Abstract
Atrial fibrillation (AF) is a condition of genuine clinical concern. This arrhythmia increases patient morbidity and mortality, most notably due to stroke, thromboembolism and heart failure. Consequentially, there is a strong impetus to acquire a greater understanding of its natural history and course in order to provide crucial evidence-based treatment and resource allocation in the future. The objective of this review article is to present a concise overview of the management of AF, with reference to the recent evidence-based National Institute of Clinical Excellence (NICE) National Clinical Guidelines for the management of AF.
Keywords: atrial fibrillation, National Institute of Clinical Excellence, guidelines
Introduction
Atrial fibrillation (AF) is the most frequently encountered and sustained cardiac arrhythmia in clinical practice, with an overall population prevalence of 0.65% in the United Kingdom (Stewart et al 2001). The incidence of AF has a male predisposition, affecting men 1.5 times more commonly than women (Benjamin 1994). The prevalence of AF is highly age dependent; occurring in less than 1% of the population aged under 50 and about 10% of those above 80 (Freestone et al 2003). Furthermore, it is present in 3%–6% of acute medical admissions (Lip et al 1994). Given an aging general population, the predominance of AF among the elderly and the improved survival of patients with cardiovascular disease, its frequency is dramatically increasing.
AF exacts a significant clinical burden. For example, AF is an independent predictor of mortality and is associated with an odds ratio for death of 1.5 for men and 1.9 in women, independent of other risk factors (Benjamin et al 1998). AF increases the risk of stroke by 4–5 fold and accounts for 10%–15% of all ischemic strokes and nearly a quarter of strokes in patients aged 80 and over (Lip et al 2001, 2005a). Furthermore, it is linked to far more severe strokes, with longer hospitalization and higher 30-day mortality compared with subjects without AF (Wolf et al 1991; Lin et al 1996). In addition, AF increases the development of heart failure and adversely affects quality of life, including cognitive function (Freestone et al 2003).
AF is most commonly classified according to its temporal pattern (Levy et al 2003). Paroxysmal AF is defined as a self-terminating AF episode lasting less than 7 days, and usually less than 48 hours. Sustained AF, lasting longer than 7 days and less than a year, is classified as persistent. Where AF persists beyond a year, it is classified as permanent, irrespective of the fact that there may have been temporary resolution after cardioversion. Paroxysmal AF may degenerate into more frequent paroxysms, or a sustained form of AF, which in turn may degenerate into permanent AF. ‘Lone AF’ is largely a diagnosis of exclusion and refers to AF occurring in the absence of concomitant cardiovascular disease (eg, hypertension), structural heart disease (normal echocardiogram), with a normal ECG and chest X-ray.
Given the substantial clinical burden of AF, its increasing frequency coupled with considerable health care costs, there is a compelling need for clinically directed evidence based clinical guidelines to improve its detection and management. This requirement is further justified by the observed wide variation in management and disagreement amongst UK consultants regarding what is considered to be the optimal management strategy for this highly complex condition.
Guidelines for the management of AF
The National Institute for Clinical Excellence (NICE [www.nice.org.uk]) is a UK-centered independent organization responsible for providing national guidance on the promotion of good health as well as the prevention and treatment of ill health. NICE commissioned the National Collaborating Centre for Chronic Conditions, based at the Royal College of Physicians, to develop a clinical guideline on AF for use in the NHS in England and Wales. This guideline offers clinical advice for the management of AF, based on the best-published evidence and expert consensus taking into account both patient choice and detailed cost efficacy analysis. Furthermore, the guideline highlights key priorities for implementation. In this article, we present a brief overview of some of the key recommendations made in this document.
Diagnosis of atrial fibrillation
The most common presenting symptoms of AF are dyspnea, palpitations, chest pain, dizziness, and fatigue (Levy et al 1999). In patients presenting with these symptoms, manual pulse palpation may determine the presence of an irregular pulse that warrants further investigation using a 12-lead ECG. The use of transthoracic echocardiography (TTE) should be limited to those in whom it is important for long-term management, such as younger patients, those being considered for a rhythm control strategy, those in whom the probability of underlying structural heart disease is high and where clinical risk stratification for antithrombotic therapy is needed. Where diagnostic uncertainty remains in patients with suspected but undiagnosed PAF, 24-hour monitoring should be used if symptomatic paroxysms occur less than 24 hours apart. An event recorder should be reserved for where the paroxysms occur more than 24 hours apart.
Treatment of atrial fibrillation
Changing trends
The treatment of AF has undergone a paradigm shift over the last decade. The traditional strategy of predominant rhythm control has been challenged by recent data comparing this to a more conservative rate control strategy. Results of the five primary rate versus rhythm control trials have shown that rate control is a non-inferior strategy to rhythm control in terms of mortality (Hohnloser et al 2001; Van gelder et al 2002, Wyse et al 2002; Carlsson et al 2003; Opolski et al 2003) or quality of life outcomes (Gronefeld et al 2003). However, in the largest of these studies (Wyse et al 2002), mortality was found to be higher for rhythm control in those with coronary heart disease, those without heart failure and those aged over 65 years old. In an additional rate versus rhythm control study by Okcun et al (2004), rhythm control was found to be preferable among patients with heart failure and dilated cardiomyopathy. However, there was significant variation in the duration, intensity and quality of anticoagulation in the rhythm control arm of this study that may have affected the incidence of stroke and thromboembolism.
