Abstract
Objective
To determine if the fixed-dose perindopril/indapamide combination (Per/Ind) normalizes blood pressure (BP) in the same fraction of hypertensive patients when treated in everyday practice or in controlled trials.
Methods
In this prospective trial, 17 938 hypertensive patients were treated with Per 2 mg/Ind 0.625 mg for 3–6 months. In Group 1 Per/Ind was initiated in newly diagnosed patients (n = 7032); in Group 2 Per/Ind replaced previous therapy in patients already treated but having either their BP still uncontrolled or experiencing side-effects (n = 7423); in Group 3 Per/Ind was added to previous treatment in patients with persistently high BP (n = 3483). BP was considered normalized when ≤ 140/90 mm Hg. A multivariate analysis for predictors of BP normalization was performed.
Results
Subjects were on average 62 years old and had a baseline BP of 162.3/93.6 mm Hg. After treatment with Per/Ind, BP normalization was reached in 69.6% of patients in the Initiation group, 67.5% in the Replacement Group, and 67.4% in the Add-on Group (where patients were more frequently at risk, diabetic, or with target organ damage). Mean decreases in systolic BP of 22.8 mm Hg and in diastolic BP of 12.4 mm Hg were recorded.
Conclusions
This trial was established to reflect everyday clinical practice, and a treatment strategy based on the Per/Ind combination, administered as initial, replacement, or add-on therapy, led to normalization rates that were superior to those observed in Europe in routine practice. These results support recent hypertension guidelines which encourage the use of combination therapy in the management of arterial hypertension.
Keywords: perindopril, indapamide, blood pressure normalization, risk factors, combination therapy
Introduction
Cardiovascular complications may, to a large extent, be prevented in hypertensive patients by lowering blood pressure (BP). International recommendations currently stress the importance of an effective control of not only diastolic BP (DBP), but also systolic BP (SBP). This is because it is now well recognized that SBP better reflects cardiovascular risk than DBP. This is especially true in patients older than 50 years (Franklin et al 2001).
Despite major efforts directed worldwide to control hypertension, BP normalization rates (SBP/DBP <140/90 mm Hg) in treated patients remain low, achieving hardly 30% in industrialized countries where patients have easily access to health care (Chamontin et al 2001; Wolf-Maier et al 2004; Roux et al 2006). These data highlight the need for more effective treatment strategies, in particular of combination therapy. It is indeed clear today that monotherapies most often do not allow BP normalization when prescribed as initial treatment (Matersan et al 1995; Hansson et al 1998; Cushman et al 2002). The need for treatment adjustments often delays the achievement of BP control and this may influence adversely the patients’ cardiovascular outcome. These concerns urged experts in Europe and United States of America to focus on the rationale and the potential usefulness of fixed-dose combinations for the management of hypertensive patients (Chobanian et al 2003; ESH-ESC 2003; Haute Autorité de Santé 2005).
The combination containing the angiotensin-converting enzyme (ACE) inhibitor perindopril (Per) and the diuretic indapamide (Ind) has been shown to control BP in a wide range of patients with different degrees of hypertension as well as in the presence of various target organ damages and risk factors (Chalmers et al 2000; Mogensen et al 2003; Mourad et al 2004; Dahlof et al 2005). Recently, the randomized STRATHE study has compared 3 widely accepted antihypertensive strategies: a first-line, fixed-dose combination therapy, a step-by-step strategy, and a sequential monotherapy. The Per/Ind combination normalized BP (<140/90 mm Hg) significantly more often (62%) compared with a step-by-step (47%, p = 0.005) and a sequential monotherapy approach (49%, p = 0.01). The greater efficacy of the fixed combination was related in particular to a higher efficacy on SBP (Mourad et al 2004). The objective of this OPTIMAX trial was to extend the findings of the STRATHE study in daily medical practice.
