Abstract
Endothelial dysfunction is the initial pathophysiological step in a progression of vascular damage that leads to overt cardiovascular and chronic kidney disease. Angiotensin II, the primary agent of the renin–angiotensin system (RAS), has a central role in endothelial dysfunction. Therefore, RAS blockade with an angiotensin receptor blocker (ARB) and/or angiotensin-converting enzyme (ACE) inhibitor provides a rational approach to reverse endothelial dysfunction, reduce microalbuminuria, and, thus, improves cardiovascular and renal prognosis. ARBs and ACE inhibitors act at different points in the RAS pathway and recent evidence suggests that there are differences regarding their effects on endothelial dysfunction. In addition to blood pressure lowering, studies have shown that ARBs reduce target-organ damage, including improvements in endothelial dysfunction, arterial stiffness, the progression of renal dysfunction in patients with type 2 diabetes, proteinuria, and left ventricular hypertrophy. The ONgoing Telmisartan Alone in combination with Ramipril Global Endpoint Trial (ONTARGET) Programme is expected to provide the ultimate evidence of whether improved endothelial function translates into reduced cardiovascular and renal events in high-risk patients, and to assess possible differential outcomes with telmisartan, the ACE inhibitor ramipril, or a combination of both (dual RAS blockade). Completion of ONTARGET is expected in 2008.
Keywords: angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, endothelial dysfunction, ONTARGET, renin–angiotensin system, telmisartan
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality worldwide, resulting in an estimated 17 million deaths each year, particularly from myocardial infarction and stroke (Ezzati et al 2003). The number of patients with renal disease who require dialysis is also currently high and is expected to double to 2 million by 2010 (Perico et al 2005). Endothelial dysfunction is the initial pathophysiologic step in the cardiovascular–renal continuum, which is a progression of vascular damage that is triggered by risk factors such as hypertension and leads to both kidney failure and overt cardiovascular disease (Brunner et al 2005). Prospective studies have demonstrated that, in the peripheral and coronary circulation, endothelial dysfunction is highly predictive of future cardiovascular morbidity (Schachinger et al 2000; Heitzer et al 2001; Perticone et al 2001). Therefore, it has been hypothesized that treatment to reverse endothelial dysfunction may lead to a reduction in cardiovascular and renal events.
Blockade of the renin–angiotensin system (RAS) with angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme (ACE) inhibitors has been shown to provide improved endothelial function in patients with type 2 diabetes and nephropathy (Karalliedde and Viberti 2006). These agents also reduce microalbuminuria, a marker of increased permeability due to endothelial dysfunction. Recent evidence suggests that there is no clear-cut difference between ARB and ACE inhibitor agents in their effects on endothelial dysfunction and low-grade albuminuria (Schmieder et al 2005). Significant prospective data are essential to investigate whether improved endothelial function translates into reduced cardiovascular and renal events in high-risk patients, and to assess possible differential outcomes with ARBs, ACE inhibitors, or a combination of both (dual RAS blockade). The ONgoing Telmisartan Alone in combination with Ramipril Global Endpoint Trial (ONTARGET) Programme is expected to provide ultimate evidence of the risk reduction that can be achieved with these treatments.
This review will discuss the pathophysiology of endothelial dysfunction, its role in the cardiovascular–renal continuum, and the potential for improvements with RAS blockers. The expectations and possible clinical implications of the wealth of data due from ONTARGET will be considered.
Pathophysiology of endothelial dysfunction and its role in the cardiovascular–renal continuum
The endothelium is a major regulator of vascular homeostasis, and its functional integrity is essential for the maintenance of blood flow and antithrombotic activity (Brunner et al 2005; Deanfield et al 2005). Endothelial cells, a monolayer between circulating blood and vascular smooth muscle, produce signaling molecules that modulate tone, monocyte adhesion, and platelet–neutrophil aggregation in the vascular smooth muscle cells. The balance between the actions of these signaling molecules is of critical importance. One important signaling molecule released by vascular endothelial cells is nitric oxide (NO), formed from L-arginine in the presence of NO synthase (Palmer et al 1988). NO is both a potent vasodilator and an inhibitor of platelet adhesion and aggregation, and exerts antifibrotic effects.
Endothelial dysfunction can be defined as an imbalance between vasodilating and vasoconstricting substances produced by (or acting on) endothelial cells (Brunner et al 2005). In particular, the dysfunctional endothelium is characterized by impaired NO synthesis or increased NO degradation. Potentially harmful effects include vasoconstriction, platelet aggregation, white blood cell adhesion, and proliferation of smooth muscle cells (Anggard 1994; Klahr 2001). The result is a reduction of regional or whole-organ perfusion, leading to target-organ damage and an increased incidence of ischemic events (Schachinger et al 2000; Heitzer et al 2001; Perticone et al 2001). Thus, endothelial dysfunction is an early marker of vascular disease, and leads to both cardiovascular and renal morbidity (Panza et al 1990; McAllister et al 1999; Raptis and Viberti 2001; Taylor 2001; Annuk et al 2003).