The AFFIRM study suggested that whilst the strategy of rate control was associated with a trend to reduced mortality compared with rhythm control, subgroup analyses showed that actually successfully achieving sinus rhythm is associated with reduced mortality, which was offset by an increased mortality with antiarrhythmic drug and digoxin usage (Wyse et al 2002; Corley et al 2004). Of note, anticoagulation usage was an independent predictor of improved survival (Corley et al 2004).
The strategies for rate control and rhythm control incorporate different pharmacological regimes. Analysis of the available evidence demonstrates that different situations may warrant adoption of either rate control or rhythm control strategy. A primary rate control strategy should be the preferred initial option in patients with persistent AF over 65 years, coronary artery disease, those with contraindications to antiarrhythmic drugs, patients unsuitable for cardioversion and among patients without congestive heart failure.
In contrast, a primary rhythm control strategy should be the preferred initial option in the following patients with persistent AF: those who are symptomatic, younger, presenting for the first time with lone AF, those with AF secondary to a treated/corrected precipitant and in those with congestive heart failure. The potential advantages and disadvantages of each strategy should be explained to patients. A section of patients with AF, who are resistant to the above management, may benefit from interventional techniques such as pulmonary vein isolation (PVI) or a surgical Maze procedure.
Rhythm control: the role of cardioversion
Rhythm control may include electrical (ECV) and pharmacological (PCV) cardioversion, as well as the use of surgical atrial debulking (Maze) and catheter ablation (eg, pulmonary PVI). No difference has been found in rates of successful cardioversion or in the risk of thromboembolism and stroke between electrical cardioversion (ECV) and PCV as the initial treatment strategy (Valencia et al 2002).
In prolonged cases of AF, ECV is the preferred option based on clinical experience and current clinical practice, whereas within 48 hours of onset, either pharmacological or electrical cardioversion could be performed. Whilst initial cardioversion is successful in achieving sinus rhythm in the majority, approximately 50% of patients are back in AF by one year (Lim et al 2004). Patients considered most likely to successfully cardiovert and maintain sinus rhythm are those with persistent AF of shorter duration (onset <12 months), those without underlying structural heart disease or AF secondary to a precipitant (eg, treated thyroid disease, fever) that has been treated. Hence, careful consideration should be made for the presence of coexisting factors that influence success. For example, a history of recurrent AF (Dmochowska-Perz et al 2002), chronic-obstructive pulmonary disease (Okcun et al 2002), heart failure (Okcun et al 2002) and cardiomegaly (Galperin et al 2003) has been found to be associated with reduced cardioversion success.
The maintenance of sinus rhythm post cardioversion is notoriously difficult and the treatments consistently influencing procedural success are limited. Consensus favors initial beta-blocker therapy followed by sotalol or flecanide in resistant cases (after ensuring good left ventricular function) and amiodarone as the final resort for patients that require antiarrythmic drugs post cardioversion.
Management strategies
Since atrial fibrillation is classified according its duration of onset, management strategies will differ based on the time-span of existence of AF. It can be seen from above that in general, patients who have AF of less than 1 year duration can be considered for cardioversion and have the highest chance of remission, in contrast to patients whose AF has lasted more than 1 year. The aim of treatment in these patients is to minimize symptoms and achieve adequate rate control.
Digoxin has traditionally been used for rate control; however it is has limited efficacy in hyperadrenergic states and with exercise (David et al 1979). Beta-blockers (Farshi et al 1999) and calcium antagonists (Maragno et al 1988) control exertional heart rate much more efficiently. Where required a combination treatment with atenolol and digoxin appears to be more effective than beta-blocker alone. Thus, evidence suggests that in patients requiring rate control, the first line of treatment should be beta-blockers or calcium antagonists with digoxin reserved for use in more sedentary patients (Li-Saw-Hee et al 1998).
When atrial fibrillation is paroxysmal, its management can be quite challenging. The aim of treatment is to reduce AF frequency, prevent complications and alleviate symptoms during the paroxysm itself. The ratio of asymptomatic versus symptomatic episodes can be highly variable and may be as high as 12:1 (Page et al 1994). Hence, treatment needs to be highly tailored to the individual patient and may vary depending on the frequency of paroxysms. Studies have evaluated different regimes of pharmacological therapy for PAF and found comparative efficacy with standard beta-blockers and sotalol (Steeds et al 1999). Amiodarone (Roy et al 2000; Kochiadakis et al 2004), in has been shown to be superior to all other agents (including class 1c agents); however, its efficacy is offset by its side-effect profile. Selected patients may be suitable for domiciliary self-treatment, using the so-called “pill in the pocket approach (Alboni et al 2004). Selection for this approach is critically important: these patients should be generally well educated, without evidence of coexistent left-ventricular dysfunction, valvular or ischemic heart disease and have infrequent episodes of paroxysmal AF.
