Abstract
Ovulation in the nematode Caenorhabditis elegans is regulated by complex signal transduction pathways and cell–cell interactions. Myoepithelial sheath cells of the proximal ovary are smooth muscle-like cells that provide contractile forces to push a mature oocyte into the spermatheca for fertilization. Although several genes that regulate sheath contraction have been characterized, basic components of the contractile apparatuses of the myoepithelial sheath have not been extensively studied. We identified major structural proteins of the contractile apparatuses of the myoepithelial sheath and characterized their nonstriated arrangement. Of interest, integrin and perlecan were found only at the dense bodies, whereas they localized to both dense bodies and M-lines in the striated body wall muscle. RNA interference of most of the myofibrillar components impaired ovulation in a soma-specific manner. Our results provide basic information that helps understanding the mechanism of sheath contraction during ovulation and establishing a new model to study morphogenesis of nonstriated muscle.
Keywords: actin, myosin, integrin, myofibrils, muscle, ovulation, contraction
Introduction
Ovulation in the nematode Caenorhabditis elegans is a highly coordinated process involving communications among sperm, oocytes, and the somatic gonad. A C. elegans hermaphrodite has a pair of U-shaped gonadal arms (Fig. 1). The germline and oocytes are surrounded by 10 sheath cells (pairs 1–5), and ovulation requires a signal from sperm that induces ovarian contraction and oocyte maturation, which are then followed by dilation of the spermatheca and fertilization (Ward and Carrel, 1979; McCarter et al., 1997, 1999; Hubbard and Greenstein, 2000; Yamamoto et al., 2006). The proximal sheath cells (pairs 3–5; Fig. 1) are smooth muscle-like cells with distinct thin and thick filaments and designated as the myoepithelial sheath (Hirsh et al., 1976; Strome, 1986; Ardizzi and Epstein, 1987; Hall et al., 1999). Contraction of the myoepithelial sheath is periodically enhanced upon maturation of the most proximal oocytes (McCarter et al., 1997, 1999). Sheath contraction is negatively regulated by ceh-18 encoding a POU-class homeoprotein (Rose et al., 1997; Miller et al., 2003) and gap junctions that are formed between the myoepithelial sheath and maturing oocytes (Whitten and Miller, 2006). On the other hand, it is enhanced by major sperm protein (Miller et al., 2001; Kosinski et al., 2005) by means of the VAB-1 Eph receptor (Miller et al., 2003). In addition, NMR-1 NMDA receptor and UNC-43 Ca2+-calmodulin–dependent kinase II negatively regulate sperm-dependent sheath contraction (Corrigan et al., 2005), whereas ITR-1 inositol triphosphate receptor (Yin et al., 2004), VAV-1 Rho/Rac guanine nucleotide exchange factor (GEF), and RHO-1 small GTPase (Norman et al., 2005) enhance contraction. Probably, precise regulation of sheath contraction by a complex mechanism is required for tight coupling of contraction with oocyte maturation and spermathecal dilation. However, functional interactions among these signaling molecules are still poorly understood.
Fig. 1.
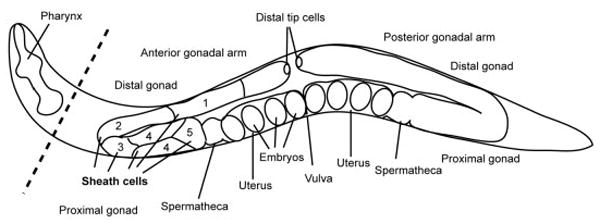
Schematic representation of the Caenorhabditis elegans hermaphroditic gonad. A hermaphrodite has two gonadal arms. The distal tip cells are located at the most distal part of the gonad. Five pairs of the sheath cells surround the germline and oocytes and are designated as pairs 1 to 5 (not all cells are shown). Pairs 3–5 are the myoepithelial sheath that has distinct thin and thick filaments and are responsible for pushing a mature oocyte into the spermatheca during ovulation. After fertilization, embryos begin development in the uterus. Note that sheath cells are shown only in the anterior gonadal arm and that the posterior gonadal arm also has the same set of sheath cells.