Endothelial dysfunction can be assessed by biochemical markers, molecular genetic tests and invasive or non-invasive techniques, although the optimal methodology is still unclear (Deanfield et al 2005; Lee et al 2006). Current biochemical markers include, for example, adhesion molecules ICAM-2, VCAM-1, E-selectin, P-selectin and sCD40L, cytokines interleukin 6, interleukin 18, hs-CRP and ET-1 (Deanfield et al 2005). Genetic markers include von Willebrand factor (vWF), angiotensin I converting enzyme (ACE), preproendothelin (ET)-1, endothelin I converting enzyme (ECE-1), endothelin B receptor, eNOS, NF-κB, ICAM-1, VCAM-1, and E-selectin (Deanfield et al 2005).
Non-invasive techniques include flow-mediated vasodilation (FMD) in the brachial or radial artery (Celermajer et al 1992). Invasive procedures are forearm blood flow (FBF) by cannulation of the brachial artery (Panza et al 1990) or for coronary circulation, an infusion of intracoronary acetylcholine (ACh) (Hasdai and Lerman 1999).
Recent evidence confirms that there is a close link between cardiovascular and renal changes, and that they are triggered by similar risk factors, such as hypertension, hyperglycemia, dyslipidemia, and obesity (Figure 1) (Hillege et al 2002; Anavekar et al 2004; Stam et al 2006). Mechanical and chemical damage resulting from these interrelated risk factors promote a general progression of vascular damage that begins with endothelial dysfunction and atherosclerosis, which leads to proteinuria, left ventricular hypertrophy, and transient ischemic attacks in the brain, then progresses to chronic kidney disease, overt cardiovascular disease, and stroke (Brunner et al 2005). This is known as the cardiovascular–renal continuum (Figure 1). It is now understood that cardiovascular prognosis is clearly reflected by measures of renal dysfunction, such as increased albuminuria and a decline in the glomerular filtration rate (GFR), with even modest changes markedly increasing the risk for cardiovascular disease (Gerstein et al 2001; Hillege et al 2002; Anavekar et al 2004; Arnlov et al 2005). Thus, renal dysfunction is a risk factor for cardiovascular death. In fact, most patients with renal impairment die from cardiovascular causes, rather than from end-stage renal disease (Gerstein et al 2001; Hillege et al 2002; Anavekar et al 2004).
Figure 1.
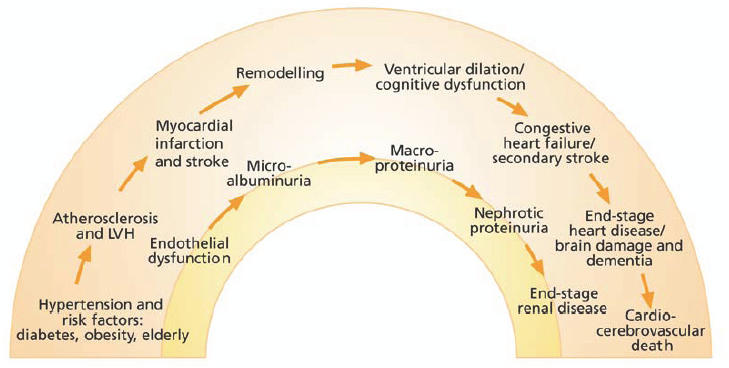
The cardiovascular–renal continuum.
Microalbuminuria occurs early in the cardiovascular–renal continuum, closely following endothelial dysfunction (Figure 1) (de Jong and de Zeeuw 2005). These small losses of protein in the urine develop when the loss of endothelial integrity in the kidney results in intraglomerular hypertension and inflammation (Klahr and Morrissey 2000). Microalbuminuria is frequently detected in individuals with atherosclerosis and has been found to be a risk factor for type 2 diabetes and cardiovascular disease (Gerstein et al 2001; Hillege et al 2002; Anavekar et al 2004; Arnlov et al 2005; Brantsma et al 2005). Thus, microalbuminuria is an early marker of widespread vascular, renal, and other target-organ damage.
The physiological actions of angiotensin II in cardiovascular, renal, neuronal, endocrine and hepatic systems are nearly all mediated by the angiotensin II receptor. Actions include, for example, the regulation of arterial blood pressure, electrolyte and water balance, and renal function (de Gasparo et al 2000). Angiotensin II is a potent arteriole vasoconstrictor which can increase total peripheral resistance thereby increasing arterial pressure. As the primary agent of the RAS, angiotensin II also has a central role in endothelial dysfunction (Figure 2) (Dzau 2001). In addition to increasing blood pressure, angiotensin II acts via the angiotensin II type 1 receptor (AT1) receptor to increase oxidative stress, causing NO breakdown (de Gasparo 2002; Zhou et al 2004). Elevated levels of Angiotensin II in the endothelium stimulates nicotinamide adenine dinucleotide phosphate (NADPH) and nicotinamide adenine dinucleotide (NADH) oxidase to generate reactive oxygen species (ROS) responsible for NO breakdown (Griendling et al 1994). This leads to endothelial dysfunction, cell growth, inflammation by the activation of nuclear factor-κB (NF-κB), monocyte chemoattractant protein-1 (MCP-1), vascular cell adhesion molecule (VCAM), and the release of the cytokines interleu-kin-6 (IL-6) and tumor necrosis factor-α (TNF-α) (Marui et al 1993; Hernandez-Presa et al 1997; Han et al 1999). VCAM and cytokine action increases the adhesiveness of the endothelium and subsequently the binding of inflammatory cells to the endothelial surface leading to vascular inflammation and thrombosis (Figure 3) (Kranzhöfer et al 1999; Schmieder 2005). Thus, angiotensin II is involved in every step of the cardiovascular–renal continuum, promoting a wide variety of deleterious effects on target organs.
