Abstract
Introduction
Hypothyroidism (HT) has been found a predictor of cardiovascular diseases. We aimed to ascertain the prevalence of HT in patients with manifest coronary heart disease (CHD), and to establish its association with conventional risk factors.
Methods
410 patients, 6–24 months after hospitalization for acute coronary syndrome, and/or revascularization, were included into the cross-sectional study.
Results
The prevalence of thyroid dysfunction was found in males and females as follows: overt HT, ie, thyroid stimulating hormone (TSH) > 3.65 mIU/L and free thyroxine (fT4) < 9 pmol/L and/or L-thyroxine substitution, in 2.6% and 8.4%, respectively; subclinical HT (TSH >3.65, fT4 9–23 and no substitution) in 4.3% and 15.0%, respectively. Higher prevalence of HT was found in females with hypercholesterolemia, and in males and females with concomitant positive thyroid peroxydase antibodies. Hypothyroid subjects had higher total homocysteine in both genders and von Willebrand factor in males only. Hypothyroid females had higher total and LDL cholesterol, and were more often treated for diabetes.
Conclusions
HT was found highly prevalent in patient with clinical coronary heart disease, mainly in females, and was associated with several cardiovascular risk factors.
Keywords: hypothyroidism, coronary heart disease, cholesterol, homocysteine, diabetes
Introduction
The association between overt hypothyroidism and coronary heart disease has been repeatedly observed (Becker 1985). Less evident is the role of asymptomatic mild elevation of thyroid stimulating hormone (subclinical hypothyroidism), notwithstanding that it represents the majority of patients with thyroid dysfunction. It was shown (Hak et al 2000) that even subclinical hypothyroidism independently doubled relative risk of myocardial infarction in females. The most frequent cause of hypothyroidism is the autoimmune thyroid disease (AITD) manifested by elevated thyroid antibodies, namely thyroid peroxydase antibodies (Samuels 1998). Thus, a sole increase in thyroid antibodies may potentially influence the coronary risk. The aim of our study was to establish the prevalence of thyroid dysfunction in a well defined sample of patients with manifest coronary heart disease and to assess its associations with other coronary risk factors.
Methods
Study sample consisted of patients with clinically manifest coronary artery disease, examined in the course of EuroAspire II survey, as Czech sample of this multinational project, methods of selection and interview of these patients were described in details elsewhere (EUROASPIRE II group 2001). Briefly, study sample selection was conducted by review of 525 hospital medical records of patients hospitalized for acute coronary event or coronary revascularization procedure. All responders were interviewed and examined by standardized methods at minimum of 6 months and maximum of 2 years after this hospitalization. Information on personal and demographic characteristics, personal and family history of coronary heart disease, lifestyle, and current pharmacotherapy were obtained at interview. The following standardized examinations were performed. Height and weight were measured in light indoor clothes without shoes using SECA 707 scales and measuring stick. Waist and hip circumferences were measured using a tape measure, and waist to hip ratio (WHR) was calculated. Blood pressure (BP) was measured twice in the sitting position on the right arm, using standard mercury sphygmomanometers to the nearest 2 mm of mercury. Information about reported current smoking was verified by breath carbon monoxide measurement using Smokerlyser device (model EC 50, Bedfont Scientific, Upchurch, UK).