Study protocol
Patients and methods
In this study, named OPTIMAX (OPTIMiser le tAuX de normalisation tensionnelle grâce à la plurithérapie de première intention), general practitioners and cardiologists, in hospital or private practice, prospectively recruited patients over a 2month period. In order to obtain a representative cross-section of patients, physicians were chosen randomly to participate in this study. Hypertension was defined as a BP >140/90 mm Hg at the physician’s office. In newly diagnosed patients as well as in-patients who had not been treated with any antihypertensive therapy for at least 3 months (“Initiation Group”), treatment was initiated with the fixed Per/Ind combination at a 2 mg/0.625 mg once daily dose. The same combination was used as replacement therapy in patients exhibiting still high BP or having experienced side-effects on the previous antihypertensive therapy (“Replacement Group”). The “Add-on Group” included patients who were treated but whose BP was only partially controlled. In this last group the Per/Ind combination was added to the existing antihypertensive treatment. Investigators were asked to enroll 6 consecutive patients, wherever possible 2 patients in each of the 3 study groups. In all patients BP readings were obtained at inclusion in the trial and again after 3–6 months of treatment with the Per/Ind combination.
Patients were excluded if they were under 18 years of age, pregnant, or presumed not to be available for follow-up during at least 6 months. Patients participating in another clinical trial were also not eligible. A detailed medical questionnaire was filled out by the physicians at the patient’s inclusion and after 3–6 months of follow-up. Information was obtained on: gender, age, baseline blood pressure, presence of end organ damage (left ventricular hypertrophy [LVH], proteinuria, and/or creatininemia between 12 and 20 mg/L), history of cardiovascular or renal disease (stroke, transient ischemic attack, myocardial infarction, angina, coronary revascularization, cardiac insufficiency, peripheral artery disease, renal insufficiency), existence of additional cardiovascular risk factors (diabetes, smoking, increased total cholesterol levels [>12.9 mmol/L], low HDL-C levels [<0.28 mmol/L], and/or increased LDL-C levels [>8.26 mmol/L]), or current use of antihypertensive drug(s).
The following efficacy and safety data were recorded during the course of the study: BP levels, occurrence of significant adverse events (defined as a cardiovascular adverse event, an unplanned hospitalization, death, or any other critical event), tolerability (estimated by the physician as very poor, poor, average, good, or very good). Brachial BP was measured in the sitting position using usual device which was in most cases semi-automated equipment. BP was considered normalized if ≤ 140/90 mm Hg.
Statistics
The primary objective was to compare the BP normalization rates observed in the 3 groups. In order to detect a 1%–2% difference in normalization rates with an anticipated 15% of non-valid questionnaires and 6 patients enrolled per physician, power calculations revealed that 20 000 patients needed to be enrolled by 4820 investigators. Investigators were arbitrarily divided into 3600 generalists, 900 private practice cardiologists, and 320 hospital cardiologists. All patients who met the inclusion criteria were included in the analyses.
BP normalization rates, between groups, were compared using two-sided tests (Mac Nemar and χ2) with an α = 5%. Changes in SBP and DBP between baseline and follow-up visits were compared between groups using ANOVA. When differences were significant, groups were compared 2 by 2 using a Bonferroni procedure. When ANOVA was invalid (as defined by Shapiro-Wilk, Qqplot, and Bartlett tests) comparisons were performed using the non-parametric Kruskall Wallis test. When changes from baseline were significant, the non-parametric Mann-Whitney test was used to compare the groups 2 by 2.
Factors contributing to BP normalization were determined using a univariate logistic regression that compared normalized and non-normalized patients. The tested variables were: gender (female vs male); age (continuous and by category ≥65 years vs <65 years and <50 years, 50–69 years, 70–79 years vs ≥80 years); SBP at inclusion (continuous and by category <120 mm Hg or ≥140 mm Hg vs 120–139 mm Hg); DBP at inclusion (continuous and by category <80 mm Hg or ≥90 mm Hg vs 80–89 mm Hg); presence vs absence of end organ damage; presence vs absence of history of cardiovascular or renal disease(s); presence vs absence of additional cardiovascular risk factor(s); and degree of treatment tolerability (by category: very poor, poor, average, or good vs very good).
A significant difference between normalized and non-normal-ized patients (χ2 test with an α =15%) was needed for a variable to be included in the multivariate analysis. The final model was built using the ascendant stepwise logistic regression program of SAS® (Statistical Analysis System, SAS-Institute, Cary NC, USA). In order to describe the profile of a normalized patient, odds ratios and 95% confidence intervals were calculated for the most significant variables. Data are reported as means ±SD.
Results
The analysis included 17 938 patients. The fixed combination Per/Ind was prescribed in 7032 subjects as initial therapy, in 7423 subjects as replacement therapy, and in 3483 patients as add-on therapy. Patients were excluded from analysis if they did not meet age requirements (n= 4), were not hypertensive, or had no BP data at baseline (n = 150).
