Abstract
Purpose
To report a new technique for the spinal component of craniospinal irradiation (CSI) in the supine position, to describe a verification procedure for this method, and to compare this technique to conventional plans.
Methods and Materials
Twelve patients were treated between 1998 and 2006 with CSI using a novel technique. Sixteen children were treated with a conventional field arrangement. All patients were followed for outcomes and toxicity. CSI was delivered using a posterior-to-anterior (PA) intensity-modulated radiation therapy (IMRT) spinal field matched to conventional, opposed lateral cranial fields. Treatment plans were generated for each patient using the IMRT technique and a standard PA field technique. The resulting dosimetry was compared to determine target homogeneity, maximum dose to normal tissues, and total monitor units delivered.
Results
Evaluation of the spinal IMRT technique compared to a standard PA technique reveals a 7% reduction in the target volume receiving ≥110% of the prescribed dose and an 8 % increase in the target volume receiving ≥ 95% of the prescribed dose. While target homogeneity was improved, the maximum dose delivered in the paraspinal muscles was increased by approximately 8.5 % with spinal IMRT compared to the PA technique. Follow-up evaluations revealed no unexpected toxicity associated with the IMRT technique.
Conclusions
A new technique of spine IMRT is presented in combination with a quality assurance method. This method improves target dose uniformity compared to the conventional CSI technique. Longer follow-up will be required to determine any benefit with regard to toxicity and disease control.
Keywords: IMRT, craniospinal, homogeneity, pediatric, supine
Introduction
This report describes a novel technique for craniospinal irradiation. Recently, several techniques have been described which exploit various technological advances or positioning verification methods in the delivery of craniospinal irradiation.1, 2 Previous reports have offered a variety of alternative methods of patient positioning, quality assurance or energy deposition.1-9 Several of these techniques adjust patient positioning for treatment in supine position in order to better access the airway for potential pediatric airway management needs and positioning reliability with thermoplastic immobilization.1, 8, 10 In addition, different applications of dosimetry have been utilized along with newer technologies for dose delivery in order to minimize long-term toxicity while simultaneously delivering homogeneous dose to the target volumes.2, 3, 7, 11 Alternative delivery methods such as helical tomotherapy (HT) 3 and proton based delivery methods have also been explored as possible methods to deliver craniospinal radiotherapy.2 Regardless of the potential benefits in regards to dose delivery with these techniques, widespread use is currently unrealistic due to the limited availability of these modalities.
The methodology employed in this report bears some similarity to previously reported techniques, but seeks to further improve both the target homogeneity associated with craniospinal irradiation in the pediatric population. This procedure makes use of relatively common technologies available within a typical academic radiation oncology department in order to deliver a time and cost-effective treatment. Furthermore, due to the ability to adjust the photon fluence at any point within the spine field, this method may offer an alternative technique to gapping cranial and spinal fields by allowing an intensity modulated match technique.
Several toxicities of craniospinal irradiation are of interest to the pediatric oncology community and survivors of pediatric malignancies. These include bone damage induced within the vertebral bodies causing closure of growth plates, other musculoskeletal abnormalities, neuroendocrine dysfunction, and neurocognitive dysfunction12-14. Radiation-induced bone damage has been evaluated with a particular interest in the pediatric population in which incomplete bone ossification at the growth plates causes significant long-term morbidity.12, 15, 16 In addition, the exit dose of photon modalities for spinal irradiation are potentially of concern in regards to late cardiac, vascular, pulmonary, and esophageal toxicity.
We report here the ability to substantially improve dose homogeneity within the thecal sac, while decreasing the absorbed dose within the thoracic vertebral bodies using a novel posterior-to-anterior spinal IMRT technique. With ongoing attempts to generally decrease craniospinal radiation doses in the pediatric population, combined with attempts to improve disease control with concurrent, intensified chemotherapy, the use of a technique which delivers a comparatively homogeneous dose to the target volume and vertebral bodies is attractive.
METHODS AND MATERIALS
Between the years 1998 and 2006, a total of twenty-seven patients were treated with craniospinal irradiation. Of these cases, twelve patients received treatment with spinal IMRT as a component of craniospinal irradiation. Patient ages ranged between two and thirty-two, with a median age of 10 years. In one case, conventional photon therapy was initiated, but due to discomfort and positioning needs, a second plan was implemented with supine positioning and IMRT in order to complete therapy. In one additional case, re-treatment with IMRT spine fields was required, due to central nervous system and testicular relapse of acute lymphocytic leukemia four years after completion of the initial, conventional craniospinal radiotherapy. Patients were evenly divided by sex, with thirteen males and females each. All children were treated as per an applicable current protocol, when not directly enrolled in a multi-institution study. Twelve patients described in this report underwent CT simulation for treatment planning and were treated with IMRT spinal fields. A conventional PA spine field was also planned for each patient in order to compare target coverage and normal tissue dosimetry with that obtained using the spinal IMRT technique.
