Abstract
Preliminary data suggest an association of posterior cortical gray matter reduction with poor outcome in schizophrenia. We made a systematic MRI assessment of regional gray and white matter volumes, parcellated into 40 Brodmann’s areas, in 104 patients with schizophrenia (51 with good outcomes, 53 with poor outcomes) and 41 normal comparison subjects, and investigated correlations of regional morphometry with outcome and severity of the illness. Schizophrenia patients displayed differential reductions in frontal and to a lesser degree temporal gray matter volumes in both hemispheres, most pronounced in the frontal pole and lateral temporal cortex. White matter volumes in schizophrenia patients were bilaterally increased, primarily in the frontal, parietal, and isolated temporal regions, with volume reductions confined to anterior cingulate gyrus. In patients with schizophrenia as a group, higher illness severity was associated with reduced temporal gray matter volumes and expanded frontal white matter volumes in both hemispheres. In comparison to good-outcome group, patients with poor outcomes had lower temporal, occipital, and to a lesser degree parietal gray matter volumes in both hemispheres and temporal, parietal, occipital, and posterior cingulate white matter volumes in the right hemisphere. While gray matter deficits in the granular cortex were observed in all schizophrenia patients, agranular cortical deficits in the left hemisphere were peculiar to patients with poor outcomes. These results provide support for frontotemporal gray matter reduction and frontoparietal white matter expansion in schizophrenia. Poor outcome is associated with more posterior distribution (posteriorization) of both gray and white matter changes, and with preferential impairment in the unimodal visual and paralimbic cortical regions.
Keywords: Schizophrenia, Poor outcome, Gray matter, White matter, Illness severity, MRI
Introduction
Schizophrenia is a complex diagnostic entity characterized by a multitude of dauntingly variable and often subjective signs and symptoms. The broad clinical picture of schizophrenia is arguably paralleled by a like diversity of pathological and neuroimaging findings. Indeed, practically every brain region has at some point been implicated in its pathophysiology (Harrison, 1999; Shenton, 2001). Not surprisingly, there have been ongoing efforts to narrow this protean semiotics into more inclusive syndromes and readily identifiable endophenotypes, - efforts that date back to Kraepelin and reached apotheosis with Karl Leonhard’s meticulous, if counterproductive, classification of endogenous psychoses (Jablensky, 2006). The thinking goes that based on the relative preponderance of these diverse clinical manifestations, several more homogeneous schizophrenic subtypes would be delineated and genetically affixed. Longitudinal outcome and severity of the illness have not been eschewed by this classificatory attention, leading to narrower, partially overlapping (Nakaya and Ohmori, 2006) categories of deficit/nondeficit and “Kraepelinian” schizophrenia (Roy et al., 2001). The latter, very poor outcome “Kraepelinian” subtype is defined by a complete existential dependence on others for maintaining basic necessities of life and thus may represent the majority of chronically institutionalized patients with schizophrenia (Keefe et al., 1987, 1988; Bralet et al., 2002).
In comparison to schizophrenia patients with more favorable outcomes, the course of the poor-outcome schizophrenia is characterized by severe dysfunctions in self-care (Keefe et al., 1996), limitations in premorbid sociosexual functioning (Keefe et al., 1989, 1990), more severe negative symptoms and formal thought disorder (Stephens, 1978; Keefe et al., 1989), poorer response to antipsychotic treatment (Harvey et al., 1991), lower association with affective symptomatology (Kilzieh et al., 2003; Rieckmann et al., 2005), excessive summertime clustering of birthdates (Bralet et al., 2002), and more extensive family history of schizophrenia spectrum disorders (Keefe et al., 1992). Some of these poor-outcome characteristics are in turn interrelated, e.g. nonresposiveness to antipsychotic treatment and increased familiality of the illness (Joober et al., 2005) or poor premorbid sociosexual functioning and severity of the negative symptoms (Salokangas, 2003). Clinical studies suggest rather significant and apparently multilobar neuropsychological impairments in this group of patients, including high incidence of age disorientation (Tapp et al., 1993; Manschreck et al., 2000), impaired performance on visual-motor processing, abstraction/flexibility (Albus et al., 1996), fine motor dexterity, and executive functioning tests (Roy et al., 2003).
Earlier computerized tomography and MRI studies observed that in comparison to patients with good outcomes, poor-outcome patients exhibited relatively larger ventricular asymmetry (Losonczy et al., 1986), as well as size (Pearlson et al., 1984; Katsanis et al., 1991; Rossi et al., 2000), and the latter not only was predictive of poor outcome (DeLisi et al., 1992) but continued to show progressive enlargement over a 5-year follow-up period (Davis et al., 1998). More recently, our group found that poor-outcome schizophrenia patients registered lower frontal/occipital metabolic ratios and regional metabolic rates in the temporal lobe, cingulate gyrus, and striatum as assessed by PET, as well as lower volumes of the putamen, especially in the right hemisphere, which may perhaps be associated with differential responsiveness to antipsychotic pharmacotherapy among the two patient groups (Buchsbaum et al., 2002, 2003). Pilot morphometric analyses, on a subsample from the current investigation, suggested a pattern of posteriorization, or more posterior distribution of cortical gray matter deficits in patients with poor outcomes. So while all patients with schizophrenia showed dorsolateral prefrontal gray matter deficits regardless of outcome, it were more posterior - temporal, parietal, and occipital - changes in gray matter volumes that differentiated between patients with varying outcomes (Mitelman et al., 2003, 2005a).
Consilient evidence for changes in white matter volumes and connectivity patterns in the poor-outcome patients group has also been recently obtained. Lower white matter volumes in poor-outcome than in good-outcome group were noted in a subsample of subjects from the current study beneath the temporal Brodmann’s area 42, parietal Brodmann’s area 31, and occipital Brodmann’s area 19 (Mitelman et al., 2003, 2005a). Differential prefrontothalamic (Mitelman et al., 2005b) and reduced prefrontotemporal (Mitelman et al., 2005c, 2005d) regional volume associations, larger decreases in ventral thalamic (Brickman et al., 2004) and anterior capsular (Brickman et al., 2006) volumes, and more extensive anisotropy reductions in the widespread white matter regions and fiber tracts (Mitelman et al., 2006, 2007) in the sample of patients with poor clinical outcomes used in the current study clearly implicate white matter changes in the prognostic subtyping of schizophrenia.
Changes in white matter volumes, however, are notoriously more difficult to appreciate because of the less clear-cut partitioning schemes and because of the relative subtlety of these changes in comparison to decreases in the co-territorial gray matter volumes (Mitelman et al., 2003). In this study, we utilized a large sample of schizophrenia patients with different outcomes, well-powered to detect these subtle morphological changes, and a Brodmann parcellation scheme aimed at a systematic and comprehensive assessment of both gray and white matter morphometry across the brain. Our goal was to confirm and extend the posteriorization in poor outcome hypothesis for gray matter from our own preliminary investigations in a much larger sample and supplement it with the analogous exploratory assessment of the regional white matter. To this end, analysis of variance (ANOVA) was done to contrast select assortments of Brodmann’s areas that reflect differential pathophysiological role accorded to anatomical regions or architectonic and information-processing supraregional divisions of the brain by various authors. Regional morphometry was then evaluated in relation to illness severity as reflected by the Positive and Negative Syndrome Scale (PANSS) general psychopathology scores (Kay et al., 1987) and in relation to longitudinal outcome of the illness by means of comparing patients with very poor outcomes (Kraepelinian schizophrenia) to those with more favorable outcomes. Finally, the use of the identical sample of participants and identical parcellation of the whole-brain white matter by its association with cortical Brodmann’s areas in the assessment of fractional anisotropy (Mitelman et al., 2006) and volumetric measures (in the current study) affords us a unique opportunity for their direct comparison.
Materials and methods
Participants
Participants of the study comprised 41 normal subjects and 104 patients with schizophrenia, divided into those with good outcomes (n=51) and with poor outcomes (n=53) based on the criteria by Keefe et al. (1987, 1988). In brief, these required that poor-outcome patients met the following criteria for at least five years prior to study contact: 1) continuous hospitalization or complete dependence on others for food, clothing, and shelter; 2) no useful employment; and 3) no evidence of symptom remission. All other schizophrenia patients were considered good-outcome. Since multiple clinical factors were considered by Keefe and colleagues (1996) in subdividing patients into the two groups, a single number to represent a severity continuum was not developed. Instead these authors emphasized naturalistic longitudinal course differences and argued “subtypologies based on differences in longitudinal course classify schizophrenic patients by criteria that are more likely than cross-sectional criteria to remain consistent throughout a patient’s illness”. For this reason, in our analyses we treated the good-outcome and poor-outcome subgroups in a dichotomized manner, rather than as a continuum of severity. See Table 1 for demographic and clinical details.
Table 1.
