Abstract
The sirolimus-eluting coronary stent received CE Mark approval in Europe in April 2002. In the US, FDA approval followed in April 2003. Since the preliminary results from the First-in-Man feasibility study were presented, several randomized, controlled trials have documented the profound antiproliferative effects of sirolimus, a macrolide antibiotic and potent cytostatic inhibitor of smooth muscle cell proliferation. Subsequently, the body of clinical evidence was increased by the second wave of evidence from trials in more complex lesions (such as in-stent restenosis, small vessels, chronic total occlusions) and “high-risk” patients such as those with diabetes. More recently we have had the opportunity to compare the two commercially available drug-eluting stents following the presentation of data from six head-to-head trials. As a result of numerous single and multi-center, national and international studies in which the safety and efficacy of sirolimus-eluting coronary stents have been subjected to close scrutiny, the global interventional cardiology community now has a wealth of evidence in support of the use of this technology resulting in dramatically improved patient outcomes after percutaneous intervention.
Keywords: angioplasty, restenosis, percutaneous coronary intervention, rapamycin, sirolimus-eluting stent
Introduction
When the findings from the first 50 patients treated with angioplasty were first published, few would have predicted the dramatic increase in the use of percutaneous coronary interventions (PCI) with the associated explosion of clinical research and attendant information (Gruntzig et al 1979). There is no doubt that after the introduction of balloon angioplasty in 1977, intracoronary stenting was the most important development in the field of percutaneous coronary revascularization. Nevertheless, the problem of post-angioplasty restenosis, or lumen re-narrowing several months after the index procedure, continued to challenge interventional cardiologists (Serruys et al 1994, 2005). The consequences of restenosis are many and varied, affecting every aspect of the perception and reality of angioplasty as a definitive therapeutic intervention. The recurrence of symptoms has a major impact on both the patient and the healthcare system. Repeat intervention (sometimes requiring coronary bypass graft surgery), repeat hospitalization, sometimes myocardial infarctions, and time off work, or in the case of the retiree, lost recreational time, impact on the quality of life of the patient, and the attendant costs all add to the economical burden of healthcare systems (Van Hout et al 1996; Morice et al 2002; Serruys et al 2005).
Pathophysiology of restenosis
Stent-induced restenosis involves a complex interplay of biological events. We now know that stent placement causes endothelial injury as well as deeper injury due to lacerations of the arterial wall. Injury stimulates smooth muscle cells to both proliferate excessively and migrate from the underlying vessel wall (Scott 2006).
Despite the scaffolding effect of the stent, the smooth muscle cells accumulate gradually, impinging upon the lumen. Tissue growth continues to be a problem because stents do not stop smooth muscle cell proliferation (Spier et al 1995). Currently, drug-eluting stents have emerged as the most promising approach in the fight against restenosis.
What is sirolimus?
Sirolimus was first isolated from a soil micro-organism, Streptomyces hygroscopius, found on Easter Island, as reported by in 1975 (Vezina et al 1975). Rapa Nui is the local name for Easter Island, inspiring the compound’s well-known common name of rapamycin.
Crystalline sirolimus was purified from fermentation media and found to be active against several strains of yeast and filamentous fungi. The produced streptomycyte was also active against some bacteria (Sehgal et al 1975; Vezina et al 1975), leading to the original classification of sirolimus as an antifungal antibiotic (Singh et al 1979; Chakraborty et al 1995).
Since sirolimus is very lipid soluble (ie, lipophilic), almost no drug is released into the bloodstream during stent placement at the lesion site, and after stent implantation, the diffusion gradient favors elution into tissue, again limiting the amounts of circulating free sirolimus.
In addition to its antibiotic activity, it became apparent that sirolimus also possessed powerful anti-proliferative and immunosuppressant properties (Chang et al 1991). Sirolimus was shown to be a novel inhibitor of cellular proliferation, distinct from cyclosporin A in a variety of in vitro and in vivo models (Chang et al 1991; Stepkowski et al 1991; Groth et al 1999). The smooth muscle anti-proliferative properties have been characterized in numerous vascular models (Marx et al 1995; Poon et al 1996; Pham et al 1998; Poston et al 1999).
In vivo studies in allograft and angioplasty models demonstrated the effectiveness of sirolimus in preventing tissue hyperplasia following vascular injury and led to is consideration as an agent for the prevention of restenosis (Gregory et al 1995; Gallo et al 1999).
Clinical studies
The First-in-Man feasibility study, conducted in Sao Paulo, Brazil and Rotterdam, the Netherlands showed the CYPHER® sirolimus-eluting stent (Cordis Corporation, Johnson & Johnson, Warren, NJ, USA) to be remarkably effective in eliminating the occurrence of restenosis (Sousa et al 2001).
These early results were followed by the unprecedented findings from the RAVEL trial, the first double blind, randomized, controlled trial of a drug-eluting stent (Morice et al 2002). These exceptional results are well known and have been replicated in three additional randomized, controlled trials – SIRIUS, E-SIRIUS, and C-SIRIUS (Moses et al 2003; Schofer et al 2003; Schampaert et al 2004).
Subsequently, the findings from the First-in-Man study study show that the efficacy and safety of sirolimus have been sustained out to 4 years (Sousa et al 2005).
Since the preliminary results from the First-in-Man feasibility study were presented, the CYPHER stent is currently available in more than 80 countries and has been used by doctors to treat more than 2 million patients worldwide (Cordis Corporation, Warren, New Jersey, USA, press release 26 April 2006). The sirolimus-eluting coronary stent (SES) is the most studied drug-eluting stent today with the largest body of clinical evidence demonstrating long-term safety and efficacy of its drug and polymer (Fig. 1). In addition, the SES was shown to yield the same strong patient outcomes with or without balloon pre-dilation, according to the comparison of intravascular ultrasound results from the multicenter, randomized E-SIRIUS and SIRIUS trials (Hoffmann et al 2005).