During simulation, patients were placed in supine position with neck extended so that a posteriorly oriented spine field could exit below the mandible. The patients were immobilized with a thermoplastic mask, marked with radio-opaque markers at the level of the tragus, and scanned from the vertex to the ischium. CT images were then forwarded to the treatment planning workstation (Eclipse, Varian Medical Systems).
Once the images were loaded and pre-processed, the brain, eyes, lenses, brainstem, and spinal canal were contoured. Care was taken to ensure that the brain contour included the cribriform plate when necessary. Treatment planning commenced with the placement of the PA spine field matched to a set of opposed lateral cranial fields, using pedestal and collimator rotation calculated to produce a non-divergent abutment of the spinal and cranial fields. An isocentric setup was used, if feasible, based on patient size, with the isocenter of the spine field placed directly inferior to that of the cranial fields by a distance calculated to provide a geometric abutment at the match line. In those cases where an isocentric treatment was not possible, an SSD setup was used. Furthermore, due to a limitation on table height, the maximum SSD for this technique was set at 110 centimeters (cm). For three cases in which it was not possible to encompass the target volume within a single field by extending the treatment distance, two adjoining PA fields were used. Table shifts were calculated from the setup point to the cranial fields, as well as from the cranial fields to the spinal fields. The match plane was placed within the lower neck in order to allow an adequate margin to accommodate sequential junction shifts, made inferiorly in five to ten millimeter (mm) increments, after each week of treatment. These shifts eliminated the need for recalculating leaf motion for the spinal IMRT field. The superior border of the spine field was reduced simply by closing the primary collimator.
In order to generate the field aperture for the cranial fields, the multi-leaf collimator was automatically positioned with the inside corner of the aperture 1.5 cm from the contoured brain. The MLC leaves were adjusted, as needed in the neck area, to ensure that the 100% isodose line encompassed the cervical cord by a suitable margin of 1-2 cm, depending on the disease process and patient size. Beam weighting was adjusted to ensure coverage of the target volume within the 100% isodose curve.
Treatment planning for the IMRT spinal field commenced with the contouring of the spinal canal superiorly to the level of the match plane and inferiorly to the S2 vertebral level, using computed tomography (CT) or, preferably, magnetic resonance imaging (MRI) guidance, if available. A 2 cm lateral margin on each side of the spinal canal was constructed, defining a target volume called the “cord band” based on the spinal canal volume. The Helios IMRT algorithm then optimized the photon fluence and the MLC dynamic leaf sequence in order to deliver a relatively uniform dose to this region. This technique only provided modulation in superior-inferior and lateral directions. Modulation of the intensity along the PA beam axis was not possible.
When two separate spinal fields were required, the overlap was determined using computer-based dosimetric parameters. The IMRT algorithm optimized the intensity distribution in order to achieve overall dose uniformity, while eliminating the “cold” and “hot” regions within the target volume. The overlap was adjusted in 5 mm increments, ranging from 4 cm to 6 cm, to determine the optimal overlap. For individuals with short necks, the superior field was treated as an asymmetric half-field, up to a maximum length of 20 cm. Beyond this length, two IMRT fields were required.
The entire cord band was treated to 100% of the prescribed dose, to ensure full coverage through the proximal neural foramina. For one patient, the prescription required delivery of a maximum of 45 Gy to the cord.