Demographic and clinical characteristics of schizophrenia patients and normal comparison subjects
| Variable | Normal Subjects | Schizophrenia Patients | ||
|---|---|---|---|---|
| Good Outcome | Poor Outcome | Total | ||
| Age | 44.2±14.7 | 40.6±12.6 | 44.8±11.4 | 43.0±12.3 |
| % women | 31.7% | 19.6% | 18.9% | 19.2% |
| Left-handed | 3 | 3 | - | 3 |
| Median years of educationa | 16.5 | 12.9 | 12.5 | 12.7 |
| MMSEb | 29.9±0.42 | 27.0±2.6 | 26.7±2.8 | 26.9±2.7 |
| PANSSc positive | 16.1±4.9 | 21.7±6.9 | 18.9±6.6 | |
| PANSS negative | 16.2±5.4 | 21.6±8.6 | 18.9±7.7 | |
| PANSS general | 32.2±7.7 | 41.8±9.3 | 37.1±9.8 | |
| Age at neuroleptic onset | 26.7±6.9 | 22.8±10.7 | 25.0±9.0 | |
| Neuroleptic exposure at scan date | ||||
| % none | 15.2 | 10.0 | 12.8 | |
| % typical | 32.6 | 17.5 | 25.6 | |
| % atypical | 39.1 | 47.5 | 43.0 | |
| % mixed | 13.0 | 25.0 | 18.6 | |
| Predominant neuroleptic exposure over preceding 3 years | ||||
| % none | 28.3 | 30.0 | 29.4 | |
| % typical | 32.6 | 15.0 | 24.4 | |
| % atypical | 26.1 | 32.5 | 29.1 | |
| % mixed | 13.0 | 22.5 | 17.4 | |
Data are expressed as means ± standard deviations
Median years of education not available for all subjects
MMSE, mini-mental status examination
PANSS, positive and negative syndrome scales
All participants were administered a semi-structured diagnostic interview with the Comprehensive Assessment of Symptoms and History (Andreasen et al., 1992) and those meeting DSM-IV criteria for a psychotic disorder were classified into the paranoid (n=25), disorganized (n=4), residual (n=5), catatonic (n=3), and undifferentiated (n=56) schizophrenia subtypes, or schizoaffective disorder (n=11). Patients with schizophrenia were recruited from the inpatient and outpatient services at Pilgrim State Psychiatric Center, Mount Sinai and Bronx VA Medical Centers, all in New York metropolitan area. The matched normal comparison subjects were recruited through advertisement. The exclusion criteria consisted of a history of licit or illicit substance abuse, head trauma, neurological illness, more than 25% exceedance of average body weight, as well as significant abnormalities on screening physical examination and laboratory tests (including urine toxicology screen, thyroid function, VDRL, B12 and folate levels). The project was approved by the institutional review board of the Mount Sinai School of Medicine and informed consent was obtained from each participant.
There were no significant age or gender differences between schizophrenia patients and normal subjects (t143=0.58, p=0.56 and χ21=0.02, p=0.11) and between patients with good and poor outcomes (t102=1.77, p=0.08 and χ21=3.24, p=0.93). Nor were there significant intergroup differences in prevailing pattern of pharmacotherapy at the time of the scan (χ21=4.36, p=0.23) and over preceding three years (unmedicated, predominantly typical, atypical, or mixture of both neuroleptic types; χ21=4.14, p=0.25). As compared to the good-outcome group, patients with poor outcomes commenced neuroleptic treatment at a younger age (t67=2.34, p=0.02) and displayed significantly more severe positive syndrome (21.7±6.9 vs. 16.1±4.9), negative syndrome (21.6±8.6 vs. 16.2±5.4), and general psychopathology (41.8±9.3 vs. 32.2±7.7) scores as assessed with PANSS (all F ratios higher than 15.00, df=1, 97, p<0.0001). Mini-Mental Status Examination scores were available for 36 normal subjects and 91 patients with schizophrenia, with significantly lower scores in patients than comparison subjects (t125=6.6, p<0.0000001) and no difference between patients with varying outcomes (t89=0.5, p=0.62). Participants in the current study have previously featured in reports on thalamic and capsular morphometry (Brickman et al., 2004; Mitelman et al., 2005a; Brickman et al., 2006), intercorrelations of regional cortical volumes (Mitelman et al., 2005b, 2005c, 2005d), and diffusion tensor imaging of the white matter (Mitelman et al., 2006, 2007; Buchsbaum et al., 2006b). A subset of 37 patients and 37 comparison subjects from the present cohort participated in previously published pilot volumetric assessments (Mitelman et al., 2003, 2005a).
Image acquisition and processing
T1-weighted MR images were acquired using a 1.5T scanner (Signa 5×, GE Medical Systems) with a 3D-SPGR sequence (TR=24 msec, TE=5 msec, flip angle=40°, matrix size 256×256, field of view 23 cm, slice thickness 1.2 mm, total slices 128, NEX=1). Images were adjusted along the AC-PC axis with a 6-parameter rigid-body transformation using SPM 1999. Brodmann map parcellation and quantification of tissue types were performed exactly as in our previous pilot report on a subsample of present participants (Mitelman et al., 2003; see also Mitelman et al., 2005c for a detailed description and validation, as well as discussed limitations of the technique). Briefly, we used a semi-automated parcellation technique based on the Perry post-mortem brain atlas (the atlas was published in Mitelman et al., 2005c). This method splits manually traced coronal brain slices into 20 radial and 10 midline sectors in each hemisphere, and each temporal lobe into 16 sectors, which are then assigned to 40 Brodmann’s areas identified in the Perry atlas. Segmentation into the white and gray tissue types is done using the FSL program FAST with bias field correction of MRIs followed by kmeans clustering and local Markov analysis at each voxel; a binary image is created for each of the tissue types, which has received extensive methodological examination (Zhang et al., 2001). Our gray matter volume ± standard deviation for area 44 (left plus right) in 41 normal subjects was 8.1±1.2 cm3, which is close to that of 7 cm3 reported in a stereological study by Uylings et al. (2005, our reading of graphs in their figure 6). For area 45 (left plus right) we obtained 8.6±1.2 cm3, which is smaller than their value of 15 cm3 (Uylings et al., 2005). We also observed the left>right asymmetry found by this research group (Uylings et al., 2006): in area 45 left=4.20±0.65 cm3, right=4.00±0.65 cm3, t=3.03, p=0.0041, consistent with the validity of our segmentation and Brodmann’s area assessment methods.
Statistical analysis
We analyzed relative tissue volumes, computed as the ratio of absolute region-of-interest volume in mm3 to total brain volume (a sum of all slices multiplied by the slice thickness in mm3). To address the issue of multiple statistical testing in assessment of the resultant dataset, multiway analysis of variance (ANOVA) with group by region and hemisphere interactions served as justification for follow-up Student’s t tests (used to isolate and confirm the ANOVA-derived patterns of intergroup differences). The Huynh-Feldt degree-of-freedom correction was used, with both uncorrected and corrected p values tabulated. Where group-by-region interactions were significant with ANOVA and/or Huynh-Feldt procedure, we proceeded with follow-up t-test display for individual Brodmann’s areas. We present t-tests with a color bar for each Brodmann’s area to allow the reader to use either a replication or exploratory probability level (e.g. reduced volumes for area 22 have been reported in several studies, hence it could be argued that it should be tested one-tailed in replication; similar arguments might be developed for medial and dorsolateral prefrontal regions). Finally, in order to evaluate the relationship between regional volumes and illness severity, we explored the Pearson’s r product-moment correlations between the PANSS scores and Brodmann’s area volumes in schizophrenia patients groups. Here, our a priori hypothesis was a negative correlation between temporal lobe volume and PANSS (based on pilot good vs. poor outcome volume analysis in Mitelman et al., 2003). We also explored white matter volume correlations with PANSS scores; the individual correlations are provided (in the figure legend) so that a priori (0.05) and corrected (0.0013) levels can be considered.
A systematic contrasting of Brodmann’s area groupings was done with repeated measures ANOVA. Analysis of co-variance (ANCOVA) was also used to control for subjects’ age at the time of the scan and at initiation of antipsychotic treatment; significant interactions are presented when distinct from ANOVA. Diagnostic categories were used as an independent group factor (schizophrenia patients vs. normal subjects and patients with good outcomes vs. patients with poor outcomes). The repeated measures were hemisphere (right, left), region (e.g. frontal, temporal, and parietal lobes), and selected sets of Brodmann’s areas constituting each region (enumerated in Table 2). These sets of Brodmann’s areas for the regional contrasts were arranged based on the anatomical and functional-architectonic considerations, anchored in the detailed review of the schizophrenia literature. Thus, anatomical ROI groupings strived to comprehensively contrast variously arranged lobar volumes (e.g. frontal vs. temporal), gross sublobar regions (e.g. lateral and medial temporal or posterior, dorsolateral, orbital, and cingulate frontal), and volumes of individual Brodmann’s areas within each lobe. Regional gray and white matter volumes in these ANOVAs were analyzed separately.
Table 2. Brodmann’s area ANOVA comparisons between patient groups and normal subjects.