Figure 1.
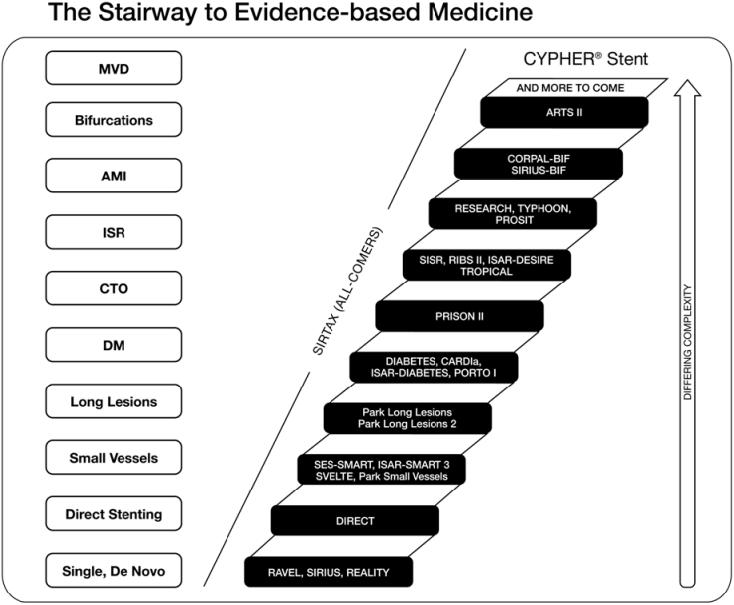
Sirolimus-eluting coronary stent is the most studied of all drug-eluting stents, with data generated from randomized controlled trials and registries. AMI, acute myocardial infarction; CTO, chronic total occlusions; DM, diabetes mellitus; ISR, in-stent restenosis; MVD, multi vessel disease.
However, the main criticism of any controlled clinical program is that by its very nature, patient entry is limited by the strict inclusion and exclusion criteria. It is against this background that several prospective registry studies have begun, in order to collect a large series of data in more challenging lesions (such as small vessels, long lesions, bifurcations, chronic total occlusions and left main disease) and patient populations (acute coronary syndromes and multi-vessel disease).
Management of complex lesions and “high-risk” patients
As operator experience with SES grows, and the clinical evidence base is enhanced following the outcomes from a series of independent, “head-to-head” comparative trials, so SES are being used to treat increasingly complex lesions and patients considered to be “high risk”. Among the factors associated with this changing face of interventional cardiology three stand out:
The rising epidemic of diabetes, more complex lesions (small vessels, more extensive and diffuse disease, multi-vessel disease, total occlusions, left main disease) (Urban et al 2006).
The rising epidemic of obesity – associated with metabolic syndrome (hypertension, dyslipidemia, high fasting glucose) and early onset of atherosclerosis.
The advent of drug-eluting stents – resulting in improved outcomes and their resultant use in a broader range of indications (including diabetes/multi-vessel disease, in-stent restenosis, chronic total occlusions, bifurcations, saphenous vein grafts, and left main stem disease).
Following the First-in-Manfeasibility study and the series of trials in which SES was compared with bare metal stents in relatively simple lesions, the body of clinical evidence was increased by additional data from trials on more complex lesions such as small vessels (Meier et al 2006; Ardissino et al 2004), chronic total occlusions (Jang et al 2006; Lotan et al 2006; Suttorp et al 2006), in-stent restenosis (Alfonso et al 2006; Holmes et al 2006), multi-vessel disease (Serruys et al 2005), acute myocardial infarction (Spaulding et al 2006), and patients with diabetes (Ortolani et al 2005; Sabate et al 2006).
Additional data from the SIRIUS trial serve to confirm the durability of outcomes with CYPHER stent and the importance of inhibiting neointimal hyperplasia (NIH) and late loss as much as possible from the start. SIRIUS follow-up results presented at ACC showed that the highly significant differences (p < 0.0001) between the CYPHER stent and bare metal control stent for all clinical event endpoints were sustained at 3-years. Furthermore, when these results are compared with the 9m follow-up data, SIRIUS demonstrates that the clinical benefit of CYPHER stent over bare metal stents increases from 9 to 12 months (by an average of 24%) and this remains virtually unchanged at 3 years with no evidence of late “catch-up” in restenosis (Moses et al 2003).
Comparative trials
More recently, we have had the opportunity to compare the SES and paclitaxel drug-eluting stents (PES) following the presentation of data from a total of ten head-to-head trials (Table 1).
Table 1.
Comparative trials: sirolimus-eluting stents and paclitaxel-eluting stents
| Author | Trial | Indication | Patients (N) | Outcomes |
|---|---|---|---|---|
| Kastrati et al 2005a | ISAR-DESIRE (RCT) | In-stent restenosis | 300s | Angiographic restenosis |
| SES: 14.3% PES: 21.7% PTCA: 44.6% | ||||
| Target vessel revascularization | ||||
| SES: 8.0% PES: 19.0% PTCA: 33.0% | ||||
| Dibra et al 2005 | ISAR-DIABTES (RCT) | Diabetes | 250 | In-segment restenosis |
| SES: 6.9% PES: 16.5% | ||||
| Target lesion revascularisation | ||||
| SES: 6.4% PES: 12.0% | ||||
| Mehilli et al 2006 | ISAR-SMART 3 (RCT) | Small coronary arteries | 360 | Angiographic restenosis |
| SES: 11.4% PES: 19.0% | ||||
| Target lesion revascularization | ||||
| SES: 6.6% PES: 14.7% | ||||
| Windecker et al 2005 | SIRTAX (RCT) | All comer | 1.012 | MACE |
| SES: 6.2% PES: 9.8% | ||||
| Target lesion revascularisation | ||||
| SES: 4.8% PES: 8.3% | ||||
| Morice et al 2006 | REALITY (RCT) | De novo lesions | 1386 | In-lesion restenosis |
| SES: 9.6% PES: 11.1% | ||||
| Pan et al 2007 | CORPAL | Bifurcation lesions | 205 | Target lesion revascularization |
| SES: 4% PES: 13% | ||||
| Lee et al 2006 | PROSIT (RCT) | AMI | 231 | MACE rates @ 9 months |
| SES: 6.9% PES: 14.8% | ||||
| Park et al 2006 | Long Lesions II | Lesion length ≥25 mm | 500 | In-segment restenosis |
| SES: 3.0% PES: 10.3% | ||||
| MACE rates @ 9 months | ||||
| SES: 3.0% PES: 7.8% | ||||
| Goy et al 2005 | TAXI (RCT) | All comer | 202 | MACE rates @ 7 months |
| SES: 6% PES: 4% | ||||
| Target lesion revascularization | ||||
| SES: 3% PES: 1% | ||||
| Kaiser et al 2005 | BASKET (RCT) | All comer | 826 | Target vessel revascularisation |
| SES: 1.5% PES: 2.6% | ||||
| Target lesion revascularization | ||||
| SES: 0.8% PES: 0.7% |
Abbreviations: RCT, randomized controlled trial; PES, paclitaxel-eluting stents; SES, sirolimus-eluting stents.