For the hemodynamically compromised AF patient, treatment strategies should be in accordance with resuscitation council guidelines with the aim being to re-establish circulation while acknowledging the potential risks of inadequate thromboprophylaxis. ECV is indicated irrespective of the duration of AF (Michael et al 1999). Intravenous amiodarone may be used where delays in ECV are expected. Importantly, in patients with known Wolf-Parkinson-White syndrome, AV nodal blocking drugs should be avoided.
AF is an important postoperative complication as it significantly increases both morbidity (eg, longer hospitalization) and mortality (Almassi et al 1997). It occurs in approximately 33% of patients post-coronary heart surgery (Vecht et al 1980). One major meta-analysis (Crystal et al 2003) helped clarify the utility of varying medical treatments, and found that for patients undergoing cardiothoracic surgery, prophylactic administration of amiodarone, standard beta-blockers and sotalol were all justified in preventing post-operative AF in high-risk patients. Evidence in favor of the rate-limiting calcium antagonist diltiazem was extracted from additional studies (Seitelberger et al 1994). Indeed, rhythm control has been shown to be superior to rate control in maintenance of sinus rhythm and decreasing length of hospital stay among patients with postoperative AF (Lee et al 2003). Where AF occurs post operatively with patients undergoing non-cardiothoracic surgery, the treatment should be similar to that of acute AF.
Failed medical treatment, pre-excitation syndromes, poorly controlled symptomatic are all indications for nonmedical interventions in AF. Various treatments including PVI (Wazni et al 2005), pacemaker therapy (Levy et al 1999), arrhythmia surgery (Kawaguchi et al 1996), atrioventricular (AV) junction catheter ablation (Ueng et al 2001), and atrial defibrillators (Ricci et al 2004) have variable benefit in these patients. Of course, all interventions have inherent procedural risks. AV junction ablation mandates the implantation of a permanent pacing system. The complications of PVI are not insignificant and include pulmonary vein stenosis. Also, the incidence of recurrence of asymptomatic AF post procedures is unknown and raises the dilemma of adequate anticoagulation. The recent NICE guidelines have not given specific guidance regarding the use of these procedures. However, NICE acknowledges their increasing role in AF management. The reasons for referral for specialist intervention or continued drug use should be explained and discussed with the patient.
Antithrombotic therapy in AF
The principal aim of antithrombotic therapy in AF is the prevention of ischemic stroke and other thromboembolic events. In prevention of stroke, there is overwhelming evidence from randomized trials and systematic reviews to suggest benefit of thromboprophylaxis in patients with AF (Lip et al 2005, Hart et al 1999).
Compared with placebo, warfarin reduces the risk of ischemic stroke and systemic embolism by two-thirds (Hart et al 1999, Benavente et al 2003). When compared with antiplatelet therapy, adjusted-dose warfarin leads to a 36% relative risk reduction in ischemic stroke (Hart et al 1999). In contrast, aspirin results in a 22% risk reduction in preventing ischemic stroke among patients with AF, compared with controls (Hart et al 1999). This result is largely driven by the Stroke Prevention in AF (SPAF-1) clinical trial, where aspirin was more effective in reducing non-disabling strokes but did not reduce severe or recurrent strokes in those aged 75 and over (SPAF study 1991).
Anticoagulation for persistent AF depends on the adoption of either a rate-control or rhythm-control treatment strategy. Where a rhythm-control treatment strategy is chosen, antithrombotic therapy is needed to prevent acute stroke or other thromboembolic events occurring during or shortly after cardioversion. It is well accepted that patients should maintain therapeutic anticoagulation (INR 2.0–3.0) with warfarin for a minimum of 3 weeks pre-cardioversion (Collins et al 1995; Klein et al 2001) and for a minimum of 4 weeks post cardioversion. In acute cases where cardioversion cannot be postponed, heparin should be administered, cardioversion performed, and warfarin be administered for at least four weeks post cardioversion. The use of trans-oesophageal echocardiography may help exclude a left atrial thrombus and thus facilitate acute cardioversion where adequate pre-procedure anticoagulation was not possible (Klein et al 2001).
Data from the rate versus rhythm-control trials suggest that consideration should be given towards long-term anticoagulation in patients at high risk of stroke and AF recurrence. This is particularly important as there may be frequent asymptomatic AF recurrences that can lead to thromboembolism in the presence of risk factors (Wyse et al 2002; Corley et al 2004; Lim et al 2004).
Among patients with permanent AF, adjusted-dose warfarin is again the most effective treatment aiming for a target international normalized ratio (INR) of 2.5 (range 2.0–3.0). Where warfarin is inappropriate, aspirin (EAFT 1993; Hart et al 1999; Lip et al 2005) should be administered at 75–300 mg/day. Aspirin should not be co-administered with warfarin as it provides no additional thromboprophylactic benefit and may in fact be harmful (Lechat et al 2001). There is not enough evidence at the moment to recommend combination antiplatelet agents for AF. Of note, a recent large clinical trial found anticoagulation to be superior to aspirin-clopidogrel combination therapy for stroke prevention in AF (ACTIVE, Lancet 2005).
The effectiveness of anticoagulation in reducing the rate of ischemic stroke is similar for patients with either paroxysmal or permanent AF (Van walraven et al 2002). Patients with paroxysmal AF have an annual stroke rate similar to permanent AF (3.3%) (Lin et al 1996). Paroxysmal AF patients also frequently have asymptomatic recurrences, and thus, they are exposed to the risk of recurrent AF without recognition. Anticoagulation for paroxysmal AF should not be based on the frequency or duration of paroxysms but on appropriate risk stratification, as for permanent AF.