Regional anatomical contrasts entering each regional contrast ANOVA are presented under “Anatomical contrasts” (first column) as regions and their constituent Brodmann’s areas (in parentheses). Significant ANOVA effects including group (g), region (r) and hemisphere (h) are listed in other columns. Italicized are interactions at a trend level of significance (p<0.06, two-tailed). Uncorrected p-values are followed by Huynh-Feldt corrected p-values in parentheses. Note that Brodmann’s areas 1, 2, 3, and 5 were combined in these analyses (hence 1/2/3/5).
| Schizophrenia vs. normal subjects | Good-outcome vs. poor-outcome patients | |||
|---|---|---|---|---|
| Anatomical contrasts | Gray matter | White matter | Gray matter | White matter |
| Interlobar | ||||
| DLPFC (44-45-46), temporal (20-21-22), parietal (7a-39-40), occipital (17-18-19) |
— | g×h×r, F3, 429=3.16, p=0.025 (0.03) | g×h, F1, 102=4.403, p=0.04 (0.04) g×r, F1, 102=3.39, p=0.02 (0.047) g×r×BA, F6, 612=3.63, p=0.0015 (0.005) |
g×r, F3, 306=2.72, p=0.045 (0.08) g×r×BA, F6, 612=2.295, p=0.034 (0.04) |
| Frontal (4-6-8-9-10-11-12-44-45-46-47), temporal (20-21-22-27-28-34-35-36-38-41-42), parietal (1/2/3/5-7a-7b-23-26-29-30-31-39-40-43) |
g×r, F2, 286=3.44, p=0.03 (0.046) g×r×BA, F20, 2860=2.32, p=0.0008 (0.05) |
g×h×r, F2, 286=1.54, p=0.006 (0.01) g×h×r×BA, F20, 2860=1.54, p=0.059 (0.16) |
g×r×BA, F20, 2040=2.04, p=0.004 (0.08) g×h×r×BA, F20, 2040=2.18, p=0.002 (0.02) |
g×h, F1, 102=10.53, p=0.0016 (0.0016) g×r×BA, F20, 2040=2.92, p=0.000015 (0.0027) g×h×r×BA, F20, 2040=3.35, p=0.0000001 (0.0018) |
| Frontal (4-6-8-9-10-11-12-24-25-32-44-45-46-47), parieto-occipital (1/2/3/5-7a-7b-23-26-29-30-31-39-40-43) |
g×r, F1, 143=6.06, p=0.015 (0.015) | g×h×r, F1, 143=5.20, p=0.024 (0.024) | g×r×BA, F13, 1326=2.34, p=0.004 (0.08) g×h×r×BA, F13, 1326=2.16, p=0.009 (0.057) |
g×h, F1, 102=7.03, p=0.009 (0.009) g×r, F1, 102=5.16, p=0.025 (0.025) g×r×BA, F13, 1326=1.86, p=0.03 (0.14) g×h×r×BA, F13, 1326=2.67, p=0.001 (0.01) |
| Temporal (20-21-22-27-28-34-35-36-38-41-42), parietal (1/2/3/5-7a-7b-23-26-29-30-31-39-40-43) |
g×r, F1, 143=3.55, p=0.06 (0.06) g×r×BA, F10, 1430=1.93, p=0.04 (0.12) |
g×r, F1, 143=5.85, p=0.02 (0.02) g×h×r×BA, F10, 1430=2.43, p=0.007 (0.057) |
g×r, F1, 102=4.02, p=0.048 (0.048) g×r×BA, F10, 1020=4.48, p=0.000003 (0.002) g×h×r×BA, F10, 1020=2.37, p=0.009 (0.035) |
g×h, F1, 102=12.95, p=0.0005 (0.0005) g×r×BA, F10, 1020=2.57, p=0.0045 (0.04) g×h×r×BA, F10, 1020=5.11, p=0.0000001 (0.0007) |
| Cingulate gyrus (23-24-25-26-29-30-31-32) | g×BA, F7, 1001=2.52, p=0.01 (0.048) | — | — | g×h, F1, 102=5.53, p=0.02 (0.02) g×h×BA, F7, 714=2.07, p=0.044 (0.08) |
| Cingulate divisions: anterior (24-25), posterior (23-31), retrosplenial (29-30) |
— | — | — | g×h, F1, 102=4.29, p=0.04 (0.04) g×h×r×BA, F2, 204=4.16, p=0.02 (0.017) |
| Monolobar | ||||
| Frontal lobe (4-6-8-9-10-11-12-24-25-32-44-45-46-47) | g×BA, F3, 1859=2.40, p=0.02 (0.14) | g×BA, F13, 1859=2.06, p=0.001 (0.13) | g×h×BA, F13, 1326=2.60, p=0.001 (0.024) |
g×h, F1, 102=3.856, p=0.052 (0.052) g×h×BA, F13, 1326=2.05, p=0.015 (0.09) |
| Frontal lobe divisions: posterior (4-6-8), DLPFC (44-45-46), cingulate (24-25-32), orbital (11-12-47) |
— | g×r, F3, 429=2.73, p=0.044 (0.098) | g×h×r, F3, 306=4.61, p=0.0036 (0.02) | g×h, F1, 102=4.93, p=0.029 (0.029) g×h×r, F3, 306=3.95, p=0.009 (0.037) gxhxrxBA, F6, 858=2.06, p=0.056 (0.55) |
| DLPFC (44-45-46) | ME, F1, 143=6.83, p=0.01 | gxh, F1, 143=3.83, p=0.05 (0.05) | — | — |
| Temporal lobe (20-21-22-27-28-34-35-36-37-38-41-42) | g×h×BA, F11, 1573=2.51, p=0.004 (0.03) | g×h×BA, F11, 1573=2.09, p=0.02 (0.08) | g×BA, F11, 1122=4.82, p=0.0000001 (0.003) g×h×BA, F11, 1122=2.73, p=0.002 (0.02) |
g×h, F1, 102=8.64, p=0.004 (0.004) g×BA, F11, 1122=3.79, p=0.00002 (0.01) g×h×BA, F11, 1122=2.84, p=0.001 (0.02) |
| Temporal lobe divisions: lateral (20-21-22-41-42), medial (27-28-34-35-36) |
g×h×r×BA, F4, 572=3.79, p=0.005 (0.016) | g×h×r×BA, F4, 572=3.51, p=0.0076 (0.02) | g×r×BA, F4, 408=6.81, p=0.0003 (0.0017) g×h×r×BA, F4, 408=2.61, p=0.035 (0.06) |
g×h, F1, 102=10.64, p=0.0015 (0.0015) g×h×r, F1, 102=6.71, p=0.01 (0.01) g×r×BA, F4, 408=5.67, p=0.0002 (0.006) |
| Parietal lobe (1/2/3/5-7a-7b-23-26-29-30-31-39-40-43) | — |
ME, F1, 143=3.53, p=0.06 g×BA, F10, 1430=1.9, p=0.04 (0.14) gxhxBA, F10, 1030=1.79, p=0.057 (0.16) |
g×BA, F10, 1020=2.39, p=0.008 (0.08) | g×h, F1, 102=9.16, p=0.003 (0.003) g×h×BA, F10, 1020=6.58, p=0.0000001 (0.0006) |
| Occipital lobe (17-18-19-37) | — | — | g×BA, F3, 306=2.67, p=0.048 (0.07) | — |
| Functional and architectonic contrasts | ||||
| Primary sensorimotor (4-17-41-42-43), unimodal association-premotor (8-18-19-20-21), heteromodal association (9-39-40-45-46), extracingulate paralimbic (12-27-28-32-38) |
— | g×r, F3, 306=3.28, p=0.021 (0.04) g×r×BA, F12, 1224=2.39, p=0.0047 (0.049) g×h×r×BA, F12, 1224=1.84, p=0.037 (0.09) |
||
| Heteromodal association cortex (9-36-37-39-40-44-45-46) | g×BA, F7, 1001=4.61, p=0.00004 (0.002) | — | ||
| Paralimbic cortex: cingulate (23-24-25-29-30), extracingulate (12-27-28-32-38) |
g×h×r×BA, F4, 572=3.024, p=0.017 (0.03) | g×r, F1, 102=5.29, p=0.024 (0.02) g×r×BA, F4, 408=2.54, p=0.04 (0.05) |
||
| Visual (17-18-19), auditory (22-41-42), sensory (1/2/3/5-7a-7b), motor (4-6-8) | — | g×r, F3, 306=2.81, p=0.04 (0.06) g×r×BA, F6, 612=5.28, p=0.00003 (0.0008) g×h×r×BA, F6, 612=2.34, p=0.03 (0.05) |
||
| Granular (10-11-36-42), dysgranular (8-9-12-38), agranular (6-25-32-39) |
ME, F1, 143=3.63, p=0.059 g×r, F2, 286=5.88, p=0.003 (0.006) g×r×BA, F6, 858=2.51, p=0.02 (0.06) g×h×r×BA, F6, 858=2.45, p=0.024 (0.044) |
g×h×r, F2, 204=4.82, p=0.009 (0.009) g×h×r×BA, F6, 612=2.20, p=0.04 (0.06) |
||
- ME
- main effect of diagnosis
- g
- group
- h
- hemisphere
- r
- region
- BA
- Brodmann’s area
- ×
- interaction (e.g. g×h×r×BA, group by hemisphere by region by Brodmann’s area interaction)
- DLPFC
- dorsolateral prefrontal cortex.
Example for navigating tables 2 and 3: A significant group by region by hemisphere interaction was found for white matter for the dorsolateral prefrontal, temporal, parietal and occipital cortical contrasts (first row), which had an F=3.16 with 3 and 429 df and p value of 0.025 (with Huynh-Feldt correction this was p=0.03). The direction of this effect is presented in table 3, which indicates an increase in left parietal, occipital and dorsolateral prefrontal cortex in the matching table position.