Figure 2.
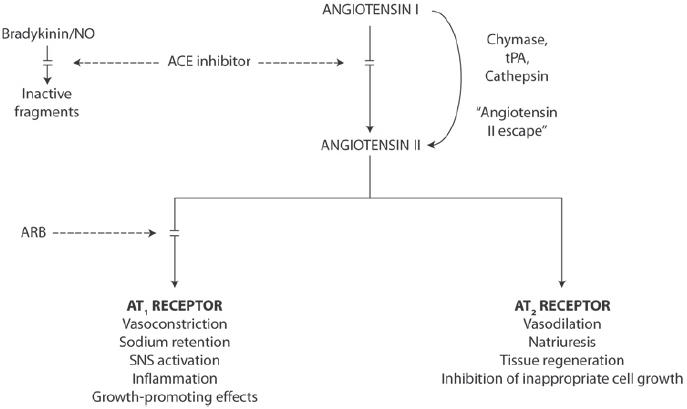
Blockade of the RAS pathway with ACE inhibitors and ARBs.
Figure 3.
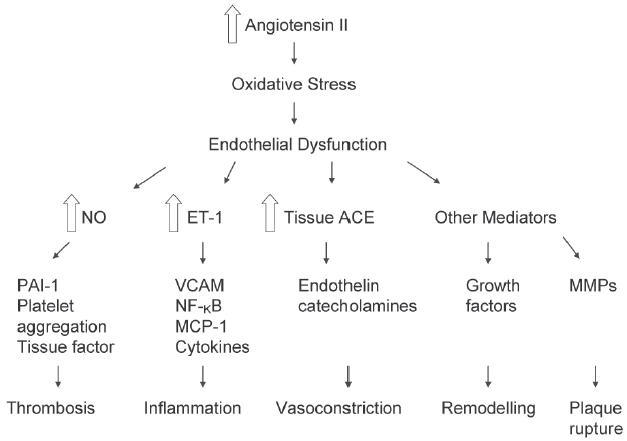
Schematic representation of the multiple effects of increased tissue production of angiotensin II. Reprinted from Schmieder RE, 2005. Mechanisms for the clinical benefits of Angiotensin II receptor blockers. Am J Hypertens, 18:720–30. Copyright © 2005, with permission from American Journal of Hypertension, Ltd.
Abbreviations: ET-1, endothelin-1; MCP-1, monocyte chemoattractant protein-I; MMP, matrix metalloproteinase; NF-kB, nuclear factor-kB; NO, nitric oxide; PAI-1, plasminogen activator type 1;VCAM, vascular cell adhesion molecule;ACE, angiotensin-converting enzyme.
RAS blockade to reverse endothelial dysfunction
In addition to blood pressure-lowering effects, RAS blockade with an ARB and/or ACE inhibitor provides a rational approach to reversing endothelial dysfunction by reducing the harmful effects of angiotensin II (Karalliedde and Viberti 2006). Such treatments may provide cardiovascular and renal protection beyond that of reducing a single cardiovascular risk factor. Indeed, current clinical guidelines recommend ARBs as first-line treatment in patients with type 2 diabetes and nephropathy (American Disease Association 2004).
ARBs and ACE inhibitors act at different points in the RAS pathway (Figure 2). ACE inhibitors prevent the generation of angiotensin II, which subsequently can activate both AT1 and angiotensin II type 2 (AT2) receptors (Burnier 2001). ACE inhibitors also inhibit the breakdown of bradykinin by kinase II, thereby increasing bradykinin levels. This may cause vasodilation, thereby decreasing blood pressure, and may improve endothelial function (Chen et al 2003). However, bradykinin and the structurally related substance P can also potentially cause cough, a side effect that many patients find unacceptable (Chen et al 2003). In addition, ACE inhibitors can allow continued activation of AT1 by angiotensin II via alternative pathways, a phenomenon known as “angiotensin II escape” (Roig et al 2000). During long-term therapy, angiotensin II concentrations can revert to pretreatment levels, thus attenuating the protective effect of ACE inhibition. Angiotensin II escape may be a particular problem for the local kidney RAS, in which up to 40% of angiotensin II formation is via non-ACE pathways (Hollenberg et al 1998). This may explain why ACE inhibitors do not reduce levels of angiotensin II in the renal interstitial fluid (Nishiyama et al 2002). ACE inhibitors and vascular diseases has recently been reviewed by Napoli and Loscalzo (2005).
In contrast to ACE inhibitors, ARBs are highly selective for the AT1 receptor, which is believed to be responsible for the pathophysiologic effects of angiotensin II (Burnier et al 2001). The AT2 receptor generally has effects opposed to those of AT1 and is abundantly expressed in endothelial cells (Ardaillou 1999) (Figure 2). ARBs do not increase bradykinin levels and are, therefore, not associated with cough. Furthermore, ARBs maintain selective blockade of AT1 and are, thus, not associated with angiotensin II escape.