Patients were on average 62 years of age (Table 1). At inclusion, they had a mean SBP of 162.3±13.1 mm Hg and a mean DBP of 93.6±9.1 mm Hg. Most patients (78%) had an SBP >150 mm Hg. Overall, newly diagnosed subjects (Initiation Group) tended to be younger and had slightly higher baseline BP values than patients who were already on therapy at the time of inclusion (Replacement and Add-on Groups). A significantly greater percentage of patients in the Replacement Group and in the Add-on Group had target organ damage, history of cardiovascular or renal disease, and/or cardiovascular risk factors compared with patients in the Initiation Group (p < 0.0001) (Table 2).
Table 1.
Baseline characteristics of the patients
| Initiation n = 7032 | Replacement n = 7423 | Add-on n = 3483 | Total n = 17938 | |
|---|---|---|---|---|
| Agea in years, mean ± SD | 58.2 ± 11.2 | 63.1 ± 11.2 | 65.2 ± 10.7 | 61.6 ± 11.5 |
| Genderb, % male | 56.9 | 51.0 | 54.8 | 54.0 |
| Previous antihypertensive treatment, % | ||||
| Diuretic | − | 27.3 | 21.9 | 25.6 |
| Beta-blocker | − | 21.0 | 47.3 | 29.4 |
| Calcium inhibitor | − | 27.9 | 48.6 | 34.5 |
| ACE inhibitor | − | 25.2 | 14.4 | 21.8 |
| ATII inhibitor | − | 13.7 | 8.3 | 11.9 |
| Central acting | − | 7.1 | 14.7 | 9.5 |
| Other Vasodilators | − | 1.9 | 3.2 | 2.3 |
| SBPb in mm Hg | ||||
| Mean ± SD | 165.2 ± 11.9 | 159.4 ± 14.0 | 162.8 ± 12.2 | 162.3 ± 13.1 |
| Severityb | ||||
| ≤140 mm Hg, % | 1.0 | 10.1 | 1.2 | 4.8 |
| >150 mm Hg, % | 86.9 | 69.2 | 79.6 | 78.1 |
| DBPb in mm Hg | ||||
| mean±SD | 95.6 ± 8.7 | 91.9 ± 9.2 | 93.2 ± 9.0 | 93.6 ± 9.1 |
| DBPb≤ 90 mm Hg, % | 35.4 | 52.8 | 47.8 | 45.0 |
Data were missing in <3% of patients.
Data were missing in <1% of patients.
Abbreviations: ACE, angiotensin converting enzyme;ATII, angiotensin-receptor II; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 2.
Target organ damage, history of cardiovascular or renal disease and cardiovascular risk factors at inclusion
| Risk factor, % | Initiation n = 7032 | Replacement n = 7423 | Add-on n = 3483 | Total n = 17938 |
|---|---|---|---|---|
| Left ventricular hypertrophy | 8.7 | 21.9 | 36.4 | 19.6 |
| Proteinuria | 4.0 | 8.4 | 15.6 | 8.1 |
| Ischemic stroke | 1.3 | 3.0 | 4.5 | 2.6 |
| Hemorrhagic stroke | 0.1 | 0.3 | 0.8 | 0.3 |
| Transient ischemic attack | 2.9 | 5.7 | 8.8 | 5.2 |
| Myocardial infarction | 0.6 | 3.3 | 8.2 | 3.2 |
| Angina | 2.5 | 7.9 | 17.7 | 7.7 |
| Coronary revascularization | 0.8 | 3.2 | 8.3 | 3.2 |
| Cardiac failure | 1.0 | 3.9 | 7.0 | 3.3 |
| Peripheral artery disease | 4.1 | 8.0 | 12.7 | 7.4 |
| Renal failure | 1.0 | 3.0 | 5.9 | 2.8 |
| Diabetes type 1 | 2.9 | 3.8 | 4.7 | 3.6 |
| Diabetes type 2 | 11.3 | 16.4 | 22.4 | 15.6 |
| Smoking | 38.7 | 28.6 | 27.4 | 32.3 |
| Total cholesterol out of range | 12.1 | 12.9 | 15.0 | 13.0 |
| HDL-C out of range | 2.5 | 2.7 | 3.1 | 2.7 |
| LDL-C out of range | 14.9 | 17.6 | 20.2 | 17.1 |
Abbreviations: CVA, cerebrovascular accident; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol.