One of the critical signals for sheath contraction is increase in the intracellular Ca2+ level (Yin et al., 2004; Xu et al., 2005). Troponin and tropomyosin function as actin-linked Ca2+ sensors in the thin filaments of the myoepithelial sheath cells and are required for ovulation (Myers et al., 1996; Ono and Ono, 2004). Thus, these nonstriated muscle cells are atypical, as troponin is generally expressed in striated muscle. Instead, vertebrate smooth muscle uses a myosin-linked system to activate contraction upon elevation of Ca2+ (Marston, 1995). However, potential regulators of myosin activity including VAV-1 Rho/Rac GEF, RHO-1, LET-502 Rho-kinase, and MEL-11 myosin phosphatase regulatory subunit are also required for ovulation (Wissmann et al., 1999; Norman et al., 2005). A biochemical study on purified actin and myosin from C. elegans suggests that there are both actin- and myosin-linked regulatory systems for actomyosin interaction (Harris et al., 1977). In addition, recent studies in vertebrate smooth muscle have shown that regulators of actin polymerization are important for contraction (Tang and Tan, 2003; Tang et al., 2005). Therefore, actomyosin contractility in the myoepithelial sheath might also be regulated by multiple mechanisms.
The myoepithelial sheath cells express MYO-3 and UNC-54 myosin heavy chains (Ardizzi and Epstein, 1987), which are also expressed in striated body wall muscle (Miller et al., 1983). Ovulation is perturbed by disturbing functions of several structural proteins, including MUP-2 troponin T (Myers et al., 1996), PAT-10 troponin C (Ono and Ono, 2004), LEV-11 tropomyosin (Ono and Ono, 2004), PAT-3 β-integrin (Lee et al., 2001), talin (Cram et al., 2003), PAT-4 integrin-linked kinase (Xu et al., 2006), and UNC-112 Mig-2 (Xu et al., 2006). However, overall organization and molecular components of the contractile apparatuses of the myoepithelial sheath are not clearly understood. In this study, we identified and localized major components of the contractile apparatuses in these nonstriated muscle cells and showed that many of these proteins are required for proper ovulation.
Results
Expression Patterns of Cytoskeletal Proteins in the C. elegans Somatic Gonad
To identify and localize the components of the contractile apparatuses of the gonadal myoepithelial sheath, antibodies against well-characterized cytoskeletal proteins were used for immunofluorescent staining of the somatic gonad. We detected expression of major cytoskeletal proteins in the myoepithelial sheath, and, interestingly, some of them were also expressed in other parts of the somatic gonad (Fig. 2). As described previously by Strome (1986), visualization of actin filaments by tetramethylrhodamine-phalloidin revealed a nonstriated meshwork of the filaments in the sheath cells and filamentous staining in other gonadal cells including germline (Fig. 2H,Q). Immunostaining of actin by mouse monoclonal (our unpublished data) or rabbit polyclonal anti-actin antibodies (Fig. 2B,E,K,N) yielded indistinguishable patterns of actin filaments in the sheath cells. Therefore, we used either tetramethylrhodamine-phalloidin or anti-actin antibody to label actin filaments, depending on the optimal fixation method. Many antibodies were optimal with fixation with cold methanol, which was not compatible with staining with tetramethylrhodamine-phalloidin.
Fig. 2.
