Abstract
Fear conditioning, including variants such as delay and trace conditioning that depend on different neural systems, is widely used to behaviorally characterize genetically altered mice. We present data from three strains of mice, C57/BL6 (C57), 129/SvlmJ (129), and a hybrid strain of the two (F1 hybrids), trained on various versions of a trace fear-conditioning protocol. The initial version was taken from the literature but included unpaired control groups to assess nonassociative effects on test performance. We observed high levels of nonassociative freezing in both contextual and cued test conditions. In particular, nonassociative freezing in unpaired control groups was equivalent to freezing shown by paired groups in the tests for trace conditioning. A number of pilot studies resulted in a new protocol that yielded strong context conditioning and low levels of nonassociative freezing in all mouse strains. During the trace–CS test in this protocol, freezing in unpaired controls remained low in all strains, and both the C57s and F1 hybrids showed reliable associative trace fear conditioning. Trace conditioning, however, was not obtained in the 129 mice. Our findings indicate that caution is warranted in interpreting mouse fear-conditioning studies that lack control conditions to address nonassociative effects. They also reveal a final set of parameters that are important for minimizing such nonassociative effects and demonstrate strain differences across performance in mouse contextual and trace fear conditioning.
The majority of animal behavioral models used to evaluate cognition and emotion were developed in rats. However, rapid advances in neurogenetics has led to explosive growth in the generation of genetically modified mice that are undergoing behavioral testing with procedures that were established in rats, and in many cases have not been fully validated in mice. Such validation is critical because studies have shown that mice often behave differently from rats in significant ways (Whishaw and Tomie 1996; Frick et al. 2000; Podhorna and Didriksen 2005; Cressant et al. 2007). Furthermore, as more information has emerged concerning mice of different strains, it has become apparent that each strain has a unique behavioral profile (Upchurch and Wehner 1988; Crawley et al. 1997; Montkowski et al. 1997; Holmes et al. 2002; Koopmans et al. 2003; Bothe et al. 2004; Brooks et al. 2004). Understanding these differences is crucial because mutants are frequently generated from mice of different background strains. Thus, it is important to undertake validation studies in mice to (1) confirm that behavioral performance shows properties that are commonly ascribed to the test based on work with other rodent species, and (2) gather data on strains that may be useful in the interpretation of the behavior of mutant mice.
One behavioral model in which a substantial amount of testing has been done in different strains of mice and those with genetic modifications is fear conditioning. Fear conditioning is a form of Pavlovian conditioning in which an initially neutral stimulus (conditioned stimulus; CS), usually a tone, is paired with the presentation of an aversive event (unconditioned stimulus; US), usually a footshock, to elicit fearful behavior characterized by robust autonomic responses and a cessation of movement or freezing (Fanselow and Poulos 2005). After a number of pairings, the fear response can be elicited by presentation of the tone alone, or by the environment or context in which the pairings occurred. Fear conditioning is an ideal behavioral paradigm for studying cognition–emotion interactions in neurogenetics, because it has direct translational relevance to human affective disorders (Schneider et al. 1999; Veit et al. 2002; Sommer et al. 2006), it is well characterized behaviorally (Phillips and LeDoux 1992, 1994; Bast et al. 2001), and it is increasingly well understood at neural and molecular levels (Maren and Quirk 2004; Fanselow and Poulos 2005). Although there are several task variants of fear conditioning, the two most commonly used variants are delay and trace conditioning. In delay conditioning, the CS precedes and overlaps with the US and the critical neural substrate of learning is the amygdala (Maren and Quirk 2004; Fanselow and Poulos 2005). In trace fear conditioning, the CS and US are separated by a stimulus-free “trace interval,” and the critical brain substrates include the hippocampus and prefrontal cortex in addition to the amygdala (McEchron et al. 1998; Runyan et al. 2004; Chowdhury et al. 2005; Misane et al. 2005). Trace fear conditioning has been used in many mutant mouse models (Huerta et al. 2000; Crestani et al. 2002; Kinney et al. 2002; Wiltgen et al. 2005) and in C57BL/6 mice (Han et al. 2003; Gould et al. 2004; Weitemier and Ryabinin 2004), but there are little or no data concerning strain differences in learning, performance, and nonassociative effects during trace fear conditioning.
Nonassociative effects are apparent in fear conditioning when the subjects show a fear response to stimuli that have not been explicitly paired with a fear-inducing event. Such nonassociative effects can be revealed in studies that include an unpaired control group. A typical unpaired control group is one in which the CS and US are presented separately at random intervals, rather than the CS always preceding the US, so that little or no association develops between the two stimuli. Nonassociative processes such as sensitization, pseudoconditioning, novelty stress, etc., can be evoked by experience with the CS and/or US per se, and an unpaired group shows whether these nonassociative effects contribute to freezing behavior, the presumed measure of the associative fear response. Yet, many mouse studies have not included such a control in fear conditioning and thus have no way to detect nonassociative effects (Gould et al. 2004; Weitemier and Ryabinin 2004; Yee et al. 2004; Misane et al. 2005). When high levels of nonassociative freezing were observed in unpaired controls in one study of trace conditioning in mice, such behavior was attributed to unavoidable environmental factors (Huerta et al. 2000).
The goal of the current study was to develop a trace fear-conditioning protocol suitable for mice that included an unpaired control group demonstrating low levels of nonassociative effects on freezing. We also wanted to examine performance across strains of mice typically used in behavioral studies of fear and in the generation of genetic models. To accomplish this goal we tested three different strains of mice (C57BL/6J; C57, 129Sv/ImJ; 129, and B6129F1, a hybrid of the previous two; F1 hybrid) on a number of trace fear-conditioning protocols. Results from our Initial Protocol showed that nonassociative effects can be quite large, and indicated that all three strains tested performed similarly. However, after several adjustments to this procedure we successfully developed a Final Protocol that minimized nonassociative effects and revealed significant strain differences in response to the trace–CS.