Venous blood samples drawn in fasting state were used for biochemical laboratory analysis. The laboratory examinations included estimation of total (TCHOL) and HDL cholesterol (HDL), triglycerides (TG), and glucose (GLU) and were provided by the central laboratories of the EUROASPIRE II study (University Department of Medicine, Manchester Royal Infirmary, Manchester, UK). Unimate 7 cholesterol, Unimate HDL Direct and Unimate triglyceride reagents (Roche Diagnostics, Mannheim, Germany) on a Cobas Mira S Autoanalyser (Roche Diagnostics) were used. During the course of the study, the coefficient of variation for total cholesterol was 1.2%, for HDL cholesterol 9.4%, and for triglycerides 2.1%. Plasma glucose was measured from lithium-heparin samples using the hexokinase method on a Bayer Axon analyser (Bayer AG, Leverkusen, Germany); coefficient of variation was 2.8%. Non-HDL cholesterol (non-HDL) was calculated as difference between TCHOL and HDL. Additional variables were estimated in our local laboratory from serum aliquots series, stored at −80°C. Thyroid stimulating hormone (TSH), free thyroxine (fT4), thyroid peroxydase antibodies (TPO-Ab), total homocysteine (tHcy), folate, and vitamin B12 were assessed using commercial fluorescent polarization immunoassay (FPIA) kits (Abbott Laboratories, Wiesbaden, Germany) and AxSym analyser (variations of these measurements were less than 1.5%). Ultrasenzitive C-reactive protein (uCRP) was estimated immunoturbidimetricaly using Orion Diagnostica (Espoo, Finland) commercial kits and Olympus AU400 analyser (coefficient of variation was 4.1%). Serum intracellular adhesion molecule-1 (ICAM-1) and vascular adhesive molecule-1 (VCAM-1) were assessed using ELISA by RD Systems (Minneapolis, USA) and von Willebrand factor (vWf) was assessed by IMTEC GmbH (Berlin, Germany) ELISA kits; coefficients of variation of these ELISA analyses were less than 9%. All procedures were done according to the Good Clinical Practice regulation and were approved by the local Ethical Committee. Informed consent was obtained from all subjects and all personal data were stored under the provisions of the Czech Data Protection Act.
Statistical analysis of the data was done using software STATA Version 8 on PC. In the present study we categorized the thyroid parameters by following definitions: euthyroid-TSH 0.58–3.65 mIU/L. and fT4 9.0–23.0 pmol/L; overt hypothyroidism—TSH > 3.65 mIU/L and fT4 less than 9.0 pmol/L and/or thyroxine supplementation; subclinical hypothyroidism—TSH > 3.65 mIU/L and fT4 9.0–23.0 pmol/L; hyperthyroidism—TSH < 0.58 mIU/L. Positive TPO-Ab (ie, autoimmune thyroid disorder) was defined as TPO-Ab ≥ 14 IU/L. The reference ranges for TSH and TPO-Ab were given for particular kits used, according to the methodology described in National Academy of Clinical Biochemistry guidelines (Baloch et al 2003): the TSH reference range was established as 95% confidence limits of log-transformed TSH of 120 healthy (60 males and 60 females), TPO-Ab negative volunteers, aged less than 30 years, without personal or family history of thyroid disorder or palpable goiter. Similarly, TPO-Ab reference range was established from 120 male healthy volunteers, aged less than 30 years, with TSH concentrations between 0.5–2.0 mU/L, without palpable goiter, personal or family history of thyroid disease or any autoimmune disorder. The significance of differences among hypothyroid and euthyroid subjects were evaluated by Fisher's χ2 test for categorical variables and by Mann-Whitney U test for continuous variables. Multiple logistic regressions were used to ascertain the adjusted odds ratios (OR) and 95% confidence intervals (CI).
Results
A total of 410 responders, aged 59.2 ± 0.39 (age range 36.6–73.8 years), 301 males and 109 females, were analyzed in thepresent study. The overall distribution of TSH is shown separately by gender in Figure 1. In females, the distribution curve is skewed towards higher values, medians of values (25th and 75th percentiles) for males and females were 1.52 (1.08, 2.07) and 1.74 (1.08, 2.99), respectively (p < 0.01; Mann-Whitney U test). The prevalence of various forms of thyroid disorder is given in Table 1. Prevalence of hypothyroidism was significantly higher in females than in males, and moreover, euthyroid females were more often TPO-Ab positive, than euthyroid males. Hypothyroid females were also more often substituted with thyroid hormones than males. We separately analyzed the prevalence and odds ratios of overt or subclinical hypothyroidism in selected particular subgroups (Table 2). Prevalence of hypothyroidism was more than 3 times higher in subjects with TPO-Ab > 14 IU/L in both genders and also significantly higher in females with total cholesterol levels greater than 7 mmol/L.
Figure 1.