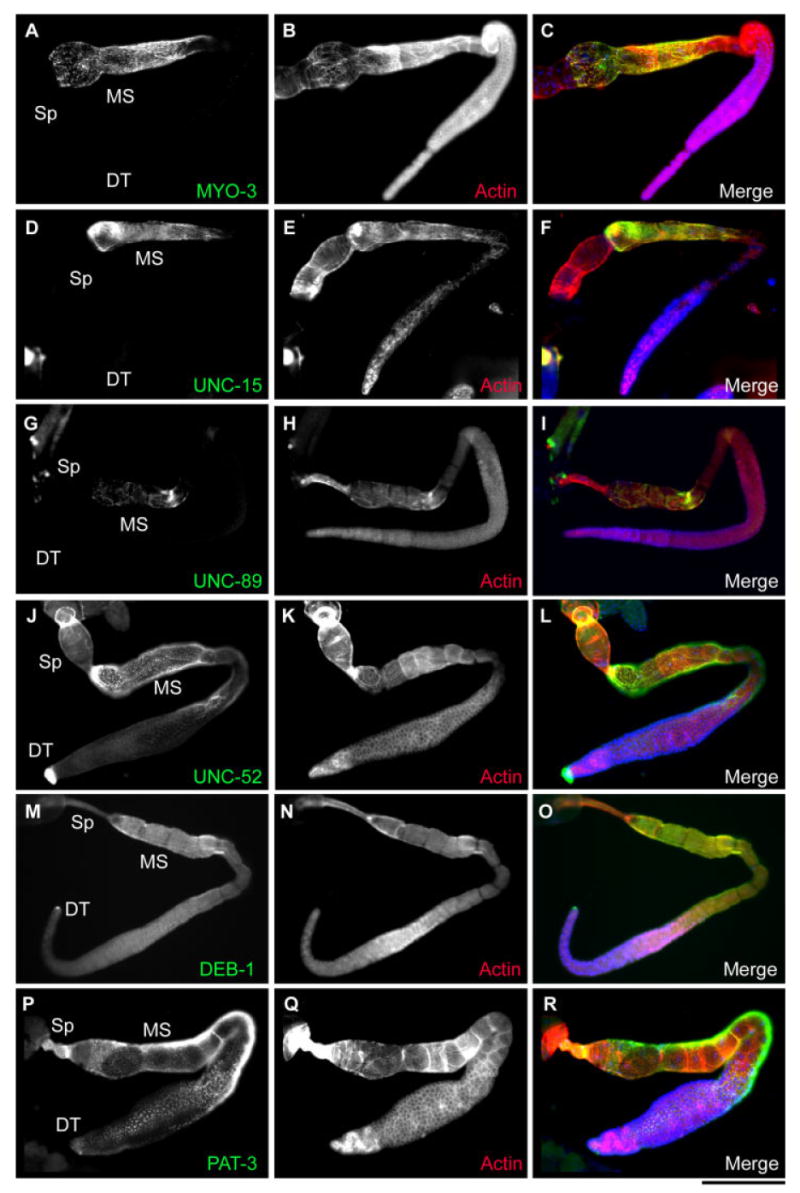
Localization of structural proteins in the somatic gonad. A,B,D,E,G,H,J,K,M,N,P,Q: Dissected hermaphroditic gonads were doubly stained for MYO-3 myosin heavy chain (A), UNC-15 paramyosin (D), UNC-89 (G), UNC-52 perlecan (J), DEB-1 vinculin (M), or PAT-3 β-integrin (P), and actin (B,E,H,K,N,Q). C,F,I,L,O,R: Merged images of actin (red), other proteins (green), and DNA (blue), are shown. Positions of the spermatheca (Sp), the myoepithelial sheath (MS), and the distal tip (DT) are indicated. Scale bar = 100 μm.
As reported previously (Ardizzi and Epstein, 1987), MYO-3 myosin heavy chain A (myoA; Fig. 2A–C) and UNC-15 paramyosin (Fig. 2D–F) were specifically expressed in the myoepithelial sheath. We previously demonstrated that PAT-10 troponin C and Ce-kettin, a large immunoglobulin-like repeat protein, were also specific in the myoepithelial sheath (Ono and Ono, 2004; Ono et al., 2006). We found that the monoclonal antibody MH42 against UNC-89, a large obscurin-like thick-filament-associated protein (Benian et al., 1996), specifically stained the proximal myoepithelial sheath (Fig. 2G–I). Although MH42 weakly stained the germline, RNAi of unc-89 specifically removed signals in the myoepithelial sheath but not in the germline (data not shown), suggesting that the germline staining was nonspecific. In contrast, components of the adhesion structures, UNC-52 perlecan, DEB-1 vinculin, and PAT-3 β-integrin, were present in all somatic gonadal cells from the distal tip to the spermatheca (Fig. 2J–R). In addition, we previously reported that Ce-tropomyosin was expressed in the proximal myoepithelial sheath and the spermatheca (Ono and Ono, 2004). All of these structural proteins, except for actin, were not significantly detected in the germline as observed at different focal planes (data not shown). As summarized in Figure 3 and Table 1, all these cytoskeletal proteins are the cytoskeletal components of the smooth muscle-like proximal myoepithelial sheath, and some proteins are limited in these cells, suggesting their specific roles in the regulation of sheath contraction. However, some are present in other somatic gonadal cells, suggesting that they are involved in multiple aspects of the gonadal activities.
Fig. 3.