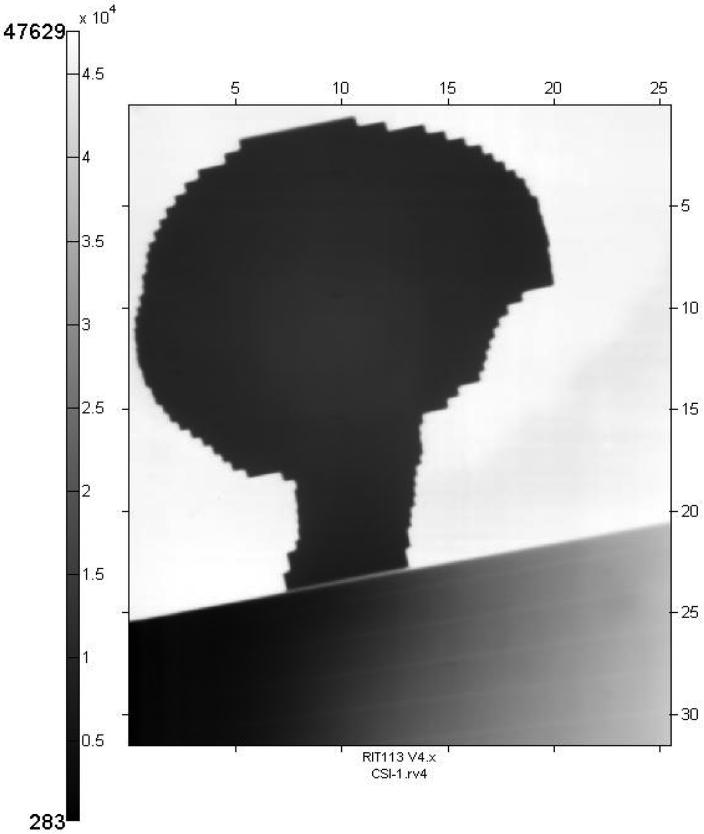
Pre-treatment quality assurance was performed using film dosimetry to verify an acceptable match between cranial and spinal fields. Since the set-up positions were marked on the mask, this was used, along with a head support, to represent the patient. Pre-calculated table shifts from the set-up points were used to position the cranial and spinal fields. Kodak EC (Enhanced Contrast) film (33 cm by 41 cm) was inserted in an EC-L Lightweight Cassette and secured to the treatment couch in the mid-sagittal plane, perpendicular to the opposed lateral fields and parallel to the plane of the PA field (Figure 1). The film position spanned the match region between the inferior margin of the brain field and the superior margin of the PA spine field. After setting the table position for the cranial field, the gantry was rotated to the lateral position and the film was exposed using 2 monitor units (MUs). Then the table was shifted to the spine field position. The gantry was rotated to the posterior vertical position and an additional 4 MUs were delivered to the film. This double-exposure technique permitted an accurate visual determination of the alignment between the lateral and diverging PA fields (Figure 2). Corrections could be quickly implemented by re-positioning the couch and refilming.
Figure 1. Cranial and spinal field match verification.
Film verification of the juxtaposition and lack of overlap or excessive gap between the cranial field and spinal field. The cranial field is filmed first (A) followed by filming of the PA spinal field (B).
Figure 2. Exposed film demonstrating juxtaposition of fields.
The juxtaposition and lack of overlap between cranial and spinal fields were verified with Kodak EC film. Note the evident match of divergence and lack of overlap between the cranial and spinal field.
Similar methods were employed in patients undergoing CT simulation with conventional PA spine fields. Following induction of anesthesia, if necessary, the neck was immobilized using a thermoplastic mask in the extended position, with care taken to ensure good reproducibility for all treatments by marking the mask at the forehead and tragus bilaterally. CT scanning commenced from the vertex through the ischium and images were forwarded to the treatment planning workstation. The necessary pre-processing and contouring of critical structures was followed by generation of a conventional PA spine field with coverage of the entire target volume.
A comparison between the dosimetric results obtained with the posterior-to anterior spinal IMRT technique and conventional single or multiple PA spine fields was performed using the overall target dose volume histogram (DVH) (Figure 3), quantitative values of the target minima, maxima, mean doses, and a comparison of the percent volumes receiving at least 95 % and greater than 110 % of the prescribed dose. (Table 1) In addition, normal tissue dosimetry was compared at the dose maxima for the single PA fields, within the posterior spinal musculature. The total number of monitor units required to deliver the IMRT spine field(s) and the standard PA spine field(s) were compared for each patient. (Table 2)
Figure 3. DVH comparison of target coverage.
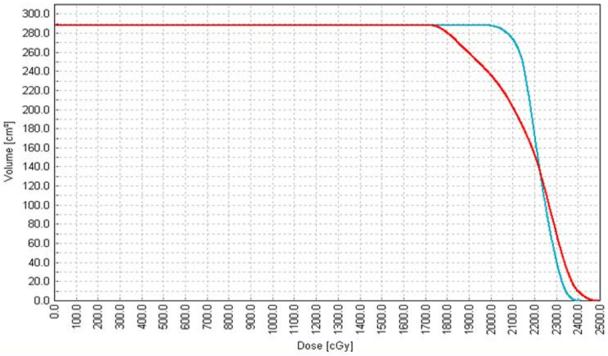
Dose volume histogram of a representative plan comparing target (cord band) coverage between the conventional PA spine technique (red curve) and the IMRT technique (blue curve). The target coverage is and homogeneity arte improved with the IMRT technique as evidenced by the decrease in maximum dose and the increase in the percentage of the volume receiving the prescription dose.