Functional architectonic groupings mainly followed the hierarchical levels of information-processing in the cortex (see Mesulam, 1999 for review) and contrasted primary sensorimotor, unimodal association, heteromodal association, and paralimbic regions, and their constituent Brodmann’s areas. Finally, cortical regions of different cytoarchitectonic types (granular, dysgranular, and agranular) were probed as well. Only gray matter measures were analyzed with these latter, architectonic groupings as this very classification only applies to gray matter. Main effects of diagnosis and regional interactions were examined to localize between-groups differences. Note, that we do not report interactions without diagnostic group or interactions where the lower level of nesting is significant while the upper level is not (e.g. Brodmann’s area interactions are significant but lobe interactions are not), since their interpretability is limited.
Results
Patients with schizophrenia had significantly lower whole-brain volume than normal comparison subjects (1153.8±125.8 vs. 1200.8±115.2 cm3; t143=2.07, p=0.04) but no differences were found between the patients with good and poor outcomes (1175.6±105.5 vs. 1132.9±140.5 cm3; t102=1.75, p=0.08). When patients with different outcomes were separately compared to normal subjects, patients with poor outcomes showed significantly lower whole-brain volume (t92=2.5, p=0.014) and patients with good outcomes did not differ from normal controls (t90=1.1, p=0.28).
Gray matter
Repeated measures ANOVA showed that patients with schizophrenia had lower gray matter volumes in comparison to normal subjects primarily in the frontal and to a lesser degree in the temporal lobes in both hemispheres, especially pronounced in the frontopolar areas 9 and 10, dorsolateral prefrontal cortex (DLPFC), and somewhat less pronounced in other prefrontal (8, 32, 12), temporal (21, 22, 36, 42, 20), and parietal (1, 2, 3, 5, 23) areas (see Table 3 for a detailed presentation of the ANOVA results). We also analyzed the intergroup differences in volumes with an ANCOVA and subject’s age as covariate. Although the results were overall similar, this analysis produced an additional significant main effect of diagnosis for monolobar assessment of frontal gray matter (F1, 141=4.42, p=0.037; lower gray matter volume in patients with schizophrenia) and trends towards lower heteromodal association (main effect of diagnosis, F1, 141=3.71, p=0.056) and paralimbic (main effect of diagnosis, F1, 141=3.89, p=0.05) gray matter volumes in patients.
Table 3. ANOVA-derived volume differences between schizophrenia patients and normal subjects and between patients with good and poor outcomes.
Narrative description of significant ANOVA interactions conforms to the following pattern: group by hemisphere and group by region interactions are presented first, followed by the higher-order interactions (e.g. group by hemisphere by region by Brodmann’s area); most pronounced volume differences are followed by less pronounced differences, i.e. presented in decreasing order of magnitude of the effect. See legend to table 2 for an example of co-joint navigation of tables 2 and 3.Analyses of the frontal lobe divisions and individual Brodmann’s areas produced similar results and are presented together, as are the temporal lobe divisions and Brodmann’s area analyses. Areas 1, 2, 3, and 5 were combined in these analyses (jointly described as 1-2-3-5 in the table). Note that ANOVAs with functional and architectonic regional contrasts were done on gray matter volumes only.
| Schizophrenia patients (N=104) | Patients with poor outcomes (n=53) | |||
|---|---|---|---|---|
| ANOVA contrasts | Gray matter | White matter | Gray matter | White matter |
| Interlobar | ||||
| DLPFC, temporal, parietal, and occipital regions of interest | — | ↑ Left parietal, occipital, DLPFC | ↓ Occipital, temporal, and parietal bilaterally (esp. left); esp. BA 21, 22, 17, 18, 19. No differences in DLPFC | ↓ Temporal, parietal, and occipital ROIs; each BA |
| Frontal, temporal, and parietal lobes | ↓ Frontal lobe, less pronounced temporal lobe; esp. BA 9 and 10 | ↑ Left frontal, followed by right and left parietal, right frontal lobes; esp. left BA 4 and 6 | ↓ Bilateral BA 21 and 22 (esp. left), left BA 6 | ↓ Right hemisphere (esp. BA 21, 22, 20) ↑ Left hemisphere (esp. BA 6, 8, 9, 1-2-3-5, 42); less right 6, 8, 9 |
| Frontal and parieto-occipital lobes | ↓ Frontal lobe | ↑ Both frontal and parieto-occipital regions bilaterally | ↓ Bilaterally BA 17, 18, 19, and left BA 6 | ↓ Parieto-occipital lobe (esp. BA 17, 18, 19) ↑ Left frontal lobe (BA 6, 8, 9) |
| Temporal and parietal lobes | ↓ More pronounced in temporal than parietal lobe (trend level); BA 21, 22, 36, 42, 1-2-3-5 | ↑ More pronounced in parietal than temporal lobe; esp. left BA 1-2-3-5, 7b, 7a and right BA 7b, 7a, 40 | ↓ More pronounced in temporal than parietal lobe; esp. BA 22 and 22 bilaterally | ↓ Right hemisphere (esp. BA 22, 21, 20) |
| Cingulate gyrus | ↓ BA 32, followed by BA 24, 23 | — | — | ↑ Left BA 32, 31, followed by left BA 24 ↓ BA 23, 29, 30 (esp. left 23) |
| Monolobar | ||||
| Frontal lobe | ↓ BA 9 and 10 | ↑ Posterior frontal (motor-premotor) region; BA 4 and 6 | ↓ Left posterior frontal (motor-premotor) region; left BA 6 | ↑ Left posterior frontal lobe; BA 6, 8, 9 (left more than right) |
| DLPFC | ↓ DLPFC | ↑ Right DLPFC (trend) | — | — |
| Temporal lobe | ↓ Bilateral BA 21, 22, 36, esp. right BA 42 | ↑ Right BA 20, 21, 22, 37 ↓ Bilateral BA 42 (esp. right) |
↓ BA 21 and 22 bilaterally, followed by BA 28, 36, 37 ↑ Left BA 42 |
↓ Right lateral, followed by right medial temporal lobe; right BA 20, 21, 22, 36, 37 ↑ Left BA 42 |
| Parietal lobe | — | ↑ 7b, followed by 7a and left 1-2-3-5 | ↑ BA 1-2-3-5 | ↓ Bilateral BA 7b, 39, right BA 40 and 1-2-3-5 ↑ Left 1-2-3-5 |
| Occipital lobe | — | — | ↓ BA 17, 18, 19, to lesser degree BA 37 | — |
| Architectonic | ||||
| Primary sensorimotor, unimodal-premotor, heteromodal, and paralimbic regions | — | ↓ Unimodal association, followed by paralimbic cortex; BA 17, 18, 19, 21 bilaterally ↑ Left BA 42 |
||
| Heteromodal association cortex | ↓ BA 9, followed by BA 36, 44, 45 | — | ||
| Paralimbic cortex | ↓ Left BA 38 and 28, bilateral BA 32 and 12 | ↓ More pronounced in extracingulate than cingulate paralimbic cortex; esp. BA 28, 38, and 24 | ||
| Visual, auditory, sensory, and motor regions | — | ↓ Visual cortex; BA 17, 18, 19, 22, left BA 6 ↑ Left BA 42, bilateral BA 1-2-3-5 |
||
| Granular, dysgranular, and agranular cortex | ↓ Most pronounced in granular, least pronounced in agranular cortex; all granular areas and BA 10, 9, 8 bilaterally, left BA 36, right BA 32 and 42 | ↓ Left agranular, less pronounced in left dysgranular, no differences in left granular; all regions in the right hemisphere; esp. left BA 6 | ||
- DLPFC
- dorsolateral prefrontal cortex
- BA
- Brodmann’s area(s)
- esp.
- especially
- ROI
- region(s) of interest
- ↑ and ↓
- respectively larger and lower volumes in patients with schizophrenia than in normal subjects or in patients with poor outcomes than in patients with good outcomes.
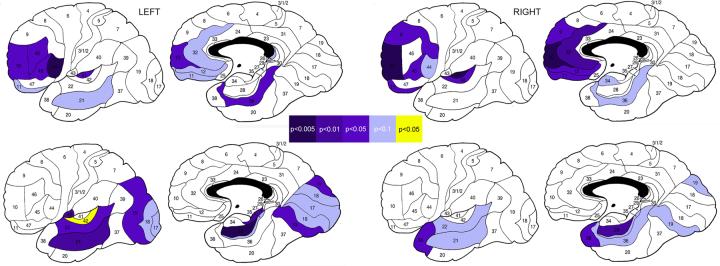
As compared to patients with good outcomes, in the poor-outcome group gray matter volumes were decreased predominantly in the temporal and occipital and to a lesser degree in the parietal lobes, as well as in the posterior frontal (motor-premotor) cortex. Functional architectonic contrasts revealed that in comparison to patients with good outcomes, overall gray matter decreases in the poor-outcome group were most eminent in the unimodal/visual and to a lesser degree in the paralimbic cortical regions, and specifically in the left temporal areas 21, 22 and bilateral occipital areas 17, 18, 19, followed by left frontal area 6 and more posterior areas 28, 36, 37 (medial temporal lobe), 7b, 39, 40 (parietal lobe), and 24, 29, 30, 31 (cingulate gyrus). In the left hemisphere, there was a propensity towards specific loss of volume in the agranular cortex.