The onset of AF is associated with a cluster of thromboembolic events (Wolf et al 1983), but the development of intra-atrial thrombi, and the immediate risk of thromboembolism, is perceived to be minimal within the first 48 hours. Thus, in patients presenting with AF, a clear history of onset is necessary in order to guide appropriate antithrombotic therapy and cardioversion. Common clinical practice is that cardioversion may be safely performed without the need for oral anticoagulation if AF duration is clearly <48 hours (Singer et al 2004).
Secondary prevention of stroke is also well established with warfarin (EAFT 1993) However in cases of an acute stroke, assessment of the risk from hemorrhagic transformation in patients with large cerebral infarcts needs to be made. There is uncertainty about the optimal timing of administration of anticoagulants following acute stroke. Patients with AF can not only have thromboembolic stroke but also develop lacunar strokes secondary to hypertension and other comorbidities, and in such cases, warfarin is less effective (Evans et al 2001). There are little data available to assess the risks and benefits of warfarin administration in the setting of acute stroke and AF. Once cerebral imaging has excluded hemorrhage, antithrombotic therapy could be commenced within two weeks after a small stroke or transient ischemic attack (TIA). In patients with AF who have had a large infarction or uncontrolled hypertension, antithrombotic therapy should be delayed until at least 2 weeks after the stroke.
Practical aspects of anticoagulation
The increasing use of oral anticoagulation therapy as thromboprophylaxis in AF has encouraged a move towards “point-of-care” (POC) and patient self-monitoring. POC for INR testing reduces INR waiting time (Murray et al 2004). Self-monitoring is shown to be safe and effective in a UK population (Fitzmaurice et al 2005). Patients undertaking anticoagulation self-monitoring should be trained by a competent healthcare professional and remain in contact with a named clinician.
Despite clear guidelines, warfarin is underused, with a prescription rate of only 15%–44% among eligible patients (Bungard et al 2000; Fang et al 2004). Physician perceptions of risk benefit are not always reliable and bleeding risks are often overestimated (Bungard et al 2000). Elderly patients are often suboptimally treated, despite the fact that this age group is most likely to benefit from anticoagulation. A careful assessment of risk benefit ratio, especially among the elderly, should be ascertained rather than simply relying on age or physician preference alone.
Improved efforts at risk stratification for thromboprophylaxis would help target “high risk” subjects for anticoagulation. However, there is variation between the various risk stratification criteria as well as inconsistencies between the published risk stratification schema (Lip and Boos 2005). For example, the initial joint ACC/AHA/ESC guidelines in 2001 (Fuster et al 2001) recommended a risk stratification schema where any “high risk” factor (previous stroke, TIA, embolism; hypertension; heart failure; age >75 years; mitral stenosis; prosthetic valve) or >1 “moderate risk” factor (age 65–75; diabetes; coronary artery disease) merits anticoagulation. Thus, a 45-year-old man with AF and hypertension would be recommended warfarin using the 2001 ACC/AHA/ESC schema, but not by other published risk stratification schemas. This schema has now been suitably modified (in 2006) to recommend anticoagulation for those with high risk of stroke such as those with prior thromboembolism and rheumatic mitral stenosis and for those patients with more than 1 moderate risk factor (age 75 years or greater, hypertension, HF, impaired LV systolic function diabetes mellitus) (Fuster et al 2006). For patients with only one moderate risk factor the choice of warfarin versus aspirin must be carefully considered in the light of the perceived risk/benefit for the individual patient.
Another well-used and validated system, the CHADS2 risk stratification scheme (Gage et al 2001) gives a numerical score to each of five risk factors (congestive heart failure, hypertension, age ≥75 years, diabetes and stroke/TIA, the latter receiving a score of 2). The total score (0–6) equates to a recognized future stroke risk (where a CHADS2 score of 0 would define a person as being at “low” risk (and suitable for aspirin) and ≥3 as a “high risk” (and warfarin is recommended). However, AF patients with previous stroke, TIA, or thromboembolism are considered to be at high risk of a further stroke or thromboembolic event. However using the CHADS2, such patients with this risk factor alone would only have a total CHADS2 score of 2, which would classify them as “moderate’ risk”.
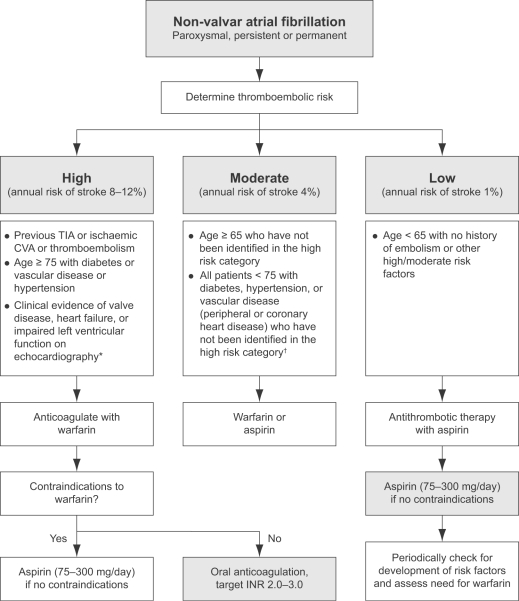
As a result of these uncertainties, the NICE guidelines has recommended an algorithm-based risk stratification schema (Figure 1), which incorporates data from other previously published risk stratification schemes and has been shown to be applicable to UK clinical practice. The NICE schema (which is based on the Birmingham risk stratification schema) has been compared with the CHADS2 schema and was found to be comparable for predicting stroke and vascular events (Lip et al 2006).
