (Fig. 2A, lanes 2 and 5). As noted above, 0.5 M sorbitol induced apoptosis in HeLa cells (Fig. 2A, lane 4). Infection with HSV-1(F) did not protect HeLa cells treated with 0.5 M sorbitol either at 37°C (Fig. 2A, lane 4) or at 39.5°C (Fig. 2A, lane 6).
(ii) Subconfluent cultures of SK-N-SH cells were protected from chromosomal DNA degradation by infection with HSV-1(F) in temperature-shift experiments. SK-N-SH cells underwent apoptosis after temperature shift (Fig. 2B, lane 8), and neither cells infected and maintained at 37°C or cells infected and subjected to temperature shift (Fig. 2B, lanes 7 and 9) exhibited detectable quantities of fragmented DNA. Whereas exposure to 1 M sorbitol at 37°C induced apoptosis (Fig. 2B, lane 10), HSV-1(F)-infected cells exposed to 1 M sorbitol and maintained at 37°C or 39.5°C were protected from this effect (Fig. 2B, lanes 11 and 12).
(iii) The effect of the physiological state of the cells is apparent from experiments on confluent cultures of SK-N-SH cells shown in Fig. 2C. Reproducibly, HSV-1(F) did not block apoptosis in these cultures exposed to 1 M sorbitol (Fig. 2C, lanes 14 and 15).
We conclude that HSV-1(F) infection does not protect HeLa cells from induction of apoptosis by either thermal shock or sorbitol. In contrast, HSV-1(F) was effective in inhibiting programmed cell death of SK-N-SH cells, but only in subconfluent cell cultures.
HSV-1-Infected Cells Are Protected from TNFα, Anti-Fas Antibody, and Ceramide-Induced Apoptosis in a Cell-Type-Dependent Fashion.
The purpose of this series of experiments was to test the ability of HSV to block apoptosis induced by anti-Fas antibody, TNFα, or ceramide. The observation that CHX was required to maximize the cytotoxic effect of both TNFα and Fas in SK-N-SH cells and of TNFα in HeLa cells presented a problem inasmuch as low concentrations of CHX could nevertheless affect viral protein synthesis and make the interpretation of the next set of experiments difficult. To circumvent this problem, we included two sets of samples in each experiment in which induction of apoptosis could be dependent on the presence of CHX. In one set, mock-infected or infected cells were exposed 0.5 hr after infection to TNFα or anti-Fas antibody alone. In the second set, the cells were exposed to inducers of apoptosis in presence of 1 μg of CHX per ml at 4 hr after infection. In other experiments (data not shown) we found that Vero cells were unresponsive to treatment with anti-Fas antibody, TNFα, or C2-ceramide. The results were as follows:
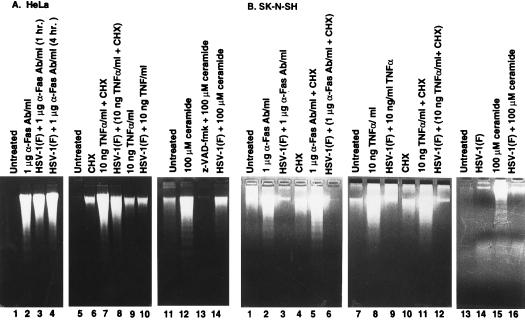
(i) Fragmented chromosomal DNA was observed in both infected and uninfected HeLa cells incubated in medium containing anti-Fas IgM for 8 hr (Fig. 3A, lanes 2 and 4), but not in untreated cultures (Fig. 3A, lane 1). The response of cultures exposed simultaneously to anti-Fas IgM and virus inoculum (Fig. 3A, lane 4) could not be differentiated from cultures exposed only to anti-Fas IgM one hr after infection (Fig. 3A, lane 3).
Figure 3.
Photograph of agarose gels containing electrophoretically separated low molecular weight DNA fractions from mock-infected or infected cells and stained with ethidium bromide. Subconfluent HeLa (A) or SK-N-SH cells (B) were mock-infected or infected with HSV-1(F) and treated by incubation in 1 μg/ml of anti-Fas IgM or 10 ng/ml TNFα both in the presence or the absence of 1 μg/ml CHX. Anti-Fas IgM or TNFα-treated HeLa cells were collected at 8 and 6 hr after treatment, respectively. The SK-N-SH cell samples were collected at 12 and 16 hr after treatment, respectively. Mock-infected and infected HeLa and SK-N-SH cells were treated with 100 μM C2-ceramide and collected at 6 hr after treatment.
