Abstract
Beta-blockers have been shown to improve survival in patients with chronic heart failure. The effect of different generations of beta blockers has been debated. Both metoprolol and carvedilol have demonstrated beneficial effects in placebo-controlled trials. In The Carvedilol Or Metoprolol European Trial (COMET) two beta blockers were compared in a double-blind randomized matter. This is the first direct comparison between metoprolol and carvedilol of long-term effect on survival in patients with chronic heart failure. The all-cause mortality was signif icantly reduced in the favour of carvedilol. The dose and formulation of metoprolol used in this trial has caused debate, and it has been questioned whether a similar beta1-blockade is obtained in the two intervention groups. At this time there is an unresolved debate as to whether carvedilol is a superior beta-blocker or whether differences in beta1-blockade explained the results of COMET.
Keywords: beta-blockers, chronic heart failure, carvedilol
Introduction
Heart failure is a growing problem with an increasing number of affected people and with an increasing burden on society (Greenberg 2004). Much has been done to improve survival in patients with heart failure. The first breakthrough came with the VHEFT-1 (Vasodilator Heart Failure Trial) trial where it was demonstrated that a combination of hydralazine and isosorbide dinitrate was able to improve survival of patients with heart failure (Cohn et al 1986). Shortly after it was demonstrated that angiotensin-converting enzyme (ACE) inhibitors could also improve survival (Pfeffer et al 1992; Kober et al 1995). But the mortality remained high and the need for further improvement was evident. The failing human heart has an increased adrenergic drive, which mediates its adverse effect through beta1- and possibly beta2- and alpha1-adrenergic receptors (Bristow 2000). This was the rationale for introducing beta-blockers in the treatment of chronic heart failure.
Beta1-receptors are down-regulated in the failing heart; the beta2-receptors are un-changed and the alpha1-receptors are increased. In patients with heart failure it was found that 50% of the adrenergic receptors in the heart were beta1-receptors and the rest were beta2- and alpha1-receptors (Bristow et al 1986, 1988, 1997; Bristow 1993).
Most of the myocardial damage observed in heart failure appears to be beta1mediated (Dorn 2002) with both pathological hypertrophy (Lowes et al 2002) as well as apoptosis (Communal et al 1999) being mediated via the beta1-receptor. Much myocardial damage in the failing human heart is mediated by norepinephrine (NE). The relative potency of NE for beta1-, beta2-, and alpha1-receptors is 20:1:2 (Bristow 1997). Norepinephrine is released from presynaptic stores and stimulates beta1-receptors preferentially (Khamssi and Brodde 1990).
On this background it is evident that beta-blockers can have a place in the treatment of heart failure, but it is unclear whether beta-1 selective blockers or non-selective blockers are preferable.
Beta-blocker treatment in heart failure
Beta-blockers have been shown to be of clinical benefit in patients with chronic heart failure (Greenberg 2004). The first study was performed by Waagstein and colleagues (1975). One patient was given alprenolol and 6 patients received practolol (Waagstein et al 1975). They all had an improved ventricular function due to the beta-blocker treatment and considerations for using beta-blockers in the treatment of heart failure began.
During the nineties an array of large randomized trials of beta-blockers for the treatment of chronic heart failure (CHF) were conducted. In 1990 the results from the Xamoterol in Severe Heart Failure Trial was published (Xamoterol 1990). Even though preliminary data had shown that treatment with the beta1-blocker xamoterol in patients with CHF was a safe and effective treatment, the result of the trial was disappointing. No beneficial effect of treatment with xamoterol was observed. In an analysis of intention-to treat 9.1% of the patients in the xamoterol-treated group died within the first 100 days from randomization, compared with 3.7% in the placebo-treated group. Xamoterol has a major intrinsic sympatomimetic effect, which is not thought beneficial for use in patients with heart failure. The failure of xamoterol is generally attributed to the intrinsic sympatomimetic activity, but another important consideration is that the treatment was initiated with a high dose from the very start in patients with extreme risk.