Table 1.
Dosimetric comparison of IMRT and conventional (Std) spinal fields
| Patient | %Vol. by 95% Dose | %Vol. > 110% Dose | Min (%) in Cord Band | Max (%) in Cord Band | Mean in Cord Band | Max in Patient Body (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMRT | Std | IMRT | Std | IMRT | Std | IMRT | Std | IMRT | Std | IMRT | Std | |
| 1 | 98.5 | 91 | 2.4 | 5.5 | 82.9 | 86.5 | 112 | 114 | 104 | 103 | 138.2 | 127.2 |
| 2 | 92.9 | 80.8 | 0 | 3.5 | 76 | 55.4 | 108.8 | 112.6 | 101.2 | 100.7 | 130.6 | 119.8 |
| 3 | 95.6 | 94 | 0 | 1.9 | 76.6 | 86.9 | 109.6 | 112.4 | 102 | 102.9 | 128.7 | 119.4 |
| 4 | 95 | 83.3 | 0 | 18.7 | 80.2 | 77.2 | 108.7 | 117.1 | 100.7 | 103 | 137.7 | 122.3 |
| 5 | 98.1 | 89.7 | 8.2 | 30.3 | 82.1 | 83 | 113.5 | 121.9 | 105.1 | 105.4 | 146.1 | 134.9 |
| 6 | 98 | 89.2 | 2.5 | 2.1 | 80.3 | 72.5 | 112.9 | 111.4 | 104.3 | 102.7 | 118.1 | 124.2 |
| 7 | 94.8 | 91.5 | 0 | 4.2 | 80.2 | 87.8 | 109.8 | 114.5 | 101.3 | 101.4 | 130.5 | 121.5 |
| 8 | 94 | 78.8 | 0.1 | 2.4 | 77.8 | 79.7 | 110.3 | 112.8 | 101.6 | 99.8 | 134.4 | 120.8 |
| 9 | 94.7 | 83.7 | 2.3 | 14.8 | 77.3 | 66.5 | 111.1 | 126.8 | 101.6 | 101.9 | 146.3 | 143.3 |
| 10 | 90.4 | 86.3 | 0 | 3.5 | 71.6 | 67 | 103 | 115.3 | 100 | 100.8 | 122.1 | 117.5 |
| 11 | 97.6 | 93.4 | 0 | 2.5 | 81.9 | 80.5 | 109.5 | 112.9 | 102.3 | 103.4 | 133.5 | 121.4 |
Table 2.
Comparison of monitor units required to deliver plans with conventional (Std) and IMRT techniques.
| Monitor Units | |||
|---|---|---|---|
| Patient | IMRT | Std | % Increase |
| 1 | 300 | 218 | 37.6 |
| 2 | 249 | 175 | 42.3 |
| 3 | 270 | 214 | 26.2 |
| 4 | 268 | 179 | 49.7 |
| 5 | 369 | 277 | 33.2 |
| 6 | 233+238 | 165+169 | 41.2 + 40.8 |
| 7 | 269 | 205 | 31.2 |
| 8 | 339 | 252 | 34.5 |
| 9 | 245+247 | 168+185 | 45.8 + 33.5 |
| 10 | 306 | 243 | 25.9 |
| 11 | 241 | 173 | 39.3 |
RESULTS
A total of twelve children received spine treatment with IMRT-based fields. Due to the length of the thecal sac in three children, two matched IMRT spine fields were required to address the entire target volume. Eleven of the twelve datasets were readily cross-comparable; in one case, the prescription required a cord-dose constraint of 45 Gy and the plan was normalized accordingly. Due to the necessity of carrying dose to within acceptable cord tolerance limits, an additional setup error of 1 mm was allowed in order to further reduce the risk of field overlap. Graphical representations of mid-sagittal plane isodose plans are included in Figures 4 and 5.