(ii) Significant levels of fragmented chromosomal HeLa cell DNA was not observed in cultures exposed to 10 ng of TNFα per ml in the absence of CHX (Fig. 3A, lane 9). Significantly higher levels of fragmentation of chromosomal DNA was observed in both infected and uninfected cultures treated with TNFα and 1 μg of CHX per ml (Fig. 3A, lanes 7 and 8, respectively).
(iii) Exposure to 100 μM C2-ceramide induced fragmentation of chromosomal DNA in uninfected cells (Fig. 3A, lane 12) and infected HeLa cells (Fig. 3A, lane 14). In this cell line z-VAD-fmk blocked DNA degradation by C2-ceramide (Fig. 3A, lane 13).
(iv) In SK-N-SH cell cultures, significant fragmentation of chromosomal DNA was induced by 1 μg of anti-Fas IgM per ml of medium in the presence or absence of CHX (Fig. 3B, lanes 2 and 5), by 10 ng of TNFα per ml of medium with or without CHX (Fig. 3B, lanes 8 and 11), and by 100 μM C2-ceramide (Fig. 3B, lane 15) in uninfected but not in infected cells. It should be noted that in some experiments, we observed moderate levels of DNA fragmentation in untreated cells (Fig. 3B, lanes 1 and 7) or in cells treated with CHX alone (Fig. 3B, lanes 4 and 10). Consequently, although the results of this experiment do not allow a definitive assessment of the effects of CHX alone, other experiments suggest that CHX has a slight but reproducible effect of accelerating the degradation of DNA (e.g., compare Fig. 3B, lanes 7 and 10).
Caspase Inhibitors Do Not Block HSV-1 d120 Mutant-Induced Apoptosis.
The studies described above indicate that viral infection is sufficient to block the TNF/Fas pathway of cell death. The TNF/Fas pathway has been shown to be dependent on the activity of caspases and therefore the question arose whether apoptosis induced by the d120 mutant could be blocked by caspase inhibitors. In this series of experiments cells were incubated in medium containing 50 μM z-VAD-fmk or 100 μM Ac-DEVD-CHO for 1 hr before infection with d120 mutant [10 plaque-forming units (pfu) per cell] and maintained in medium containing the same concentrations of inhibitor for the length of the experiment. The results were as follows:
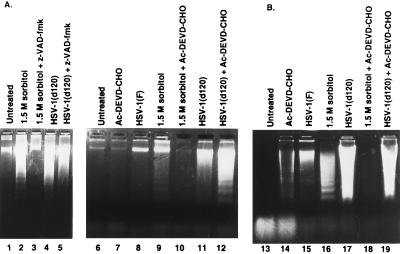
(i) Preincubation with Ac-DEVD-CHO was sufficient to block induction of apoptosis in HeLa cells treated with 1.5 M sorbitol (Fig. 4B, lanes 16 and 18), but not by infection with the d120 mutant (Fig. 4B, lanes 17 and 19). Ceramide-induced apoptosis in HeLa cells could also be blocked by the general inhibitor of cysteine proteases, z-VAD-fmk (Fig. 3A, lanes 12 and 13).
Figure 4.
(A) Photograph of agarose gels containing electrophoretically separated low molecular weight DNA fractions from mock-infected cells or cells infected with d120 mutant and stained with ethidium bromide. Subconfluent Vero (A) or HeLa cells (B) were either left untreated or treated with 50 μM z-VAD-fmk or 100 μM Ac-DEVD-CHO (Vero cells) or treated with 100 μM Ac-DEVD-CHO (HeLa cells) and then exposed to 1.5 M sorbitol or infected with 10 pfu of d120 mutant per cell and harvested at 5 hr after treatment and 24 hr after infection, respectively. Samples were processed as described in the legend to Fig. 2.
(ii) Pretreatment with either z-VAD-fmk or Ac-DEVD-CHO blocked apoptosis induced in Vero cells by incubation with 1.5 M sorbitol (Fig. 4A, lanes 2 and 3 and 9 and 10, respectively), but not by infection with the d120 mutant (Fig. 4A, lanes 4 and 5 and 11 and 12, respectively).