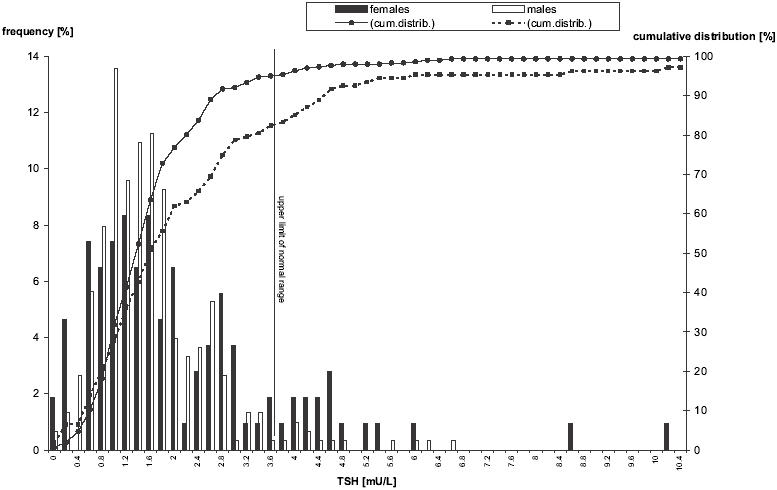
Distribution of thyroid stimulating hormone concentrations in males and females with clinical coronary heart disease (left axis for frequency, right axis for cumulative distribution).
Table 1.
Prevalence of thyroid function categories in males and females with clinical coronary heart disease [n (%)]
| Total sample | Males | Females | p | |
|---|---|---|---|---|
| All patients | 410 | 303 | 107 | — |
| Euthyroid | 341 (83.2%) | 265 (87.5%) | 76 (71.0%) | — |
| TPO-Ab negative | 312 (76.1%) | 252 (83.2%) | 60 (56.0%) | — |
| TPO-Ab positive | 29 (7.1%) | 13 (4.3%) | 16 (15.0 %) | <0.0001b |
| Hypothyroid | 46 (11.2%) | 21 (6.9%) | 25 (23.4%) | <0.0001a |
| subclinical | 29 (7.1%) | 13 (4.3%) | 16 (15.0%) | — |
| overt untreated | 9 (2.2%) | 7 (2.3%) | 2 (1.9%) | — |
| overt treated | 8 (1.9%) | 1 (0.3%) | 7 (6.5%) | <0.03b |
| Hyperthyroidc | 23 (5.6%) | 17 (5.6%) | 6 (5.6%) | 0.67a |
gender differences in prevalence of hypo- or hyperthyroidism.
gender differences in proportion of subcategories (χ2 test).
overt or subclinical.
Table 2.
Prevalence and odds ratio (OR) of hypothyroidism (overt or subclinical) by conventional risk factors sub-categories
| Males | Females | |||
|---|---|---|---|---|
| prevalence(%) | OR (95% CI)† | prevalence(%) | OR (95% CI)† | |
| Age | ||||
| • <55 years | 4.0 | 1 | 21.7 | 1 |
| • >55 years | 9.1 | 2.39 (0.78–7.31) | 25.6 | 1.24 (0.41–3.78) |
| Smoking | ||||
| • non-smokers | 7.0 | 1 | 28.6 | 1 |
| • smokers | 8.6 | 1.31 (0.46–3.77)a | 12.5 | 2.88 (0.60–13.90)a |
| Positivity of TPO-Ab | ||||
| • TPO-Ab < 14 IU/L | 6.3 | 1 | 16.7 | 1 |
| • TPO-Ab > 14 IU/L | 23.5** | 4.07 (1.17–14.10)a | 44.8** | 3.95 (1.50–10.40)a |
| Elevated blood pressure | ||||
| • BP < 140/90 mmHg | 7.1 | 1 | 23.2 | 1 |
| • BP ≥ 140/90 mmHg | 7.9 | 1.03 (0.42–2.57)b | 25.0 | 0.99 (0.39–2.51)b |
| Hypercholesterolemia | ||||
| • TCHOL < 5 mmol/L | 11.8 | 1 | 12.5 | 1 |
| • TCHOL 5–6.99 mmol/L | 5.5 | 0.41 (0.16–1.03)c | 27.1 | 2.87 (0.59–13.88)c |
| • TCHOL ≥7 mmol/L | 8.7 | 1.03 (0.42–2.57)c | 39.1 | 6.93 (1.02–45.20)c |
| Diabetes type IId | ||||
| • non-diabetic | 6.6 | 1 | 22.4 | 1 |
| • diabetic | 9.7 | 1.48 (0.57–3.84)a | 32.0 | 1.47 (0.53–4.07)a |
adjusted for age.