Follow-up t tests for individual Brodmann’s areas confirmed a pattern of frontotemporal volume loss in patients with schizophrenia and temporo-occipital differences among schizophrenia patients with different outcomes (Fig. 1). We also conducted t test gray matter comparisons on a subsample of patients with schizophrenia (n=67), which excluded those who participated in our previously published, preliminary pilot assessment (Mitelman et al., 2003). Brodmann’s areas with significant differences between schizophrenia patients and normal subjects in this analysis followed a frontotemporal distribution similar to the pilot assessment (left hemisphere areas 10, 11, 21, 44, and 45; right hemisphere areas 10, 11, 12, 32, 39, 41, 44, and 45).
Fig. 1.
Significant differences in regional gray matter volumes between schizophrenia patients and normal comparison subjects (upper row) and between schizophrenia patients with good outcomes and patients with poor outcomes (lower row)
Color bar illustrates p levels of significance as determined by Student’s t test for independent samples. Blue are Brodmann’s areas with lower gray matter volumes in schizophrenia patients than in normal subjects or in patients with poor outcomes than in patients with good outcomes. Yellow is the area with larger volume in schizophrenia patients with poor outcomes than in patients with good outcomes.
White matter
Regional white matter volumes were generally larger in patients with schizophrenia than in normal subjects, particularly in the left frontal lobe, to a lesser degree in both parietal lobes, followed by the right frontal lobe. The largest increases were seen in white matter associated with areas 4 and 6 (frontal lobe), followed by areas 1, 2, 3, 5, 7a, 7b, 31, 39, 40 (parietal lobe), and 20, 21, 22, 37 (temporal lobe). White matter volumes were modestly lower in patients with schizophrenia than in normal subjects only in association with areas 42 and 24. Follow-up t tests confirmed increases of white matter volume in patients with schizophrenia associated with Brodmann’s areas 4, 7a, and 44 in the left hemisphere and 7a, 7b, 39, and 40 in the right hemisphere (p<0.05, two-tailed).
In patients with poor outcomes, white matter volumes were overall lower than in the good-outcome group in the right hemisphere (in particular in the temporal, parietal, and occipital lobes) and somewhat larger than in the good-outcome group in the left hemisphere (in particular in the posterior frontal and anterior cingulate regions). Higher-order interactions suggested that the regions with lower volumes in the poor-outcome group comprised white matter associated with areas 20, 21, 22 (temporal lobe), 17, 18, 19 (occipital lobe), 1, 2, 3, 5, 7b, 31, 39, 40 (parietal lobe) in the right hemisphere and areas 23, 29, 30 (posterior cingulate) in both hemispheres. Regions with larger volumes in the poor-outcome group in both hemispheres (more prominently on the left) included white matter associated with areas 6, 8 (posterior frontal lobe) and, to a lesser degree, circumjacent areas 9 (bilateral) and 1, 2, 3, 5 (left), as well as temporal area 42 and cingulate areas 24, 31, and 32 in the left hemisphere only. Follow-up t tests confirmed that in comparison to patients with good outcomes, white matter volumes in the poor-outcome group were lower in association with right areas 21, 22, 26, 39 and left posterior cingulate areas 26, 29; larger volumes in patients with poor outcomes were confirmed for left areas 8 and 42 (p<0.05, two-tailed).
Correlations with PANSS
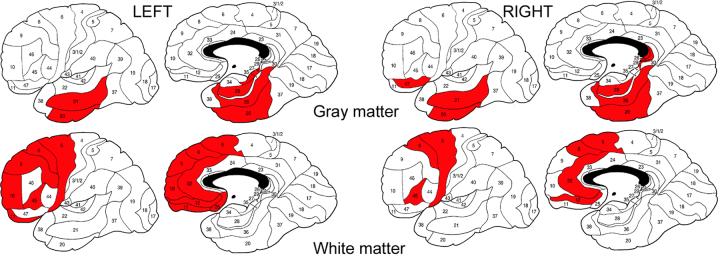
PANSS general psychopathology scores in patients with schizophrenia inversely correlated with the temporal gray matter volumes and directly correlated with the frontal white matter volumes in each hemisphere (Fig. 2).
Fig. 2.
Significant correlations between PANSS general psychopathology scores and regional volumes of gray matter (upper row, negative correlations) and white matter (lower row, positive correlations) in schizophrenia patients
Red color denotes Brodmann’s areas with Pearson’s r product-moment correlations significant at p<0.05 (r>0.19). Note that all significant PANSS correlations with regional gray matter volumes were negative whereas all significant PANSS correlations with regional white matter volumes were positive but both are presented in red to stress the temporal vs. frontal contrast in topology.
Pearson’s r values: left hemisphere gray matter - BA 20 (-0.24), BA 21 (-0.20), BA 27 (-0.21), BA 28 (-0.23), BA 36 (-0.20); right hemisphere gray matter - BA 20 (-0.21), BA 21 (-0.27), BA 26 (-0.20), BA 28 (-0.30), BA 29 (-0.20), BA 36 (-0.32), BA 47 (-0.24); left hemisphere white matter - BA 6 (0.24), BA 8 (0.29), BA 9 (0.22), BA 10 (0.22), BA 11 (0.27), BA 12 (0.23), BA 25 (0.25), BA 32 (0.29), BA 45 (0.24); right hemisphere white matter - BA 6 (0.22), BA 8 (0.27), BA 12 (0.20), BA 32 (0.29), BA 45 (0.25).
Discussion
In this study we replicate extensive literature on frontotemporal gray matter reductions in schizophrenia patients and present a novel finding of expansion in regional white matter as partitioned by its association with cortical Brodmann’s areas. We also confirm our previous preliminary finding of posteriorization of cortical gray matter deficits with poor outcome in schizophrenia. While all schizophrenia patients as a diagnostic group display reductions in the frontotemporal gray matter volumes, the differences between patients with varying outcomes lie overwhelmingly in the posterior, primarily temporal and occipital regions (Fig. 1). We propose a potential relationship between the cortical granularity and gray matter reduction in schizophrenia overall and in patients with varying outcomes in particular. Regions with higher granularity (granular and to a lesser extent dysgranular cortex) were affected in all patients with schizophrenia as a group, whereas regions with lower granularity (agranular and to a much lesser degree dysgranular cortex) in the left hemisphere were preferentially affected in patients with poor functional outcomes. These results are subject to significant individual variability of the cortical Brodmann map and other limitations inherent in atlas-based morphometric analyses (as discussed in detail elsewhere Mitelman et al., 2005a, 2005c), yet their cohesiveness with the solid body of neuroimaging literature and our own multimodal evaluations of these patients is encouraging and may guide additional research in the future. Mutual consistency among findings from the multiple regional ANOVA contrasts and post-hoc t-tests in this study should also be emphasized. Below, in addition to detailing the regional gray matter deficits in schizophrenia, we further extend our previous findings into the white matter morphometry and report on the correlations between the illness severity and temporal gray matter and frontal white matter volumes (Fig. 2).
Regional morphometry in patients with schizophrenia
In comparison to normal subjects, patients with schizophrenia displayed lower whole-brain volumes and gray matter volumes of the frontal lobe and dorsolateral prefrontal cortex. More localized decreases in gray matter volume were prominent in the frontopolar areas 9 and 10 in both hemispheres, and also observed in surrounding prefrontal (8, 11, 12), including anterior cingulate (24 and 32), areas. In the temporal lobe, gray matter volume reductions appeared more widespread in the left than right hemisphere, comprising Brodmann’s areas 22, 21, 20, 36, 42, 28, and 38. In the parietal lobe, gray matter volume reductions were only noted in the postcentral areas 1, 2, 3, and 5 (which were summarily evaluated in this study for methodological reasons) and left posterior cingulate area 23. Granular cortex was preferentially affected by gray matter volume loss, with less extensive involvement of transitional (dysgranular) and mostly spared agranular cortical areas, and there was a trend towards statistically significant volume reductions in the heteromodal association and paralimbic cortices.
Regional white matter volumes were almost universally increased in schizophrenia patients as compared to normal subjects, most prominently in the frontal and parietal lobes. The largest increases centered on the posterior frontal white matter (areas 4 and 6) in both hemispheres and circumjacent frontal (8 and 9) and upper parietal (1, 2, 3, and 5) areas in the left hemisphere, extending into parietal areas 7a, 7b, 39, 40, and 31, more saliently in the right than left hemisphere. A second, less marked node of expanded white matter volume was registered in association with the temporal areas 20, 21, 22, and 37 in the right hemisphere. Only two small areas of decreased regional white matter volumes in schizophrenia patients were detected - in the right upper temporal lobe (area 42) and ventral anterior cingulate gyrus (area 24). It must be stressed, that all changes in white matter were of lesser proportions in comparison to changes in the co-territorial gray matter volumes, which may explain our limited ability to detect them in a much smaller subsample of the subjects engaged in the current study (Mitelman et al., 2003).