Results
Initial Protocol
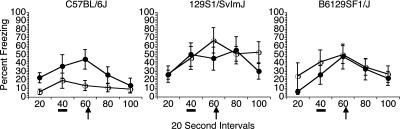
Trace fear conditioning under the Initial Protocol involved 3 d of behavioral experimentation, with a pre-exposure session on day 1, training on day 2, and tests for conditioned freezing on day 3. A total of 36 subjects (12 from each strain of C57, 129, and F1 hybrid mice) were assigned to either paired or unpaired groups. The paired groups received four trials in the training session on day 2, each consisting of a tone CS (85 dB, 20-sec duration) and a shock US (0.5 mA, 2-sec duration) with those stimuli separated by a trace interval of 18 sec. The unpaired groups received four tone-alone presentations during the day 1 pre-exposure session and four shock-alone presentations during the day 2 session. Tests for conditioned freezing to the training context and to the tone cue, the latter assessed in a novel context, occurred during the third session for all groups; no shocks were given during testing. Results from the Initial Protocol are shown in Figures 1 and 2. Trace conditioning, as indexed by freezing to the tone–CS for each of the strains is depicted in Figure 1. Although four tone–CS tests were given, data from only the first test trial was analyzed and depicted in the figures. This was because freezing levels remained high over all test trials subsequent to the first tone presentation, therefore, the first trial most accurately reflects the response of the mice to the tone–CS presentation and the subsequent trace interval. Notably, little or no difference was evident between the paired and unpaired groups in each strain. Indeed, a three-way analysis of variance (ANOVA) that included Training Group (paired vs. unpaired) as one factor yielded no main effect of Training Group overall (Groups, F(1,30) = 0.001, P = 0.972) and no interaction of Training Group with other factors (Strain and Interval). That ANOVA only revealed significant main effects of Strain (F(2,30) = 5.218, P = 0.011) and Interval (F(4,120) = 8.232, P < 0.0001), the latter due to an overall increase in freezing during the periods when the tone was presented (40-sec time block, underscored in Fig. 1) and immediately following the tone presentation (60-sec time block). Overall freezing rates for the Pre-CS period (20-sec baseline interval) were elevated after the first trial, but analysis of data averaged across all four trials revealed identical results to those in trial 1 in all three strains across both groups (Table 1). Thus, paired vs. unpaired training had no significant influence on the response of the mice to the tone cue in tests for trace conditioning under the initial training protocol.
Figure 1.
Test of trace conditioning to the tone–CS under the Initial Protocol. These data were analyzed using 3 (Strain) × 2 (Training group) × 5 (Interval) factorial ANOVA (see text for the statistical results). All paired groups of mice responded with a high level of freezing to the tone–CS. However, of the unpaired groups, only the C57BL/6J mice exhibited somewhat lower levels of freezing to the tone–CS. There was no significant difference between paired and unpaired overall (no main effect of Training Group) and no interaction between Training Group and Strain. Behavior during the interval prior to presentation of the tone–CS (pre-CS period) is indicated at the 20-sec datapoint. The black bar at the 40-sec interval indicates when the tone–CS was presented; the 60-sec interval encompasses the empty trace interval until the shock occurred during training (black arrow). (●) Paired mice; (○) unpaired mice.
Figure 2.
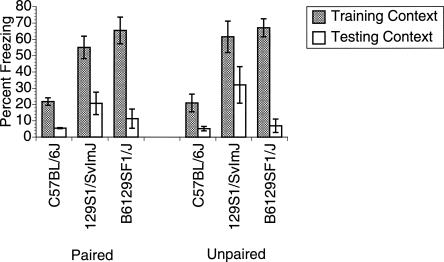
Test of contextual conditioning under the Initial Protocol. There was no overall difference between Paired (left) and Unpaired (right) groups, but the 129S1/SvImJ mice exhibited higher levels of freezing to the testing context compared with the C57BL/6J and B6129F1/J hybrid mice. Data are presented as mean ± SE.
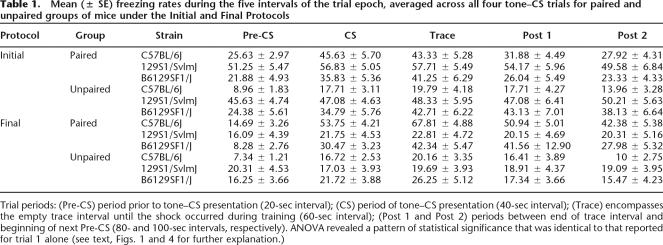
Table 1.
Mean (± SE) freezing rates during the five intervals of the trial epoch, averaged across all four tone–CS trials for paired and unpaired groups of mice under the Initial and Final Protocols
Trial periods: (Pre-CS) period prior to tone–CS presentation (20-sec interval); (CS) period of tone–CS presentation (40-sec interval); (Trace) encompasses the empty trace interval until the shock occurred during training (60-sec interval); (Post 1 and Post 2) periods between end of trace interval and beginning of next Pre-CS (80- and 100-sec intervals, respectively). ANOVA revealed a pattern of statistical significance that was identical to that reported for trial 1 alone (see text, Figs. 1 and 4 for further explanation.)
Figure 2 depicts the level of freezing exhibited by each strain of mice in both the training context (shaded bars) and in the novel test context prior to tone presentation (open bars) for the paired (Fig. 2, left) and unpaired (Fig. 2, right) groups. Contextual conditioning would be expected to occur in the training context for all groups and the associative specificity of that learning would be reflected in the difference in freezing between contexts (training context, where shock occurred, and novel test context, in which no shock was delivered). An ANOVA with a mixed 3 (strain) × 2 (groups) × 2 (context) factorial design yielded a significant main effect of Context (F(1,30) = 125.852, P < 0.0001), as well as a main effect of Strain (F(2,30) = 18.036, P < 0.0001) and a significant Context × Strain interaction (F(2,30) = 14.506, P < 0.0001). Simple comparisons revealed that the interaction was the result of the C57 mice exhibiting significantly lower levels of freezing compared with the other two strains in the training context (P < 0.0001), and the 129 mice exhibiting significantly more freezing compared with the other two strains in the testing context (P < 0.03). These data demonstrate that under the Initial Protocol, background strain had an influence on response to the two contexts, but Training Group status (paired or unpaired) did not.
Our comparison between paired and unpaired strains of mice using the Initial Protocol produced some unexpected results. In trace conditioning specifically, what might appear to be conditioning in the paired mice did not differ from the nonassociative sensitization observed in the unpaired mice during tone cue testing. Futhermore, all mice conditioned to the context, but strain differences were evident.
Intermediate Protocols
At the conclusion of the Initial Protocol we set out to develop a procedure that would reduce nonassociative effects but still yield strong contextual and tone cue (trace) conditioning. To achieve this goal, several pilots were carried out in which various parameters were manipulated either individually or in combination. These intermediate pilots utilized an additional 117 mice. The general result of all of the intermediate pilots was that, depending on the parametric manipulation, conditioning to the context typically ranged from moderate to strong; freezing to the training context typically ranged from 40% to 80% and freezing to the testing context generally ranged from 10% to 20%. However, freezing to the tone cue remained in the 30%–60% range in both the unpaired and paired groups of mice of each strain during many of the intermediate protocols.