Figure 1.
Practical guidelines for antithrombotic therapy in non-valvular atrial fibrillation. Assess risk, and reassess regularly.
Note: Risk factors are not mutually exclusive, and are additive to each other in producing a composite risk. An echocardiogram not needed for routine risk assessment but refines clinical risk stratification in case of moderate or severe left ventricular dysfunction and valve disease. †Owing to lack of sufficient clear cut evidence, treatment may be decided on an individual basis, and the physician must balance the risks and benefits of warfarin versus aspirin; as stroke risk factors are cumulative, warfarin may (for example) be used in the presence of 2 or more risk factors. Referral and echocardiography may help in cases of uncertainty. *Since the incidence of stroke and thromboembolic events in patients with thyrotoxicosis appears similar to other aetiologies of AF, antithrombotic therapies should be chosen based on the presence of validated stroke risk factors.
Abbreviations: INR, international normalized ratio; CVA, cerebrovascular accident;TIA, transient ischemic attack.
Conclusions
The NICE guidelines for AF management are designed to assist in decision-making and are based on current best available evidence. In no way are they designed to replace clinical judgment and patient choices. Indeed, these guidelines are an attempt to ease the burden on physicians and hospitals in managing this common yet enigmatic disease. The future of AF heralds interesting prospects with novel therapy targeting incidence of AF and improved and newer anticoagulation regimes.
Disclosures
GL has received funding for research, educational symposia, consultancy, and lecturing from different manufacturers of drugs used for the treatment of atrial fibrillation and thrombosis. He is Clinical Adviser to the Guideline Development Group writing the United Kingdom National Institute of Clinical Excellence (NICE) Guidelines on atrial fibrillation management (www.nice.org.uk).
References
- Alboni P, Botto GL, Baldi NL, et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Eng J Med. 2004;351:2384–91. doi: 10.1056/NEJMoa041233. [DOI] [PubMed] [Google Scholar]
- Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–11. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonielli E, Pizzuti A, Palinkas A, et al. Clinical value of left atrial appendage flow for prediction of long-term sinus rhythm maintenance in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 39:1443–9. doi: 10.1016/s0735-1097(02)01800-4. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Ahn C, Kronzon I, et al. Risk factors for new thromboembolic stroke in patients ≥62 years of age with chronic atrial fibrillation. Am J Cardiol. 1998;82:119–21. doi: 10.1016/s0002-9149(98)00247-1. [DOI] [PubMed] [Google Scholar]
- Benavente O, Hart R, Koudstaal P, et al. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. The Cochrane Library. 2003 doi: 10.1002/14651858.CD001927. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population based cohort:the Framingham heart study. J Am Med Assoc. 1994;271:840–4. [PubMed] [Google Scholar]
- Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–6. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- Burton JH, Vinson DR, Drummond K, et al. Electrical cardioversion of emergency department patients with atrial fibrillation. Ann Emerg Med. 2004;44:20–30. doi: 10.1016/j.annemergmed.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Carlsson J, Miketic S, Windeler JS, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the strategies of treatment of atrial fibrillation (STAF) Study. J Am Coll Cardiol. 2003;41:1690–6. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- Collins LJ, Silverman DI, Douglas PS, et al. Cardioversion of nonrheumatic atrial fibrillation. Reduced thromboembolic complications with 4 weeks of precardioversion anticoagulation are related toatrial thrombus resolution. Circulation. 1995;92:160–3. doi: 10.1161/01.cir.92.2.160. [DOI] [PubMed] [Google Scholar]
- Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. Circulation. 2004;109:1509–13. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- Crystal E, Connolly SJ, Ginger T, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. The Cochrane Library. 2003 doi: 10.1002/14651858.CD003611.pub2. [DOI] [PubMed] [Google Scholar]
- David D, Di Segni E, Klein HO, Kaplinsky E, et al. Inefficiency of digitalis in the control of heart rate in patients with chronic atrial fibrillation: beneficial effects of an added beta-adrenergic blocking agent. Am J Cardiol. 1979;44:1378–1382. doi: 10.1016/0002-9149(79)90456-9. [DOI] [PubMed] [Google Scholar]
- Dmochowska-Perz M, Loboz-Grudzien K, Sokalski L, et al. Factors predicting recurrence of atrial fibrillation after cardioversion. Kardiologia Polska. 2002;57:501–11. [PubMed] [Google Scholar]
- Dogan A, Avsar A, Ozturk M, et al. P-wave dispersion for predicting maintenance of sinus rhythm after cardioversion of atrial fibrillation. Am J Cardiol. 200;93:368–71. doi: 10.1016/j.amjcard.2003.09.064. [DOI] [PubMed] [Google Scholar]