ISAR-DESIRE was the first randomized trial to show that drug-eluting stents provide results superior to those achieved with standard percutaneous transluminal coronary angioplasty (PTCA) in the treatment of in-stent restenosis. Secondary analysis also suggests an advantage of SES over PES in terms of clinical restenosis rates in this indication (SES: 14.3%, PES: 21.7%) (Kastrati et al 2005a).
Results from SIRTAX, a Swiss study involving 1005 patients, revealed that when compared with TAXUS, the CYPHER stent had significantly lower rates of death, myocardial infarction (MI), or target lesion revascularization (TLR) at 9 months – the composite primary endpoint (6.2% vs 10.8%, p < 0.009). What makes these findings all the more impressive is the fact that SIRTAX is an “all-comers” trial with a very complex patient population which included those with acute coronary syndromes, chronic total occlusions and bifurcation lesions (Windecker et al 2005).
Following the pattern set by earlier comparisons of drug-eluting stents, diabetic patients treated in ISAR-DIABETES trial, revealed CYPHER to have a significantly superior suppression of neointimal hyperplasia, as measured by both in-stent and in-segment late lumen loss (Dibra et al 2005). This finding corresponds to a significant reduction in restenosis rates (16.5% in-segment restenosis in the paclitaxel group versus 6.9% in the sirolimus group) and a much lower need for repeat intervention with CYPHER stent. Target lesion revascularization rates were 12.0% and 6.4% for the paclitaxel and sirolimus groups respectively (Dibra et al 2005).
The results from the REALITY trial show no significant differences in the primary end point of binary restenosis at 8 months among the 1386 patients treated with either the CYPHER or the TAXUS. Late loss and diameter stenosis were significantly less in the CYPHER-treated patients, but this did not translate into differences in the secondary end points of target lesion and target vessel revascularizations at one year. The composite end point of cardiac death, MI, coronary artery bypass grafting (CABG), or repeat percutaneous coronary intervention (PCI) (MACE) at one year was no different between the two groups. REALITY also does not settle the question of stent thrombosis, which trended higher in the Taxus-treated patients (Morice et al 2006). The issue of stent thrombosis is discussed below.
Similarly, TAXI, a prospective randomized comparison between PES and SES in the real world of interventional cardiology confirmed that the high success rate obtained with both stents in randomized trials can be replicated in routine clinical practice. A total of 202 patients were enrolled into the study. One hundred patients were treated with a PES and 102 received an SES. Target lesion revascularisation rates were low in both groups: 1% with paclitaxel and 3% with sirolimus. The investigators acknowledged that in this small group of patients they were unable to show any advantage of one stent over the other (Goy et al 2005).
ISAR-SMART 3 involved a total of 360 patients undergoing PCI for de novo lesions in native coronary arteries with a diameter of <2.80 mm. They were randomly assigned to receive either an SES or a PES. The primary endpoint was in-stent late luminal loss, the primary endpoint, was 0.32 mm, which was greater than that in the SES group, failing to show non-inferiority of the PES to the SES. Angiographic restenosis was reported in 19.0% of the PES cohort as compared with 11.4% in those treated with the SES. Similarly, target lesion revascularization rates were 14.7% and 6.6% for the paclitaxel and sirolimus groups respectively (Mehilli et al 2006).
Conducted at Cordoba and Las Palmas in Spain, the CORPAL trial evaluated 1182 lesions in 910 patients identified as being at high risk for restenosis. Consecutive patients with documented myocardial ischemia secondary to coronary lesions were randomized to either SES or PES. There were no significant differences in terms of immediate or 1-month follow-up. However, late evaluation (15 ± 8 months) did reveal differences in terms of restenosis rates (15% vs 23% for SES and PES respectively, and target lesion revascularization (4% vs 7%) (Suarez de Lezo et al 2005).
Data from the multi-centre, prospective, randomized controlled LONG-DES II trial revealed that patients treated with SES had significantly less in-stent late loss than those treated with paclitaxel-eluting stents (0.05 ± 0.22 mm vs 0.25 ± 0.35 mm). Major adverse cardiac event rates were 3% for sirolimus vs 10.3% for paclitaxel (Hong et al 2006).
Results from PROSIT, the prosective, randomized, independent, controlled trial, in acute MI patients, show that rates of major adverse cardiac events (MACE) at 9-month follow-up were 6.9% (sirolimus) and 14.8% (paclitaxel). The MACE rates in the paclitaxel cohort were driven by death (7.8%) and TLR (7.8%). 231 patients were enrolled into the study, randomized to either SES (n = 116) or PES stents (n = 115) (Lee et al 2006).