Since freezing to the tone in the unpaired groups of mice was high, we wanted to determine whether the observed sensitization was the result of the tone, the shock, or both. Therefore, in Pilot 1 we tested three groups of F1 hybrid mice: a paired group (received tone and shock on day 2), a tone only group (received only tones on days 1 and 2; no shocks) and a shock only group (received shock on day 2; no tone was presented). All other parameters remained the same as in the Initial Protocol. The paired group of mice exhibited high levels of freezing to the training context (80%) and low levels to the test context (10%), and displayed high levels of freezing to the tone cue at test (90%). The tone-only group did not generally exhibit a freezing response to the tone or either of the contexts during final testing. The shock-only group exhibited high levels of freezing to both contexts and to the tone cue, even though they had not had any previous experience with the tone. Thus, the experience of being shocked alone was sufficient to induce sensitization that influenced responding to many test stimuli in the mice.
In an attempt to minimize nonassociative effects in the unpaired groups of mice, the second intermediate pilot (Pilot 2) included only an unpaired group. Modifications to the parameters included increasing the total trial length to 210 sec, while keeping the tone and shock durations the same as in the Initial Protocol. In order to keep the length of each day’s session similar to that of the Initial Protocol, the acclimation time of the mice on day 1 and day 2 was reduced to 6 min before trials began. Acclimation time during the day 3 session and all other parameters remained unchanged. These manipulations produced results similar to those of the Initial Protocol with a large amount of nonassociative freezing (50%–60%).
A subsequent pilot study asked whether the decibel level of the tone (85 dB) contributed to sensitization. Observations of mice in the prior protocols during training revealed a pronounced startle response to the 85-dB tone and in the unpaired groups considerable freezing occurred after the termination of tone presentation. Loudness of the tone cue was lowered from 85 to 70 dB. Pilot 3 was carried out in paired and unpaired groups of all three strains. All other parameters were as in Pilot 2. This eliminated startle responses to the tone, but also reduced the level of conditioning to the training context (25%–30%) and was not sufficient to lower levels of freezing during the tone cue test (30%–60%) in the unpaired groups.
To address the weakened context conditioning observed in Pilot 3, the octagon chambers were further altered to better distinguish them from the square chambers in pilot 4. These alterations consisted of adding black and white tiles to four of the eight angled walls of the octagon chambers, giving these walls a checkered pattern that contrasted with the solid Plexiglas walls of the square chambers. All other parameters were the same as in Pilot 3. This modification substantially strengthened conditioning to the training context in all mice (40%–80%), but also increased the freezing response to the testing context in the unpaired groups of mice (50%–60%). In addition, freezing to the tone cue in the unpaired groups still remained comparable to the paired groups (∼50%).
In Pilots 5 and 6, white noise at 70 dB replaced the 2-KHz pure tone as the CS cue during training and testing, acclimation time in the testing context on day 3 was reduced to 3 min, the number of training trials was increased to six, while trial length was reduced to 150 sec. However, these modifications did not reduce nonassociative effects.
Pilot 7 used the 2-KHz 70 dB pure tone as the CS in addition to the modifications made in Pilots 5 and 6 (increased trials, reductions in trial length, and day 3 acclimation time). These parameters yielded excellent contextual learning, e.g., high levels of freezing to the training context (55%–80%) and low levels to the testing context (5%–25%). However, substantial nonassociative effects were still apparent in tests for trace conditioning (40%–60%).
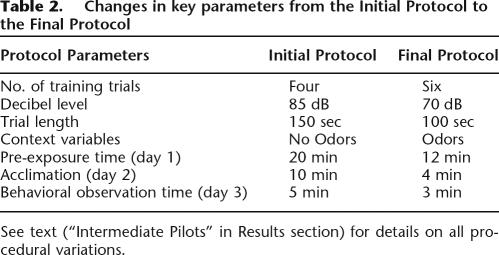
We next further distinguished the two contexts using olfactory cues. This procedural modification in addition to those outlined in Pilot 7 became the Final Protocol detailed in the Materials and Methods section, which yielded optimal data for trace conditioning while maintaining strong context conditioning. Table 2 highlights the key differences between the Initial Protocol and the Final Protocol.
Table 2.
Changes in key parameters from the Initial Protocol to the Final Protocol
See text (“Intermediate Pilots” in Results section) for details on all procedural variations.
Final Protocol
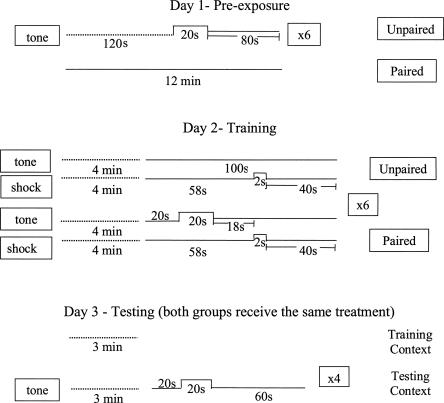
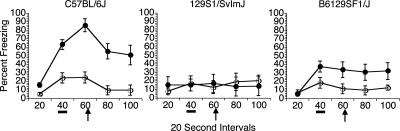
The Final Protocol was tested with a new set of 48 mice (16 of each strain), and is the protocol we continue to use in the Johns Hopkins Neurogenetics and Behavior Center with reliable results consistent with the findings reported here. A schematic of the Final Protocol is depicted in Figure 3. The results from the Final Protocol are shown in Figures 4and 5. As shown in Figure 4, the test of trace conditioning to the tone–CS yielded low levels of freezing in the unpaired groups, indicating little sensitization or pseudoconditioning. Both C57BL/6J and F1 hybrids showed evidence of associative learning as indicated by greater responses in the paired groups relative to unpaired groups for those strains. However, the 129S1/SvImJ mice failed to exhibit trace conditioning with low levels of freezing in the paired group, not differing from performance in the unpaired group. This was confirmed by a mixed four-way factorial ANOVA, which revealed significant main effects of Strain (F(2,36) = 5.382, P = 0.009), Group (F(1,36) = 14.105, P = 0.001), Odor Order (F(1,36) = 0.037), and Interval (F(4,144) = 18.931, P < 0.001), as well as significant interactions of Strain × Group (F(2,36) = 5.221, P = 0.010), Strain × Interval (F(8,144) = 5.671, P < 0.001), Interval × Group (F(4,144 = 4.726, P = 0.001), and Strain × Group × Interval (F(8,144) = 2.991, P = 0.004). The three-way interaction was mainly due to the Strain × Group effect. Both the paired C57 and paired F1 mice increased freezing at the second sampling interval (40 sec) when the tone was presented, freezing that remained high throughout the entire trial epoch. However, the level of freezing in the paired 129 mice remained low for the entire trial epoch. Indeed, further analysis of the Strain × Training Group interaction showed that, among the paired groups of mice, the C57 mice froze significantly more compared with the F1 mice (P = 0.0482) and the 129 mice (P = 0.0028). In contrast, there were no significant strain differences among any of the unpaired groups of mice. Post hoc comparisons of the paired and unpaired mice by strain revealed a significant group difference in the C57 mice (P < 0.0001), and the F1 mice (P = 0.0183), but there was no difference between the paired and unpaired groups of 129 mice. As in the Initial Protocol, only the first of the four tone–CS trials was included in the analysis. In contrast to the Initial Protocol, averages of all four trials under the Final Protocol (cf. Table 1 and Fig. 4) show that the overall freezing rates for the Pre-CS period were not elevated after the first trial. Similar to the Initial Protocol, results of ANOVA of data averaged across all four trials were identical with those of trial 1.