Telmisartan is a potent selective once-daily ARB that provides a sustained blood pressure-lowering effect over 24 hours (Battershill and Scott 2006). As discussed below, studies have shown that telmisartan also reduces target-organ damage, including improvements in endothelial dysfunction (Svolis et al 2002; Schmieder et al 2005; Symeonides et al 2006), arterial stiffness (Asmar et al 2002; Uchida et al 2004), the progression of renal dysfunction in patients with type 2 diabetes (Barnett et al 2004), proteinuria (Redón et al 2005; Ryšavá et al 2005; Sengul et al 2006), and left ventricular hypertrophy (Galzerano et al 2004; Ivanova et al 2005). In clinical trials, other ARBs have also demonstrated effective renoprotection in patients with type 2 diabetes and renal disease (Brenner et al 2001; Lewis et al 2001; Parving et al 2001; Viberti and Wheeldon 2002; Klingbeil et al 2003). These trials showed that ARBs can reverse microalbuminuria, suppress the progression of albuminuria and loss of renal function, and prevent progression to end-stage renal disease.
RAS blockade with ACE inhibitors may demonstrate favorable effects on the endothelium. In short-term clinical studies, ACE inhibitors reduced microalbuminuria and, in the longer term, they are superior to non-RAS-targeting antihypertensive agents in maintaining normal renal function (ACE inhibitors in diabetic nephropathy trialist group 2001). In one study, hypertensive patients receiving ACE inhibitors displayed improved maximal forearm blood flow response to hyperemia that was significantly greater (p < 0.05) than the response in patients treated with calcium channel blockers, β-blockers, or diuretics (Higashi et al 2000).
Improved endothelial function with telmisartan
The Telmisartan versus Ramipril in renal ENdothelial DYsfunction (TRENDY) study showed that both telmisartan and ramipril improved endothelial function (increased NO activity) in patients with type 2 diabetes and hypertension (Schmieder et al 2005). TRENDY was the first head-to-head comparison of the effect of an ARB versus an ACE inhibitor on endothelial function. In a prospective, randomized, double-blind, forced-titration design, 96 patients with type 2 diabetes, normoalbuminuria or microalbuminuria, thus early-stage nephropathy, were randomized to 9 weeks’ treatment with telmisartan 40–80 mg or ramipril 5–10 mg, with add-on therapy (hydrochlorothiazide, metoprolol, or atenolol) to achieve blood pressure control. The primary end point was renal plasma flow in response to N-monomethyl-L-arginine (L-NMMA) infusion. Telmisartan significantly increased the response of renal plasma flow to L-NMMA infusion (p < 0.001 vs baseline) taken as an indicator of NO activity (Figure 4) (Schmieder et al 2005). At rest (ie, without the stimulus), telmisartan also significantly improved (p < 0.05) renal plasma flow (by 27.3 mL/min) and reduced renal vascular resistance (by 7%). In addition, although levels of albuminuria were low at baseline, telmisartan significantly improved (p < 0.05) albuminuria (reduced from 9.0 to 7.2 mg/24 hours). Ramipril also increased the response of renal plasma flow to L-NMMA infusion (p < 0.02 versus baseline) but, in contrast to telmisartan, the ACE inhibitor ramipril did not improve renal plasma flow or reduced renal vascular resistance significantly.
Figure 4.
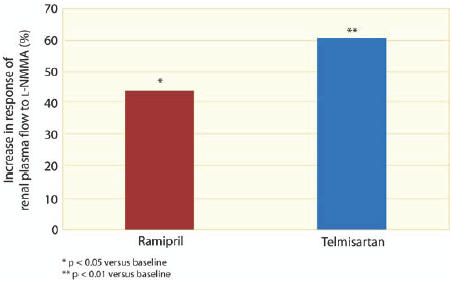
Improved endothelial function with telmisartan compared with ramipril in the TRENDY study. From Schmieder et al. 2005. Effects of telmisartan versus ramipril on endothelium function of the renal vasculature in type 2 diabetes. J Hypertens, 23:S147.
In a 3-month, double blind, cross-over study, endothelial function was improved by telmisartan 40 mg but to a lower extent by ramipril 2.5 mg in 40 non-hypertensive patients with controlled type 2 diabetes without coronary artery disease, left ventricular dysfunction, or microalbuminuria (Symeonides et al 2006). Brachial artery flow-mediated dilation was improved 96% by telmisartan compared with only 36% by ramipril. Interestingly, the combination of telmisartan and ramipril increased dilation by 111%. Tissue plasminogen activator and von Willebrand factor, measures of endothelial health and fibrinolytic status, were improved in this study, with the combination of the ARB and the ACE inhibitor again producing the greatest benefit. Telmisartan has also been shown to improve endothelial function in treatment-naïve hypertensive patients with chronic heart failure (Svolis et al 2002).