Similarly, data from a meta-analysis, in which the results from six randomized controlled trials were combined so as to compare the Cypher and Taxus drug-eluting stents. The six trials included in the meta-analysis were CORPAL, ISAR-DIABETES, ISAR-DESIRE, REALITY, SIRTAX, and TAXI. A total of 3669 patients with 4878 lesions were treated with either CYPHER or TAXUS stents in the seven trials. The analysis revealed that patients receiving SES had a significantly lower risk of restenosis and target vessel revascularization compared with those receiving PES. Rates of death, death or MI, and stent thrombosis were similar (Kastrati et al 2006b).
Further data on the use of CYPHER stents to treat complex lesions come from a Danish study. The ScandStent (The Stenting of Coronary Arteries in Non-Stress/Benestent Disease Trial) study is a multicentre trial randomized 322 patients with complex coronary lesions to either the CYPHER stent or bare metal control stent. It provides independent confirmation that the superiority of CYPHER over bare metal stents seen in the pivotal trials in less complex lesions also holds for more complex lesions (Kelbaek et al 2006).
Follow-up results from ARTS II (Arterial Revascularization Therapies Study part II of the CYPHER SES in the treatment of patients with multi-vessel de novo coronary artery lesions) add to the growing evidence in support of the use of SES in the treatment of multi-vessel disease. However, as with all “new” technologies, continued careful investigation will be an essential aid to appropriate patient selection and treatment when treating patients with more complex lesions and multi-vessel disease (Serruys et al 2005).
Table 2.
Rate of late-stent thrombosis: sirolimus-eluting, paclitaxel-eluting, drug-eluting, and bare metal stents
| Author | Trial | Patients (N) | Length of follow-up | Thrombosis rates by stent type (SES/PES/DES/BMS) | |
|---|---|---|---|---|---|
| Kereiakes et al 2006 | Pooled analysis | SES: 337 | 1080 days | SES: 0% | |
| BMS: 0.4% | BMS: 238 | ||||
| Moreno et al 2005 | Meta-analysis | DES: 2602 | — | DES: 0.58% | SES: 0.11% |
| BMS: 2428 | BMS: 0.54% | PES: 0.29% | |||
| Park et al 2006 | Single center, all comers | SES: 1545 | 18 months | DES: 0.8% | |
| PES: 366 | |||||
| Schampaert et al 2004 | Pooled analysis | SES: 758 | 2 years | SES: 0.4% | |
| BMS: 752 | BMS: 0.5% | ||||
| Bavry et al 2005 | Meta-analysis | SES: 1515 | 13.5 months | SES: 0.07% | |
| BMS: 1448 | BMS: 0.48% | ||||
| Weisz et al 2006 | SIRIUS trial | SES: 533 | 361–720 days | SES: 0.9% | |
| BMS: 525 | BMS: 1.5% | ||||
| Urban et al 2006 | e-CYPHER registry | SES: 15157 | 12 months | SES: 0.19% | |
| lakovou 2005 | Prospective observational cohort study | SES: 1062 | 9 months | SES: 0.8% | |
| PES: 1167 | PES: 1.7% | ||||
Abbreviations: BMS, bare metal stents; DES, drug-eluting stents; PES, paclitaxel-eluting stents; SES, sirolimus-eluting stents.
Late loss: a key measurement in differentiating drug-eluting stents (DES)
Late loss is the angiographic metric that allows post-stent neointimal hyperplasia to be most accurately and reliably quantified. It reflects the ability of DES to inhibit the inflammatory and hyperplastic processes that translate into adverse clinical outcomes such as binary restenosis and target lesion revascularization (Mauri et al 2005).
When the CYPHER vs TAXUS head-to-head trials are organized in order of increasing patient and lesion complexity (REALITY → SIRTAX → ISAR-DIABETES → ISAR-DESIRE) a trend emerges showing an association between lower late loss and superior clinical outcomes (restenosis and TLR). As the patient population becomes more complex, the gap between CYPHER and TAXUS in terms of late loss and restenosis widens, showing an increasing benefit with the CYPHER stent (Fig. 2).
Figure 2.
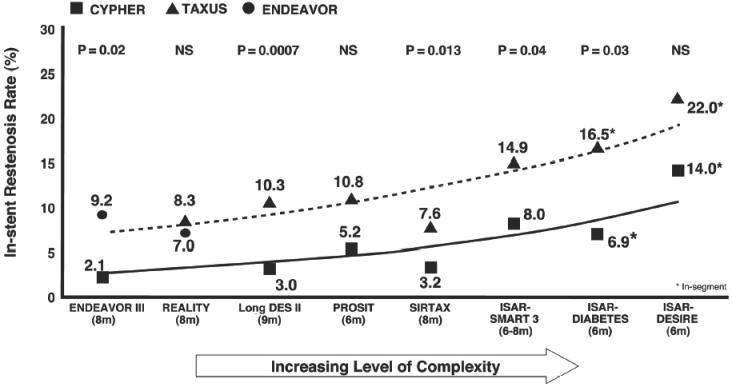
In higher risk cohorts, differences in late loss are more strongly associated with risk of target lesion revascularization.
It has been seen that there is an association between late loss, restenosis, and complexity in CYPHER vs TAXUS studies. Late loss and in-stent restenosis increase as patient populations become more complex, and these increases are consistently higher in TAXUS populations. This trend is expressed as a “complexity curve”. When the ENDEAVOR III results for late loss are mapped onto this complexity curve it can be seen that the late loss and restenosis rate are high considering the relatively straightforward patient population evaluated in ENDEAVOR III (Fig. 3).
Figure 3.