Figure 3.
Schematic representation of the 3-d trace fear-conditioning procedure for the Final Protocol. Note that the times represented by the dashed lines are not repeated.
Figure 4.
Test of trace conditioning to the tone–CS under the Final Protocol. All unpaired groups of mice exhibited low nonassociative effects and low levels of freezing to the tone cue. Strain differences were evident under the Final Protocol. Only the C57BL/6J mice exhibited robust conditioning to the tone–CS, while the B6129SF1/J hybrid mice exhibited conditioning, but at a lower level. The 129S1/SvImJ mice failed to condition to the tone–CS. The 20-sec interval represents the 20 sec prior to presentation of the tone–CS (pre-CS period); the black bar (40-sec interval) indicates when the tone–CS was presented; the 60-sec interval encompasses the trace interval and the time period when the shock occurred during training (black arrow). Note that freezing levels peaked at this time point. (●) Paired mice; (○) unpaired mice.
Figure 5.
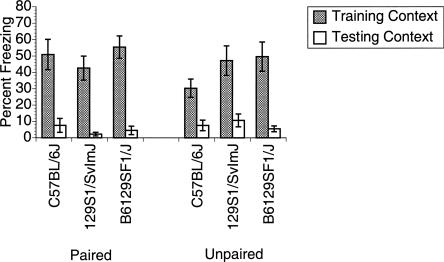
Test of contextual conditioning under the Final Protocol. All three strains of mice and both Paired (left) and Unpaired (right) groups exhibited high levels of freezing to the training context and low levels to the testing context, indicating low levels of nonassociative effects.
Odor Order was included as a factor in the analysis of the data from the Final Protocol to determine whether or not this variation affected the results. Analysis shows that this factor altered levels of freezing as a main effect, but it did not have an impact on strain differences on trace conditioning, as it did not significantly interact with any other factor.
Figure 5 shows the level of freezing exhibited by the paired and unpaired groups of mice for each strain during testing in both the training (shaded bars) and testing (open bars) contexts for paired (Fig. 5, left) and unpaired groups (Fig. 5, right). In contrast to the results of trace conditioning, context conditioning was robust for all strains of mice. The ANOVA (mixed four-way factorial design) yielded only a significant main effect of Context (F(1,36) = 149.473, P < 0.001). Thus, all mice, regardless of strain or training group, responded with equivalent high levels of freezing in the training context and similar low levels of freezing in the testing context. In contrast to the Initial Protocol, strain differences in contextual freezing were not present with the modified protocol.
Thus, with the Final Protocol, we were able to (1) eliminate nonassociative effects in tone trace fear conditioning, (2) eliminate strain differences in contextual fear conditioning, (3) disassociate strain effects on contextual vs. trace fear conditioning.
Discussion
The parameters of the Initial Protocol produced strain differences in contextual conditioning whereby C57 mice showed less associative freezing and 129 mice showed more nonassociative freezing than their counterparts in the other strains. The Initial Protocol also produced a freezing response to the tone cue, but this effect was predominantly the result of nonassociative effects of CS–US presentations, as the unpaired groups of mice also exhibited a robust response to presentation of the tone cue during testing. After modifying several experimental parameters, we generated a protocol that produced high levels of freezing in the training context and low levels of freezing in the testing context across all three strains. Moreover, this protocol eliminated nonassociative effects in response to the tone cue, and revealed a difference between the paired and unpaired groups and between strains during the trace-conditioning test.
Modification of experimental parameters reduced nonassociative effects
In developing the Final Protocol, experimental parameters were not manipulated in a fully systematic, stepwise fashion. Experimental parameters were manipulated both one at a time and in combination. In some cases experimental parameters were manipulated more than once, either alone or in combination with others. As a result, our data do not conclusively indicate which parameter(s) contributed most to the different outcomes between the Initial and Final Protocols. However, the data suggest that tone intensity and the addition of odor to the context were each important modifications. It is also notable that in both protocols a period of pre-exposure to the training context was included, and that CS and US were unpaired by presenting them on separate days.
We reduced the tone intensity from 85 to 70 dB because we observed that at the higher decibel level the response of the mice resembled unconditioned fear to loud noise or a startle response (Bullock et al. 1997; Logue et al. 1997; Willott et al. 2003). That is, when an 85-dB tone was presented, mice frequently responded by freezing independent of a history with shock. Thus, immediately after tone presentation, mice in the paired group would freeze before presentation of the shock, and mice in the unpaired group would freeze even though no shock had been presented. The 70-dB tone provided a cue that was salient but that did not elicit an unconditioned freezing and/or startle response. Thus, the associative properties of the CS were maintained while eliminating nonassociative aversive properties of the auditory cue.
It has been reported that some strains of mice, particularly C57 mice, experience some loss of hearing as they age (Zheng et al. 1999; Johnson et al. 2000; Davis et al. 2001; Francis et al. 2003). This loss is typically restricted to frequencies above 16 kHz starting at 6 mo of age (White et al. 2000; Prosen et al. 2003). In the current study, the tone cue was presented at 2 kHz to ensure that it was detectable by all strains tested. Moreover, this frequency is within the range of frequencies commonly used in fear conditioning to test both rats and mice (0.8–5 kHz) (Schafe et al. 1999; Nader et al. 2000; Holmes et al. 2002; Kinney et al. 2002; Quinn et al. 2002; Laxmi et al. 2003; Chowdhury et al. 2005; Fendt et al. 2005). It is also unlikely that there are strain differences in sensitivity to the shock US. The fact that all three strains showed robust contextual conditioning indicates that all mice detected and could process the shock as an aversive event.
Adding an odor to one of the two contexts also likely had an impact on the differing outcomes between the Initial and Final Protocols. This is an element of context that only a handful of laboratories have used (Cook et al. 2002; Wrenn et al. 2002; Balogh and Wehner 2003; Bothe et al. 2004; Gould et al. 2004), and we believe it reduced effects unrelated to tone-shock associations by making the contexts more distinguishable. Although the visual (checkered vs. solid colored walls) and tactile elements (smooth vs. grid floor; square vs. octagon shape) yielded discriminable contexts in the Initial Protocol, as reflected in the different levels of freezing to the two contexts in the initial study, the addition of odor to one of the two contexts during the Final Protocol provided a distinguishing element in a sensory modality that is highly salient to mice (Doty 1986; Beauchamp and Yamazaki 2003; Breer 2003). The effectiveness of this manipulation suggests that it further reduced generalization across the training and test contexts, generalization that may have played a role in the nonassociative responding observed in the Initial Protocol.