Efficacy
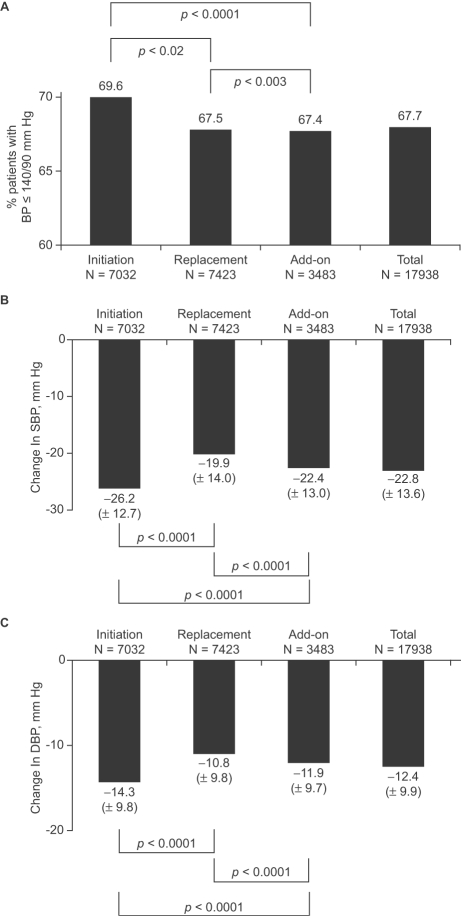
Most patients were prescribed Per 2 mg/Ind 0.625 mg alone at inclusion (Table 3). BP normalization was reached in 69.6% (Initiation Group), 67.5% (Replacement Group) and 67.4% (Add-on Group) of patients (Figure 1A). After treatment with Per/Ind, most patients had a SBP ≤140 mm Hg (70.8%, 68.5%, and 65.5% of patients in the Initiation, Replacement, and Add-on Groups, respectively) and few patients retained a SBP >150 mm Hg (7.0%, 8.4%, and 9.6% of patients in the Initiation, Replacement, and Add-on groups, respectively). After treatment, most patients had a DBP ≤90 mm Hg (93.5%, 93.9%, and 93.3% of patients in the Initiation, Replacement, and Add-on Groups, respectively).
Table 3.
Antihypertensive drug regimen at inclusion
| Initiation n = 7032 | Replacement n = 7423 | Add-on n = 3483 | Total n = 17938 | |
|---|---|---|---|---|
| Per/Ind alone, n (%) | 6857 (97.5) | 6588 (88.8) | − | 13445 (75.0) |
| Per/Ind + 1 additional drug, n (%) | 81 (1.2) | 583 (7.9) | 2603 (74.2) | 3267 (18.2) |
| Per/Ind + ≥2 additional drugs, n (%) | 94 (1.3) | 252 (3.4) | 880 (25.3) | 1226 (6.8) |
Abbreviations: Per/Ind, perindopril/indapamide combination.
Figure 1.
Blood pressure after 3–6 months of treatment with the fixed perindopril/in-dapamide (Per/Ind) combination. Per/Ind was initiated in newly diagnosed hypertensive patients (Initiation Group, n = 7032), replaced previous treatment in patients whose blood pressure (BP) was uncontrolled at inclusion and/or who experienced side-effects (Replacement Group, N = 7,423), or added to previous treatment in patients who were treated but only partially controlled (Add-on Group, n = 3483). Panel A: BP normalization was defined as a systolic BP ≤140 mm Hg and a diastolic BP ≤90 mm Hg. Panel B: Changes in SBP; Panel C: Changes in DBP. Data were missing in 1% of patients in each group.
Changes from baseline in SBP and DBP were also evaluated (Figure 1B-C). The largest decrease in SBP was recorded in the Initiation Group (26.2±12.7 mm Hg). In the Replacement and Add-on Groups the corresponding decreases averaged 19.9±14.0 and 22.4±13.0 mm Hg. The changes from baseline in SBP were statistically significant in all groups (p vs Baseline <0.0001). The differences between groups were statistically significant (p between Groups <0.0001). Similarly, the decrease in DBP was the greatest in the Initiation Group (14.3±9.8 mm Hg) and the smallest in the Replacement group (10.8±9.8 mm Hg). Changes from baseline in DBP were statistically significant in all groups (p vs Baseline <0.0001). The differences between groups were also statistically significant (p between Groups <0.0001).