adjusted for age and treatment with antihypertensives.
adjusted for age and treatment with lipid-lowering drugs.
fasting glucose ≥6.8 mmol/l and/or antidiabetic treatment
*p < 0.05,
p < 0.01,
***p < 0.001; differences in proportion of hypothyroidism and euthyroidism between selected subgroups (χ2test).
by multiple logistic regression, presence of overt or subclinical hypothyroidism as dependent variable
Abbreviations: BP, blood presssure; CI, confidence interval;TCHOL, total cholesterol; OR, odds ratio.
We compared a large set of coronary risk factors by thyroid status and presence of positive TPO-Ab (Table 3). As expected, males and females with hypothyroidism (both, overt or subclinical, but without L-thyroxine substitution) had significantly higher TSH and TPO-Ab, while lower fT4 concentrations, than the euthyroid TPO-Ab negative subjects (as can be expected). Hypothyroidism was associated with increased tHcy in both genders and the significance of association remained significant even after adjustement for potential confounders (age, current smoking, fibrate treatment, creatinine, folate, and B12 concentrations). Males with hypothyroidism showed increased levels of vWf, and again, the significance of this association also persisted, if adjusted for potential confounders (age, current smoking, systolic blood pressure, LDL-cholesterol, glucose, and tHcy concentrations). On the other hand, the significance of association between VCAM-1 and hypothyroidism disappeared after adjustment for the same confounders.
Table 3.
Differences in selected factors between euthyroid and hypothyroid subjects with regard to thyroid antibodies
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Euthyroid TPO-Ab negative | Euthyroid TPO-Ab positive | Hypothyroid | Euthyroid TPO-Ab negative | Euthyroid TPO-Ab positive | Hypothyroid | |
| Age (years) | 58.1±0.49 | 62.5±1.85 | 60.5±1.99 | 60.7±0.98 | 60.1±2.01 | 64.3±1.55 |
| Smoking (%) | 19.1 | 23.1 | 20.0 | 28.3 | 25.0 | 6.3 |
| Body mass index (kg/m2) | 28.9±0.25 | 29.2±0.99 | 29.5±0.70 | 31.0±0.81 | 32.3±1.47 | 29.2±1.17 |
| Waist circumference (cm) | 100.5±0.64 | 101.2±1.93 | 100.7±1.16 | 96.0±1.70 | 96.8±3.81 | 90.7±2.94 |
| Systolic BP (mmHg) | 136.5±2.07 | 133.8±5.72 | 138.8±6.26 | 137.1±2.45 | 146.6±6.65 | 137.7±6.10 |
| Diastolic BP (mmHg) | 81.4±0.67 | 81.2±3.11 | 79.9±1.82 | 78.6±1.51 | 83.1±2.00 | 77.7±2.82 |
| Heart Rate (beats/min) | 66.3±0.64 | 66.7±2.54 | 64.0±2.58 | 67.0±1.53 | 67.9±2.33 | 67.3±2.07 |
| antihypertensives (%) | 88.0 | 76.9 | 80.0 | 91.7 | 87.5 | 94.4 |
| Total cholesterol (mmol/L) | 5.54±0.07 | 5.25±0.18 | 5.30±0.27 | 6.04±0.17 | 6.06±0.44 | 6.73±0.30* |
| Triglycerides (mmol/L) | 1.74±0.06 | 1.51±0.20 | 2.01±0.29 | 1.67±0.11 | 1.69±0.24 | 1.97±0.14 |
| HDL cholesterol (mmol/L) | 1.18±0.02 | 1.15±0.07 | 1.15±0.07 | 1.45±0.06 | 1.40±0.12 | 1.50±0.15 |
| LDL cholesterol (mmol/L) | 3.60±0.06 | 3.41±0.14 | 3.32±0.26 | 3.88±0.14 | 3.89±0.41 | 4.51±0.28* |