Decline in GFR is an important sign of renal damage. The Diabetics Exposed to Telmisartan And enalaprIL (DETAIL) study showed that both telmisartan and the ACE inhibitor enalapril similarly reduced the decline in GFR over 5 years in patients with hypertension and early type 2 diabetic nephropathy (Barnett et al 2004). In a prospective, randomized, double-blind, forced-titration design, 250 patients were randomized to telmisartan 40–80 mg or enalapril 10–20 mg, each with add-on therapy to control blood pressure. After the first year, both treatments reduced GFR decline with a consistent, year-on-year effect. By year 3, the annual decline in GFR stabilized to approximately 2 mL/min/1.73 m2. This is substantially lower than the annual decline of 10–12 mL/min/1.73 m2 that is observed in untreated diabetic patients with proteinuria (Barnett 2005). There were no significant differences in blood pressure lowering between the groups.
Clinical studies have consistently shown that telmisartan reduces proteinuria (Redón et al 2005; Ryšavá et al 2005; Sengul et al 2006). A 12-month study of 206 patients assessed the interactions between RAS gene polymorphisms and telmisartan therapy in patients with mild-to-moderate hypertension (Redón et al 2005). Microalbuminuria was present in 28% of patients, and mean urinary albumin excretion was 32.7 mg/day. Telmisartan treatment significantly reduced albuminuria by 69% by month 12. The effects of 3 months’ treatment with telmisartan 40–80 mg/day were examined in 92 patients with hypertension (60 with diabetes), serum creatinine <350 µmol/L, and proteinuria >1 g/day (Ryšavá et al 2005). Telmisartan reduced proteinuria by 21%; corresponding to a decrease of 0.76 g/24 hours from 3.62 g/24 hours.
Telmisartan, when given in addition to the ACE inhibitor lisinopril, provided further reductions in the albumin excretion compared with ACE inhibitor monotherapy (Sengul et al 2006). Patients with type 2 diabetes and micro-albuminuria (n = 219) had been receiving ACE inhibitors for at least 6 months previously, then received either telmisartan 80 mg or lisinopril 20 mg monotherapy for a further 6 months. Half of the patients then received combination therapy with telmisartan 80 mg/lisinopril 20 mg. At 6 months, reductions in albuminuria were 31% with telmisartan and 37% with lisinopril. After 12 months, telmisartan had reduced albuminuria by an additional 30%. Similar results were seen in the group in which lisinopril was added to telmisartan. Both treatments produced comparable reductions in blood pressure, supporting the notion that the further reduction of proteinuria was blood pressure independent.
Arterial stiffness is an early sign of atherosclerosis and an important risk factor for cardiovascular disease (Safar et al 2003). In one study of arterial stiffness, 24 patients with hypertension received telmisartan 40 mg for 3 months (Uchida et al 2004). Brachial-ankle pulse wave velocity was reduced by 12% from 18.92 m/s at baseline to 16.72 m/s (p < 0.01). The improvement in pulse wave velocity was greater than predicted on the basis of the reduction in blood pressure observed. In another study, telmisartan significantly reduced arterial stiffness, measured by carotid-femoral pulse wave velocity, compared with placebo in 28 patients with type 2 diabetes and hypertension (Asmar et al 2002).
The ONTARGET trial
The ONTARGET Trial Programme is comparing telmisartan, ramipril, and the combination of both antihypertensive agents in the prevention of cardiovascular morbidity and mortality in patients at high risk (Teo et al 2004). Telmisartan was the ARB selected for The ONTARGET Trial Programme because it provides sustained antihypertensive activity effect over the 24-hour period between doses (Battershill and Scott 2006). The comparator, the ACE inhibitor ramipril, was selected because in the Heart Outcomes Protection Evaluation (HOPE) trial, it was proven to reduce the incidence of cardiovascular events in a similar patient population (55 years of age or older, with evidence of vascular disease or diabetes plus one other cardiovascular risk factor and who were not known to have a low ejection fraction or heart failure) (Yusuf et al 2000). Patients enrolled in ONTARGET have vascular disease (coronary artery disease, peripheral arterial occlusive disease, or stroke), or diabetes with end-organ damage (Teo et al 2004). The primary outcome is a composite end point of cardiovascular death, stroke, acute myocardial infarction, and hospitalization for chronic heart failure. A variety of renal end points have also been included. Telmisartan Randomized AssessmeNt Study in ACE-I-iNtolerant subjects with cardiovascular Disease (TRANSCEND) is a parallel study within The ONTARGET Trial Programme that is comparing the cardiovascular protective effect of telmisartan with placebo in patients intolerant to ACE inhibitors (Teo et al 2004).
ONTARGET has several important features. The trial has recruited 25620 patients from 800 centers in 40 countries throughout Europe, North America, Africa, Australasia, and Asia (Teo et al 2004), and findings will be based on more than 100 000 patient-years of data. This makes ONTARGET the largest ever randomized clinical trial to test the effects of an ARB versus an ACE inhibitor and of combination treatment of an ARB with an ACE inhibitor on cardiovascular risk reduction. Results from previous trials (see above) suggested that treatment with telmisartan or ramipril reduces the cardiovascular outcome to similar extent, with the combination to be superior. Thus, ONTARGET should provide the ultimate evidence regarding the cardiovascular benefit of different strategies to block the RAS in high-risk patients. Furthermore, TRANSCEND, which has enrolled 5926 patients, is the largest trial to evaluate the cardiovascular protective effects of telmisartan in patients with ACE inhibitor intolerance.