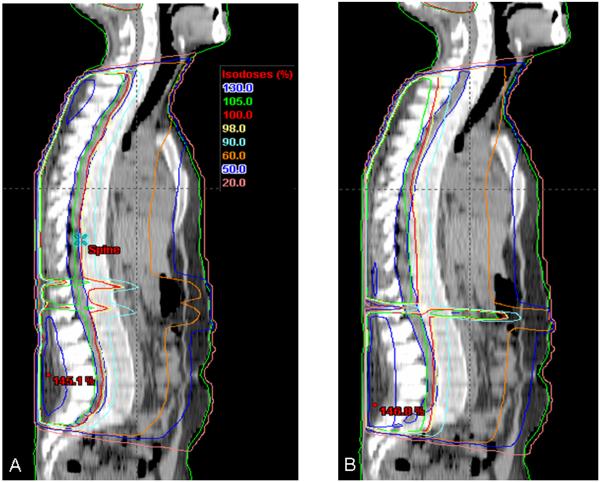
Figure 4. Comparison of mid-sagittal isodose curves for plans including a single PA spine field.
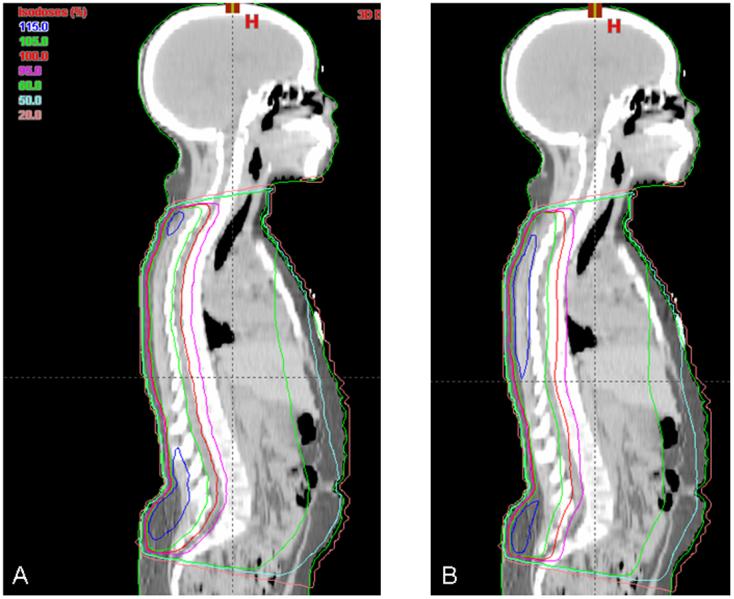
In smaller patients, extended distance treatments allowed planning of a single PA spine field. Mid-sagittal isodose curves from representative plans are pictured from the same patient with a single PA spine IMRT field (A) and a conventional PA spine field (B). Note the improved coverage of the target volume in the cervical and lumbosacral regions.
Figure 5. Comparison of mid-sagittal isodose curves for plans requiring matched PA spinal fields.
Mid-sagittal isodose curves are pictured from the same patient planned with two IMRT spine fields (A) and two conventional spine fields (B). Note the improved coverage of the cervical and lumbosacral spine with the IMRT plan compared to the conventional plan.
The IMRT method showed significant improvement in dose coverage in the thecal sac and cord band structure compared with the conventional uncompensated PA field. Table 1 demonstrates a consistent improvement in dose homogeneity within the target structure in eleven comparative cases. In those cases, the mean percent target volume covered by the 95 % dose level varied from 87.4 % with a conventional field to 95.4 % with an IMRT field. The mean percent target volume receiving greater than 110 % of the dose decreased from 8.1 % with the conventional PA field to 1.4 % with the spinal IMRT field. The reason for the improved target dose homogeneity with the IMRT spine fields arises from the natural lordosis and kyphosis in the lumbosacral and cervicothoracic spine, respectively. This anatomy resulted in more dose to the posterior lumbosacral vertebral body elements in the IMRT plans due to the often greater depths of tissue traversed with dose delivery to the target at this site. The greatest difference appears in the lumbosacral spine and results in greater thecal sac coverage at the expense of greater dose to the posterior elements of the lumbosacral vertebral bodies in the IMRT plans. Conversely, the conventional plans appear to deliver higher doses to the vertebral bodies at the high thoracic level, likely due to the shallow depth of the target structure at this point. Likewise, IMRT plans suggest a more consistent dose gradient in the posterior to anterior direction through the vertebral bodies compared to conventional plans. In each case, the maximum target volume depth received a more homogeneous dose with the spinal IMRT technique; however, increased dose was deposited in regions requiring dose to deeper targets, namely, the posterior spinous muscles and bones.