While our gray matter findings are entirely in vein of the existing schizophrenia literature, that primarily implicates frontotemporal gray matter deficits in its pathophysiology, the findings of regional white matter expansion in patients with schizophrenia are novel. Although there have been several reports of focal decreases in white matter density (Suzuki et al., 2002; Shapleske et al., 2002; Spaletta et al., 2003; Hulshoff Pol et al., 2004; Davatzikos et al., 2006), regional white matter volumes proper in patients with schizophrenia have not been frequently assessed (Shenton et al., 2001; Kubicki et al., 2005). This is primarily related to difficulties with its parcellation, given a lack of consensual anatomical landmarks or whole-brain partition schemes. Not surprisingly, findings thus far have been overall inconclusive, with reports of reductions (Cannon et al., 1998; Wright et al., 2000; Okugawa et al., 2002; Antonova et al., 2005) or no intergroup differences (Lim et al., 1998; Gur et al., 1999; Hulshoff-Pol et al., 2002; Marcelis et al., 2003; Bassitt et al., 2006) for global white matter volumes and regional decreases in some (Breier et al., 1992; Buchanan et al., 1998; Paillere-Martinot et al., 2001; Sigmudsson et al., 2001; Okugawa et al., 2002; Maric et al., 2003; Riffkin et al., 2005; Price et al., 2006) but not other (Suddath et al., 1990; Wibble et al., 1995; Baare et al., 1999; Staal et al., 2000; Matsumoto et al., 2001; Yamasue et al., 2004) publications. Increased volumes in schizophrenia have been reported for whole-brain white matter (Lim et al., 1996), as well as for the parietal and occipital lobes (Zipursky et al., 1992; Lim et al., 1996; Highley et al., 2003), posterior superior temporal gyri (Taylor et al., 2005), and cerebellar vermis (Lee et al., 2006) in both hemispheres, with greater increases in cerebral white matter volume over the first year of follow-up predictive of higher PANSS positive syndrome subscale score at 5-year follow-up (Cahn et al., 2006). Adding to this evidence of decreased gray matter and increased white matter in schizophrenia, neuroleptic treatment was shown to alleviate these contrasting alterations in volumes, resulting in gray matter expansion and white matter reduction in the parietal and occipital lobes, so that the effect may be viewed as a trend towards normalization of volumetric deficits with treatment (Molina et al., 2005). Our results, therefore, are supportive of this latter group of studies in that patients with schizophrenia exhibited increased regional white matter volumes. Moreover, they appear to imply that in the case of white matter assessment global or lobar bulk volumetric measures may not be productive since the areas of its expansion in patients are relatively circumscribed yet transcend lobar boundaries.
Diffusion tensor imaging analysis in the participants of the current study and using identical parcellation scheme (Mitelman et al., 2006) found a widespread pattern of decreased anisotropy in patients as compared to normal subjects. This finding appears to parallel similarly widespread increases in regional white matter volumes in the present analysis. While there was no exact region-to-region correspondence in the topography of the anisotropy and volume changes in schizophrenia patients, some regions did display such corresponding changes, e.g. anterior cingulate area 24 in both hemispheres was amongst the few areas with somewhat higher anisotropy and amongst only two areas with lower white matter volumes. In this group of patients, widespread decreases in white matter anisotropy appear to parallel widespread increases in bulk white matter volumes, suggesting that increases in bulk white matter volumes may be related to disruptions of white matter microstructural integrity. In addition, there is recent evidence from our laboratory of higher metabolic activity in white matter in schizophrenia patients (Buchsbaum et al., 2006a), hence it may be more broadly speculated that white matter in schizophrenia displays regional increases in volume and metabolic activity with regional decreases in density and fiber directionality (as inferred from the anisotropy measures). This speculation clearly merits further independent validation by multimodal assessment of white matter in one group of subjects.
Morphometric correlates of clinical outcome in schizophrenia
Whole-brain volume in patients with poor outcomes was significantly lower than in normal comparison subjects, but did not differ significantly from whole-brain volume in the good-outcome schizophrenia group. As compared to the good-outcome group, patients with poor clinical outcomes display lower regional gray matter volumes in the temporal (areas 21, 22, 27, 28, 36, 37, 38), occipital (17, 18, 19), and to some extent posterior frontal (motor-premotor) and parietal (7b, 39, 40, 43) cortices, more salient in the left than right hemisphere. In the cingulate gyrus, lower gray matter volumes in the poor-outcome group were evidenced in the left dorsal posterior (area 31) and bilateral retrosplenial (areas 29, 30) regions, to a lesser degree in the ventral anterior area 24. These differences in gray matter volumes in patients with varying outcomes primarily involved unimodal association (visual) and paralimbic (especially extracingulate) cortex. Whereas all patients with schizophrenia regardless of outcome revealed volume decreases in the granular cortex, there was a preferential impairment in the agranular cortical areas of the left hemisphere in those with poor outcomes.
This pattern of posterior brain distribution of cortical deficits relates poor outcome in schizophrenia to several cognitive domains of certain heuristic value. Thus, more pronounced involvement of the temporal lobe may signify more persistent hallucinatory experiences (Ho et al., 2003) and, along with posterior cingulate deficits, a potential cognitive decline (Antonova et al., 2004; Mitelman et al., 2005a). Decreased volumes in the unimodal cortical regions, specifically in the occipital lobe, may implicate impaired early-stage processing of sensory (and especially visual) information in poor clinical outcome (Butler et al., 2005). Involvement of the motor-premotor and adjacent parietal cortex may point to impairment in planning of voluntary activity (so-called motor intention) (Fogassi and Luppino, 2005), i.e. to abulia that has often been described in context of institutionalization in patients with schizophrenia (Krasik and Logvinovich, 1977, 1983; Krasik and Meshcheriakova, 1987). All these structure-outcome associations deserve further investigation.
White matter volume in patients with poor outcomes was lower than in patients with good outcomes in the right hemisphere and somewhat larger in the left hemisphere. In particular, lower white matter volumes were noted in the right lateral (and to a lesser degree medial) temporal lobe, as well as the parietal and occipital lobes. More localized areas of lower white matter volumes were confirmed in association with areas 20, 21, 22, 36, 37, 38 (temporal lobe), 17, 18, 19 (occipital lobe), 1, 2, 3, 5, 7b, 31, 39, 40 (parietal lobe), 45, 46, 47 (frontal lobe) in the right hemisphere. Only in the posterior cingulate gyrus (areas 23, 29, 30) were areas of lower white matter volumes bilateral. In the left hemisphere, more localized posterior frontal (6, 8, 9), cingulate (24, 31, 32), and temporal (37, 42) white matter volumes in patients with poor outcomes were actually larger than in those with better outcomes.
Our prior diffusion tensor imaging evaluation of these patients suggested an inverse relationship among regional anisotropy and white matter volumes in patients with good outcomes, and proposed that it may be compensatory (Mitelman et al., 2006). That is larger white matter volumes in better-outcome patients may compensate for lower regional anisotropy (lower fiber directionality). As seen in the present study, these patients tend to exhibit larger regional white matter volumes, which in contrast to the poor-outcome group are not associated with overall symptom severity (PANSS general psychopathology score). This may indicate that the white matter expansion in the bilateral posterior cingulate, right temporal, parietal, and occipital lobes may indeed be a successful compensatory reaction, inherent in the better outcome to the illness.
Morphometric indicators of illness severity in schizophrenia
In patients with schizophrenia, symptomatic severity of the illness as assessed with PANSS general psychopathology score was inversely associated with temporal gray matter volumes and directly associated with frontal white matter volumes in both hemispheres (Fig. 2). In other words, higher severity of the illness is associated with more pronounced reduction of the temporal gray matter and with more pronounced expansion of the frontal white matter. Temporal gray matter volumes were reduced in patients with schizophrenia as a group with more pronounced reductions in those with poor outcomes, as could be expected given their higher PANSS general psychopathology scores (41.8±9.3 in poor-outcome patients vs. 32.2±7.7 in good-outcome patients). Analogously, frontal white matter volumes were increased in all patients with schizophrenia, but significantly more so in patients with poor outcomes (again in line with their higher general PANSS scores) than in the good-outcome group. In fact, this very relationship between the white matter volumes and PANSS general psychopathology scores was only detected in patients with poor outcomes and not observed in the good-outcome group. These findings provide further evidence that increases in regional white matter volumes are associated with more severe clinical presentation.
In conclusion, the association of more severe temporal gray matter reductions and more pronounced frontal white matter expansion with both the poor-outcome subgroup of patients and with illness severity in the overall schizophrenia sample adds to the relationship amongst longitudinal outcome and symptomatic severity in this illness. Yet, cortical impairments in association with poor outcome are broader than those associated with illness severity, indicating that poor outcome may only partially be explained by more severe clinical manifestations and that other cognitive mechanisms may be at play. A combination of neuropsychological and imaging assessments will be paramount in their future delineation.