In CIBIS I (The Cardiac Insufficiency Bisoprolol Study), bisoprolol was compared with placebo in 641 patients with CHF, New York Heart Association (NYHA) Classification III–IV, with a follow-up period of almost 2 years. No significant difference was seen in the rate of death (CIBIS 1994), but the study was underpowered. A few years later bisoprolol was once again tested in the CIBIS II trial. 2647 patients were randomized to receive either bisoprolol or placebo, and the follow-up period lasted for about 1 year (CIBIS 1999). This time a significant reduction in all cause mortality as well as cardiovascular death and hospitalization for cardiovascular reasons was observed. Actually the benefit in CIBIS I and II were identical and the differences in significance are explained by the different sample sizes of the studies.
The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) with 3991 patients enrolled, examined the effect of metoprolol in patients with CHF, NYHA II–IV, and as for bisoprolol, metoprolol succinate showed a favourable effect on all-cause mortality compared with placebo (MERIT-HF 1999).
Another study was performed in a population with many black patients, where patients with CHF, NYHA III-IV were randomized to receive either bucindolol or placebo (BEST 2001). A non-significant difference in all-cause mortality was observed, 15% of the patients in the group treated with bucindolol died compared with 17% in the group treated with placebo. The results of this trial have caused some debate. There have been speculations that the relative predominance of black patients influenced the results of the trial but this speculation has not been substantiated. In a subsequent retrospective study, it was demonstrated that there was a subset of patients who experienced marked sympatholysis with bucindolol (Bristow et al 2004) and who had an increased risk of death. There has also been an unresolved debate as to whether bucindolol is associated with intrinsic sympathomimetic activity (Andreka et al 2002; Maack et al 2003). Should this be the case it might explain the failure of bucindolol similarly to the xamoterol trial described above.
Impressive results have been obtained with carvedilol. The results from the US Carvedilol Heart Failure Study were published in 1996. In this study 1064 patients with CHF were randomized to receive either carvedilol or placebo (Packer et al 1996). The trial was designed to evaluate the safety of treatment with carvedilol and mortality was observed as a secondary f inding. As for the primary end point, a significant reduction in the risk of hospitalization was found and worsening of heart failure was reduced in the group treated with carvedilol. A risk reduction of 65% was found in favour of carvedilol. The problem with interpreting the results of this study was that it was stopped early and was a meta-analysis of a group of studies designed to demonstrate symptomatic relief of carvedilol. These problems became secondary with the study of carvedilol in patients with severe heart failure. The Carvedilol Prospective Randomized Cumulative Survival Study (COPERNICUS) trial was performed. 2289 patients with severe heart failure were randomized to receive carvedilol or placebo for a mean treatment period of 10.4 months (Packer et al 2001). A decrease of 35% in risk of death was shown. The effect, previously seen in the group with mild or moderate heart failure, was thereby reproduced in patients with severe heart failure.
In the Carvedilol Post-Infarction Survival Control in LV Dysfunction (CAPRICORN) study, effect of carvedilol in patients who have had an acute myocardial infarction was investigated (Dargie 2001). Mean Left Ventricular Ejection Fraction (LVEF) was 32.8% and most of the patients were already treated with both ACE-inhibitors and diuretics. A 23% reduction in all-cause mortality was seen. This percentage in reduction is comparable with the results seen in the large trials on ACE-inhibitors after Acute Myocardial Infarction (AMI): SAVE (the Survival and Ventricular Enlargement trial), AIRE (the Acute Infarction Ramipril Efficacy study) and TRACE (the Trandolapril Cardiac Evaluation study) (Pfeffer et al 1992; Kober et al 1995). In spite of the result it is debated whether CAPRICORN was a positive or a negative study. The initial primary endpoint of this study was all-cause mortality. During the trial the steering committee noted that all-cause mortality was lower than expected, and they changed the primary endpoint to the combination of all-cause mortality and all-cause hospitalization. Eventually the original endpoint came out positive and the new endpoint was neutral. To regulatory authorities, CAPRICORN was therefore a negative study, to a general scientific interpretation there are no specific rules and many consider the trial positive.
As a net result it is widely accepted that beta-blocker therapy has substantial benefit to patients with heart failure, with, and without myocardial infarction. One of many important questions that remain is whether different beta-blockers have different value.