| lipid lowering drugs | 57.1 | 46.2 | 50.0 | 63.3 | 56.3 | 61.1 |
| Glucose (mmol/L) | 6.90±0.15 | 6.66±0.40 | 7.00±0.43 | 6.79±0.33 | 7.61±0.83 | 7.03±0.50 |
| antidiabetics (%) | 10.7 | 7.7 | 20.0 | 8.3 | 18.8 | 33.3* |
| Homocysteine (μmol/L) | 13.4±0.32 | 14.4±1.53 | 17.9±4.54** | 12.9±0.65 | 12.9±1.05 | 17.2±2.70** |
| Serum folate (ng/mL) | 9.41±1.72 | 7.13±0.90 | 6.98±0.57 | 7.26±0.31 | 7.52±0.48 | 6.73±0.44 |
| Serum B12 (pg/mL) | 350.0±10.5 | 329.5±47.8 | 378.6±41.3 | 375.4±29.5 | 340.9±31.6 | 390.3±40.5 |
| ultrasensitive CRP (mg/L) | 2.28±0.13 | 2.45±0.42 | 1.75±0.38 | 2.67±0.29 | 2.10±0.43 | 1.77±0.28 |
| ICAM-1 (ng/mL) | 308.0±9.32 | 350.3±42.9 | 364.0±57.7 | 358.9±19.4 | 378.7±47.3 | 297.1±27.7 |
| VCAM-1 (ng/mL) | 922.1±29.1 | 976.2±145.8 | 1189.0±155.3* | 776.2±46.1 | 713.6±120.2 | 743.7±63.6 |
| vonWillebrand factor (%) | 187.7±4.94 | 193.1±14.5 | 251.5±34.2** | 187.2±9.81 | 188.8±25.5 | 170.3±19.2 |
| TSH (mlU/L) | 1.65±0.04 | 1.76±0.17 | 6.05±1.44*** | 1.65±0.10 | 2.01±0.21 | 8.82±3.07*** |
| Free thyroxine (pmol/L) | 13.9±0.16 | 13.7±0.33 | 11.4±0.52*** | 13.8±0.26 | 13.9±0.55 | 12.2±0.84*** |
| TPO-Ab (IU/L) | 5.72±0.12 | 186.7±85.6*** | 130.8±65.4*** | 5.71±0.26 | 321.1±98.6*** | 169.2±74.2*** |
p < 0.001,
p < 0.01,
p < 0.05, adjusted for age.
Hypothyroid females showed significantly higher total and LDL cholesterol (also if adjusted for lipid lowering treatments), and were more often treated for diabetes (Table 3).
Euthyroid, TPO-Ab positive subjects did not differ in any examined characteristics (with the exception of the sole TPO-Ab) from euthyroid TPO-Ab negative ones (Table 3).
Hypothyroid, TPO-Ab positive patients were further compared with hypothyroid, TPO-Ab negative ones (data not shown). They showed significantly higher TSH, than hypothyroid TPO-Ab negative patients (10.3 ± 3.44 vs 4.24 ± 0.65 mU/L; p = 0.02 by Mann-Whitney U test); however, they did not differ in any other examined risk factors.
Discussion
In our sample of patients with manifest coronary heart disease we found the overall prevalence of hypothyroidism 11.5%. It was about 4 times more prevalent in females, than in males (23.4% vs 6.9 %, respectively). In comparison, the prevalence of hypothyroidism in general population of the same geographical area was found in 6.8% males and 13.8% females (Mayer et al 2005). The review of 12 population-based studies (Vanderpump and Tunbridge 1996) found subclinical or overt hypothyroidism in about 5% of the general population. Prevalence of hypothyroidism was shown to gradually increase between age 45 and 60 years and to be higher in females, than in males (Canaris et al 2000; Hollowell et al 2002). In contrast to these findings, the prevalence of hypothyroidism in females was in our sample similar in both, aged ≥55 or younger than 55 years. Therefore, the screening of thyroid functions seems to be warranted in female coronary patients without regard to age.