In the Replacement Group, when changes in SBP were subdivided according to the type of previous antihypertensive treatment, SBP reductions of 18.5 mm Hg (vs calcium antagonist) to 24.9 mm Hg (vs vasodilator) were observed (Table 4). In the Add-on Group, additional decreases in SBP of 20.4 mm Hg (plus ACE inhibitor) to 28.3 mm Hg (plus vasodilator) were recorded. The changes in DBP in relation to the previous (Replacement Group) or concomitant (Add-on Group) therapy are also shown in Table 4.
Table 4.
Additional changes in blood pressure according to the type of previous antihypertensive treatment
| Replacement n = 7423 | Add-on n = 3483 | |||||
|---|---|---|---|---|---|---|
| Previous AH tt | n | SBP mean ± SD | DBP mean ± SD | n | SBP mean ± SD | DBP mean ± SD |
| Diuretic | 1015a | −21.1 ± 2.3 | −11.5 ± 9.5 | 68 | −24.0 ± 14.4 | −11.7 ± 10.2 |
| Beta-blocker | 850 | −18.8 ± 14.1 | −11.0 ± 10.3 | 739b | −23.0 ± 13.3 | −12.7 ± 9.0 |
| Calcium inhibitor | 1378b | −18.5 ± 14.2 | −10.2 ± 9.5 | 765b | −22.4 ± 12.1 | −12.0 ± 9.0 |
| ACE inhibitor | 1271 | −20.2 ± 12.2 | −11.2 ± 8.8 | 25 | −20.4 ± 12.8 | −8.8 ± 8.5 |
| ATII inhibitor | 684 | −20.5 ± 12.2 | −11.4 ± 9.7 | 67 | −21.8 ± 14.1 | −14.3 ± 10.4 |
| Central AH | 234a | −21.3 ± 13.7 | −11.6 ± 8.8 | 75 | −22.4 ± 11.4 | −11.7 ± 8.1 |
| Vasodilator | 60 | −24.9 ± 14.4 | −12.9 ± 10.1 | 25 | −28.3 ± 10.5 | −15.3 ± 6.4 |
SBP data missing for 1 patient
DBP data missing for 1–4 patients.
Abbreviations: ACE, angiotensin converting enzyme;AH, antihypertensive;ATII, angiotensin-receptor II; DBP, diastolic blood pressure; SBP, systolic blood pressure; tt, treatment.
Safety
The treatment was overall well tolerated; at the end of follow-up, the vast majority of patients (83%) continued treatment with the low-dose Per/Ind combination (2 mg/0.625 mg). In 10% of patients, the doses had to be increased to 4 mg/1.250 mg; the Per/Ind combination was replaced by another treatment in 5% of patients and discontinued in another 2% of patients. In the Initiation and Replacement Groups, very few patients were given an additional antihypertensive treatment (2%–4%).
Predictors of blood pressure normalization
After univariate analysis, age, baseline SBP, baseline DBP, LVH, proteinuria/creatinuria, angina, renal insufficiency, peripheral artery disease, type 1 and 2 diabetes, total levels were significantly less likely to normalize their BP after cholesterol, LDL-C, treatment, and treatment tolerance were treatment than patients under 70 years of age, with low SBP selected to be included in a multivariate stepwise regression at inclusion, no LVH, no peripheral artery disease, or normal (data not shown). Results of the multivariate analysis revealed total cholesterol levels, respectively (Figure 2). Patients in that patients over the age of 80, with high SBP at inclusion, whom tolerance was good or very good (Figure 2) had a with LVH, peripheral artery disease, or high total cholesterol probability of normalizing their BP 3 and 5 times greater, respectively, than those who tolerated treatment very poorly. Interestingly, the presence of type 2 diabetes mellitus had no influence on the rate of BP normalization in response to the Per/Ind treatment.
Figure 2.