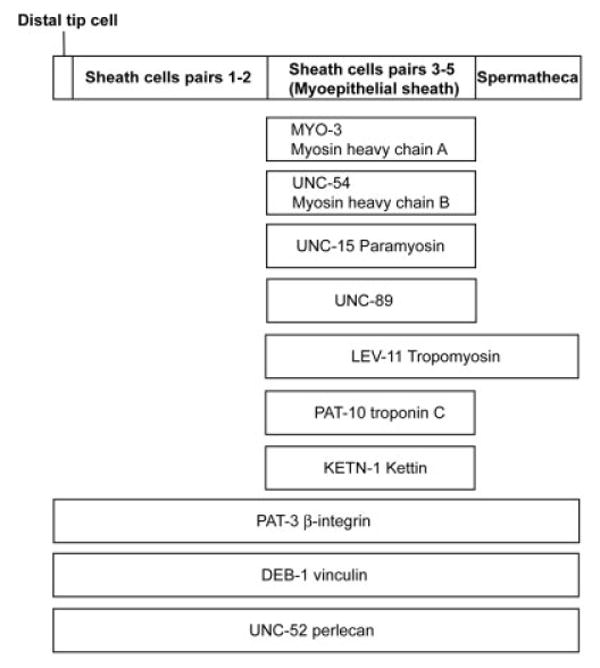
Schematic representation of the distribution patterns of structural proteins in the somatic gonad. Results of this study and previous studies (Ardizzi and Epstein, 1987; Ono and Ono, 2004; Ono et al., 2006) are summarized.
TABLE 1. Fixation and Staining Conditions for Fluorescence Microscopya.
| Figure | Panel | Protein | Fixationb | Primary antibodies/probe | Secondary antibodiesc | Primary antibodies | Zenon-IgG2ad |
|---|---|---|---|---|---|---|---|
| 2 & 4 | A-C | MYO-3 | A | 5-6 | A488-GAM | NA | NA |
| Actin | AAN01 | Cy3-DAR | |||||
| D-F | UNC-15 | A | 5-23 | A488-GAM | NA | NA | |
| Actin | AAN01 | Cy3-DAR | |||||
| G-I | UNC-52 | B | MH2 | A488-GAM | NA | NA | |
| Actin | TRITC-phalloidin | - | |||||
| J-L | UNC-89 | A | MH42 | A488-GAM | NA | NA | |
| Actin | AAN01 | Cy3-DAR | |||||
| M-O | DEB-1 | C | MH24 | A488-GAM | NA | NA | |
| Actin | AAN01 | Cy3-DAR | |||||
| P-R | PAT-3 | B | MH25 | A488-GAM | NA | NA | |
| Actin | TRITC-phalloidin | - | |||||
| 5 | A-C | UNC-15 | A | 5-23 | Cy3-DAM | - | - |
| MYO-3 | - | - | 5-14 | A488 | |||
| D-F | UNC-89 | A | MH42 | Cy3-DAM | - | - | |
| MYO-3 | - | - | 5-14 | A488 | |||
| 6 | A-D, | Actin | A | AAN01 | Cy3-DAR | - | - |
| Q-T | PAT-3 | - | - | MH25 | A488 | ||
| MYO-3 | 5-6 | A647-GAM | - | - | |||
| E-H | Actin | A | AAN01 | Cy3-DAR | - | - | |
| PAT-3 | - | - | MH25 | A488 | |||
| UNC-89 | MH42 | A647-GAM | - | - | |||
| I-L | Actin | A | AAN01 | Cy3-DAR | - | - | |
| PAT-3 | - | - | MH25 | A488 | |||
| DEB-1 | MH24 | A647-GAM | - | - | |||
| M-P | Actin | A | AAN01 | Cy3-DAR | - | - | |
| PAT-3 | - | - | MH25 | A488 | |||
| UNC-52 | MH2 | A647-GAM | - | - |
NA, not applicable
Fixation methods: A, methanol (5 min, –20 °C); B, 4% paraformaldehyde in cytoskeleton buffer (138 mM KCl, 3 mM MgCl2, 2 mM EGTA, and 10 mM MES-KOH, pH 6.1) containing 0.32 M sucrose (20 min at room temperature) followed by phosphate-buffered saline containing 0.5% Triton X-100 and 30 mM glycine (20 min at room temperature); and C, methanol (5 min, –20 °C) followed by acetone (5 min, –20 °C).