Hypothyroidism has been generally considered as cardiovascular risk factor in majority of studies, mainly because of its association with elevated serum total and LDLcholesterol. Hypercholesterolemia in hypothyroidism probably results from reduced catabolism of lipoproteins, a phenomenon that may be explained by a decreased expression of lipoprotein receptors (Thomson et al 1981; Scarbottolo et al 1986). We found that females with untreated hypothyroidism (both overt and subclinical) had significantly higher total and LDL cholesterol. Females with total cholesterol greater than 7 mmol/L had about a 7 times higher risk of hypothyrodism. Surprisingly, no such association was found in males and this discrepancy cannot be explained by differences in lipid-lowering drug intake.
In our study we also ascertained that females with hypothyroidism were significantly more often treated for type 2 diabetes. Several studies found thyroid dysfunction highly prevalent in type 1 diabetes patients; reported prevalence was 12%–24% in females and 6% in males (Gray et al 1979; Duckworth et al 1986; Umpierrez et al 2003). The prevalence of thyroid dysfunction in type 2 diabetic patients (3%–6%) was found very to be close to the estimates in the general population. On the other hand, a recent small clinical study reported that L-thyroxine substitution improved the ability of insulin to stimulate glucose disposal, ie, beneficially influenced insulin resistance, the main pathophysiological mechanism of type 2 diabetes (Rochon et al 2003). Increased insulin resistance in hypothyroid subjects may be the cause of poorly compensated diabetes and consequently increased administration of anti-diabetic drugs, as observed in the present study.
We also found that hypothyroid males and females showed significantly higher tHcy concentrations by about 4–5 μmol/L. Homocysteine was established as a significant predictor of vascular diseases. In a recent meta-analysis of 20 prospective studies, Wald et al (2002) calculated that a 5 μmol/L increase in tHcy raised the risk for coronary event by 32% and for stroke by 59%. Our finding is in line with that of Christ-Crain and colleagues (2003), who observed elevated tHcy in patients with overt hypothyroidism, but without coronary heart disease. The pathogenesis of elevated tHcy in hypothyroidism is generally attributed to the reduction of glomerular filtration rate, because of the well established effect of hypothyroidism on the kidney (Kreisman et al 1999). An alternative explanation of this effect could be that concentration of tHcy can also rise through reduced activity of methylenetetrahydrofolate reductase activity (MTHFR), the key enzyme for re-methylation of homocysteine to methionine. Hypothyroid individuals can be defective in converting riboflavin to the co-enzyme flavin-adenin dinucleotide and, consequently, deficient in MTHFR activity (Rivlin et al 1966; Shane et al 1985; Cimino et al 1987).
The association of hypothyroidism (both overt and subclinical) with increased uCRP levels has also been reported (Christ-Crain et al 2003). In contrast, in our sample we did not find any differences in uCRP or other inflammatory markers (such as ICAM-1 and VCAM-1); these parameters might have been influenced by concomitant therapies with aspirin and statins.
Hypothyroidism has been observed to be associated with endothelial dysfunction, measured by impaired endothelium-dependent vasodilatation; it could be reversed by L-thyroxine supplementation (Taddei et al 2004). In the present study we used vWf as a marker of endothelial dysfunction and it appeared significantly increased in hypothyroid males (but not females). This association was completely independent of other factors, which are known to potentially impair the endothelial functions, notably from the observed increase of tHcy.
We also examined whether autoimmune thyroid disorder per se (TPO-Ab positivity) influenced the coronary risk factors. Despite high prevalence of positive TPO-Ab (about 24% of males and 45% of females), no influence on any included cardiovascular risk factor in either euthyroid or hypothyroid subjects was found.
The question remains whether L-thyroxine supplementation in mild hypothyroid patients could reduce coronary risk. Several placebo-controlled randomized trials of L-thyroxine substitution have been performed (Meier et al 2001; Danese et al 2000; Caraccio et al 2002). Some of these studies have shown that L-thyroxine may favorably influence symptoms of hypothyroidism as well as dyslipidemias. A quantitative review of 13 studies with L-thyroxine substitution in mild thyroid failure done by Danese et al (2000) found that the mean decrease of TCHOL was 0.20 mmol/L (with median of changes from baseline value −5%) and mean decrease of LDL was 0.26 mmol/L, leaving HDL cholesterol and TG uninfluenced. A recently published population-based cohort study (Flynn et al 2006) reported, that total mortality among patients with treated thyroiddysfunction (hypo- or hyperthyroidism) did not differ from rates in the general population.