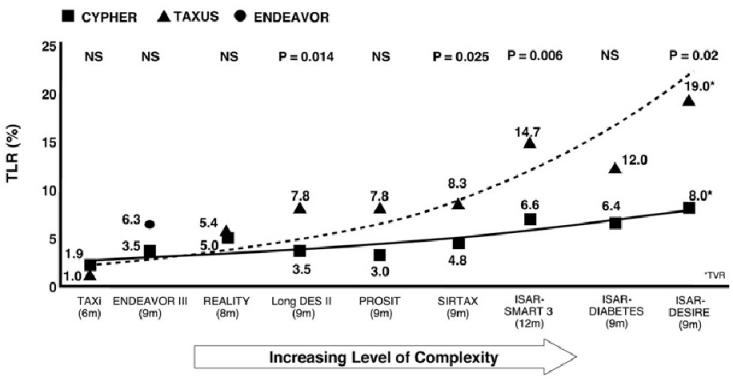
In higher risk cohorts, differences in late loss are more strongly associated with risk of restenosis.
Abbreviations: TLR, target lesion revascularization.
Key characteristics of an ideal DES
To be successful, DES must combine several key characteristics. The first requirement is for a drug that reliably blocks smooth muscle cell hyperplasia and reduces vascular inflammation while allowing healing to occur. The second requirement is for a stent design that permits homogenous delivery of optimal drug dosages using a system, which delivers the drug in a predictable fashion. The CYPHER SES brings together nearly ideal solutions that meet all three criteria. The unique combination of the highly effective anti-proliferative agent, sirolimus, a closed-cell design, and a biocompatible polymer results in exceptional outcomes. This combination of three elements produces optimal drug delivery; controlled, precise drug release; and marked reduction of late loss with a favorable safety profile.
Sirolimus – safety and tolerability
Safety of a broad therapeutic window
Sirolimus has been shown to have a broad therapeutic window. In pre-clinical animal trials, sirolimus has demonstrated biological activity in doses ranging from 18 to 1200 µg without displaying toxicity to the vessel wall (Cordis Corporation, Warren, New Jersey, USA, data on file).
The highest dose now tested is approximately 6 times that of the CYPHER stent, with no adverse effects (no evidence of necrosis, aneurysm, or other pathology with 1200 µg in an exaggerated dose study.
The broad therapeutic profile of sirolimus means that overlapping stents can be deployed without the risk of toxicity due to overdosing (Suzuki et al 2001). SIRIUS data from over 27% of patients with 2 or more overlapping stents demonstrate the safety and efficacy when overlapping SES (Moses et al 2003; Munoz et al 2004). Additional data come from the RESEARCH registry, based on a study population of patients who had a combination of ≥2 overlapping stents at a minimum length of 41 mm (ie, one 33 mm SES overlapping an 8 mm SES) to treat native de novo coronary lesions. The investigators monitored the incidence of major cardiac adverse events (death, non-fatal MI, and TLR). Clinical follow-up was available for all patients at a mean of 320 days (range 265–442). Angiographic follow-up at 6 months was obtained in 67 patients (71%). Binary restenosis rate was 11.9% and in-stent late loss was 0.13 +/− 0.47 mm. At long-term follow-up (mean 320 days), there were 2 deaths (2.1%), and the overall incidence of major cardiac events was 8.3%. The investigators concluded that SES implantation appears safe and effective for de novo coronary lesions requiring multiple stent placement over a very long vessel segment (Aoki et al 2005).
The flat dose-response curve of sirolimus demonstrates a consistent, homogeneous anti-proliferative and anti-inflammatory effect at all doses evaluated, with no indication of cytotoxicity (Cordis Corporation, data on file).
Stent thrombosis
Since their introduction, more than 2 million patients, often with complex lesions, diabetes, and acute MI, have been treated with an SES. Although the published data support the claim that DES are safe and effective there have been concerns raised about the incidence of very late stent thrombosis (more than 1-year after implantation) compared with the use of bare metal stents (Iakovou et al 2005; Colombo and Corbett 2006). This concern has been fuelled by individual case reports and new long-term data from TAXUS-II, -IV, and -V and the Basel Stent Cost-Effectiveness Trial (BASKET) studies (American College of Cardiology Scientific Sessions 2006). However, despite these concerns over the long-term safety of DES, the actual incidence of stent thrombosis after 1-year is unknown (Park et al 2006).
In the absence of an internationally accepted definition of late stent thrombosis or any fact-based evidence concerning the incidence of stent thrombosis, a review of the literature suggests that the incidence of late stent thrombosis with SES is comparable with that of bare metal stents (Bavry et al 2005; Iakovou et al 2005; Moreno et al 2005; Kereiakes et al 2006; Weisz et al 2006; Park et al 2006; Schampaert et al 2006; Urban et al 2006). The individual trials/analyses are shown in Table 1.
It has been suggested that treatment with a drug-eluting stent results in delayed arterial healing when compared with bare metal stents of similar implant duration. It has also been postulated that the cause of late stent thrombosis associated with DES is multifactorial, with delayed healing in combination with other clinical and procedural risk factors playing a role (Joner et al 2006). The available evidence indicates that the predictors of stent thrombosis are premature anti-platelet therapy interruption, primary stenting in acute MI, and total stent length. However, if we are to gain a better understanding of the problems of DES thrombosis it would appear that an extended period of follow-up in a randomized, controlled trial or a large registry such as e-SELECT will be necessary. Thankfully, the incidence of late stent thrombosis appears to be very rare. Nevertheless, its impact can be tragic.
Closed cell design
The distribution of an eluted drug in the tissue of a vessel wall is not at all homogenous, and this might reflect the pattern of the stent struts. While the dose distribution may be sub-therapeutic in one spot, it may be toxic in the direct vicinity of the struts. Homogenous drug distribution would also require a symmetric deployment of a stent, which does not necessarily happen in the real world. Overlapping stents may lead to doubling of the intended dose, and longitudinally the drug tissue levels may vary considerably from proximal to distal end. An open cell versus a closed cell stent design has different characteristic patterns of apposition to the cell wall, leading again to a difference in the delivered dose, with a closed cell design appearing to offer better drug distribution.