Baseline characteristics are now available for all patients recruited into the ONTARGET study (Safar et al 2003). Importantly, patients have controlled hypertension (mean blood pressure at randomization was 134/77 mm Hg) and cardiovascular risk factors at baseline, and are thus representative of those seen in everyday clinical practice. Compared with the HOPE study, patients in ONTARGET have greater ethnic diversity, are slightly older, are more likely to have cerebrovascular disease and hypertension, and have increased statin use (60.7% and 28.9%, respectively). These differences reflect the changing approach to cardiovascular disease prevention.
The ONTARGET Trial Programme is the first long-term prospective trial to investigate the efficacy of an ARB/ACE inhibitor combination (dual RAS blockade). Due to the different mechanisms of action of telmisartan and ramipril, there is the potential for differential cardiovascular outcomes, independent of their effects on blood pressure. The combination may be able to fully suppress the deleterious effects of angiotensin II and, at the same time, provide additive improvements in renal bradykinin and cyclic guanosine monophosphate, two molecules that improve endothelial function (Siragy et al 2001). Therefore, the combination may offer superior cardiovascular protection compared with either monotherapy as a result of their complementary effects.
Predefined substudies should provide greater understanding of differences in the cardiovascular risk reduction achieved by RAS blockade with telmisartan, ramipril, and the combination (Safar et al 2003). The incidence and time course of erectile dysfunction is being evaluated during drug treatment in 1500 patients from both ONTARGET and TRANSCEND. Previous studies have shown that erectile dysfunction is caused by premature atherosclerosis and such antihypertensive drugs as β-blockers and diuretics (Brunner et al 2005). Therefore, the choice of antihypertensive medication appears to be critical. Furthermore, drugs known to improve or delay endothelial dysfunction may have a positive impact on erectile dysfunction. The substudy of ONTARGET/TRANSCEND will assess whether erectile dysfunction is related to cardiovascular risk factors and to the use of cardioprotective drugs. The primary end point is the onset of change of erectile dysfunction, assessed using the modified “Cologne Male Survey” questionnaire, in patients receiving placebo, telmisartan, ramipril, or both active agents.
In another substudy, 284 patients enrolled in TRANSCEND will undergo evaluation of arterial stiffness. As mentioned above, telmisartan has proven benefits on arterial stiffness in patients with type 2 diabetes and hypertension (Asmar et al 2002; Uchida et al 2004). The arterial stiffness substudy of TRANSCEND will answer the question of whether changes in arterial stiffness correlate with clinical outcome. The primary end point is change in aortic stiffness determined by carotid-femoral pulse wave velocity. As a secondary end point, the predictive value of early, short-term changes in arterial stiffness on the long-term clinical outcome of patients is being evaluated.
Conclusions
The ONTARGET Trial Programme is due to be completed in 2008, and results are expected to provide clinicians with a wealth of data to improve evidence-based treatment of patients at high risk of cardiovascular events. By blocking the RAS, ACE inhibitor and ARB treatment may, in addition to blood-pressure lowering, have positive effects on endothelial function, the initial step in vascular damage. This should provide greater benefits than treating individual cardiovascular risk factors. With over 100000 patient-years of data, the pro-gram is expected to provide the ultimate evidence for whether improved endothelial function does indeed translate into reduced cardiovascular and renal events in high-risk patients. In addition, the trial will establish whether treatment with the ARB telmisartan, the ACE inhibitor ramipril, and dual RAS blockade with both agents is associated with differential cardiovascular/renal outcomes. Due to its high selectivity for the AT1 receptor, telmisartan may prove to exert excellent cardiovascular protection. The complementary mechanisms of action of telmisartan and ramipril may lead to superior cardiovascular protection with the combination compared with either monotherapy. Thus, the ONTARGET data are expected to have a substantial impact on clinical practice by allowing physicians to choose the optimal treatment regimen to protect their patients at risk of future cardiovascular or renal morbidity and mortality.