- Evans A, Perez I, Yu G, Kalra L. Should stroke subtype influence anticoagulation decisions to prevent recurrence in stroke patients with atrial fibrillation? Stroke. 2001;32:2828–32. doi: 10.1161/hs1201.099520. [DOI] [PubMed] [Google Scholar]
- [EAFT] European Atrial Fibrillation Study Group. Secondary prevention in non rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255–62. [PubMed] [Google Scholar]
- Ezekowitz MD, Laupacis A, Boysen G, et al. Echocardiographic predictors of stroke in patients with atrial fibrillation: A prospective study of 1066 patients from 3 clinical trials. Arch Intern Med. 1998;158:1316–20. doi: 10.1001/archinte.158.12.1316. [DOI] [PubMed] [Google Scholar]
- Fang MC, Stafford RS, Ruskin JN, et al. National trends in antiarryhthmic and anti-thrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164:55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- Farshi R, Kistner D, Sarma JS, et al. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: a crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33:304–10. doi: 10.1016/s0735-1097(98)00561-0. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice DA, Murray ET, McCahon D, et al. Self management of oral anticoagulation: randomised trial. BM J. 2005;331:1057. doi: 10.1136/bmj.38618.580903.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone B, Lip GYH. Epidemiology and costs of Cardiac Arrhythmias. In: Lip GYH, Godtfredson J, editors. Cardiac arryhythmias: a clinical approach. Edinburgh: Mosby; 2003. pp. 3–24. [Google Scholar]
- Frick M, Frykman V, Jensen-Urstad M, et al. Factors predicting success rate and recurrence of atrial fibrillation after first electrical cardioversion in patients with persistent atrial fibrillation. Clin Cardiol. 2001;24:238–44. doi: 10.1002/clc.4960240313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin J, Elizari MV, Chiale PA, et al. Pharmacologic reversion of persistent atrial fibrillation with amiodarone predicts long-term sinus rhythm maintenance. J Cardiovas Pharmacol Ther. 2003;8:179–86. doi: 10.1177/107424840300800302. [DOI] [PubMed] [Google Scholar]
- Fuster V, Ryden LE, Asinger RW, et al. American College of Car-diology/American Heart Association/European Society of Cardiology Board. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary a report of the American College of Cardiology/American heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for practice guidelines and policy conferences (committee to develop guidelines for the management of patients with atrial fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Circulation. 2001;104:2118. [PubMed] [Google Scholar]
- Fuster V, Rydén L.E, David S, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients With atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With atrial fibrillation): Developed in Collaboration With the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- Go AS, Hylek EM, Phillips KA. Prevalence of diagnosed atrial fibrillation in adults, national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- Gronefeld GC, Lilienthal J, Kuck KH, et al. Pharmacological intervention in atrial fibrillation. Impact of rate versus rhythm control on quality of life in patients with persistent atrial fibrillation. Results from a prospective randomized study. Eur Heart J. 2003;24:1430–6. doi: 10.1016/s0195-668x(03)00261-6. [DOI] [PubMed] [Google Scholar]
- Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- Hart RG, Pearce LA, Rothbart RM, et al. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Car-diol. 2000;35:183–7. doi: 10.1016/s0735-1097(99)00489-1. [DOI] [PubMed] [Google Scholar]
- Hohnloser SH, Kuck KH. Randomized trial of rhythm or rate control in atrial fibrillation: the Pharmacological intervention in atrial fibrillation trial (PIAF) Eur Heart J. 2001;22:801–2. doi: 10.1053/euhj.2001.2596. [DOI] [PubMed] [Google Scholar]
- Kamp O, Verhorst PMJ, Welling RC, et al. Importance of left atrial appendage flow as a predictor of thromboembolic events in patients with atrial fibrillation. Eur Heart J. 1999;20:979–85. doi: 10.1053/euhj.1998.1453. [DOI] [PubMed] [Google Scholar]
- Kawaguchi AT, Kosakai Y, Sasako Y, et al. Risks and benefits of combined maze procedure for atrial fibrillation associated with organic heart disease. J Am Coll Cardiol. 1996;28:985–90. doi: 10.1016/s0735-1097(96)00275-6. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Leitch JW, Neil A, et al. Cardiac event recorders yield more diagnoses and are more cost-effective than 48-hour Holter monitoring in patients with palpitations. A controlled clinical trial. Ann Inter Med. 1996;124:16–20. doi: 10.7326/0003-4819-124-1_part_1-199601010-00003. [DOI] [PubMed] [Google Scholar]
- Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–20. doi: 10.1056/NEJM200105103441901. [DOI] [PubMed] [Google Scholar]
- Kochiadakis GE, Igoumenidis NE, Hamilos MI, et al. Long-term maintenance of normal sinus rhythm in patients with current symptomatic atrial fibrillation: amiodarone vs propafenone, both in low doses. Chest. 2004;125:377–83. doi: 10.1378/chest.125.2.377. [DOI] [PubMed] [Google Scholar]