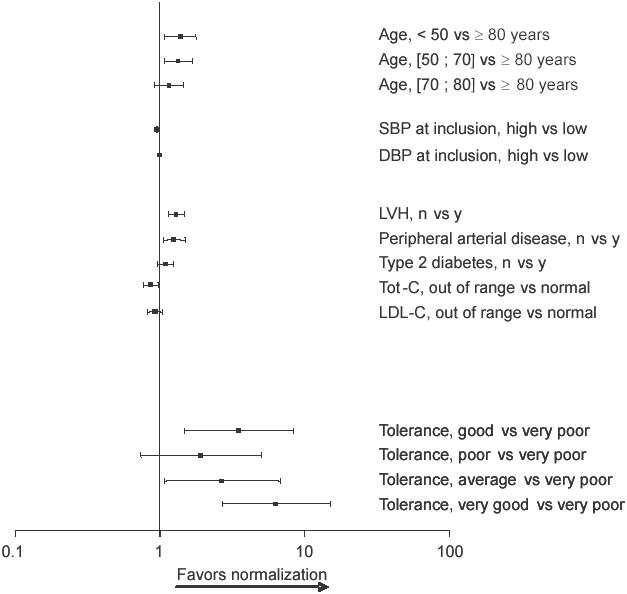
Multivaritate analysis of factors affecting the normalization of blood pressure. Odds ratios and 95% confidence intervals are presented.
Abbreviations: DBP, diastolic blood pressure; LDL-C, low density lipoprotein-cholesterol; LVH, left ventricular hypertrophy; SBP, systolic blood pressure;Tot-C, total cholesterol.
Discussion
Hypertension guidelines have been adapted to emphasize the role of combination treatments for more effective blood pressure control in current medical practice (Chobanian et al 2003; ESH-ESC 2003). The value of this therapeutic strategy has been tested recently using the combination containing perindopril and indapamide. In a randomized controlled trial performed in France (STRATHE trial), 62% of hypertensive patients had their BP normalized (<140/90 mm Hg) after 6–9 months of Per/Ind treatment (Mourad et al 2004). A significantly smaller percentage of patients allocated to conventional strategies reached the target BP. Such strategies included the sequential mono-therapy (BP normalization rate=49%) and the step-by-step (BP normalization rate= 47%) approaches. A major finding of this study was that the Per/Ind-based strategy allowed BP normalization in about twice as many patients as expected (33%) from a recent epidemiological study performed in France (Chamontin et al 2001). A high BP normalization rate (51%) has also been recently obtained with the Per/Ind combination in a large observational study (PRIMUS Study) (Holzgreve et al 2006).
The present study was planned to assess whether the BP control achieved using the Per/Ind combination in the STRATHE and PRIMUS studies can also be reached using the same preparation in a real-life setting. The Per/Ind combination reduced BP to ≤140/90 mm Hg in more than two thirds of patients, whether they were previously treated or not. This high BP control rate is equal to that observed in large interventional trials in which it was mandatory to adjust various drug regimens until BP normalization (ALL-HAT 2003; Julius et al 2004). Notably, in these morbidity–mortality trials, combination therapy was also required in most patients to reach both the systolic and the diastolic BP targets. The recognition of the need for combination therapy in hypertensive patients led experts in Europe and the USA to consider combination therapy in their guidelines (Chobanian et al 2003; ESH-ESC 2003; Haute Autorité de Santé 2005). Co-administering two drugs lowering BP by different mechanisms may have advantages: enhancement of antihypertensive efficacy, and improved tolerability.
This study observed a high BP normalization rate in patients included in the Add-on Group. These patients were older, at higher cardiovascular risk and had already more evidence of target organ damage than patients included in the Initial and in the Replacement Groups. Recent surveys have shown that it becomes more and more difficult to control BP as the global cardiovascular risk increases (Amar et al 2002; Mancia et al 2004). This study noted the ability of Per/Ind to normalize BP in such hypertensive patients, even when they were prone to be treatment-resistant. Moreover, the Per/Ind combination can be prescribed with other classes of BP-lowering drugs, especially calcium antagonists and β-blockers.
In this study the Per/Ind combination normalized systolic BP, which is known to be more difficult to control than diastolic BP. This was confirmed by the results of a subanalysis of the STRATHE study: diastolic BP was <90 mm Hg in nearly all patients in whom systolic BP could be brought <140 mm Hg (Waeber and Mourad 2006). An improved control of systolic BP compared with monotherapies (the β-blocker atenolol and the ACE inhibitor enalapril) has also been obtained with the Per/Ind combination in hypertensive patients with left ventricular hypertrophy or with type 2 diabetes mellitus (Mogensen et al 2003; de Luca et al 2004; Dahlof et al 2005).