The first developed beta-blockers, the so-called first generation beta-blockers, was a non-specific beta-blocker that blocks both beta1- and beta2-adrenoceptors. Propranolol, the traditionally used first-generation beta-blocker, is not recommended for use in heart failure patients: it has not been tested. Metoprolol and bisoprolol belongs to the group of second generation beta-blockers with a specific beta1-blockade. The third-generation beta-blockers include carvedilol and bucindolol and have a non-selective beta-blocking effect on both beta1- as well as beta2adrenoceptors. Carvedilol further has an alpha-blocking effect which results in a vasodilating property. Bucindolol also has a vasodilating property. The reason for the vasodilating property of bucindolol has not clearly been demonstrated, but it may be because of an intrinsic sympatomimetic effect. In addition to the adrenergic blocking properties carvedilol it has an antioxidant effect and suppresses endothelin biosynthesis (Yue et al 1992; Ohlstein et al 1998; Lysko et al 2000; Arumanayagam et al 2001).
The Carvedilol Or Metoprolol European Trial
In The Carvedilol Or Metoprolol European Trial (COMET) two generations of beta-blockers were compared. Metoprolol, a second-generation beta-blocker was compared with the third-generation beta-blocker carvedilol (Poole-Wilson et al 2003). Both beta-blockers have been proven beneficial for use in a group of patients with heart failure (Packer et al 1996, 2001). The aim of the trial from the outset was to study whether the extra effects of carvedilol mattered clinically and therefore the trial was designed to ensure as close as possible beta-1 blocking effect in the two study groups.
3029 patients with CHF, mean ejection fraction 26%, and NYHA II–IV, were randomized to receive metoprolol tartrate with a target dose of 50mg twice daily or carvedilol with a target dose of 25mg twice daily. Both beta-blockers were well tolerated, and no differences were seen in terms of side-effects or withdrawals (Torp-Pedersen, Poole-Wilson, et al 2005). A significant reduction in mortality was observed in favour of the group treated with carvedilol, with an absolute reduction in mortality of 5.7% over a 5 year follow-up period (Figure 1). Similarly to the reduction in mortality, a reduction in mortality due to cardiovascular reasons, stroke, sudden death, and death caused by circulatory failure was seen in the group treated with carvedilol. No differences were seen between the two groups as it comes to worsening in heart failure, hospitalizations, and cause-specific hospitalizations (Torp-Pedersen, Poole-Wilson, et al 2005). As an interesting finding, carvedilol reduced the prevalence of new-onset diabetes by 22% compared with metoprolol (Torp-Pedersen, Cleland, et al 2005). Mean duration of follow-up was 58 months, but the benefit, in concern of reduced mortality among the patients treated with carvedilol, appeared already at 6 months of observation.
Figure 1.
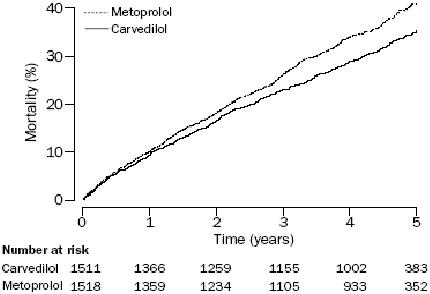
All cause mortality in the COMET trial. Copyright © 2003. Reproduced with permission from Poole-Wilson PA, Swedberg K, Cleland JG, et al. 2003. Comparison of carvedilol and metoprolol on clinical outcomes in patients withchronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET):randomised controlled trial. Lancet, 362:7–13.
Abbreviations: COMET, The Carvedilol Or Metoprolol European Trial.