Within the cord band structure, the spinal IMRT technique resulted in an average minimum dose of 79 %, compared with 77 % for the conventional PA spine field. The relative cord band maxima between the IMRT technique and the conventional technique were 110 % and 113 % respectively, while the patient’s body maxima were 134 % and 121 %. Using the IMRT spine technique, the non-target hotspots characteristically appeared in the superficial tissues of the superior and inferior borders of the treatment field, corresponding with regions in which the target volume extended the maximum anterior distance from the posterior skin surface. The apparent benefit of the spinal IMRT technique was a more homogeneous dose delivered to the cord band structure.
The use of IMRT required additional monitor units (MUs) to be delivered per fraction. (Table 2) Spinal IMRT fields utilized between 26 % and 86 % more MUs per fraction in twelve patients. The case requiring the most MUs received a prescription with a maximum dose of 45 Gy; otherwise all IMRT plans utilized less than 50 % more MUs relative to conventional spine fields. All patients treated with a single PA IMRT spine field required less than 40 % more monitor units. In the three children treated with two PA IMRT matched fields, between 34 % and 50 % more MUs were used compared to plans generated with conventional PA fields. In each of these three cases, the superior PA IMRT field required either the same MUs or up to 29 % more than the corresponding inferior PA spine field.
Neurologic evaluations at routine followup in the delayed acute and long-term setting revealed no case of new focal motor or sensory long-tract symptoms attributable to the spinal field or match of the spinal field with the cranial field.
DISCUSSION
We report a novel technique utilizing supine setup, single field spinal IMRT treatment, and setup film verification to deliver CSI. This method offers the distinct advantage of supine positioning and improved target homogeneity at the expense of minimally increased treatment time and an increase in paraspinal dose maximum.
A major advantage of this technique compared to more complex delivery methods is the ease and rapidity of planning and implementing therapy with an IMRT spinal field. Cord band contouring is readily accomplished with a segmentation mask or an expansion of the initial contour to create an elliptical structure. Calculation of dynamic leaf sequence and fluence mapping adds little to the workload and time constraints of the dosimetry staff, often requiring no more than thirty to forty-five minutes for complete planning of the entire craniospinal plan with current planning technologies. Most of the quality assurance is completed during the initial virtual simulation, prior to initiation of therapy, thus minimizing the time an anesthetized patient or young child must be immobilized for final verification.
In the past decade, several groups have described a variety of alternative means for CSI positioning, quality assurance or energy deposition.1-9 Improved patient comfort, airway access, and positioning reliability have been demonstrated with some of these techniques.1, 8, 10 The therapeutic goal has been to deliver more homogeneous dose while minimizing late toxicities.2, 3, 7, 11
Michalski et al, demonstrated the use of film dosimetry in order to verify field matching using a square 6 inch Kodak XOMAT V film beneath a supinely positioned patient’s neck during each treatment in order to confirm positioning with respect to CT-based digital reconstructions.1 This technique offers a quick, reproducible method for documenting patient position and making adjustments on a daily basis. The film verification process is reliable and quickly establishes necessary corrections to patient position, thereby minimizing patient discomfort and time required at simulation. In addition, this procedure allows for a quick, visual verification that all calculated matches are appropriately performed and that no misapplications of this complex procedure are implemented at the time of therapy.
Using this methodology, the initial positioning verification has been documented prior to treatment at least once. Both patient comfort and ease of monitoring positioning have been consistently improved. Indeed, one patient required re-simulation and planning in the supine position due to discomfort in the prone position. Supine positioning has been particularly helpful at our institution for airway management and patient monitoring in pediatric patients requiring anesthesia.
One issue pertinent to three field matching and dose homogeneity is the gap between cranial and spinal fields and adjacent spinal fields. This is of particular concern for craniospinal irradiation, where systematic errors could result in under- or over-dosing of critical structures. The “feathered” match technique has become the generally accepted method for avoiding this possibility; however, nearly 60 % of 243 Children’s Oncology Group member institutions abut brain and spine fields directly at the skin.17 In addition, a traditional gap between matched fields ensures no overdose to the cord, while shifting the match point by sequentially shrinking and growing the cranial or spine fields at set points throughout the treatment is widely accepted to result in a more homogeneous dose throughout the cervical spinal cord and thecum within the match region.17 While such a rationale is sound, the development of newer technologies may obviate the need for conventional junctional matching and allow for quicker alternative techniques which deliver homogeneous dose throughout the target volume.