Pre-exposure was included as part of both the Initial and Final Protocols because it allowed the mice to acclimate to the training environment before training began. By reducing the pre-exposure time and the acclimation time before the training and test phases, we reduced the amount of time that the mice had to explore when first introduced into the chambers on all days. This modification may have favorably contributed to the results, because less reduction in exploratory activity provided a better baseline for assessing the tendency to freeze. In addition, we avoided unpaired presentations of the CS and US within the same training session (“same day” method), in favor of an unpaired procedure involving presentation of the CS during the first session on day 1, and US during the second session on day 2. We chose this “separate day” method because pilot studies suggested that it substantially reduces nonassociative freezing to the novel context (from 80% to 40%; data not shown).
One important parameter that we did not change throughout testing was the length of the trace interval. Many studies have used short trace intervals ranging from 1 to 2.5 sec (Crestani et al. 2002; Holmes et al. 2002; Kinney et al. 2002; Wrenn et al. 2002), but evidence suggests that intervals this short do not engage the hippocampus and may therefore be more similar to delay fear conditioning, in which there is no interval between CS and US presentation (Chowdhury et al. 2005; Misane et al. 2005; Wanisch et al. 2005). Still others have used longer trace intervals ranging from 20 to 30 sec (Huerta et al. 2000; Weitemier and Ryabinin 2003; Gould et al. 2004; Yee et al. 2004; Wiltgen et al. 2005). We chose an 18-sec trace interval because studies that have examined the role of the hippocampus in trace fear conditioning at multiple interval lengths (1–60 sec) have reported that a 15–20 sec interval is most effective at engaging the hippocampus (Chowdhury et al. 2005; Misane et al. 2005; Wanisch et al. 2005).
As mentioned above, our data do not conclusively indicate which of the parameters contributed most to the different outcomes between the Initial and Final protocols. Therefore, it would be worthwhile to examine each of the parameters more systematically in future studies, particularly the addition of odor cues and the intensity of the tone–CS.
Inclusion of an unpaired control group may help to more clearly identify differences between mouse strains
The inclusion of an unpaired control group is not new, but most of these studies have been in rats (Phillips and LeDoux 1994; McEchron et al. 1998; Contarino et al. 2002; Majchrzak et al. 2006), and to date, only one other study has included an unpaired control group in the examination of trace fear conditioning in mice. Huerta et al. (2000) examined trace fear conditioning in knockout mice lacking NMDA receptors in the CA1 pyramidal cells of the hippocampus. As a pseudo-conditioning control (unpaired), these investigators trained mice using a “same day” procedure to deliver 10 random presentations each of tone and shock. The control group (paired) received 10 CS–US pairings. Their results showed that during testing in a novel context, the unpaired controls exhibited a 50% freezing level. Although this was statistically lower than the reported freezing level of the KO mice (80%), a 50% freezing level strongly suggests high levels of nonassociative effects. In addition, when tested for conditioning to the tone cue, the unpaired (pseudo-control) mice exhibited an average of 40% freezing over three tone presentations. The performance of the pseudo-conditioning control group in the Huerta et al. (2000) study is similar to that of the unpaired group of the current study under the Initial Protocol. Huerta and colleagues suggest that this is due to “unavoidable factors in the experimental environment.” However, our results under the Final Protocol show that such affects are avoidable.
Several studies have compared the performance of various strains of mice on fear conditioning. All have included C57 mice, many include one or several substrains of 129 mice, and a few have included hybrids of 129 and C57 (Paylor et al. 1994; Owen et al. 1997; Stiedl et al. 1999; Balogh et al. 2002; Cook et al. 2002; Balogh and Wehner 2003; Bothe et al. 2005). For the most part, these studies have focused on delay conditioning and have reported strain-dependent differences in conditioning either to the context, the auditory cue, or both. To date, only one study has examined trace fear conditioning in different strains, but no unpaired control groups were included (Holmes et al. 2002). In the Holmes et al. (2002) study, males and females of three strains (C57, DBA, and 129S6) were trained with four CS–US pairings using an 80-dB tone and a short 2.5-sec trace interval between presentation of CS and US. The contexts in that study differed not only in visual (color) and tactile (shape, texture) elements, but also included an olfactory element. Those procedures are similar to the current study with the exception that Holmes and colleagues included odor only in the testing context, whereas in the current study, presence of odor was counterbalanced across both testing and training contexts (see Materials and Methods). They reported no conditioning in DBA mice, but found that the 129 and C57 mice conditioned to the context and the trace–CS cue. Moreover, they reported that freezing in the testing context was <20%. Their context conditioning results are similar to those of the current study, but the cue (trace) conditioning results differ in that we observed differences between the strains and they did not. Since no unpaired control group was included in the Holmes et al. (2002) study, it is possible that nonassociative effects contributed to their findings. However, it should be noted that the two studies tested different substrains of 129 mice, and it is also possible that the difference in cue trace conditioning was mediated by genetic background. Under the Final Protocol of the current study, all three strains of mice conditioned equally well to the context but conditioned differently to the trace–CS. This difference is not altogether surprising, as it is well documented that each strain of mice has a different behavioral profile (Upchurch and Wehner 1988; Voikar et al. 2001; Wolff et al. 2002; Brooks et al. 2004).
Strain differences in hippocampal processing of trace interval
In trace fear conditioning, two different types of hippocampal-dependent conditioning take place. One is the conditioning to the context as a result of associations between the context and the US, and the other is conditioning to the discrete CS over a trace interval (Phillips and LeDoux 1992; McEchron et al. 1998; Quinn et al. 2002). It has been established in rabbits (Moyer et al. 1990; Kim et al. 1995; McEchron et al. 2000), rats (Fendt et al. 2005; Quinn et al. 2005; Bangasser et al. 2006), and more recently in mice (Weitemier and Ryabinin 2004; Chowdhury et al. 2005; Misane et al. 2005; Wanisch et al. 2005) that conditioning to the trace–CS requires the hippocampus. In the current study, all three strains of mice tested, regardless of group status (paired or unpaired), exhibited robust conditioned response (freezing) to the context, indicating that this aspect of hippocampal function is intact. However, in trace conditioning we observed a strain difference (Final Protocol). We found that the F1 hybrid mice exhibited less conditioning than the C57 mice, and that the 129 mice failed to condition to the trace–CS at all. Moreover, the C57 mice exhibited peak levels of freezing during the trace interval period. That all of the strains conditioned to the context equally, but exhibited differences in trace conditioning, raises the possibility that these two aspects of hippocampal-dependent associative learning can be dissociated, that other brain systems may be involved in the difference, or that the two tasks are differentially sensitive to hippocampal disruption.