Acknowledgement
The authors are grateful to Drs. King-Wai Chu and Tse-Chung Wei for the programming support; Rachel Bloom, Jesse Brand, and Jimcy Platholi for their assistance with study implementation and participants recruitment. This study was supported by Merit Award 2571-005 from the Department of Veterans Affairs (L.S.), grants P50 MH 66392-01 (NIMH Conte Center “White Matter Abnormalities in Schizophrenia”), MH 60023 (M.S.B.), and by Young Investigator Award from National Alliance for Research on Schizophrenia and Depression (S.A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albus M, Hubmann W, Ehrenberg C, Forcht U, Mohr F, Sobizack N, Wahlheim C, Hecht S. Neuropsychological impairment in first-episode and chronic schizophrenic patients. Eur. Arch. Psychiatry Clin. Neurosci. 1996;246:249–255. doi: 10.1007/BF02190276. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr. Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, Sharma T. The relationship of structural alterations to cognitive deficits in schizophrenia: A voxel-based morphometry study. Biol. Psychiatry. 2005;58:457–467. doi: 10.1016/j.biopsych.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Baare WFC, Hulshoff Pol HE, Hijman R, Mali WPT, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive functioning and symptomatology. Biol. Psychiatry. 1999;45:1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- Bassitt DP, Rodrigues M, Neto L, Campi de Castro C, Busatto GF. in press Insight and regional brain volumes in schizophrenia Eur. Arch. Psychiatry Clin. Neurosci [DOI] [PubMed] [Google Scholar]
- Bralet MC, Loas G, Yon V, Marechal V. Clinical characteristics and risk factors for Kraepelinian subtype of schizophrenia: replication of previous findings and relation to summer birth. Psychiatry Res. 2002;111:147–154. doi: 10.1016/s0165-1781(02)00148-8. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirpatrick B, Gellad F. Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch. Gen. Psychiatry. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Shihabuddin L, Byne W, Newmark RE, Brand J, Ahmed S, Mitelman SA, Hazlett EA. Thalamus size and outcome in schizophrenia. Schizophr. Res. 2004;71:473–484. doi: 10.1016/j.schres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Ivanov Z, Borod JC, Foldi NS, Hahn E, Mitelman SA, Hazlett EA, Lincoln SJ, Newmark RE, Shihabuddin L. Internal capsule size in good and poor outcome schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2006;18:364–376. doi: 10.1176/jnp.2006.18.3.364. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Vladar K, Barta PE, Pearlson GD. Structural evaluation of the prefrontal cortex in schizophrenia. Am. J. Psychiatry. 1998;155:1049–1055. doi: 10.1176/ajp.155.8.1049. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Francis A, Arango C, Miller K, Lefkowitz DM, McMahon RP, Barta PE, Pearlson GD. Morphometric assessment of the heteromodal association cortex in schizophrenia. Am. J. Psychiatry. 2004;161:322–331. doi: 10.1176/appi.ajp.161.2.322. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Hazlett EA, Schroder J, Haznedar MM, Powchik P, Spiegel-Cohen J, Wei T, Singer MB, Davis KL. Kraepelinian and non-Kraepelinian schizophrenia subgroup differences in cerebral metabolic rate. Schizophr. Res. 2002;55:25–40. doi: 10.1016/s0920-9964(01)00206-7. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Brickman AM, Miozzo R, Prikryl R, Shaw R, Davis KL. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr. Res. 2003;64:53–62. doi: 10.1016/s0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Buchsbaum BR, Brickman AM, Haznedar MM, Mitelman SA, Schneiderman J, Shihabuddin L. White matter in schizophrenia: larger in volume, more disorganized, and more metabolically active. Ann. Gen. Psychiatry. 2006a;5:S27. doi: 10.1186/1744-859X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Schoenknecht P, Torosjan Y, Newmark RE, Chu KW, Mitelman SA, Brickman AM, Shihabuddin L, Haznedar MM, Hazlett EA, Ahmed S, Tang TY. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Ann. Gen. Psychiatry. 2006b;5:19. doi: 10.1186/1744-859X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch. Gen. Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn W, van Haren NEM, Hulshoff Pol HE, Schnack HG, Caspers E, Laponder DAJ, Kahn RS. Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. Br. J. Psychiatry. 2006;189:381–382. doi: 10.1192/bjp.bp.105.015701. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Gur RE, Yan M. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch. Gen. Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, Hughett P, Turetsky BI, Gur RE. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch. Gen. Psychiatry. 2006;62:1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- Davidson M, Harvey P, Welsh KA, Powchik P, Putnam KM, Mohs RC. Cognitive functioning in late-life schizophrenia: a comparison of elderly schizophrenic patients and patients with Alzheimer’s disease. Am. J. Psychiatry. 1996;153:1274–1279. doi: 10.1176/ajp.153.10.1274. [DOI] [PubMed] [Google Scholar]
- Davis KL, Buchsbaum MS, Shihabuddin L, Spiegel-Cohen J, Metzger M, Frecska E, Keefe RSE, Powchik P. Ventricular enlargement in poor-outcome schizophrenia. Biol. Psychiatry. 1998;43:783–793. doi: 10.1016/s0006-3223(97)00553-2. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Stritzke P, Riordan H, Holan V, Boccio A, Kushner M, McClelland J, van Eyl O, Anand A. The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol. Psychiatry. 1992;31:241–254. doi: 10.1016/0006-3223(92)90047-4. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr. Opin. Neurobiol. 2005;15:626–631. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Bilker WB, Gur RC. Reduced gray matter volume in schizophrenia. Arch. Gen. Psychiatry. 1999;56:905–911. doi: 10.1001/archpsyc.56.10.905. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Putnam KM, Davidson M, Kahn RS, Powchik P, McQueeney R, Keefe RSE, Davis KL. Brief neuroleptic discontinuation and clinical symptoms in Kraepelinian and non-Kraepelinian chronic schizophrenic patients. Psychiatry Res. 1991;38:285–292. doi: 10.1016/0165-1781(91)90018-k. [DOI] [PubMed] [Google Scholar]
- Highley JR, DeLisi LE, Roberts N, Webb JA, Relja M, Razi K, Crow TJ. Sex-dependent effects of schizophrenia: an MRI study of gyral folding, and cortical and white matter volume. Psychiatry Res. Neuroimaging. 2003;124:11–23. doi: 10.1016/s0925-4927(03)00076-3. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch. Gen. Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, Cahn W, Collins DL, Evans AC, Kahn RS. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. NeuroImage. 2004;21:27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Jablensky A. Subtyping schizophrenia: implications for genetic research. Mol. Psychiatry. 2006;11:815–836. doi: 10.1038/sj.mp.4001857. [DOI] [PubMed] [Google Scholar]
- Joober R, Rouleau GA, Lal S, Bloom D, Lalonde P, Labelle A, Benkelfat C. Increased prevalence of schizophrenia spectrum disorders in relatives of neuroleptic-nonresponsive schizophrenic patients. Schizophr. Res. 2005;77:35–41. doi: 10.1016/j.schres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, Beiser M. Relationship of lateral ventricular size to psychophysiological measures and short-term outcome. Psychiatry Res. 1991;37:115–129. doi: 10.1016/0165-1781(91)90069-2. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Kendler KS, Horvath TB, Nora R, Davis KL. Characteristics of very poor outcome schizophrenia. Am. J. Psychiatry. 1987;144:889–895. doi: 10.1176/ajp.144.7.889. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Davidson M, Losonczy MF, Silverman JM, Lesser JC, Horvath TB, Davis KL. Kraepelinian schizophrenia: a subgroup of schizophrenia? Psychopharmacol. Bull. 1988;24:56–61. [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Horvath TB, Davis KL. Premorbid sociosexual functioning and long-term outcome in schizophrenia. Am. J. Psychiatry. 1989;146:206–211. doi: 10.1176/ajp.146.2.206. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Silverman JM, Losonczy MF, Davidson M, Horvath TB, Davis KL. Characteristics of Kraepelinian schizophrenia and their relation to premorbid sociosexual functioning. In: Angrist B, Schulz SC, editors. The neuroleptic nonresponsive patients: Characterization and treatment. American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- Keefe RSE, Lobel DS, Mohs RC, Silverman JM, Harvey PD, Davidson M, Losonczy MF, Davis KL. Diagnostic issues in chronic schizophrenia: kraepelinian schizophrenia, undifferentiated schizophrenia, and state-independent negative symptoms. Schizophr. Res. 1991;4:71–79. doi: 10.1016/0920-9964(91)90026-n. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Frescka E, Apter SH, Davidson M, Macaluso JM, Hirschowitz J, Davis KL. Clinical characteristics of Kraepelinian schizophrenia: replication and extension of previous findings. Am. J. Psychiatry. 1996;153:806–811. doi: 10.1176/ajp.153.6.806. [DOI] [PubMed] [Google Scholar]
- Kilzieh N, Wood AE, Erdmann J, Raskind M, Tapp A. Depression in Kraepelinian schizophrenia. Compr. Psychiatry. 2003;44:1–6. doi: 10.1053/comp.2003.50002. [DOI] [PubMed] [Google Scholar]
- Krasik ED, Logvinovich GV. Клиническая структура госпитализма у больных шизофренией [Clinical structure of institutionalism in schizophrenic patients (rehabilitation aspect)] Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova. 1977;77:1711–1715. [PubMed] [Google Scholar]
- Krasik ED, Logvinovich GV. Госпитализм при шизофрении (клинико-реабилитационные аспекты) [Schizophrenia hospitalism (clinico-rehabilitative aspects)] Tomsk University Publishing; Tomsk, USSR: 1983. [Google Scholar]