Much debate has been taking place since the COMET trial was first published. Criticism has been focused on the choice of formulation of metoprolol. In the COMET trial immediate-release (IR) metoprolol was used instead of the controlled-release (CR) metoprolol which was proven beneficial in the MERIT-HF trial (MERIT-HF 1999). But when COMET was designed in 1996, the only clinical trial available was the Metoprolol in Dilated Cardiomyopathy (MDC) study (Waagstein et al 1993). The question is then whether this formulation can improve mortality and is worthy of comparison with carvedilol. In the MDC trial, treatment with IR metoprolol in patients with idiopathic dilated cardiomyopathy resulted in a 34% reduction in primary end-point, which was of all-cause mortality or need for heart transplantation. Need for transplantation was used as an expression for deterioration of heart failure. No reduction in all-cause mortality was observed, possibly due to a very low number of deaths in the trial. But when follow up was extended a beneficial effect on mortality was indeed observed with IR metroprolol (MDC 1998). The basic question is, whether the apparent superior effect on mortality seen with carvedilol compared with IR metoprolol, has any relevance to a higher dose and more sustained blockade that is obtained with 200mg of CR metoprolol as was used in MERIT-HF. Was there simply insufficient beta-1 blockade with IR metroprolol in the COMET study?
To assess the degree of beta1-blockade the most accepted method is to study exercise-induced increase in heart rate (HR). In a review of such studies it was found that a 20% reduction in exercise-induced tachycardia could be obtained with the dose of 50 mg, 100 mg and 150 mg daily with carvedilol, IR metoprolol, and CR metoprolol respectively (Packer 2003). In the COMET trial no evaluation of the relative beta1-blockade of the two beta-blockers used was performed. The only information collected was the resting HR. A significant albeit small difference in the reduction of HR obtained was observed in the carvedilol arm, no difference in HR was seen beyond the f irst year of observation (Figure 2) (Packer 2003). The CR metoprolol delivers 33% less metoprolol into the bloodstream than the IR metoprolol (Sandberg et al 1988). A significantly higher beta1-blockade over 24 hours was seen with 200 mg of CR metoprolol than with IR metoprolol tartrate 50 mg × 3 (Andersson et al 2001). As carvedilol exert different adrenoceptor blocking properties besides its antioxidative effect, it is of interest to know which mechanism gives carvedilol its favorable effect in superiority to metoprolol on mortality.
Figure 2.
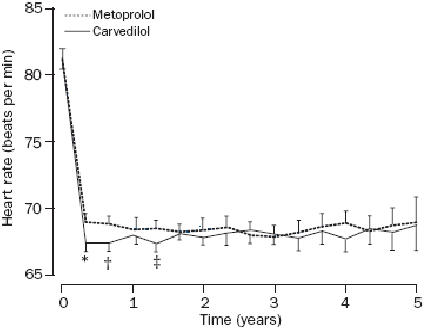
Heart rate during treatment in the COMET trial. Copyright © 2003. Reproduced with permission from Poole-Wilson PA, Swedberg K, Cleland JG, etal. 2003. Comparison of carvedilol and metoprolol on clinical outcomes inpatients with chronic heart failure in the Carvedilol Or Metoprolol EuropeanTrial (COMET): randomised controlled trial. Lancet, 362:7–13.
Abbreviations: COMET, The Carvedilol Or Metoprolol European Trial. *p=0.0022, †p=0.034, ‡p=0.0040.
The alpha-blocking properties have been tested. Doxazosin, a selective alpha1-blocker, versus placebo was tested in 73 patients with CHF (DiBianco et al 1991). Both investigators and patients assessment of symptomatic change was improved after treatment with doxazosin. When doxazosin was added to the beta1-blocker metoprolol in patients with heart failure, no improvement in hemodynamic measurements were seen, when compared with patients only treated with metoprolol (Kukin et al 1996). Even though carvedilol does not have the same pharmacokinetic properties as the combination of doxazosin and metoprolol, this study indicates that the beneficial effect seen with carvedilol is less likely to be caused solely by the alpha1-blocking properties of carvedilol.
The beta2-blocking effect affects the pre-synaptic release of NE, which reduces the amount of NE that can stimulate the beta1-receptors. carvedilol also exerts a more pronounced beta1-binding, and all together this reduces the deleterious effect of NE in heart failure (Bristow 2000).
Sub-studies from the COMET trial showed a decreased number of new-onset diabetes in the carvedilol arm, this sub-study is published as an abstract (Torp-Pedersen, Cleland, et al 2005). This was in contrast to earlier findings from beta-blocker trials showing an increased number of new-onset diabetes when treated with traditional beta1-receptor blockers (Dahlof et al 2002). That carvedilol might have a beneficial effects on the glucose metabolism was already shown in a study by Jacob and colleagues (1996). In this study metoprolol was compared with carvedilol in a group of patients with hypertension. An improvement in insulin sensitivity was seen among those treated with carvedilol. Guigliano (1997) also showed improved insulin sensitivity when treated with carvedilol. The changes were shown by making an insulin clamp testing.