The effect of the Per/Ind combination on systolic BP may be related to beneficial structural and functional changes of the vasculature, both on large arteries and microcirculation. The Per/Ind combination was shown to reduce central systolic BP more effectively than brachial systolic BP (Asmar et al 2001). As Per/Ind slows pulse wave velocity and decreases the aortic augmentation index, it reflects changes in arterial stiffness and wave reflections issued from arteriolar territory where Per/Ind is known to improve vessel wall structure (Asmar et al 2001; London et al 2004). Notably, in the CAFE trial (an ancillary study of the ASCOT trial), central aortic systolic BP was substantially lower during the amlodipine/perindopril treatment than during the atenolol/thiazide treatment. This differential response of central BP appears clinically relevant as it might have played a determinant role in the better protection against stroke afforded by the amlodipine/perindopril compared with the atenolol/thiazide drug regimen (Williams et al 2006).
The presence of diabetes in patients treated with the Per/Ind combination was of interest as this combination has been previously shown to have beneficial effects on albuminuria and cardiovascular events in hypertensive patients with type 2 diabetes (Mogensen et al 2003). These data are consistent with the current understanding of the direct action of ACE inhibitors on the renin-angiotensin system. Not only do ACE inhibitors improve renal and cardiovascular outcomes in diabetic patients, but these effects have been shown to extend beyond those attributable to blood pressure control and may be linked to an increase in tissue perfusion (Heart Outcomes Prevention Evaluation Study Investigators 2000; Mourad et al 2003; Renauld et al 2004; Kawata et al 2006). The use of the Per/Ind combination in diabetic patients is being further investigated in the ADVANCE study, which will evaluate the impact of tight glucose control and changes in blood pressure through combination treatment (Per/Ind and a modified-re-lease formulation of gliclazide) on both macrovascular and microvascular endpoints (ADVANCE 2001).
The results of the present trial should be interpreted with caution as the study protocol was adapted to be meaningful in everyday practice. Because of the large sample size this study demonstrated that hypertension can be successfully controlled in most patients with various risk factors, co-morbidities and degrees of BP elevation. These results could assist physicians in the management of blood pressure control in general practice.
Conclusion
An antihypertensive strategy based on the first-line Per/Ind combination, administered as initial, replacement, or add-on therapy, achieved the desired BP normalization rates. These exceeded those observed in clinical studies in a broad range of patients with various added risk factors and treatments, including the elderly, diabetic, or with target organ damage.
Acknowledgments
The authors would like to thank all the investigators who participated in the study and SERVIER for sponsoring the study.
References
- ADVANCE management committee. Study rationale and design of ADVANCE: action in diabetes and vascular disease–Preterax and Diamicron MR controlled evaluation. Diabetologia. 2001;44:1118–20. doi: 10.1007/s001250100612. [DOI] [PubMed] [Google Scholar]
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Diuretic versus α-blocker as first-step antihypertensive therapy. Final results from the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) Hypertension. 2003;42:239–46. doi: 10.1161/01.HYP.0000086521.95630.5A. [DOI] [PubMed] [Google Scholar]
- Amar J, Vaur L, Perret M, et al. On behalf of the PRATIK study investigators. Hypertension in high-risk patients : beware of the underuse of effective combination therapy (results of the PRATIK study) J Hypertens. 2002;20:779–84. doi: 10.1097/00004872-200204000-00038. [DOI] [PubMed] [Google Scholar]
- Asmar RG, London GM, O’Rourke ME, et al. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patients: a comparison with atenolol. Hypertension. 2001;38:922–6. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- Chalmers J, Castaigne A, Morgan T, et al. Long-term efficacy of a new, fixed, very-low-dose angiotensin-converting enzyme-inhibi-tor/diuretic combination as first-line therapy in elderly hypertensive patients. J Hypertens. 2000;18:327–37. doi: 10.1097/00004872-200018030-00013. [DOI] [PubMed] [Google Scholar]