- Krasik ED, Meshcheriakova EI. Прогностическое значение мотивационных характеристик больных шизофренией [Prognostic value of the motivational characteristics of schizophrenic patients] Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova. 1987;87:66–71. [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Shenton ME. Evidence for white matter abnormalities in schizophrenia. Curr. Opin. Psychiatry. 2005;18:121–134. doi: 10.1097/00001504-200503000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Farrow TFD, Parks RW, Newton LD, Mir NU, Egleston PN, Brown WH, Wilkinson ID, Woodruff PWR. (in press) Increased cerebellar vermis white-matter volume in men with schizophrenia J. Psychiatric Res 2007 [DOI] [PubMed] [Google Scholar]
- Lim KO, Sullivan EV, Zipursky RB, Pfefferbaum A. Cortical gray matter volume deficits in schizophrenia: a replication. Schizophr. Res. 1996;20:157–164. doi: 10.1016/0920-9964(95)00081-x. [DOI] [PubMed] [Google Scholar]
- Lim KO, Adalsteinsson E, Spielman D, Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Proton magnetic resonance spectroscopic imaging of cortical gray and white matter in schizophrenia. Arch. Gen. Psychiatry. 1998;55:346–352. doi: 10.1001/archpsyc.55.4.346. [DOI] [PubMed] [Google Scholar]
- Losonczy MF, Song IS, Mohs RC, Small NA, Davidson M, Johns CA, Davis KL. Correlates of lateral ventricular size in chronic schizophrenia, I: Behavioral and treatment response measures. Am. J. Psychiatry. 1986;143:976–981. doi: 10.1176/ajp.143.8.976. [DOI] [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Winzig L, Candela SF, Beaudette S, Boshes R. Age disorientation in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2000;12:350–358. doi: 10.1176/jnp.12.3.350. [DOI] [PubMed] [Google Scholar]
- Marcelis M, Suckling J, Woodruff PW, Hofman P, Bullmore ET, van Os J. Searching for a structural endophenotype in psychosis using computational morphometry. Psychiatry Res. 2003;122:153–167. doi: 10.1016/s0925-4927(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Maric N, Kamer T, Schneider Axmann T, Dani I, Jasovic Gasic M, Paunovic VR, Falkai P. [Volumetric analysis of gray matter, white matter and cerebrospinal fluid space in schizophrenia] Spr. Arh. Celok. Lek. 2003;131:26–30. doi: 10.2298/sarh0302026m. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Simmons A, Williams S, Hadjulis M, Pipe R, Murray R, Frangou S. Superior temporal gyrus abnormalities in early-onset schizophrenia: similarities and differences with adult-onset schizophrenia. Am. J. Psychiatry. 2001;158:1299–1304. doi: 10.1176/appi.ajp.158.8.1299. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann’s areas of the cortex in patients with schizophrenia with good and poor outcomes. Am. J. Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr. Res. 2005a;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark RE, Chu KW, Buchsbaum MS. Correlations between MRI-assessed volumes of the thalamus and cortical Brodmann’s areas in schizophrenia. Schizophr. Res. 2005b;75:265–281. doi: 10.1016/j.schres.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. NeuroImage. 2005c;27:753–770. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Buchsbaum MS. Cortical intercorrelations of temporal area volumes in schizophrenia. Schizophr. Res. 2005d;76:207–229. doi: 10.1016/j.schres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Newmark RE, Torosjan Y, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. White matter fractional anisotropy and outcome in schizophrenia. Schizophr. Res. 2006;87:138–159. doi: 10.1016/j.schres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. (in press) Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: A diffusion tensor imaging survey Schizophr. Res 2007 [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Sanz J, Palomo T, Benito C, Sanchez J, Sarramea F, Pascau J, Desco M. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr. Res. 2005;80:61–71. doi: 10.1016/j.schres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Ohmori K. Kraepelinian subtype and deficit syndrome in chronic schizophrenia. Psychiatry Res. 2006;144:221–225. doi: 10.1016/j.psychres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Sedvall GC, Agartz I. Reduced grey and white matter volumes in the temporal lobe of male patients with chronic schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2002;252:120–123. doi: 10.1007/s00406-002-0370-9. [DOI] [PubMed] [Google Scholar]
- Paillere-Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, Recasens C, Attar-Levy D, Martinot JL. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr. Res. 2001;50:19–26. doi: 10.1016/s0920-9964(00)00137-7. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Garbacz DJ, Breakey WR, Ahn HS, DePaulo JR. Lateral ventricular enlargement associated with persistent unemployment and negative symptoms in both schizophrenia and bipolar disorder. Psychiatry Res. 1984;12:1–9. doi: 10.1016/0165-1781(84)90133-1. [DOI] [PubMed] [Google Scholar]
- Rieckmann N, Reichenberg A, Bowie CR, Parrella M, White L, Friedman JI, Harvey PD. Depressed mood and its functional correlates in institutionalized schizophrenia patients. Schizophr. Res. 2005;77:179–187. doi: 10.1016/j.schres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Riffkin J, Yucel M, Maruff P, Wood SJ, Soulsby B, Olver J, Kyrios M, Velakoulis D, Pantelis C. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Res. 2005;138:99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Rossi A, Bustini M, Prosperini P, Marinangeli MG, Splendiani A, Daneluzzo E, Stratta P. Neuromorphological abnormalities in schizophrenic patients with good and poor outcome. Acta Psychiatr. Scand. 2000;101:161–166. doi: 10.1034/j.1600-0447.2000.900666.x. [DOI] [PubMed] [Google Scholar]
- Roy MA, Merette C, Maziade M. Subtyping schizophrenia according to outcome or severity: a search for homogeneous subgroups. Schizophr. Bull. 2001;27:115–138. doi: 10.1093/oxfordjournals.schbul.a006851. [DOI] [PubMed] [Google Scholar]
- Roy MA, Lehoux C, Emond C, Laplante L, Bouchard RH, Everett J, Merette C, Maziade M. A pilot neuropsychological study of Kraepelinian and non-Kraepelinian schizophrenia. Schizophr. Res. 2003;62:155–163. doi: 10.1016/s0920-9964(02)00481-4. [DOI] [PubMed] [Google Scholar]
- Salokangas RKR. Symptom dimensions and outcome in schizophrenia. World Psychiatry. 2003;2:172–178. [PMC free article] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Chitnis XA, Suckling J, Simmons A, Bullmore ET, Woodruff PW, David AS. A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb. Cortex. 2002;12:1331–1341. doi: 10.1093/cercor/12.12.1331. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizopr. Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore ET, Greenwood K, Fukuda R, Ron M, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am. J. Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Tomaiuolo F, Marino V, Bonaviri G, Trequattrini A, Caltragirone C. Chronic schizophrenia as a brain misconnection syndrome: a white matter voxel-based morphometry study. Schizophr. Res. 2003;64:15–23. doi: 10.1016/s0920-9964(03)00010-0. [DOI] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn MLC, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am. J. Psychiatry. 2000;157:416–421. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- Stephens JH. Long-term prognosis and followup in schizophrenia. Schizophr. Bull. 1978;4:25–47. doi: 10.1093/schbul/4.1.25. [DOI] [PubMed] [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N. Engl. J. Med. 1990;32:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nohara S, Hagino H, Kurokawa K, Yotsutsuji T, Kawasaki Y, Takahashi T, Matsui M, Watanabe N, Seto H, Kurachi M. Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr. Res. 2002;55:41–54. doi: 10.1016/s0920-9964(01)00224-9. [DOI] [PubMed] [Google Scholar]
- Tapp A, Tandon R, Scholten R, Dudley E. Age disorientation in Kraepelinian schizophrenia: frequency and clinical correlates. Psychopathology. 1993;26:225–228. doi: 10.1159/000284826. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Blanton RE, Levitt JG, Caplan R, Nobel D, Toga AW. Superior temporal gyrus differences in childhood-onset schizophrenia. Schizophr. Res. 2005;73:235–241. doi: 10.1016/j.schres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Malofeeva LI, Bogolepova IN, Jacobsen AM, Amunts K, Zilles K. No postnatal doubling of neurons in human Broca’s areas (Brodmann areas 44 and 45)? A stereological study. Neuroscience. 2005;136:715–728. doi: 10.1016/j.neuroscience.2005.07.048. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Jacobsen AM, Zilles K, Amunts K. Left-right asymmetry in volume and number of neurons in adult Broca’s area. Cortex. 2006;42:652–658. doi: 10.1016/s0010-9452(08)70401-5. [DOI] [PubMed] [Google Scholar]
- Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW. Prefrontal cortex and schizophrenia. A quantitative magnetic resonance imaging study. Arch. Gen. Psychiatry. 1995;52:279–288. doi: 10.1001/archpsyc.1995.03950160029007. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, Davis AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am. J. Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Iwanami A, Hirayasu Y, Yamada H, Abe O, Kuroki N, Fukuda R, Tsujii K, Aoki S, Ohtomo K, Kato N, Kasai K. Localized volume reduction in prefrontal, temporolimbic, and paralimbic regions in schizophrenia: an MRI parcellation study. Psychiatry Res. 2004;131:195–207. doi: 10.1016/j.pscychresns.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch. Gen. Psychiatry. 1992;49:195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]