Recently a large randomized trial in which the two beta-blockers were compared concerning their abilities in affecting the metabolic control in patients with diabetes and hypertension was published (Bakris et al 2004). An increase in glycosylated hemoglobin was seen in the metoprolol arm, whereas no change was seen in the carvedilol arm. Improvement in insulin sensitivity was seen in the carvedilol group and no change was seen among the patients treated with metoprolol.
This supports the findings from the COMET trial. In both trials the IR metoprolol was used. The beneficial role of carvedilol is speculated to be caused by its alpha-blocking properties and thereby causing vasodilation. Peripheral vasodilation facilitates glucose uptake in skeletal muscle and thereby improves insulin sensitivity (Smith and Warren 1982). Other anti-hypertensives that also increase peripheral blood-flow have the same benef icial effect on insulin sensitivity (Lind and Lithell 1993).
Whether the increased peripheral blood-flow is solely to explain the benefit of carvedilol on insulin sensitivity is doubtful. The combined effect of carvedilol, including its antioxidative properties, is more likely to be the explanation.
Perspective of the COMET trial
The perspective of COMET can be divided in 3 parts: the importance of beta-1 inhibition, the importance of other effects of carvedilol and the clinical consequence.
Three beta-blockers with beta-1 activity have been shown to reduce mortality in CHF. One interpretation of COMET is that a more effective inhibition by carvedilol, either because of a higher dose being given or because carvedilol has a higher affinity for the beta-1 receptor, explained the marked difference. Thus effective beta-1 inhibition with as high a dose as possible is clearly important for the treatment of CHF.
The mortality difference in COMET was marked even though the difference in resting pulse was small. Therefore, it is likely that other effects of carvedilol compared with metoprolol are important for the treatment of heart failure. Further research is necessary to clarify whether beta-2 inhibition, alpha-1 inhibition, antioxidative properties of, inhibition of endothelin-1biosynthesis, or yet another effect is beneficial (Table 1).
Table 1.
The possible effect of the adrenoreceptors in the progression of heart failure. Copyright © 2004. Reproduced with permission from Metra M, Cas LD, di Lenarda A, et al. 2004. Beta-blockers in heart failure: are pharmacological differences clinically important? Heart Fail Rev, 9:123–30.
 |
As for the clinical consequence at this time what remains is a continuing debate. carvedilol protagonists (including the authors of this paper) will claim that a superiority of carvedilol compared with metoprolol is likely and that this drug should therefore be preferred for the treatment of CHF. Metoprolol protagonists dismiss the results of COMET and insist that a high dose of ER metoprolol should serve equal benefit as carvedilol for patients with CHF.
Acknowledgments
Grant was awarded by The Danish Heart Foundation.