A488-GAM, Alexa488-labeled goat anti-mouse IgG; A647-GAM, Alexa647-labeled goat anti-mouse IgG; Cy3-DAM, Cy3-labeled donkey anti-mouse IgG; Cy3-DAR, Cy3-labeled donkey anti-rabbit IgG.
Zenon Alexa Fluor mouse IgG2a labeling reagents (Invitrogen).
Myofibrillar Components of the Gonadal Myoepithelial Sheath
We focused on the proximal myoepithelial sheath and examined localization of the cytoskeletal proteins at a higher magnification. Thin and thick filaments were arranged in a nonstriated manner, and major components of the adhesion structures were only associated with the thin filaments but not with the thick filaments.
Thin filaments
As described previously by Strome (1986), actin filaments are arranged in a nonstriated meshwork in the sheath cells (Fig. 4B,E,H,K,N,Q). The filaments were apparently continuous, and breaks at the thick filaments and dense bodies were not observed (Fig. 4B,E,H,K,N,Q) as clearly as in the striated body wall muscle (Waterston, 1988; Moerman and Fire, 1997). This finding suggests that the thin filaments in the myoepithelial sheath may have variable length and that the filament ends may not be tightly aligned. We have previously shown that tropomyosin (CeTM) and PAT-10 troponin C localize to the thin filaments in the myoepithelial sheath (Ono and Ono, 2004). Ce-kettin (MH44 antigen) also localizes to the thin filaments in the myoepithelial sheath, but it is limited to a narrow region near the dense bodies (Ono et al., 2005, 2006).
Fig. 4.
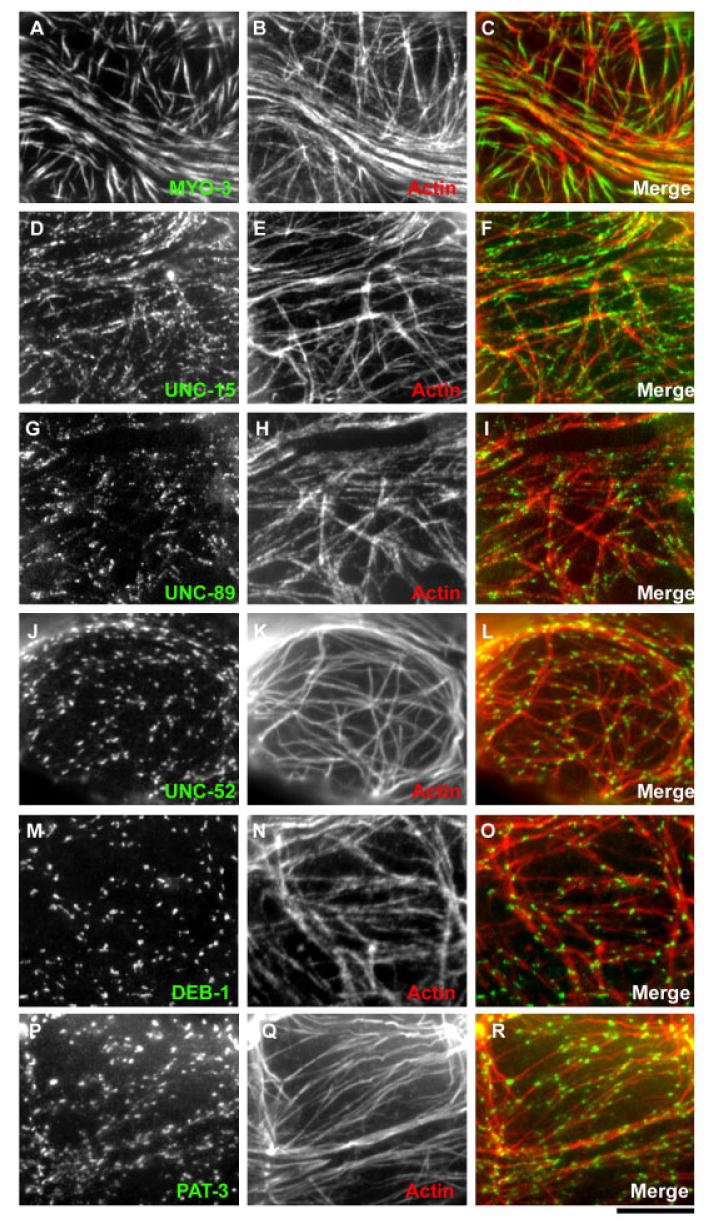
Localization of structural proteins in the myoepithelial sheath cells. A,B,D,E,G,H,J,K,M,N,P,Q: Dissected hermaphroditic gonads were doubly stained for MYO-3 myosin heavy chain (A), UNC-15 paramyosin (D), UNC-89 (G), UNC-52 perlecan (J), DEB-1 vinculin (M), or PAT-3 β-integrin (P), and actin (B,E,H,K,N,Q), and the myoepithelial sheath was observed at a high magnification. C,F,I,L,O,R: Merged images of actin (red) and other proteins (green) are shown. Scale bar = 10 μm.