The important determinant of thyroid function is current iodine status. Because iodine supply in our population (according to the Czech Health Statistical Yearbook, about 150 μmg/day in 2005) is within recommended limits, our patients have been assumed to have no iodine deficit. The question remains as to what extent the thyroid parameters were influenced by iodine-containing compounds, such as amiodarone or contrast media. Angiography was performed in about two-third of the patients at least 6 months before the interview, when the thyroid parameters were estimated. It was proved that application of iodine contrast media leads to a small but significant decrease in fT4 and an increase in TSH. The peak of this effect is on day 3 and depends on preexisting thyroid status (Gartner et al 2004). The long-term effect on thyroid status remains questionable. In addition, 10 patients in our sample were being treated with amiodarone. Four showed increased fT4 and could be misclassified as hyperthyroid; however amiodarone treatment did not influence the prevalence of hypothyroidism.
There are several limitations of this study. Because of the cross-sectional design, it is impossible to consider the causality of hypothyroidism in coronary heart disease etiology. We also have no evidence as to whether thyroid dysfunction appeared before the coronary event or in the first months thereafter. Only patients surviving the acute coronary event for least 6 months were included according to the protocol; the prevalence of hypothyroidism can be potentially underestimated by this bias. Finally, the definition of thyroid function categories was based on single laboratory estimation, while from clinical point of view, it is necessary to confirm the diagnosis by repeated measurements.
In conclusion, thyroid dysfunction has to be considered a highly prevalent condition, mainly in females, which could potentially contribute to the overall coronary risk and may be amenable to secondary preventive intervention. The evidence of benefit of L-thyroxine substitution in addition to conventional therapies on morbidity and mortality of coronary patients remains to be elucidated by randomized pharmacological trials.
Acknowledgments
The present study was funded by Grant Agency of Czech Ministry of Health, grant no. 7534-3. We acknowledge the help of Abbott Laboratories Ltd, providing some free-of-charge laboratory kits. We also used several data from EUROASPIRE II survey, carried out under auspices of European Society of Cardiology and we would like to acknowledge the cooperation of all investigators and coworkers who participated on the EUROASPIRE II survey in Czech Republic.
References
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- Becker C. Hypothyroidism and atherosclerotic heart disease: pathogenesis, medical management, and the role of coronary artery bypass surgery. Endocr Rev. 1985;6:432–40. doi: 10.1210/edrv-6-3-432. [DOI] [PubMed] [Google Scholar]
- Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab. 2002;87:1533–8. doi: 10.1210/jcem.87.4.8378. [DOI] [PubMed] [Google Scholar]
- Cimino JA, Jhangiani S, Schwarz E, et al. Riboflavin metabolism in the hypothyroid human adult. Proc Soc Exp Biol Med. 1987;184:151–3. doi: 10.3181/00379727-184-42459. [DOI] [PubMed] [Google Scholar]
- Christ-Crain M, Meier C, Guglielmetti M, et al. Elevated C-reactive protein and homocysteine values: cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial. Atherosclerosis. 2003;166:379–86. doi: 10.1016/s0021-9150(02)00372-6. [DOI] [PubMed] [Google Scholar]
- Danese MD, Ladenson PW, Meinert CL, et al. Effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: A quantitative review of the literature. J Clin Endocrinol Metab. 2000;85:2993–3000. doi: 10.1210/jcem.85.9.6841. [DOI] [PubMed] [Google Scholar]
- Duckworth WC, Badlissi J, Kitabchi AE. Thyroid function in diabetes. In: Vanmiddleworth L, editor. The thyroid gland. Chicago: Year Book Medical; 1986. pp. 247–61. [Google Scholar]