- Lardoux H, Mallet A, et al. FFAACS (Fluindione, Fibrillation Auriculaire, Aspirin et Contraste Spontane) Investigators. Anticoagulant (fluindione)-aspirin combination in patients with high-risk atrial fibrillation. A randomized trial (Fluindione, Fibrillation Auriculaire, Aspirin et Contraste Spontane, FFAACS) Cerebrovasc Dis. 2001;12:245–52. doi: 10.1159/000047711. [DOI] [PubMed] [Google Scholar]
- Lee JK, Klein GJ, Krahn AD, et al. Rate-control versus conversion strategy in postoperative atrial fibrillation: trial design and pilot study results. Card Electrophysiol Rev. 2003;7:178–84. doi: 10.1023/a:1027428003609. [DOI] [PubMed] [Google Scholar]
- Levy T, Walker S, Rochelle J, et al. Evaluation of biatrial pacing, right atrial pacing, and no pacing in patients with drug refractory atrial fibrillation. Am J Cardiol. 1999;84:426–9. doi: 10.1016/s0002-9149(99)00327-6. [DOI] [PubMed] [Google Scholar]
- Li-Saw-Hee FL, Lip GYH. Digoxin Revisted. Q JM. 1998;91:259–64. doi: 10.1093/qjmed/91.4.259. [DOI] [PubMed] [Google Scholar]
- Lim HS, Hamaad A, Lip GY. Clinical management of atrial fibrillation—rate control versus rhythm control. Crit Care. 2004;8:271–9. doi: 10.1186/cc2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation: the Framingham study. Stroke. 1996;27:1760–4. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- Lip GY, Edwards SJ. Stroke prevention with aspirin, warfarin and ximelgatran in patients with atrial fibrillation: a systematic review and meta-analysis. Thromb Res. 2006;118:321–33. doi: 10.1016/j.thromres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lip GY, Boos CJ. Antithrombotic therapy for atrial fibrillation. Heart. 2005;92:155–61. doi: 10.1136/hrt.2005.066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip GY, Tean KN, Dunn FG. Treatment of atrial fibrillation in a district general hospital. Br Heart J. 1994;71:92–5. doi: 10.1136/hrt.71.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip GY. Does atrial fibrillation confer a hypercoagulable state? Lancet. 1995;346:1313–14. doi: 10.1016/s0140-6736(95)92339-x. [DOI] [PubMed] [Google Scholar]
- Lip GY, Lane D, Van Walraven C, et al. Additive role of plasma von Willebrand factor levels to clinical factors for risk stratification of patients with atrial fibrillation. Stroke. 2006;37:2294–300. doi: 10.1161/01.STR.0000236840.00467.84. [DOI] [PubMed] [Google Scholar]
- Maragno I, Santostasi G, Gaion RM, et al. Low- and medium-dose diltiazem in chronic atrial fibrillation: comparison with digoxin and correlation with drug plasma levels. Am Heart J. 1998;116:385–92. doi: 10.1016/0002-8703(88)90610-2. [DOI] [PubMed] [Google Scholar]
- Michael JA, Stiell IG, Agarwal S, et al. Cardioversion of paroxysmal atrial fibrillation in the emergency department. Ann Emerge Med. 1999;33:379–87. doi: 10.1016/s0196-0644(99)70300-8. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Ito T, Suwa M, et al. Role of transesophageal echocardiography in the prediction of thromboembolism in patients with chronic nonvalvular atrial fibrillation. Jpn Circ J. 2001;65:874–8. doi: 10.1253/jcj.65.874. [DOI] [PubMed] [Google Scholar]
- Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK generalpractice. Br J Gen Pract. 2002;52:373–80. [PMC free article] [PubMed] [Google Scholar]
- Murray ET, Fitzmaurice DA, McCahon D. Point of care testing for INR monitoring: where are we now? Br J Haematol. 2004;127:373–8. doi: 10.1111/j.1365-2141.2004.05154.x. [DOI] [PubMed] [Google Scholar]
- Okcun B, Yigit Z, Arat A. Comparison of rate and rhythm control in patients with atrial fibrillation and nonischemic heart failure. Jpn Heart J. 2004;45:591–601. doi: 10.1536/jhj.45.591. [DOI] [PubMed] [Google Scholar]
- Okcun B, Yigit Z, Kucukoglu MS, et al. Predictors for maintenance of sinus rhythm after cardioversion in patients with nonvalvular atrial fibrillation. Echocardiography. 2002;19:351–7. doi: 10.1046/j.1540-8175.2002.00351.x. [DOI] [PubMed] [Google Scholar]
- Opolski G, Torbicki A, Kosior D, et al. Rhythm control versus rate control in patients with persistent atrial fibrillation. Results of the HOT CAFE Polish Study. Kardiologia Polska. 2003;59:1–16. [PubMed] [Google Scholar]
- Page RL, Wilkinson WE, Clair WK, et al. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–7. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- Paraskevaidis IA, Dodouras T, Tsiapras D. Prediction of successful cardioversion and maintenance of sinus rhythm in patients with lone atrial fibrillation. Chest. 2005;127:488–94. doi: 10.1378/chest.127.2.488. [DOI] [PubMed] [Google Scholar]
- Reiffel JA, Schwarzberg R, Murry M. Comparison of autotriggered memory loop recorders versus standard loop recorders versus 24-hour holter monitors for arrhythmia detection. Am J Cardiol. 2005;95:1055–9. doi: 10.1016/j.amjcard.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Ricci R, Quesada A, Pignalberi C, et al. Dual defibrillator improves quality of life and decreases hospitalizations in patients with drug refractory atrial fibrillation. J Interv Card Electrophysiol. 2004;10:85–92. doi: 10.1023/B:JICE.0000011490.32755.40. [DOI] [PubMed] [Google Scholar]