A non-gapped, directly matched three field setup has been used with an intensity modulated spinal field for several years with no unexpected toxicities in treated children related to cord over- or under-dosing. These children have been followed to document neurologic outcomes and musculoskeletal development; longer followup will be necessary to meaningfully assess these endpoints in relation to the IMRT spinal technique. Neurologic and developmental endpoints continue to be followed routinely, and potential toxicities of a non-gapped three-field junction will be monitored with respect to individual outcomes.
We are not the first group to report craniospinal therapy with a more caudal location of match between the spinal and cranial field. The positioning of the match site has been evaluated by Paulino et al 18 in a retrospective dosimetric review of five patients who were planned with high C1-C2 versus low neck cranial-spinal junctions. This review revealed that while cervical spinal dosing can be modestly reduced by approximately five percent with use of a high junction, the trade-off results in significantly more dose to the thyroid, mandible, pharynx and larynx.18 In our own series, low cervical neck matching attempts to account for the higher monitor units required to deliver therapy, thereby lessening overall dose to the critical structures of the head and neck.
The development of helical tomotherapy (HT) provides yet another solution for delivery of dose to an extensive target volume. Bauman et al provide a detailed overview of planning considerations using HT in comparison with standard conformal radiation3. Certain clear benefits may exist with HT, however, long-term potential clinical benefits may be clouded by closely related dose-delivery issues. If the entire craniospinal axis receives therapy simultaneously, the complications of multiple field matching and shifting is eliminated along with providing daily mega-voltage computed tomographic localization data. However, a narrower fan-beam width is necessary to provide adequate coverage without overdosing critical structures. Most difficulties were noted in avoiding orbital structures while providing full dose to the cribriform plate. A 10 mm fan-beam width results in a 40 minute treatment time, while a 25 mm fan-beam width results in a fifteen minute treatment (similar to a standard linear accelerator based plan) but results in poorer dose-shaping around the orbital structures. Using two separate plans to address both spine and cranium respectively, requires junctional matching, defeating one of the major benefits of tomotherapy.
Delivery of therapy with HT also requires more extensive planning in order to formulate a complete cost-benefit dose delivery calculation. At least the target volume, lungs, liver, bowel, heart and kidneys must be contoured if the spine alone is to be treated with HT.3, 7 Additional structures are necessary to address the cranium. Planning then also requires an extensive cost-benefit function analysis to yield a fully optimized plan. Consequently, one must question the total integral dose throughout a developing body. Integral doses of 3-10 Gy are postulated, and while this may not be enough to induce a marked increase in secondary malignancy, doses between 0.1 and 2.5 Seivert are known to induce cancer with a positive linear relationship.19, 20 Certainly, this may well be enough dose to cause effects in a wide variety of normal tissues.
Proton therapy continues to rapidly develop into a major new technological modality in this country, with the number of treating institutions likely to double in the next few years. While the radiobiologic effective dose corresponds to a similar value to standard mega-voltage x-rays, the true clinical outcomes remain to be proven in larger clinical trials.19 Regardless, the major perceived benefit of proton therapy is the reduced exit dose that is thought to be responsible for many of the late toxicities of spinal radiation, including second malignancies of visceral organs.
The implications for carcinogenesis at these total body absorbed doses are worrisome. A young child undergoing a full course of radiotherapy may have a lifetime risk of second malignancy ten-fold higher than a fully mature adult, based on both increased susceptibility to mutations and an overall greater time-span in which to express them.20 This must be considered when utilizing techniques such as IMRT to increase spinal homogeneity due to the requirement of an increased number of MUs delivered.
CONCLUSION
The use of a PA spine field with intensity modulation functioning as an electronic tissue compensator allows both a time- and cost-effective strategy for delivery of quality assured radiation with improved dose homogeneity to the thecal target volume while adding minimal toxicities. This method also enhances airway access, patient comfort and can be delivered with readily verifiable dosimetric tools offering a simple intuitive process for ensuring appropriate dose delivery. While other technologies have rapidly progressed into the realm of clinical practice, they can introduce new concerns for long-term toxicity, particularly in the pediatric population.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, NCI.