The CYPHER stent’s closed-cell design results in uniform vessel coverage, making it an optimal platform for drug delivery. With a closed-cell design, when the stent is deployed in a tortuous site, cell size is minimally affected either on the outer aspect or inner aspect of the bend, and uniform vessel coverage and dosing are maintained. In contrast, with open-cell design, tortuosity can cause dramatic changes in cell sizes. This may result in both excessively large cells on the outer side of the bend and small cell sizes on the inner surface of the bend. Consequently, there is non-uniform coverage of the vessel wall and non-uniform dosing, both with potential under dosing and over dosing. Closed-cell design results in optimal drug delivery to tortuous anatomy, for example, in lesions of the right coronary artery and in eccentric lesions, as encountered in highly asymmetric proximal left anterior descending plaque.
Polymer
For drug distribution and safety one needs to consider the relationship between the stent design and the drug tissue concentration. Currently used polymers for stent coatings have been proven safe. They release drugs at predictable rates and it is interesting to observe that fast and slow release polymers lead to similar tissue concentrations. The tissue penetration depends more on the hydrophobic or hydrophilic properties of the drug. A hydrophobic or lipophilic drug will easily penetrate and be found in high concentrations regardless of slow or fast release. The difference between slow and fast release may lie in the tissue toxicity; a high tissue level, built up quickly, may have toxic necrotic effects, as seen with paclitaxel. This can lead to thrombus formation; the stent may no longer be adherent to the necrotic wall. Overall, clinical and histological toxicity is a concern.
Controlled release is crucial to the efficacy of DES. The CYPHER stent has a unique polymer coating, which allows for localized delivery of sirolimus precisely to the site of the lesion. It contains a specific concentration of sirolimus and the polymer ensures that the drug does not wash off during the most time-intensive procedures. The polymer also ensures that there is no rapid “dumping” of the drug, but rather tightly regulated drug release over a defined period of time. Essentially all the drug is delivered in the first 3 months after implantation.
Impact on patients
Over the past 25 years coronary angioplasty has developed into a highly sophisticated series of techniques that has the potential to match surgery, and in many cases surpass it. Implantation of SES has revolutionized the field of percutaneous coronary angioplasty with an impressive reduction of in-stent restenosis compared with bare metal stents. This advantage translates into fewer repeat treatments for the patient, a reduction in the need for surgical intervention, and the ability to treat more patients. Thankfully, the incidence of stent thrombosis appears to be in line with that of bare metal stents. That being said, the ability to identify the patient who is at risk of stent thrombosis is a major and urgent challenge.
Conclusions and place in therapy
The introduction of SES was a major breakthrough for interventional cardiology. Many large, randomized, clinical trials using SES have shown a remarkable reduction in angiographic restenosis and target vessel revascularization compared with bare metal stents. The results of these trials also appear to be supported by evidence from everyday practice and non-controlled clinical trials. However, the expanded applications of SES, especially in treating complex lesions such as left main disease, acute MI, and saphenous vein graft lesions, are still under evaluation with ongoing studies. The adoption of SES in all percutaneous coronary intervention may become a reality in the near future.
References
- Alfonso F, Perez-Vazcayno MJ, Hernandez R, et al. A randomized comparison of sirolimus-eluting stent with balloon angioplasty in patients with in-stent restenosis: results of the Restenosis Intrastent: Balloon Angioplasty Versus Elective Sirolimus-Eluting Stenting (RIBS-II) trial. J Am Coll Cardiol. 2006;47:2152–60. doi: 10.1016/j.jacc.2005.10.078. [DOI] [PubMed] [Google Scholar]
- Aoki J, Ong A, Rodriguez Granillo GA, et al. “Full metal jacket” (stented length >r = 64 mm) using drug-eluting stents for de novo coronary artery lesions. Am Heart J. 2005;150:994–9. doi: 10.1016/j.ahj.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Ardissino D, Cavallini C, Bramucci E, et al. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA. 2004;292:2727–34. doi: 10.1001/jama.292.22.2727. [DOI] [PubMed] [Google Scholar]
- Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL. Risk of thrombosis with the use of sirolimus-eluting stents for percutaneous coronary intervention (from registry and clinical trial data) Am J Cardiol. 2005;95:1469–72. doi: 10.1016/j.amjcard.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Chakraborty TK, Weber HP, Nicolaou KC. Design and synthesis of a rapamycin-based high affinity binding FKBR12 ligand. Chem Biol Mar. 1995;2:157–61. doi: 10.1016/1074-5521(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Chang JY, Sehgal SN, Bansbach CC. FK506 and rapamycin novel pharmacological probes of the immune response. Trends Pharmacol Sci. 1991;12:218–23. doi: 10.1016/0165-6147(91)90555-7. [DOI] [PubMed] [Google Scholar]
- Colombo A, Corbett SJ. Drug-eluting stent thrombosis: increasingly recognized but too frequently overemphasized. J Am Coll Cardiol. 2006;48:203–5. doi: 10.1016/j.jacc.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Dibra A, Kastrati A, Mehilli J, et al. Paclitaxel-eluting or sirolimuseluting stents to prevent restenosis in diabetic patients. N Engl J Med. 2005;353:663–70. doi: 10.1056/NEJMoa044372. [DOI] [PubMed] [Google Scholar]
- Gallo R, Padurean A, Jayaraman T, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–70. doi: 10.1161/01.cir.99.16.2164. [DOI] [PubMed] [Google Scholar]
- Goy JJ, Stauffer JC, Siegenthaler M, et al. A prospective randomized comparison between paclitaxel and sirolimus stents in the real world of interventional cardiology: the TAXi trial. J Am Coll Cardiol. 2005;45:308–11. doi: 10.1016/j.jacc.2004.10.062. [DOI] [PubMed] [Google Scholar]
- Gregory CR, Huang X, Pratt RE, et al. treatment with rapamycin and mycophenolic acid reduces arterial intimal thickening produced by mechanical injury and allows endothelial replacement. Transplantation. 1995;59:655–61. doi: 10.1097/00007890-199503150-00002. [DOI] [PubMed] [Google Scholar]
- Groth CG, Bachman L, Morales JM, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999;67:1036–42. doi: 10.1097/00007890-199904150-00017. [DOI] [PubMed] [Google Scholar]
- Gruntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transradial coronary angioplasty. N Engl J Med. 1979;301:61–8. doi: 10.1056/NEJM197907123010201. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Guagliumi G, Musumeci G, et al. Vascular response to sirolimus-eluting stents delivered with a nonaggressive implantation technique: comparison of intravascular ultrasound results from the Multicenter, randomized E-SIRIUS, and SIRIUS trials. Catheter Cardiovasc Interv. 2005;66:499–506. doi: 10.1002/ccd.20542. [DOI] [PubMed] [Google Scholar]
- Holmes DR, Jr, Teirstein P, Satler L, et al. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA. 2006;295:1264–73. doi: 10.1001/jama.295.11.1264. [DOI] [PubMed] [Google Scholar]
- Hong MK, Park SJ, et al. American College of Cardiology Annual Scientific Sessions 2005. 2005. Oral presentation.