- Roijer A, Meurling CJ, Eskilsson J, et al. Left atrial appendage outflow velocity index is superior to conventional criteria for prediction of maintenance of sinus rhythm after cardioversion. Scand Cardiovas J. 2001;35:119–24. doi: 10.1080/140174301750164817. [DOI] [PubMed] [Google Scholar]
- Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of atrial fibrillation investigators. N Engl J Med. 2000;342:913–20. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- Seitelberger R, Hannes W, Gleichauf M, et al. Effects of diltiazem on perioperative ischemia, arrhythmias, and myocardial function in patients undergoing elective coronary bypass grafting. J Thorac Cardiovasc Surg. 1994;107:811–21. [PubMed] [Google Scholar]
- Shinokawa N, Hirai T, Takashima S, et al. A transesophageal echocardiographic study on risk factors for stroke in elderly patients with atrial fibrillation: a comparison with younger patients. Chest. 2001;120:840–6. doi: 10.1378/chest.120.3.840. [DOI] [PubMed] [Google Scholar]
- Singer DE, Albers GW, Dalen JE, et al. Antithrombotic Therapy in atrial fibrillation: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:429S–56S. doi: 10.1378/chest.126.3_suppl.429S. [DOI] [PubMed] [Google Scholar]
- Stroke Prevention in atrial fibrillation investigators. The Stroke Prevention in atrial fibrillation (SPAF) study: final results. Circulation. 1991;84:527–539. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- Steeds RP, Birchall AS, Smith M, et al. An open label, randomised, crossover study comparing sotalol and atenolol in the treatment of symptomatic paroxysmal atrial fibrillation. Heart. 1999;82:170–5. doi: 10.1136/hrt.82.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasberg B, Arditti A, Sclarovsky S. Efficacy of intravenous amiodarone in the management of paroxysmal or new atrial fibrillation with fast ventricular response. Int J Cardiol. 1985;7:47–55. doi: 10.1016/0167-5273(85)90172-x. [DOI] [PubMed] [Google Scholar]
- Stewart S, Hart CL, Hole DJ, et al. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–21. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt D, Hobbs R, Fitzmaurice D, et al. A randomised controlled trial and cost effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in the over 65s: (SAFE) BMC Cardiovasc Disord. 2004;4:12. doi: 10.1186/1471-2261-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow M, Rodgers H, Kenny RA, et al. Identification of patients with atrial fibrillation in generalpractice: a study of screening methods. BMJ. 1998;317:327–8. doi: 10.1136/bmj.317.7154.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueng KC, Tsai TP, Tsai CF, et al. Acute and long-term effects of atrioventricularjunction ablation and VVIR pacemaker in symptomatic patients with chronic lone atrial fibrillation and normal ventricular response. J Cardiovasc Electrophysiol. 2001;12:303–9. doi: 10.1046/j.1540-8167.2001.00303.x. [DOI] [PubMed] [Google Scholar]
- Valencia MJ, Climent PV, Marin O, et al. The efficacy of scheduled cardioversion in atrial fibrillation: Comparison of two schemes of treatment: Electrical versus pharmacological cardioversion. Revista Espanola de Cardiologia. 2002;55:113–20. doi: 10.1016/s0300-8932(02)76570-6. [DOI] [PubMed] [Google Scholar]
- van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–8. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003;163:936–43. doi: 10.1001/archinte.163.8.936. [DOI] [PubMed] [Google Scholar]
- Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- Vecht RJ, Nicolaides EP, Ikweuke JK. Incidence and prevention of supraventricular tachyarrhythmias after coronary bypass surgery. Int J Cardiol. 1986;13:125–34. doi: 10.1016/0167-5273(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Wang HE, O’connor RE, Megargel RE, et al. The use of diltiazemfor treating rapid atrial fibrillation in the out-of-hospital setting. Ann Emerge Med. 2001;37:38–45. doi: 10.1067/mem.2001.111518. [DOI] [PubMed] [Google Scholar]
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: A randomized trial. JAMA. 2005;293:2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- Wolf PA, Benjamin EJ, Belanger AJ, et al. Secular trends in the prevalence of atrial fibrillation: the Framingham study. Am Heart J. 1996;131:790–5. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- Wolf PA, Belanger AJ, Kannel WB, et al. Probabilty of stroke: A risk profile from Framingham study. Stroke. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- Wolf PA, Kannel WB, McGee DL, et al. Duration of atrial fibrillation and imminence of stroke: the Framingham study. Stroke. 1983;14:664–7. doi: 10.1161/01.str.14.5.664. [DOI] [PubMed] [Google Scholar]
- Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- Zarifis J, Beevers G, Lip GY. Acute admissions with atrial fibrillation in a British multiracial hospital population. Br J Clin Pract. 1997;51:91–6. [PubMed] [Google Scholar]