Thick filaments
Previous studies by others have shown that the myoepithelial sheath cells express MYO-3 (myoA; Fig. 4A–C) and UNC-54 (myoB) myosin heavy chains (Ardizzi and Epstein, 1987) that are also expressed in the striated body wall muscle and that UNC-54 localizes to the outer regions, whereas MYO-3 is limited to the central part of the thick filaments (Rose et al., 1997) as observed in the thick filaments in the striated body wall muscle (Miller et al., 1983). UNC-15 paramyosin was also expressed in the myoepithelial sheath (Ardizzi and Epstein, 1987; Fig. 4D–F). Staining with the monoclonal anti–UNC-15 antibody (5-23) often resulted in punctate patterns (Fig. 4D). Double staining of UNC-15 paramyosin and MYO-3 showed that most of the UNC-15-spots (Fig. 5A, and red spots in C, arrowheads) are associated with the surface, not in the core, of the MYO-3 filaments (Fig. 5B, and green filaments in C, arrowheads). UNC-15 paramyosin is one of the core components of the nematode thick filaments (Harris and Epstein, 1977; Epstein et al., 1985, 1988). However, a previous biochemical study has demonstrated the existence of a population of paramyosin that can be readily dissociated from the thick filaments by a high-salt buffer (Deitiker and Epstein, 1993). Therefore, these spots of UNC-15 may represent the epitope of a subset of UNC-15 that is exposed on the surface of the thick filaments.
Fig. 5.
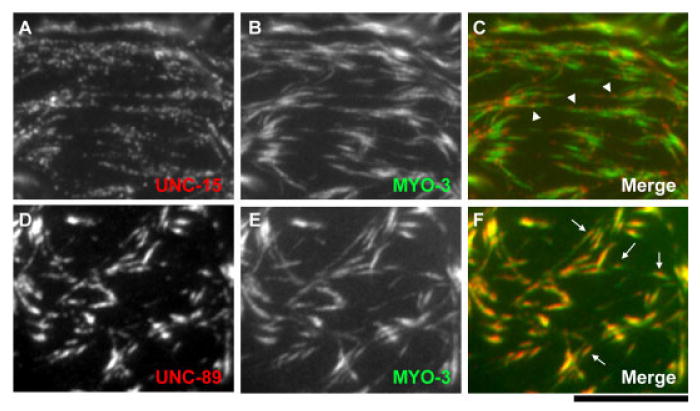
Localization of UNC-15 paramyosin, UNC-89, and MYO-3 myosin heavy chain on the thick filaments. A,B,D,E: The proximal myoepithelial sheath was doubly stained for UNC-15 paramyosin (A) or UNC-89 (D), and MYO-3 myosin heavy chain (B,E). C,F: Merged images are shown (MYO-3 in green and others in red). Arrowheads in C show typical MYO-3 filaments with punctate UNC-15 staining. Arrows in F indicate representative MYO-3 filaments with concentration of UNC-89 in the center. Scale bar = 10 μm.
UNC-89, a component of the M-lines in the striated muscles (Benian et al., 1996), was expressed in the myoepithelial sheath and accumulated into spots (Fig. 4G–I). Double staining of UNC-89 and MYO-3 showed that these proteins colocalize on the thick filaments (Fig. 5D–F), whereas UNC-89 was often highly accumulated at the center of the MYO-3 filaments (Fig. 5F, arrows). Because these central structures of the thick filaments are not organized into M-lines as in striated muscle, we designate these UNC-89–containing structures as the M-spots.