Induction of Apoptosis by HSV-1.
d120 virus expresses primarily α genes. Over a long interval of time, the infected cells also express very small amounts of proteins normally made later in infection. One hypothesis to explain the induction of apoptosis by d120 mutant is that the induction event, in contrast to the event that blocks apoptosis, takes place very early in infection, either before viral gene expression or immediately thereafter. HSV-1(HFEM)tsB7 at the nonpermissive temperature infects cells, the capsids are transported to the nuclear pores but viral DNA is not released from the capsids. As a consequence the synthesis of viral gene products does not ensue. Viral gene expression is restricted to the function of viral proteins packaged in virions and introduced into the cell during infection. To test one aspect of this hypothesis, we examined the DNA of cells infected with HSV-1(HFEM)tsB7 in Vero and SK-N-SH cells. The results were as follows:
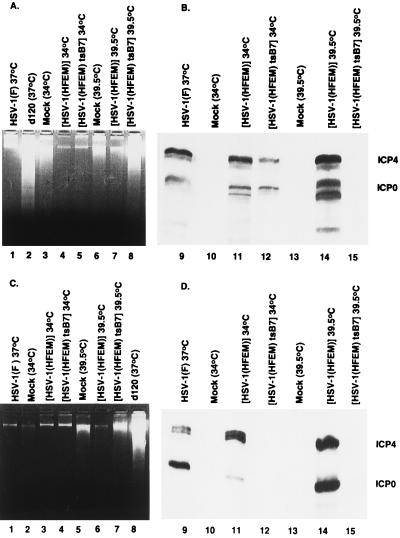
(i) Vero cells exposed to 10 pfu of HSV-1(HFEM)tsB7 per cell and maintained at 39.5°C exhibited significant amounts of fragmented DNA (Fig. 5A, lane 8) whereas maintenance of infected cells at 34°C failed to yield detectable fragmented DNA (Fig. 5A, lane 5). The parental strain, HSV-1(HFEM), failed to induce fragmentation of Vero cell DNA at either 34°C (Fig. 5A, lane 4) or 39.5°C (Fig. 5, lane 7). In these experiments HSV-1(F) and d120 served as known negative and positive controls, respectively (Fig. 5A, lanes 1 and 2, respectively). It is noteworthy that mock-infected cells exhibited moderate amounts of DNA fragmentation that were not detectable in any of the samples infected with wild-type viruses [HSV-1(F) or HSV-1(HFEM)] at either 34°C or 39.5°C. To verify that HSV-1(HFEM)tsB7 behaved as described (26), replicate cultures of Vero cells treated in the same fashion as those used for examination of DNA fragmentation were probed for the synthesis of α proteins. Fig. 5B, lanes 9–15, shows a photograph of an immunoblot of electrophoretically separated, denatured lysates of infected Vero cells reacted with mAbs to two α proteins, infected cell proteins no. 4 and 0 (ICP4 and ICP0). The results indicate that ICP4 and ICP0 were made in cells infected with the wild-type parent at both 34°C and 39.5°C (Fig. 5B, lanes 11 and 14) by HSV-1(HFEM)tsB7 at 34°C (Fig. 5B, lane 12), but not by HSV-1(HFEM)tsB7 at 39.5°C (Fig. 5B, lane 15). We should note that the multiplicity of bands observed in Vero cells reflects protease activities reported earlier (31).
Figure 5.
(A) Photograph of agarose gels containing electrophoretically separated, ethidium bromide stained low molecular weight DNA (A and C) or of immunoblots (B and D). Subconfluent Vero cells (A) or SK-N-SH cells (C) were mock-infected or infected with 10 pfu of HSV-1(F), d120, HSV-1(HFEM), or HSV-1(HFEM)tsB7 per cell and maintained at the indicated temperature. The samples were processed as described in the legend to Fig. 2. Replicate Vero cultures (B) or SK-N-SH cells (D) each containing 5 × 105 cells treated as described in corresponding panels B or D were solubilized, subjected to electrophoresis in SDS/12% polyacrylamide gels, transferred to nitrocellulose sheets and reacted first with mixtures of monoclonal antibodies to ICP0 and ICP4 and subsequently with anti-mouse antibody and visualized as described elsewhere (40).
(ii)The experiment described above was repeated with SK-N-SH cells with diametrically opposite results (Fig. 5C, lanes 1–8). In this cell line the only virus able to induce significant amounts of DNA degradation was d120 (Fig. 5C, lane 8). In this cell line, control experiments also showed that at nonpermissive temperature ICP4 and ICP0 were made in cells infected with control viruses [HSV-1(F), HSV-1(HFEM)] (Fig. 5D, lanes 9, 11, and 14) by HSV-1(HFEM)tsB7 at 34°C (Fig. 5D, lane 12), but not by HSV-1(HFEM)tsB7 at the nonpermissive temperature (Fig. 5D, lane 15).
We conclude from these studies that HSV-1 can induce apoptosis in cells before de novo transcription of viral genes but that this phenomenon is cell-type dependent.