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- Jang JS, Hong MK, Lee CW, et al. Comparison between sirolimus- and paclitaxel-eluting stents for the treatment of chronic total occlusion. J Invasive Cardiol. 2006;18:205–8. [PubMed] [Google Scholar]
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kaiser C, Brunner-La Rocca HP, Buser PT, et al. BASKET Investigators Incremental cost-effectiveness of drug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: randomised Basel Stent Kosten Effektivitats Trial (BASKET) Lancet. 2005;366:921–9. doi: 10.1016/S0140-6736(05)67221-2. [DOI] [PubMed] [Google Scholar]
- Kastrati A, Dibra A, Eberle S, et al. Sirolimus-eluting stents vs paclitaxel-eluting stents vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis; a randomized controlled trial. JAMA. 2005a;293:165–71. doi: 10.1001/jama.293.2.165. [DOI] [PubMed] [Google Scholar]
- Kastrati A, Dibra A, Eberle S, et al. Sirolimus-eluting stents vs paclitaxel-eluting stents in patients with coronary artery disease: meta-analysis of randomized trials. JAMA. 2005b;294:819–25. doi: 10.1001/jama.294.7.819. [DOI] [PubMed] [Google Scholar]
- Kastrati A, Dibra A, Mehilli J, et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006;113:2293–300. doi: 10.1161/CIRCULATIONAHA.105.601823. [DOI] [PubMed] [Google Scholar]
- Kelbaek H, Thuesen L, Helqvist S, et al. The Stenting Coronary Arteries in Non-stress/benestent Disease (SCANDSTENT) trial. J Am Coll Cardiol. 2006;47:449–55. doi: 10.1016/j.jacc.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Kereiakes DJ, Wang H, Popma JJ, et al. Periprocedural and late consequences of overlapping cypher sirolimus-eluting stents. J Am Coll Cardiol. 2006;48:21–31. doi: 10.1016/j.jacc.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim HS, Lee SW, et al. Prospective Randomized Trial of SES vs. PES for the Treatment of Acute ST-Elevation Myocardial Infarction. 2006. American College of Cardiology Annual Scientific Sessions 2006: Late Breaking Trial Presentation.
- Lotan C, Almagor Y, Kuiper K, et al. Sirolimus-Eluting Stent in Chronic Total Occlusion: The SICTO Study. J Interv Cardiol. 2006;19:307–12. doi: 10.1111/j.1540-8183.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- Marx SO, Jayarman T, Go LO, et al. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–7. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- Mauri L, Orav EJ, Kuntz RF. Late loss in lumen diameter and binary restenosis for drug-eluting stent comparison. Circulation. 2005;111:3435–42. doi: 10.1161/CIRCULATIONAHA.104.513952. [DOI] [PubMed] [Google Scholar]
- Mehilli J, Dibra A, Kastrati A, et al. Randomized trial of paclitaxel-and sirolimus-eluting stents in small coronary vessels. Eur Heart J. 2006;27:260–6. doi: 10.1093/eurheartj/ehi721. [DOI] [PubMed] [Google Scholar]
- Meier B, Sousa E, Guagliumi G, et al. Sirolimus-eluting stents in small vessels. Am Heart J. 2006;151:1019–27. doi: 10.1016/j.ahj.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Moreno R, Fernandez C, Hernandez R, et al. Drug-Eluting Stent Thrombosis: Results From a Pooled Analysis Including 10 Randomised Studies. J Am Coll Cardiol. 2005;45:954–9. doi: 10.1016/j.jacc.2004.11.065. [DOI] [PubMed] [Google Scholar]
- Morice MC, Serruys PW, Sousa JE, et al. The RAVEL study: a randomized comparison of a sirolimus eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–80. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- Morice MC, Colombo A, Meier B, et al. Sirolimus- vs paclitaxeleluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA. 2006;295:895–904. doi: 10.1001/jama.295.8.895. [DOI] [PubMed] [Google Scholar]
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- Munoz JS, Abizaid A, Mintz GS, et al. Intravascular ultrasound study of effects of overlapping sirolimus-eluting stents. Am J Cardiol. 2004;93:470–3. doi: 10.1016/j.amjcard.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Ortolani P, Ardissino D, Cavallini C, et al. Effect of sirolimus-eluting stents in diabetic patients with small coronary arteries (A SES-SMART sub-study) Am J Cardiol. 2005;96:1393–8. doi: 10.1016/j.amjcard.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Park D-W, Park S-W, Park K-H, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol. 2006;98:352–6. doi: 10.1016/j.amjcard.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Pan M, Suarez de Lezo J, Medina A, et al. Drug-eluting stents for the treatment of bifurcation lesions: a randomized comparison between paclitaxel and sirolimus stents. Am Heart J. 2007;53:15.e1–7. doi: 10.1016/j.ahj.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Pham SM, Shears LL, Kawaharda N, et al. High local production of nitric oxide as a possible mechanism by which rapamycin prevents transplant arteriosclerosis. Transplant Proc. 1998;30:953–4. doi: 10.1016/s0041-1345(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Poon M, Marx SO, Gallo R, et al. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2777–83. doi: 10.1172/JCI119038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston RS, Billingham M, Hoyt EG, et al. Rapamycin reverses chronic graft vascular disease in a novel cardiac allograft model. Circulation. 1999;100:67–74. doi: 10.1161/01.cir.100.1.67. [DOI] [PubMed] [Google Scholar]