References
- ACE inhibitors in diabetic nephropathy trialist group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med. 2001;134:370–9. doi: 10.7326/0003-4819-134-5-200103060-00009. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Nephropathy in diabetes. Diabetes Care. 2004;27:79S–83S. [Google Scholar]
- Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–95. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- Anggard E. Nitric oxide: mediator, murderer, and medicine. Lancet. 1994;343:1199–206. doi: 10.1016/s0140-6736(94)92405-8. [DOI] [PubMed] [Google Scholar]
- Annuk M, Zilmer M, Fellström B. Endothelium-dependent vasodilation and oxidative stress in chronic renal failure: Impact on cardiovascular disease. Kidney Int. 2003;63(Suppl 84):S50–3. doi: 10.1046/j.1523-1755.63.s84.2.x. [DOI] [PubMed] [Google Scholar]
- Ardaillou R. Angiotensin II receptors. J Am Soc Nephrol. 1999;10(Suppl 11):S30–9. [PubMed] [Google Scholar]
- Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–75. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- Asmar R, Gosse P, Topouchian J, et al. Effects of telmisartan on arterial stiffness in Type 2 diabetes patients with essential hypertension. J Renin Angiotensin Aldosterone Syst. 2002;3:176–80. doi: 10.3317/jraas.2002.038. [DOI] [PubMed] [Google Scholar]
- Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–61. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- Barnett AH. Preventing renal complications in diabetic patients: the Diabetics Exposed to Telmisartan And enalaprIL (DETAIL) study. Acta Diabetol. 2005;42(Suppl 1):S42–9. doi: 10.1007/s00592-005-0180-4. [DOI] [PubMed] [Google Scholar]
- Battershill AJ, Scott LJ. Telmisartan: a review of its use in the management of hypertension. Drugs. 2006;66:51–83. doi: 10.2165/00003495-200666010-00004. [DOI] [PubMed] [Google Scholar]
- Brantsma AH, Bakker SJ, Hillege HL, et al. Urinary albumin excretion and its relation with C-reactive protein and the metabolic syndrome in the prediction of type 2 diabetes. Diabetes Care. 2005;28:2525–30. doi: 10.2337/diacare.28.10.2525. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–46. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Burnier M. Angiotensin II type 1 receptor blockers. Circulation. 2001;103:904–12. doi: 10.1161/01.cir.103.6.904. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–15. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Chen R, Iwai M, Wu L, et al. Important role of nitric oxide in the effect of angiotensin-converting enzyme inhibitor imidapril on vascular injury. Hypertension. 2003;42:542–7. doi: 10.1161/01.HYP.0000092440.52239.39. [DOI] [PubMed] [Google Scholar]
- Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, et al. International Union of Phamacology. XXIII. The Angiotensin II receptors. Pharmacol Rev. 2000;52:415–72. [PubMed] [Google Scholar]
- de Gasparo M. Angiotensin II and nitric oxide interaction. Heart Fail Rev. 2002;7:347–58. doi: 10.1023/a:1020714518246. [DOI] [PubMed] [Google Scholar]
- de Jong PE, de Zeeuw D. Renoprotective therapy: is it blood pressure or albuminuria that matters? Lancet. 2005;365:913–4. doi: 10.1016/S0140-6736(05)71055-2. [DOI] [PubMed] [Google Scholar]
- Dzau VJ. Tissue angiotensin and the pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–52. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Hoorn SV, Rodgers A, et al. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet. 2003;362:271–80. doi: 10.1016/s0140-6736(03)13968-2. [DOI] [PubMed] [Google Scholar]
- Galzerano D, Tammaro P, Cerciello A, et al. Freehand three-dimensional echocardiographic evaluation of the effect of telmisartan compared with hydrochlorothiazide on left ventricular mass in hypertensive patients with mild-to-moderate hypertension: a multicentre study. J Hum Hypertens. 2004;18:53–9. doi: 10.1038/sj.jhh.1001637. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–6. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res. 1999;84:695–703. doi: 10.1161/01.res.84.6.695. [DOI] [PubMed] [Google Scholar]
- Hasdai D, Lerman A. The assessment of endothelial function in the cardiac catheterization laboratory in patients with risk factors for atherosclerotic coronary artery disease. Herz. 1999;24:544–7. doi: 10.1007/BF03044226. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- Hernandez-Presa M, Bustos C, Ortego M, et al. Angiotensin-converting enzyme innibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atheroslerosis. Circulation. 1997;95:1532–41. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, et al. A comparison of angiotensin-converting enzyme inhibitors, calcium antagonists, beta-blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: a multicenter study. J Am Coll Cardiol. 2000;35:284–91. doi: 10.1016/s0735-1097(99)00561-6. [DOI] [PubMed] [Google Scholar]
- Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK, Fisher ND, Price DA. Pathways for angiotensin II generation in intact human tissue: evidence from comparative pharmacological interruption of the renin system. Hypertension. 1998;32:387–92. doi: 10.1161/01.hyp.32.3.387. [DOI] [PubMed] [Google Scholar]
- Ivanova OV, Fomicheva OA, Sergakova LM, et al. Angiotensin II receptor blocker telmisartan: Effect on blood pressure profile and left ventricular hypertrophy in patients with arterial hypertension. J Int Med Res. 2005;33(Suppl 1):S21A–9. doi: 10.1177/14732300050330S104. [DOI] [PubMed] [Google Scholar]
- Karalliedde J, Viberti G. Evidence for renoprotection by blockade of the renin-angiotensin-aldosterone system in hypertension and diabetes. J Hum Hypertens. 2006;20:239–53. doi: 10.1038/sj.jhh.1001982. [DOI] [PubMed] [Google Scholar]