DISCUSSION
The fundamental premises underlying these studies are 2-fold, that is (i) induction of apoptosis by HSV-1 mutants but not by wild-type virus would indicate that a viral gene product induces apoptosis and that another gene product present in the wild-type virus blocks it, and (ii) the ability of the virus to block apoptosis induced by exogenous nonviral factors indicates that viral gene products act on the cellular products involved in the signaling pathway leading to apoptosis. Given these premises, it is convenient to review the results presented in this report in the light of the following working hypotheses:
(i) Multiple and diverse gene products induce apoptosis by different signaling pathways and also, multiple viral gene products block apoptosis.
(ii) The interdiction of apoptosis by the viral gene products may involve interaction with cellular products that may or may not be present in different cell lines.
The evidence bearing on this hypothesis is based on the following: (i) The induction of apoptosis by d120 indicates that a viral function induces apoptosis and that another function shown to require expression of the US3 gene blocks the signaling pathway activated by the virus. This pathway appears to function in HeLa and SK-N-SH cells as shown in this study and in Vero cells as reported (24). The mutant HSV-1(HFEM)tsB7 also induced apoptosis at the nonpermissive temperature in Vero cells whereas the parent virus did not induce apoptosis. The argument that the gene product that induces apoptosis and the product that blocks apoptosis in HSV-1(HFEM)tsB7-infected cells are different from those operating in d120 mutant-infected cells is based on the observation that induction of apoptosis at the nonpermissive temperature was restricted to Vero cells and did not take place in SK-N-SH cells (Fig. 5) or HEp-2 cells (data not shown). The data exclude the role of US3 protein kinase in cells infected with HSV-1(HFEM)tsB7 inasmuch as this protein is not packaged in the virion and the expression of α genes is required for its synthesis in infected cells. It follows that in Vero cells infected with HSV-1(HFEM)tsB7 and maintained at the nonpermissive temperature the inducer is present, but the protective function is ineffective, that in other cell lines either the inducer is nonfunctional or the protecting gene product is functional, and that at the permissive temperature in Vero cells other viral gene products capable of blocking apoptosis (e.g., US3 protein kinase) may become available. It is noteworthy that whereas the ts lesion maps in the UL36 gene of HSV-1(HFEM)tsB7, the virus carries other non-ts mutations that affect viral gene expression subsequent to initiation of infection (25).
HSV-1 brings to the infected cell a plethora of tegument proteins that create a suitable environment for viral gene expression in addition to the nine glycoproteins (gB, gC, gD, gE, gG, gH, gI, gL, and gM) and four membrane proteins (UL20, UL34, UL43, and UL45) that may also interact with cellular proteins. The tegument proteins include α-TIF (VP16) that interacts with cellular transcriptional factors to induce transcription of α genes (26), UL46 and UL47 that modulate expression of α genes (32), UL13 protein kinase whose one known cellular substrate is elongation factor 1δ (33), UL41 that induces degradation of mRNA (34), US9 that is ubiquitinated and binds to proteasomes (35), US11 that binds to polyribosomes (36), etc. In cells infected with the d120 mutant virus the predominant viral gene products are the α proteins. Among these ICP0 binds to and stabilizes cyclin D3 (37), a ubiquitin-specific protease, and the elongation factor 1δ (38), ICP27 binds to spliceosomes and blocks the maturation of spliced mRNAs (34), and ICP22 has been reported to affect the phosphorylation of RNA polymerase II at least in some cells (39). It would not be surprising if each set of proteins is capable of triggering the signaling pathway that leads to apoptosis.
(ii) It is expected that the viral gene products that block apoptosis could act by interacting either with the elements of the signaling pathway that induces apoptosis or as a compensatory factor for the cellular elements that normally block the pathway. The observation that the wild-type virus is effective in blocking apoptosis induced by various agents in SK-N-SH cells but not in HeLa cells must be viewed in the context of the observation that apoptosis by these agents is blocked by caspases (Fig. 4). The data suggest that the cellular proteins that would be expected to interact with the viral gene products to block apoptosis in HeLa cells are different from the targets of caspase inhibitors and are either unavailable or defective.
(iii) The evidence that in SK-N-SH cells both wild-type virus and caspase inhibitors block apoptosis induced by exogenous agents but that the same inhibitors do not block apoptosis induced by the d120 mutant supports the hypothesis that the virus induces apoptosis by several different signaling pathways, that some of these pathways are shared with exogenous inducers tested here and others that are not, and that at the same time HSV has evolved gene functions that block all pathways. Because in cells infected with d120 mutant the expression of viral genes is limited largely to the set of proteins introduced into the cell during infection and the α proteins made in abundance in these cells, the data are consistent with the hypothesis that these gene products induce apoptosis by more than one pathway.