- Sabate M, Jimenez-Quevedo P, Angiolillo DJ, et al. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-elutin stent (DIABTETES) trial. Circulation. 2005;112:2175–83. doi: 10.1161/CIRCULATIONAHA.105.562421. [DOI] [PubMed] [Google Scholar]
- Schofer J, Schluter M, Gershlick AH, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomized controlled trial (E-SIRIUS) Lancet. 2003;362:1093–9. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- Schampaert E, Cohen EA, Schluter M, et al. The Canadian Study of the Sirolimus-eluting Stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS) J Am Coll Cardiol. 2004;43:1110–15. doi: 10.1016/j.jacc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Schampaert E, Moses JW, Schofer J, et al. Sirolimus-eluting stents at two years: a pooled analysis of SIRIUS, E-SIRIUS, and C-SIRIUS with emphasis on late revascularizations and stent thromboses. Am J Cardiol. 2006;98(1):36–41. doi: 10.1016/j.amjcard.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Scott NA. Restenosis following implantation of bare metal coronary stents: Pathophysiology and pathways involved in the vascular response to injury. Adv Drug Deliv Rev. 2006;58:358–76. doi: 10.1016/j.addr.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Sehgal SN, Naker H, Vezina C. Rapamycin (AY-22, 989), a new antifungal antibiotic. II Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28:727–32. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- Serruys PW, de Jaegere P, Kiemeneiji F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Stusy Group. N Engl J Med. 1994;331:489–95. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Ong A, Morice MC, et al. Arterial Revascularization Therapies Study Part II – Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroInterv. 2005;2:147–56. [PubMed] [Google Scholar]
- Singh K, Sun S, Vezina C. Rapamycin (AY-22, 989), a new antifungal antibiotic. IV. Mechanism of action. J Antibiot (Tokyo) 1979;32:620–45. doi: 10.7164/antibiotics.32.630. [DOI] [PubMed] [Google Scholar]
- Sousa JE, Costa MA, Abizaid AC, et al. Lack of neointimal proliferation after implantation of sirolimus coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103:192–5. doi: 10.1161/01.cir.103.2.192. [DOI] [PubMed] [Google Scholar]
- Sousa JE, Costa MA, Abizaid A, et al. Late four-year angiographic and intravascular ultrasound follow-up of patients treated with sirolimuseluting stents. Circulation. 2005;111:2326–9. doi: 10.1161/01.CIR.0000164271.01172.1A. [DOI] [PubMed] [Google Scholar]
- Spier E, Huang ES, Modali R, et al. Interaction of human cytomegalovirus with p53: possible role in coronary restenosis. Scand J Infact Dis Suppl. 1995;99:78–91. [PubMed] [Google Scholar]
- Stepkowski SM, Chen H, Daloze P, et al. Rapamycin, a potent immunosuppressive drug for vascularized heart, kidney, and small bowel transplantation in the rat. Transplanation. 1991;51:22–6. doi: 10.1097/00007890-199101000-00002. [DOI] [PubMed] [Google Scholar]
- Suarez De Lezo J, Pan M, et al. Cordoba and Las Palmas Drug-eluting Stent Study. J Am Coll Cardiol. 2005;45(Suppl A):75A. [Google Scholar]
- Spaulding C, Henry P, Teiger E, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355:1093–104. doi: 10.1056/NEJMoa062006. [DOI] [PubMed] [Google Scholar]
- Suttorp MJ, Laarman GJ, Rahel BM, et al. Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II) Circulation. 2006;114:921–8. doi: 10.1161/CIRCULATIONAHA.106.613588. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kopia G, Hayashi S, et al. Stent based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001;104:1188–93. doi: 10.1161/hc3601.093987. [DOI] [PubMed] [Google Scholar]
- Urban P, Gershlick AH, Guagliumi G, et al. Safety of coronary sirolimus-eluting stents in daily practice: one-year follow-up of the e-CYPHER registry. Circulation. 2006;113:1434–41. doi: 10.1161/CIRCULATIONAHA.104.532242. [DOI] [PubMed] [Google Scholar]
- Van Hout BA, van der Woude T, de Jaegere PP, et al. Cost-effectiveness of stent implantation versus PTCA: the BENESTENT experience. Semin Interv Cardiol. 1996;1:263–8. [PubMed] [Google Scholar]
- Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22, 989), a new antifungal antibiotic. I. Taxonomy pf the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–6. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Weisz G, Leon MB, Holmes Dr, Jr, et al. Two-year outcomes after sirolimus-eluting stent implantation: results from the Sirolimus-Eluting Stent in de Novo Native Coronary Lesions (SIRIUS) trial. J Am Coll Cardiol. 2006;47:1350–5. doi: 10.1016/j.jacc.2005.11.077. [DOI] [PubMed] [Google Scholar]
- Windecker S, Remondino A, Eberli FR, et al. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N Engl J Med. 2005;353:653–62. doi: 10.1056/NEJMoa051175. [DOI] [PubMed] [Google Scholar]