References
- [AIRE] Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993;342:821–8. [PubMed] [Google Scholar]
- Andersson B, Aberg J, Lindelow B, et al. Dose-related effects of metoprolol on heart rate and pharmacokinetics in heart failure. J Card Fail. 2001;7:311–17. doi: 10.1054/jcaf.2001.28230. [DOI] [PubMed] [Google Scholar]
- Andreka P, Aiyar N, Olson LC, et al. Bucindolol displays intrinsic sympathomimetic activity in human myocardium. Circulation. 2002;105:2429–34. doi: 10.1161/01.cir.0000016050.79810.18. [DOI] [PubMed] [Google Scholar]
- Arumanayagam M, Chan S, Tong S, et al. Antioxidant properties of carvedilol and metoprolol in heart failure: a double-blind randomized controlled trial. J Cardiovasc Pharmacol. 2001;37:48–54. doi: 10.1097/00005344-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–36. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- [BEST] Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- Bristow MR. Changes in myocardial and vascular receptors in heart failure. J Am Coll Cardiol. 1993;22(4 Suppl A):61A–71A. doi: 10.1016/0735-1097(93)90465-d. [DOI] [PubMed] [Google Scholar]
- Bristow MR. Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol. 1997;80:26L–40L. doi: 10.1016/s0002-9149(97)00846-1. [DOI] [PubMed] [Google Scholar]
- Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–69. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Abraham WT, Yoshikawa T, et al. Second- and third-generation beta-blocking drugs in chronic heart failure. Cardiovasc Drugs Ther. 1997;11(Suppl 1):291–6. doi: 10.1023/a:1007748131847. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Umans V, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Krause-Steinrauf H, Nuzzo R, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation. 2004;110:1437–42. doi: 10.1161/01.CIR.0000141297.50027.A4. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Minobe W, Rasmussen R, et al. Alpha-1 adrenergic receptors in the nonfailing and failing human heart. J Pharmacol Exp Ther. 1988;247:1039–45. [PubMed] [Google Scholar]
- [CIBIS] Cardiac Insufficiency Bisoprolol Study II. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- [CIBIS] CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). CIBIS Investigators and Committees. Circulation. 1994;90:1765–73. doi: 10.1161/01.cir.90.4.1765. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodialtor therapy on mortality in chronic congestive heart failure: results of a Veterans Administration cooperative study. N Engl J Med. 1986;314:1547–52. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- Communal C, Singh K, Sawyer DB, et al. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–12. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- DiBianco R, Parker JO, Chakko S, et al. Doxazosin for the treatment of chronic congestive heart failure: results of a randomized double-blind and placebo-controlled study. Am Heart J. 1991;121(1 Pt 2):372–80. doi: 10.1016/0002-8703(91)90875-i. [DOI] [PubMed] [Google Scholar]
- Dorn GW., 2nd Adrenergic pathways and left ventricular remodeling. J Card Fail. 2002;8(6 Suppl):S370–3. doi: 10.1054/jcaf.2002.129267. [DOI] [PubMed] [Google Scholar]
- Gilbert EM, Abraham WT, Olsen S, et al. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996;94:2817–25. doi: 10.1161/01.cir.94.11.2817. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Acampora R, Marfella R, et al. Metabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension. A randomized, controlled trial. Ann Intern Med. 1997;126:955–9. doi: 10.7326/0003-4819-126-12-199706150-00004. [DOI] [PubMed] [Google Scholar]
- Greenberg B. Nonselective versus selective beta-blockers in the management of chronic heart failure: clinical implications of the carvedilol or Metoprolol European Trial. Rev Cardiovasc Med. 2004;5(Suppl 1):S10–17. [PubMed] [Google Scholar]
- Heilbrunn SM, Shah P, Bristow MR, et al. Increased beta-receptor density and improved hemodynamic response to catecholamine stimulation during long-term metoprolol therapy in heart failure from dilated cardiomyopathy. Circulation. 1989;79:483–90. doi: 10.1161/01.cir.79.3.483. [DOI] [PubMed] [Google Scholar]
- Hjalmarson A, Elmfeldt D, Herlitz J, et al. Effect on mortality of metoprolol in acute myocardial infarction. A double-blind randomised trial. Lancet. 1981;2:823–7. doi: 10.1016/s0140-6736(81)91101-6. [DOI] [PubMed] [Google Scholar]
- Jacob S, Rett K, Wicklmayr M, et al. Differential effect of chronic treatment with two beta-blocking agents on insulin sensitivity: the carvedilol-metoprolol study. J Hypertens. 1996;14:489–94. [PubMed] [Google Scholar]