- Klahr S, Morrissey J. The role of vasoactive compounds, growth factors and cytokines in the progression of renal disease. Kidney Int. 2000;57:7–14. [PubMed] [Google Scholar]
- Klahr S. The role of nitric oxide in hypertension and renal disease progression. Nephrol Dial Transplant. 2001;16(Suppl 1):60–2. doi: 10.1093/ndt/16.suppl_1.60. [DOI] [PubMed] [Google Scholar]
- Klingbeil AU, John S, Schneider MP, et al. Effect of AT1 receptor blockade on endothelial function in essential hypertension. Am J Hypertens. 2003;16:123–8. doi: 10.1016/s0895-7061(02)03154-0. [DOI] [PubMed] [Google Scholar]
- Kranzhöfer R, Schmidt J, Pfeiffer CA, et al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscier Thromb Vasc Biol. 1999;19:1623–9. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- Lee KW, Blann AD, Lip GY. Inter-relationships of indices of endothelial damage/dysfunction [circulating endothelial cells, von Willebrand factor and flow-mediated dilatation] to tissue factor and interleukin-6 in acute coronary syndromes. Int J Cardiol. 2006;111:302–8. doi: 10.1016/j.ijcard.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- McAllister AS, Atkinson AB, Johnston GD, et al. Basal nitric oxide production is impaired in offspring of patients with essential hypertension. Clin Sci (Lond) 1999;97:141–7. [PubMed] [Google Scholar]
- Marui N, Offerrnann MK, Swenick R, et al. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin lnvest. 1993;92:1866–74. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Loscalzo J. New challenges for ACE-inhibitors in vascular diseases. Drug Design Reviews–Online. 2005;2:485–93. [Google Scholar]
- Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2002;13:2207–12. doi: 10.1097/01.asn.0000026610.48842.cb. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Panza JA, Quyyumi AA, Brush JE, Jr., et al. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- Parving HH, Lehnert H, Bröchner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- Perico N, Codreanu I, Schieppati A, et al. The future of renoprotection. Kidney Int Suppl. 2005:S95–101. doi: 10.1111/j.1523-1755.2005.09716.x. [DOI] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–6. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- Raptis AE, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S424–37. doi: 10.1055/s-2001-18600. [DOI] [PubMed] [Google Scholar]
- Redón J, Luque-Otero M, Martell N, et al. Renin-angiotensin system gene polymorphisms: relationship with blood pressure and microalbuminuria in telmisartan-treated hypertensive patients. Phamacogenomics J. 2005;5:14–20. doi: 10.1038/sj.tpj.6500280. [DOI] [PubMed] [Google Scholar]
- Roig E, Perez-Villa F, Morales M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- Ryšavá R, Tesar V, Merta M. Effect of telmisartan on blood pressure control and kidney function in hypertensive, proteinuric patients with chronic kidney disease. Blood Press Monit. 2005;10:207–13. doi: 10.1097/01.mbp.0000172708.97534.15. [DOI] [PubMed] [Google Scholar]
- Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–9. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Schmieder RE. Mechanisms for the clinical benefits of Angiotensin II receptor blockers. Am J Hypertens. 2005;18:720–30. doi: 10.1016/j.amjhyper.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Schmieder RE, Delles C, Mimran A, et al. Effects of telmisartan versus ramipril on endothelium function of the renal vasculature in type 2 diabetes. J Hypertens. 2005;23:S147. [Google Scholar]
- Sengul AM, Altuntas Y, Kurklu A, et al. Beneficial effect of lisinopril plus telmisartan patients with type 2 diabetes, microalbuminuria and hypertension. Diabetes Res Clin Pract. 2006;71:210–9. doi: 10.1016/j.diabres.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Siragy HM, de Gasparo M, El Kersh M, et al. Angiotensin-converting enzyme inhibition potentiates angiotensin II type 1 receptor effects on renal bradykinin and cGMP. Hypertension. 2001;38:183–6. doi: 10.1161/01.hyp.38.2.183. [DOI] [PubMed] [Google Scholar]
- Stam F, van Guldener C, Becker A, et al. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. J Am Soc Nephrol. 2006;17:537–45. doi: 10.1681/ASN.2005080834. [DOI] [PubMed] [Google Scholar]
- Svolis KA, Lemboussi DSp, Svolis AA, et al. Telmisartan, an angiotensin II type 1 receptor blocker, improves endothelial function in patients with chronic heart failure [abstract] J Am Coll Cardiol. 2002;39(9 [Suppl B]):266B. (Abstract 3536) [Google Scholar]
- Symeonides P, Koulouris S, Triantafyllou K, et al. Favourable pleiotropic effects of ramipril and telmisartan on vascular endothelium of diabetics. J Am Coll Cardiol. 2006;45:428A. [Google Scholar]
- Taylor AA. Pathophysiology of hypertension and endothelial dysfunction in patients with diabetes mellitus. Endocrinol Metab Clin North Am. 2001;30:983–97. doi: 10.1016/s0889-8529(05)70223-1. [DOI] [PubMed] [Google Scholar]
- Teo K, Yusuf S, Anderson C, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease. (ONTARGET/TRANSCEND) trials. Am Heart J. 2004;148:52–61. doi: 10.1016/j.ahj.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Uchida H, Nakamura Y, Kaihara M, et al. Practical efficacy of telmisartan for decreasing morning home blood pressure and pulse wave velocity in patients with mild-to-moderate hypertension. Hypertens Res. 2004;27:545–50. doi: 10.1291/hypres.27.545. [DOI] [PubMed] [Google Scholar]
- Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation. 2002;106:672–8. doi: 10.1161/01.cir.0000024416.33113.0a. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Zhou MS, Schulman IH, Raij L. Nitric oxide, angiotensin II, and hypertension. Semin Nephrol. 2004;24:366–78. doi: 10.1016/j.semnephrol.2004.04.008. [DOI] [PubMed] [Google Scholar]