Definitive evidence of multiple pathways for induction of apoptosis in HSV-infected cells requires identification of the individual inducers and ultimately, of the signaling pathways that they activate. It seems appropriate even at this stage of the investigation to note that HSV may turn out to be a very powerful probe of cellular metabolic pathways whose derangement leads to cell death.
Acknowledgments
We thank Patricia L. Ward and Rosario Leopardi for useful discussions and advice and Alice P. W. Poon for careful review of the manuscript. These studies were aided by grants from the National Cancer Institute (CA47451) and (CA71933).
ABBREVIATIONS
- TNFα
tumor necrosis factor α
- HSV
herpes simplex virus
- pfu
plaque-forming units
- CHX
cycloheximide
- ICP
infected-cell protein
- ts
temperature sensitive
References
- 1.Ellis R E, Yuan J-Y, Horvitz H R. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y, Shenk T E. Curr Biol. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhu H, Shen Y, Shenk T. J Virol. 1995;69:7960–7970. doi: 10.1128/jvi.69.12.7960-7970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leopardi R, Roizman B. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sieg, S., Yildrim, Z., Smith, D., Kayagaki, N., Yagata, H., Huang, Y. & Kaplan, D. (1996) J. Virol. 8747–8751. [DOI] [PMC free article] [PubMed]
- 6.Koyama A H, Miwa Y. J Virol. 1997;71:2567–2571. doi: 10.1128/jvi.71.3.2567-2571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastino A, Sciortino A T, Medici M, Peri D, Ammendikia M G, Grelli S, Amici C, Pernice A, Guglielmino S. Cell Death Differ. 1997;4:629–638. doi: 10.1038/sj.cdd.4400289. [DOI] [PubMed] [Google Scholar]
- 8.Hu S, Vincenz C, Buller M, Dixit V M. J Biol Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- 9.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leopardi R, Van Sant C, Roizman B. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagi D, Hengartner H. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 12.Enari M, Talanian R V, Wong W W, Nagata S. Nature (London) 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 13.Wong B, Choi Y. Curr Opin Immunol. 1997;9:358–364. doi: 10.1016/s0952-7915(97)80082-9. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Zou H, Slaughter C, Wang X. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 15.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 16.Chinnaiyan A M, O’Rourke K, Lane B R, Dixit V M. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 17.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, et al. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi M, Singh S, Jaffrezou J-P, Aggarwal B B. J Immunol. 1996;157:297–304. [PubMed] [Google Scholar]
- 19.Watts J D, Gu M, Polverino A J, Patterson S D, Aebersold R. Proc Natl Acad Sci USA. 1997;94:7292–7296. doi: 10.1073/pnas.94.14.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosette C, Karin M. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 21.Koyama A H, Miwa Y. J Virol. 1997;71:2567–2571. doi: 10.1128/jvi.71.3.2567-2571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ejercito P M, Kieff E D, Roizman B. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 23.DeLuca N A, McCarth A, Schaffer P A. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leopardi R, Roizman B. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batterson W, Furlong D, Roizman B. J Virol. 1983;54:397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batterson W, Roizman B. J Virol. 1983;46:371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong G H W, Goeddel D V. J Immunol. 1994;152:1751–1755. [PubMed] [Google Scholar]
- 28.Tewari M, Dixit V M. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 29.Itoh M, Yonehara S, Ishii A, Yonehara M, Mizushima S I, Samashima M, Hase A, Seto Y, Nagata S. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 30.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 31.Pereira L, Dondero D, Roizman B. J Virol. 1982;44:88–97. doi: 10.1128/jvi.44.1.88-97.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Sirko D A, McKnight J L. J Virol. 1991;65:829–841. doi: 10.1128/jvi.65.2.829-841.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawaguchi Y, Van Sant C, Roizman B. J Virol. 1998;72:1731–1736. doi: 10.1128/jvi.72.3.1731-1736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roizman B, Sears A E. In: Virology. 3rd Ed. Fields B N, Knipe D M, Howley P, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. New York: Lippincott-Raven; 1996. pp. 2231–2295. [Google Scholar]
- 35.Brandimarti R, Roizman B. Proc Natl Acad Sci USA. 1997;94:13973–13978. doi: 10.1073/pnas.94.25.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roizman B. Proc Natl Acad Sci USA. 1996;93:11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawaguchi Y, Van Sant C, Roizman B. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawaguchi Y, Bruni R, Roizman B. J Virol. 1997;71:1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice S A, Long M C, Lam V, Schaffer P A, Spencer C A. J Virol. 1995;69:5550–5559. doi: 10.1128/jvi.69.9.5550-5559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purves F C, Spector D, Roizman B. J Virol. 1991;65:5757–5764. doi: 10.1128/jvi.65.11.5757-5764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]