Summary and conclusions
In summary, we have shown that typical fear-conditioning procedures in mice can engage nonassociative processes that can be misleading if nonassociative control conditions are not included in the experimental designs. Here we demonstrate the development of a protocol for trace fear conditioning in mice that uses unpaired controls and produces strong contextual conditioning in all mouse strains tested, and clear trace fear conditioning in paired groups relative to unpaired control groups during trace–CS testing, in some commonly used strains. This protocol revealed strain differences in trace–CS conditioning; C57 and F1 hybrid mice exhibited trace conditioning, but the 129 mice failed to condition to the trace–CS. Given that the C57 mice conditioned well to both context and the trace–CS, it is fair to say that the Final Protocol is optimized for C57 mice. Therefore, it is still possible that a protocol could be better optimized for 129 or F1 hybrids, such that each would show improved trace–CS conditioning. The parameters in the current study were also optimized for use in a trace fear-conditioning procedure; therefore, we can draw no conclusions about how well the same set of parameters would serve conditioning in a delay fear procedure. These considerations highlight the need to include adequate analysis in the selected strains used in neurogenetics and behavioral studies.
It is sometimes argued that including an unpaired control in every test of mouse conditioning is impractical based on the added cost, especially when testing transgenic or knockout mice that are only available in limited supply. However, our results show that nonassociative effects are prevalent in mouse conditioning and this can produce both false positives and negatives in behavioral outcomes. Such imprecision in behavioral characterization could prove even more costly over the long run, as genetically modified mice are increasingly used in studies designed to discover the cellular and molecular mechanisms of behavior.
Materials and Methods
Subjects
A total of 84 mice were used for the initial and final experiments. The subjects were male mice, 14 each of the strains C57BL/6 (C57), 129S1/SvImJ (129), and a hybrid strain B6129S1 (F1 Hybrids) derived from crossing the former two strains. Mice were obtained from Jackson Laboratory at 6–8 wk of age and were 8–10 wk of age at the time of testing. Food and water were available ad libitum. The vivarium was maintained at 25°C and on a 12:12 h light:dark cycle with lights on at 07:00 h. All animal procedures were in accordance with approved institutional animal care procedures and NIH guidelines.
Initial Protocol
Apparatus
The trace fear-conditioning task was carried out in square (17.78 cm W × 17.78 cm D × 30.48 cm H, Coulbourn) and octagonal (rad 21.59 cm, 30.48 cm H, Coulbourn) chambers. During training each chamber was equipped with a grid floor through which a footshock could be delivered. During testing, the grid floors were replaced with a solid, black-colored wooden floor coated with a clear sealant. The walls of the chambers were made of clear Plexiglas. All of the chambers were mounted within specially designed sound-attenuating shells constructed of polypropylene and PVC. Each shell was equipped with an exhaust fan (which also served as a background noise generator), a speaker mounted on the back wall through which a tone could be delivered, and a red ambient (7 W) overhead house light. The tone and shock were created via a peripheral Coulbourn programmable tone generator (model #A69-20) and Coulbourn programmable precision-regulated animal shocker (model #H13-16), and all stimuli onset and duration were controlled by a PC interfaced with Coulbourn Graphic State software. All chambers were cleaned with 70% ethanol before and after each use.
Procedure
Procedures for the Initial Protocol involved three phases: (1) pre-exposure, which allowed the mice to acclimate and become familiar with the training chamber, (2) training, during which the mice were presented with the CS and or US stimuli, and (3) testing, in which mice were observed for freezing in response to each context and to the tone and trace–CS. Each phase occurred at 24-h intervals.
Pre-exposure
On day 1, all mice were individually placed into a training chamber for 20 min. The mice assigned to the unpaired groups (six each C57, 129, and F1 hybrids) were allowed to acclimate for 10 min. After the acclimation period, an 85-Db tone was presented for 20 sec, followed by a 130-sec interval. This was repeated four times for a total of four tone presentations over a total time period of 10 min. The mice assigned to the paired groups (six each C57, 129, and F1 hybrids) were allowed to acclimate for 20 min, and no tones were presented during this time.
Training
On the training day (day 2), mice were individually placed into the same training chamber as on day 1, and all mice were allowed to acclimate for 10 min, after which conditioning trials began. Training consisted of four 150-sec conditioning trials. For the unpaired groups of mice, each trial began with a 58-sec interval, followed by presentation of a 2-sec footshock (0.5 mA), followed by another interval period of 90 sec. No tones were presented to these mice. For the paired groups of mice, each trial began with a 20-sec baseline interval, followed by presentation of a 20-sec tone (85 dB; conditioning stimulus, CS), followed by an 18-sec trace interval, followed by presentation of a 2-sec footshock (0.5 mA; unconditioning stimulus, US), followed by a 90-sec post shock interval. At the end of the last trial, all mice were removed from the chamber and returned to their home cage.
Testing
On the testing day (day 3) mice were individually placed into the same chamber (familiar context) as on the training day (day 2) and allowed to move about freely for 5 min (training context test). Mice were then removed and returned to the home cage for 2 min. Mice were then placed in the test chamber, which was different from the chamber of the training day (novel context; i.e., if a mouse was trained in a square chamber, it was tested in an octagonal chamber, or vice versa) and allowed to move about freely for 5 min (novel context test). Following this, CS-alone test trials were run to assess responses to the tone cue (against the novel context “background”), as a measure of trace conditioning. All mice received four 150-sec test trials. For all mice, each trial began with a 20-sec baseline interval, followed by presentation of a 20-sec tone (85 dB), followed by a 110-sec interval (20-sec trace, 90-sec post-trace). At the end of the last trial, all mice were removed from the chamber and returned to their home cage.
Scoring
All scoring was done at the conclusion of testing from video by a trained observer blind to experimental conditions. During this time the amount of freezing was observed in 1-sec increments throughout each context test exposure and each 150-sec trial epoch (20-sec baseline, 20-sec tone, 20-sec trace, 90-sec post-trace period). Scoring occurred throughout the entire trial epoch and intertrial interval.
Final Protocol
Apparatus
The training and testing chambers for the Final Protocol were identical to those used under the Initial Protocol with the following noted modifications: the octagonal chambers were further altered such that the Plexiglas walls were interspersed with opaque black and white tiles to form a checkered pattern along four of the eight angles. Furthermore, half of the square chambers and half of the octagonal chambers were scented with three drops of vanilla extract added to the drop pan.