Footnotes
Conflicts of Interest Notification:
No actual or potential conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Michalski J, Klein EE, Gerber R. Method to plan, administer, and verify supine craniospinal irradiation. Journal of applied clinical medical physics. 2002;3:310–316. doi: 10.1120/jacmp.v3i4.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St. Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. International Journal of Radiation Oncology*Biology*Physics. 2004;58:727–734. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 3.Bauman G, Yartsev S, Coad T, Fisher B, Kron T. Helical tomotherapy for craniospinal radiation. Br J Radiol. 2005;78:548–552. doi: 10.1259/bjr/53491625. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins RB. A simple method of radiation treatment of craniospinal fields with patient supine. International Journal of Radiation Oncology*Biology*Physics. 2001;49:261–264. doi: 10.1016/s0360-3016(00)01367-5. [DOI] [PubMed] [Google Scholar]
- 5.Culp L, Bayouth J, Endres E, Smith A, Colman M. Craniospinal irradiation-use of new technology to achieve dose uniformity. International Journal of Radiation Oncology*Biology*Physics. 2002;54:239. [Google Scholar]
- 6.Lee CT, Bilton SD, Famiglietti RM, et al. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: How do protons compare with other conformal techniques? International Journal of Radiation Oncology*Biology*Physics. 2005;63:362–372. doi: 10.1016/j.ijrobp.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Swanson E, Jursinic P, Herman J, Lee W, Firat SY. A Dosimetric Comparison of 3-Dimentional Conformal Radiotherapy (3DCRT) and Helical Tomotherapy for Craniospinal Irradiation; International Journal of Radiation Oncology*Biology*Physics. Proceedings of the American Society for Therapeutic Radiology and Oncology 47 th Annual Meeting; 2005.p. S265. [Google Scholar]
- 8.Thomadsen B, Mehta M, Howard S, Das R. Craniospinal treatment with the patient supine. Medical Dosimetry. 2003;28:35–38. doi: 10.1016/S0958-3947(02)00239-X. [DOI] [PubMed] [Google Scholar]
- 9.Roback DM, Johnson JM, Khan FM, Engeler GP, McGuire WA. The use of tertiary collimation for spinal irradiation with extended SSD electron fields. International Journal of Radiation Oncology*Biology*Physics. 1997;37:1187–1192. doi: 10.1016/s0360-3016(97)00108-9. [DOI] [PubMed] [Google Scholar]
- 10.Michalski J. Novel Method to Plan, Administer, and Verify Craniospinal Axis Irradiation. International Journal of Radiation Oncology*Biology*Physics. 1997;39:334. [Google Scholar]
- 11.Yuh GE, Loredo LN, Yonemoto LT, et al. Reducing toxicity from craniospinal irradiation: using proton beams to treat medulloblastoma in young children. Cancer Journal, The. 2004;10:386–390. doi: 10.1097/00130404-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell M, Logan P. Radiation-induced changes in bone. Radiographics. 1998;18:1125–1136. doi: 10.1148/radiographics.18.5.9747611. [DOI] [PubMed] [Google Scholar]
- 13.Grill J, Renaux VK, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. International Journal of Radiation Oncology*Biology*Physics. 1999;45:137–145. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 14.Heikens J, Michiels EMC, Behrendt H, Endert E, Bakker PJM, Fliers E. Long-term neuro-endocrine sequelae after treatment for childhood medulloblastoma. European Journal of Cancer. 1998;34:1592–1597. doi: 10.1016/s0959-8049(98)00212-3. [DOI] [PubMed] [Google Scholar]
- 15.Probert J, Parker B. The effects of radiation therapy on bone growth. Radiology. 1975;114:155–162. doi: 10.1148/114.1.155. [DOI] [PubMed] [Google Scholar]
- 16.Roebuck DJ. Skeletal Complications in Pediatric Oncology Patients. Radiographics. 1999;19:873–885. doi: 10.1148/radiographics.19.4.g99jl01873. [DOI] [PubMed] [Google Scholar]
- 17.Urie M, FitzGerald TJ, Followill D, Laurie F, Marcus R, Michalski J. Current calibration, treatment, and treatment planning techniques among institutions participating in the Children’s Oncology Group. International Journal of Radiation Oncology*Biology*Physics. 2003;55:245–260. doi: 10.1016/s0360-3016(02)03827-0. [DOI] [PubMed] [Google Scholar]
- 18.Narayana A, Jeswani S, Paulino AC. The cranial-spinal junction in medulloblastoma: does it matter? International Journal of Radiation Oncology*Biology*Physics. 1999;44:81–84. doi: 10.1016/s0360-3016(98)00479-9. [DOI] [PubMed] [Google Scholar]
- 19.Hall EJ, Giaccia, Amato J. Radiobiology for the Radiologist. Lippincott Williams & Wilkins; 2005. p. 656. [Google Scholar]
- 20.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. International Journal of Radiation Oncology*Biology*Physics. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]