- Chamontin B, Lang T, Vaisse B, et al. [Regional management of arterial hypertension in France. Report of a survey of general practitioners] Arch Mal Coeur Vaiss. 2001;94:823–7. [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens (Greenwich) 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Gosse P, Gueret P, et al. Perindopril/indapamide combination more effective than enalapril in reducing blood pressure and left ventricular mass: the PICXEL study. J Hypertens. 2005;23:2063–70. doi: 10.1097/01.hjh.0000187253.35245.dc. [DOI] [PubMed] [Google Scholar]
- de Luca N, Mallion JM, O’Rourke MF, et al. Regression of left ventricular mass in hypertensive patients treated with perindopril/in-dapamide as a first-line combination: the REASON echocardiography study. Am J Hypertens. 2004;17:660–7. doi: 10.1016/j.amjhyper.2004.03.681. [DOI] [PubMed] [Google Scholar]
- [ESH-ESC] 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–53. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–9. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- Haute Autorité de Santé. Prise en charge des patients adultes atteints d’hypertension artérielle essentielle (Management of adult patients with essential arterial hypertension. Recommendations)—Recommenda-tions. 2005 Update [online] 2005 Accessed:July 2006. URL: http://www.sfhta.org/pdf/2RecoHTA2005.pdf. [Google Scholar]
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICROHOPE substudy. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- Holzgreve H, Risler T, Trenkwalder P. Efficacy and tolerability of the perindopril/indapamide combination therapy for hypertension: the PRIMUS study. Curr Med Res Opin. 2006;22:1849–58. doi: 10.1185/030079906X132433. [DOI] [PubMed] [Google Scholar]
- Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomized trial. Lancet. 2004;363:2022–31. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- Kawata T, Masao D, Rei H, et al. Effect on coronary flow velocity reserve in patients with type 2 diabetes mellitus: Comparison between angiotensin-converting enzyme inhibitor and angiotensin II type 1 receptor antagonist. Am Heart J. 2006;151:798.e9–798.e15. doi: 10.1016/j.ahj.2005.09.014. [DOI] [PubMed] [Google Scholar]
- London CM, Asmar RG, O’Rourke M, et al. Mechanism(s) of selective Systolic Blood pressure reduction after a low-dose combination of perindopril/Indapamide in hypertensive subjects: comparison with atenolol. J Am Coll Cardiol. 2004;43:92–9. doi: 10.1016/j.jacc.2003.07.039. [DOI] [PubMed] [Google Scholar]
- Mancia G, Pessina AC, Trimarco B, et al. SILVIA (Studio Italiano Longitudinale sulla valutazione della Ipertensione Arteriosa nel 2000) Study group Blood pressure control according to new guidelines targets in low-to high-risk hypertensives managed in specialist practice. J Hypertens. 2004;22:2387–96. doi: 10.1097/00004872-200412000-00022. [DOI] [PubMed] [Google Scholar]
- Materson BJ, Reda DJ, Cushman WC, et al. Results of combination anti-hypertensive therapy after failure of each of the components. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Hum Hypertens. 1995;9:791–6. [PubMed] [Google Scholar]
- Mogensen CE, Viberti G, Halimi S, et al. Effect of low-dose perin-dopril/indapamide on albuminuria in diabetes: Preterax in albuminuria regression: PREMIER. Hypertension. 2003;41:1063–71. doi: 10.1161/01.HYP.0000064943.51878.58. [DOI] [PubMed] [Google Scholar]
- Mourad JJ, Hanon O, Deverre JR. Improvement of impaired coronary vasodilator reserve in hypertensive patients by low-dose ACE inhibi-tor/diuretic therapy : a pilot PET study. J Renin Angiotens Aldoster Syst. 2003;4:94–5. doi: 10.3317/jraas.2003.018. [DOI] [PubMed] [Google Scholar]
- Mourad JJ, Waeber B, Zannad F, et al. Comparison of different therapeutic strategies in hypertension: a low-dose combination of perin-dopril/indapamide versus a sequential monotherapy or a stepped-care approach. J Hypertens. 2004;22:2379–86. doi: 10.1097/00004872-200412000-00021. [DOI] [PubMed] [Google Scholar]
- Renaud IM, Chainey A, Belair MF, et al. Long-term protection of obese Zucker rat kidneys from fibrosis and renal failure with an angi-otensin-converting enzyme inhibitor/diuretic combination. Fundament Clin Pharmacol. 2004;18:437–47. doi: 10.1111/j.1472-8206.2004.00264.x. [DOI] [PubMed] [Google Scholar]
- Roux O, Chapellier M, Czernichow S, et al. Determinants of hypertension control in a large French population of treated hypertensive subjects. Blood Press. 2006;15:6–13. doi: 10.1080/08037050500450114. [DOI] [PubMed] [Google Scholar]
- Waeber B, Mourad JJ. Targeting systolic blood pressure: the key to controlling combined systolic/diastolic hypertension. Am J Hypertens. 2006;19:985–6. doi: 10.1016/j.amjhyper.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- Wolf-Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]