- Khamssi M, Brodde OE. The role of cardiac beta1- and beta2 adrenoceptor stimulation in heart failure. J Cardiovasc Pharmacol. 1990;16(Suppl 5):S133–7. [PubMed] [Google Scholar]
- Kober L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–6. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- Kukin ML, Kalman J, Mannino M, et al. Combined alpha-beta blockade (doxazosin plus metoprolol) compared with beta blockade alone in chronic congestive heart failure. Am J Cardiol. 1996;77:486–91. doi: 10.1016/s0002-9149(97)89342-3. [DOI] [PubMed] [Google Scholar]
- Lind L, Lithell H. Decreased peripheral blood flow in the pathogenesis of the metabolic syndrome comprising hypertension, hyperlipidemia, and hyperinsulinemia. Am Heart J. 1993;125(5 Pt 2):1494–7. doi: 10.1016/0002-8703(93)90446-g. [DOI] [PubMed] [Google Scholar]
- Lowes BD, Gilbert EM, Abraham WT, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–65. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- Lysko PG, Webb CL, Gu JL, et al. A comparison of carvedilol and metoprolol antioxidant activities in vitro. J Cardiovasc Pharmacol. 2000;36:277–81. doi: 10.1097/00005344-200008000-00020. [DOI] [PubMed] [Google Scholar]
- Maack C, Bohm M, Vlaskin L, et al. Partial agonist activity of bucindolol is dependent on the activation state of the human beta1adrenergic receptor. Circulation. 2003;108:348–53. doi: 10.1161/01.CIR.0000080325.94345.8B. [DOI] [PubMed] [Google Scholar]
- [MDC] Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. 3-year follow-up of patients randomised in the metoprolol in dilated cardiomyopathy trial. The Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet. 1998;351:1180–1. [PubMed] [Google Scholar]
- [MERIT-HF] Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- Metra M, Cas LD, di Lenarda A, et al. Beta-blockers in heart failure: are pharmacological differences clinically important? Heart Fail Rev. 2004;9:123–30. doi: 10.1023/B:HREV.0000046367.99002.a4. [DOI] [PubMed] [Google Scholar]
- Ohlstein EH, Arleth AJ, Storer B, et al. Carvedilol inhibits endothelin-1 biosynthesis in cultured human coronary artery endothelial cells. J Mol Cell Cardiol. 1998;30:167–73. doi: 10.1006/jmcc.1997.0582. [DOI] [PubMed] [Google Scholar]
- Packer M. Do beta-blockers prolong survival in heart failure only by inhibiting the beta1-receptor? A perspective on the results of the COMET trial. J Card Fail. 2003;9:429–43. doi: 10.1016/j.cardfail.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E, moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- Sandberg A, Blomqvist I, Jonsson UE, et al. Pharmacokinetic and pharmacodynamic properties of a new controlled-release formulation of metoprolol: a comparison with conventional tablets. Eur J Clin Pharmacol. 1988;33(Suppl):S9–14. doi: 10.1007/BF00578406. [DOI] [PubMed] [Google Scholar]
- Smith RS, Warren DJ. Effect of acute oral beta adrenergic blockade on muscle blood flow in man. Cardiovasc Res. 1982;16:205–8. doi: 10.1093/cvr/16.4.205. [DOI] [PubMed] [Google Scholar]
- Torp-Pedersen C, Cleland JG, Di Lenarda A, et al. Carvedilol reduces the risk for new onset of diabetes related adverse events in heart failure compared to metoprolol: Results of the COMET study [abstract] JACC. 2005;45(Suppl 1):187A. [Google Scholar]
- Torp-Pedersen C, Poole-Wilson PA, Swedberg K, et al. Effects of metoprolol and carvedilol on cause-specific mortality and morbidity in patients with chronic heart failure—COMET. Am Heart J. 2005;149:370–6. doi: 10.1016/j.ahj.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Waagstein F, Bristow MR, Swedberg K, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet. 1993;342:1441–6. doi: 10.1016/0140-6736(93)92930-r. [DOI] [PubMed] [Google Scholar]
- Waagstein F, Hjalmarson A, Varnauskas E, et al. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J. 1975;37:1022–36. doi: 10.1136/hrt.37.10.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Port JD, Asano K, et al. Cardiac adrenergic receptor effects of carvedilol. Eur Heart J. 1996;17(Suppl B):8–16. doi: 10.1093/eurheartj/17.suppl_b.8. [DOI] [PubMed] [Google Scholar]
- Yue TL, Cheng HY, Lysko PG, et al. Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J Pharmacol Exp Ther. 1992;263:92–8. [PubMed] [Google Scholar]
- [Xamoterol] Xamoterol in Severe Heart Failure Study Group. Xamoterol in severe heart failure. The Xamoterol in Severe Heart Failure Study Group. Lancet. 1990;336:1–6. [PubMed] [Google Scholar]