- Hak AE, Pols HAP, Visser TJ, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women. The Rotterdam study. Ann Intern Med. 2000;132:270–8. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- EUROASPIRE II Group. Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries. Principal results from EUROASPIRE II. Eur Heart J. 2001;22:554–72. doi: 10.1053/euhj.2001.2610. [DOI] [PubMed] [Google Scholar]
- Flynn RW, MacDonald TM, Jung RT, et al. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J Clin Endocrinol Metab. 2006;91:2159–64. doi: 10.1210/jc.2005-1833. [DOI] [PubMed] [Google Scholar]
- Gartner W, Weissel M. Do iodine-containing contrast media induce clinically relevant changes in thyroid function parameters of euthyroid patients within the first week? Thyroid. 2004;14:521–4. doi: 10.1089/1050725041517075. [DOI] [PubMed] [Google Scholar]
- Gray RS, Irvine WJ, Clarke BF. Screening for thyroid dysfunction in diabetics. Br Med J. 1979;2:1439. doi: 10.1136/bmj.2.6202.1439-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollowell J, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- Kreisman SH, Hennessey JV. Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Arch Intern Med. 1999;159:79–82. doi: 10.1001/archinte.159.1.79. [DOI] [PubMed] [Google Scholar]
- Mayer O, Jr, Simon J, Hrbkova J, et al. Epidemiological study of hypothyroidism as cardiovascular risk in population. Cas Lek Cesk. 2005;144:459–64. [PubMed] [Google Scholar]
- Meier C, Staub JJ, Roth CB, et al. TSH-controlled L-thyroxine therapy reduces cholesterol levels, clinical symptoms in subclinical hypothyroidsm? A double blind, placebo-controlled trial (Basel Thyroid Study) J Clin Endocrinol Metab. 2001;86:4860–6. doi: 10.1210/jcem.86.10.7973. [DOI] [PubMed] [Google Scholar]
- Perros P, McCrimmon RJ, Shaw G, et al. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med. 1995;12:622–7. doi: 10.1111/j.1464-5491.1995.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Rivlin RS, Landon RG. Regulation of hepatic FAD levels by thyroid hormone. Adv Enzyme Regul. 1966;4:45–58. doi: 10.1016/0065-2571(66)90006-9. [DOI] [PubMed] [Google Scholar]
- Rochon C, Tauveron I, Dejax C. Response of glucose disposal to hyperinsulinaemia in human hypothyroidism and hyperthyroidism. Clin Sci. 2003;104:7–15. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Samuel MH. Subclinical thyroid disease in the elderly. Thyroid. 1998;8:803–13. doi: 10.1089/thy.1998.8.803. [DOI] [PubMed] [Google Scholar]
- Scarbottolo L, Trezze E, Roma P, et al. Experimental hypothyroidism modulates the expression of the low density lipoprotein receptor by the liver. Atherosclerosis. 1986;59:329–33. doi: 10.1016/0021-9150(86)90129-2. [DOI] [PubMed] [Google Scholar]
- Shane B, Stokstad EL. Vitamin B12–folate interrelationships. Ann Rev Nutr. 1985;5:115–41. doi: 10.1146/annurev.nu.05.070185.000555. [DOI] [PubMed] [Google Scholar]
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasadilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab. 2004;88:3731–7. doi: 10.1210/jc.2003-030039. [DOI] [PubMed] [Google Scholar]
- Thomson GR, Souter AK, Spengel FA, et al. Defects of receptor-mediated low density lipoprotein catabolism in homozygous familial hypercholesterolemia and hypothyroidism in vivo. Proc Natl Acad Sc USA. 1981;78:2591–5. doi: 10.1073/pnas.78.4.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umpierrez GE, Kashif AL, Murphy MB. Thyroid dysfunction in patients with Type 1 Diabetes A longitudinal study. Diabetes Care. 2003;26:1181–5. doi: 10.2337/diacare.26.4.1181. [DOI] [PubMed] [Google Scholar]
- Vanderpump MPJ, Tunbridge WMG. The epidemiology of thyroid disease. In: Braveman LE, Utiger RD, editors. The Thyroid. 9th ed. Piladelphia: Lippincott-Raven Publisher; 1996. pp. 474–82. [Google Scholar]
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Br Med J. 2002;325:1202–6. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]