Procedure
To arrive at the final protocol, several parameters from the initial protocol were manipulated either in combination or alone. These manipulations took place over a series of seven pilot studies that occurred between the experiments described under the Initial Protocol and those described under the Final Protocol (see “Intermediate Pilots” in Results). Procedures for the Final Protocol were similar to those for the Initial protocol with the following noted exceptions:
Pre-exposure
During pre-exposure (day 1) the acclimation period was shortened to 2 min before tone presentation began for the mice assigned to the unpaired groups (eight each for C57, 129, and F1 hybrids). For those mice, presentation of the 20-sec tone cue was followed by an 80-sec interval, and was repeated a total of six times. For the mice assigned to the paired groups (eight each C57, 129, and F1 hybrids) the acclimation period was 12 min, corresponding to the duration of the pre-exposure session for the unpaired groups.
Training
On the training day (day 2) the acclimation period was 4 min, six conditioning trials involving a 70-dB tone–CS and a 2-sec, 0.5 mA shock US were run in trial epochs that lasted 100 sec (including the intertrial interval). For the paired groups of mice, each trial consisted of a 20-sec “baseline” interval, a 20-sec tone presentation, an 18-sec trace interval, a 2-sec shock, and a 40-sec postshock interval. Trials were the same for the unpaired groups of mice except that the tone was omitted (i.e., a 58-sec interval, a 2-sec shock, and 40-sec postshock interval).
Testing
On the testing day (day 3), mice were allowed to move about freely in the training (familiar) and test (novel; i.e., if a mouse was trained in a square unscented chamber, it was tested in an octagonal scented chamber) contexts for 3 min. All mice received four 100-sec testing trials. For all mice, each trial began with a 20-sec interval and the 20-sec 70 dB tone presentation was followed by a 60-sec interval.
Scoring
Scoring was carried out throughout the 100-sec trial epoch as described under the Initial Protocol.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.614807
References
- Balogh S.A., Wehner J.M. Inbred mouse strain differences in the establishment of long-term fear memory. Behav. Brain Res. 2003;140:97–106. doi: 10.1016/s0166-4328(02)00279-6. [DOI] [PubMed] [Google Scholar]
- Balogh S.A., Radcliffe R.A., Logue S.F., Wehner J.M. Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: Context discrimination and the effects of retention interval. Behav. Neurosci. 2002;116:947–957. doi: 10.1037//0735-7044.116.6.947. [DOI] [PubMed] [Google Scholar]
- Bangasser D.A., Waxler D.E., Santollo J., Shors T.J. Trace conditioning and the hippocampus: The importance of contiguity. J. Neurosci. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T., Zhang W.N., Feldon J. Hippocampus and classical fear conditioning. Hippocampus. 2001;11:828–831. doi: 10.1002/hipo.1098. [DOI] [PubMed] [Google Scholar]
- Beauchamp G.K., Yamazaki K. Chemical signalling in mice. Biochem. Soc. Trans. 2003;31:147–151. doi: 10.1042/bst0310147. [DOI] [PubMed] [Google Scholar]
- Bothe G.W., Bolivar V.J., Vedder M.J., Geistfeld J.G. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 2004;3:149–157. doi: 10.1111/j.1601-183x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- Bothe G.W., Bolivar V.J., Vedder M.J., Geistfeld J.G. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp. Med. 2005;55:326–334. [PubMed] [Google Scholar]
- Breer H. Sense of smell: Recognition and transduction of olfactory signals. Biochem. Soc. Trans. 2003;31:113–116. doi: 10.1042/bst0310113. [DOI] [PubMed] [Google Scholar]
- Brooks S.P., Pask T., Jones L., Dunnett S.B. Behavioural profiles of inbred mouse strains used as transgenic backgrounds. I: Motor tests. Genes Brain Behav. 2004;3:206–215. doi: 10.1111/j.1601-183X.2004.00072.x. [DOI] [PubMed] [Google Scholar]
- Bullock A.E., Slobe B.S., Vazquez V., Collins A.C. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behav. Neurosci. 1997;111:1353–1360. doi: 10.1037//0735-7044.111.6.1353. [DOI] [PubMed] [Google Scholar]
- Chowdhury N., Quinn J.J., Fanselow M.S. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav. Neurosci. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Contarino A., Baca L., Kennelly A., Gold L.H. Automated assessment of conditioning parameters for context and cued fear in mice. Learn. Mem. 2002;9:89–96. doi: 10.1101/lm.43002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M.N., Bolivar V.J., McFadyen M.P., Flaherty L. Behavioral differences among 129 substrains: Implications for knockout and transgenic mice. Behav. Neurosci. 2002;116:600–611. [PubMed] [Google Scholar]
- Crawley J.N., Belknap J.K., Collins A., Crabbe J.C., Frankel W., Henderson N., Hitzemann R.J., Maxson S.C., Miner L.L., Silva A.J., et al. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Cressant A., Besson M., Suarez S., Cormier A., Granon S. Spatial learning in Long-Evans Hooded rats and C57BL/6J mice: Different strategies for different performance. Behav. Brain Res. 2007;177:22–29. doi: 10.1016/j.bbr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Crestani F., Keist R., Fritschy J.M., Benke D., Vogt K., Prut L., Bluthmann H., Mohler H., Rudolph U. Trace fear conditioning involves hippocampal α5 GABA(A) receptors. Proc. Natl. Acad. Sci. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.R., Newlander J.K., Ling X., Cortopassi G.A., Krieg E.F., Erway L.C. Genetic basis for susceptibility to noise-induced hearing loss in mice. Hear. Res. 2001;155:82–90. doi: 10.1016/s0378-5955(01)00250-7. [DOI] [PubMed] [Google Scholar]
- Doty R.L. Odor-guided behavior in mammals. Experientia. 1986;42:257–271. doi: 10.1007/BF01942506. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Poulos A.M. The neuroscience of mammalian associative learning. Annu. Rev. Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Fendt M., Fanselow M.S., Koch M. Lesions of the dorsal hippocampus block trace fear conditioned potentiation of startle. Behav. Neurosci. 2005;119:834–838. doi: 10.1037/0735-7044.119.3.834. [DOI] [PubMed] [Google Scholar]
- Francis H.W., Ryugo D.K., Gorelikow M.J., Prosen C.A., May B.J. The functional age of hearing loss in a mouse model of presbycusis. II. Neuroanatomical correlates. Hear. Res. 2003;183:29–36. doi: 10.1016/s0378-5955(03)00212-0. [DOI] [PubMed] [Google Scholar]
- Frick K.M., Stillner E.T., Berger-Sweeney J. Mice are not little rats: Species differences in a one-day water maze task. Neuroreport. 2000;11:3461–3465. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- Gould T.J., Feiro O., Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav. Brain Res. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Han C.J., O'Tuathaigh C.M., van Trigt L., Quinn J.J., Fanselow M.S., Mongeau R., Koch C., Anderson D.J. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc. Natl. Acad. Sci. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A., Wrenn C.C., Harris A.P., Thayer K.E., Crawley J.N. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Huerta P.T., Sun L.D., Wilson M.A., Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Johnson K.R., Zheng Q.Y., Erway L.C. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Clark R.E., Thompson R.F. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav. Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kinney J.W., Starosta G., Holmes A., Wrenn C.C., Yang R.J., Harris A.P., Long K.C., Crawley J.N. Deficits in trace cued fear conditioning in galanin-treated rats and galanin-overexpressing transgenic mice. Learn. Mem. 2002;9:178–190. doi: 10.1101/m.49502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans G., Blokland A., van Nieuwenhuijzen P., Prickaerts J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol. Behav. 2003;79:683–693. doi: 10.1016/s0031-9384(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Laxmi T.R., Stork O., Pape H.C. Generalisation of conditioned fear and its behavioural expression in mice. Behav. Brain Res. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Logue S.F., Owen E.H., Rasmussen D.L., Wehner J.M. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: Implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Majchrzak M., Ferry B., Marchand A.R., Herbeaux K., Seillier A., Barbelivien A. Entorhinal cortex lesions disrupt fear conditioning to background context but spare fear conditioning to a tone in the rat. Hippocampus. 2006;16:114–124. doi: 10.1002/hipo.20138. [DOI] [PubMed] [Google Scholar]
- Maren S., Quirk G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McEchron M.D., Bouwmeester H., Tseng W., Weiss C., Disterhoft J.F. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McEchron M.D., Tseng W., Disterhoft J.F. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus. 2000;10:739–751. doi: 10.1002/1098-1063(2000)10:6<739::AID-HIPO1011>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Misane I., Tovote P., Meyer M., Spiess J., Ogren S.O., Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Montkowski A., Poettig M., Mederer A., Holsboer F. Behavioural performance in three substrains of mouse strain 129. Brain Res. 1997;762:12–18. doi: 10.1016/s0006-8993(97)00370-3. [DOI] [PubMed] [Google Scholar]
- Moyer J.R., Deyo R.A., Disterhoft J.F. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Nader K., Schafe G.E., Le Doux J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Owen E.H., Logue S.F., Rasmussen D.L., Wehner J.M. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: Implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Paylor R., Tracy R., Wehner J., Rudy J.W. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav. Neurosci. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn. Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- Podhorna J., Didriksen M. Performance of male C57BL/6J mice and Wistar rats in the water maze following various schedules of phencyclidine treatment. Behav. Pharmacol. 2005;16:25–34. doi: 10.1097/00008877-200502000-00003. [DOI] [PubMed] [Google Scholar]
- Prosen C.A., Dore D.J., May B.J. The functional age of hearing loss in a mouse model of presbycusis. I. Behavioral assessments. Hear. Res. 2003;183:44–56. doi: 10.1016/s0378-5955(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Quinn J.J., Oommen S.S., Morrison G.E., Fanselow M.S. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Quinn J.J., Loya F., Ma Q.D., Fanselow M.S. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Runyan J.D., Moore A.N., Dash P.K. A role for prefrontal cortex in memory storage for trace fear conditioning. J. Neurosci. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G.E., Nadel N.V., Sullivan G.M., Harris A., LeDoux J.E. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn. Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Schneider F., Weiss U., Kessler C., Muller-Gartner H.W., Posse S., Salloum J.B., Grodd W., Himmelmann F., Gaebel W., Birbaumer N. Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biol. Psychiatry. 1999;45:863–871. doi: 10.1016/s0006-3223(98)00269-8. [DOI] [PubMed] [Google Scholar]
- Sommer M., Hajak G., Dohnel K., Schwerdtner J., Meinhardt J., Muller J.L. Integration of emotion and cognition in patients with psychopathy. Prog. Brain Res. 2006;156:457–466. doi: 10.1016/S0079-6123(06)56025-X. [DOI] [PubMed] [Google Scholar]
- Stiedl O., Radulovic J., Lohmann R., Birkenfeld K., Palve M., Kammermeier J., Sananbenesi F., Spiess J. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav. Brain Res. 1999;104:1–12. doi: 10.1016/s0166-4328(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Upchurch M., Wehner J.M. Differences between inbred strains of mice in Morris water maze performance. Behav. Genet. 1988;18:55–68. doi: 10.1007/BF01067075. [DOI] [PubMed] [Google Scholar]
- Veit R., Flor H., Erb M., Hermann C., Lotze M., Grodd W., Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci. Lett. 2002;328:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Voikar V., Koks S., Vasar E., Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol. Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Wanisch K., Tang J., Mederer A., Wotjak C.T. Trace fear conditioning depends on NMDA receptor activation and protein synthesis within the dorsal hippocampus of mice. Behav. Brain Res. 2005;157:63–69. doi: 10.1016/j.bbr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Weitemier A.Z., Ryabinin A.E. Alcohol-induced memory impairment in trace fear conditioning: A hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- Weitemier A.Z., Ryabinin A.E. Subregion-specific differences in hippocampal activity between Delay and Trace fear conditioning: An immunohistochemical analysis. Brain Res. 2004;995:55–65. doi: 10.1016/j.brainres.2003.09.054. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q., Tomie J.A. Of mice and mazes: Similarities between mice and rats on dry land but not water mazes. Physiol. Behav. 1996;60:1191–1197. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]
- White J.A., Burgess B.J., Hall R.D., Nadol J.B. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear. Res. 2000;141:12–18. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Willott J.F., Tanner L., O'Steen J., Johnson K.R., Bogue M.A., Gagnon L. Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behav. Neurosci. 2003;117:716–727. doi: 10.1037/0735-7044.117.4.716. [DOI] [PubMed] [Google Scholar]
- Wiltgen B.J., Sanders M.J., Ferguson C., Homanics G.E., Fanselow M.S. Trace fear conditioning is enhanced in mice lacking the δ subunit of the GABAA receptor. Learn. Mem. 2005;12:327–333. doi: 10.1101/lm.89705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M., Savova M., Malleret G., Segu L., Buhot M.C. Differential learning abilities of 129T2/Sv and C57BL/6J mice as assessed in three water maze protocols. Behav. Brain Res. 2002;136:463–474. doi: 10.1016/s0166-4328(02)00192-4. [DOI] [PubMed] [Google Scholar]
- Wrenn C.C., Marriott L.K., Kinney J.W., Holmes A., Wenk G.L., Crawley J.N. Galanin peptide levels in hippocampus and cortex of galanin-overexpressing transgenic mice evaluated for cognitive performance. Neuropeptides. 2002;36:413–426. doi: 10.1016/s0143-4179(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Yee B.K., Hauser J., Dolgov V.V., Keist R., Mohler H., Rudolph U., Feldon J. GABA receptors containing the α5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur. J. Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Zheng Q.Y., Johnson K.R., Erway L.C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]