Adhesion structures
In the body wall muscle, myofibrils are attached to the plasma membrane at the dense bodies and the M-lines (Waterston, 1988; Moerman and Fire, 1997). The ultrastructural analysis of the myoepithelial sheath identified the “hemi-adherens junctions,” which are dense body-like structures (Hall et al., 1999). However, the molecular composition of the adhesion structures in the myoepithelial sheath has not been characterized. We found UNC-52 perlecan (Fig. 4J–L), DEB-1 vinculin (Fig. 4M–O), and PAT-3 β-integrin (Fig. 4P–R; Francis and Waterston, 1985) were expressed in the myoepithelial sheath and localized to dot-like structures. Surprisingly, α-actinin, a major component of the dense bodies in the body wall muscle, was not detected by two different monoclonal antibodies MH35 and MH40 (Francis and Waterston, 1985; Table 2, data not shown). Double staining for actin and UNC-52 perlecan, DEB-1 vinculin, or PAT-3 β-integrin showed that these proteins were associated with the actin filaments (Fig. 4J–R), suggesting strongly that these dot-like structures are dense bodies and link the thin filaments to the plasma membrane. However, the spots of PAT-3 β-integrin did not colocalize with MYO-3 myosin heavy chain that is located in the center of the thick filaments (Fig. 6A–D, see absence of overlap between green spots [PAT-3 β-integrin] [arrows] and blue filaments [MYO-3] [arrowheads] in D). This result was further confirmed by triple staining for actin, PAT-3 β-integrin, and UNC-89 (Fig. 6E–H) in which PAT-3 β-integrin (Fig. 6H, arrows) and UNC-89 (Fig. 6H, arrowheads), a marker of the M-spots, do not colocalize (Fig. 6H). These results strongly suggest that PAT-3 β-integrin does not link the M-spots and the thick filaments to the plasma membrane. These were unexpected results, as PAT-3 β-integrin localizes to the M-lines in the body wall muscle (Francis and Waterston, 1985, 1991). This is not likely to be an artifact of the fixation/staining conditions because, under the same fixation/staining conditions, the monoclonal antibody against PAT-3 β-integrin (MH25) stained only the dense bodies in the myoepithelial sheath (Fig. 6A–D). However, it stained both the dense bodies and the M-lines in the body wall muscle (Fig. 6Q–T).
TABLE 2. Components of the Contractile Apparatuses in the Gonadal Myoepithelial Sheath and Body Wall Muscle.
| Gene | Protein | Antibody | MSa location | Reference | BWMb location | Reference |
|---|---|---|---|---|---|---|
| act-?c | Actin | C4 or AAN01 | Thin filaments | (Strome, 1986) | Thin filaments | (Waterston et al., 1984) |
| myo-3 | Myosin heavy chain A | 5-6 | Thick filaments (core) | (Ardizzi and Epstein, 1987) | Thick filaments (core) | (Miller et al., 1983) |
| unc-54 | Myosin heavy chain B | 5-8 | Thick filaments (outer region) | (Ardizzi and Epstein, 1987) | Thick filaments (outer region) | (Miller et al., 1983) |
| unc-15 | Paramyosin | 5-23 | Thick filaments (core) | (Ardizzi and Epstein, 1987) | Thick filaments (core) | (Epstein et al., 1985) |
| unc-89 | UNC-89 | MH42 | M-spots | This study | M-lines | (Benian et al., 1996) |
| lev-11 | Tropomyosin | Anti-CeTM | Thin filaments | (Ono and Ono, 2004) | Thin filaments | (Ono and Ono, 2002) |
| pat-10 | Troponin C | Anti-PAT-10 | Thin filaments | (Ono and Ono, 2004) | Thin filaments | (Terami et al., 1999) |
| ketn-1 | Kettin | MH44 | Thin filaments (near dense bodies) | (Ono et al., 2006) | Thin filaments (near dense bodies) | (Francis and Waterston, 1985) |
| pat-3 | β-integrin | MH25 | Dense bodies | This study | Dense bodies and M-lines | (Francis and Waterston, 1985) |
| deb-1 | Vinculin | MH24 | Dense bodies | This study | Dense bodies | (Francis and Waterston, 1985) |
| unc-52 | Perlecan | MH2 | Dense bodies | This study | Dense bodies and M-lines | (Francis and Waterston, 1991) |
| atn-1 | β-actinin | MH35 or MH40 | Absent | This study | Dense bodies | (Francis and Waterston, 1985) |
| unc-60B | ADF/cofilin | Anti-UNC-60B | Absent | This study | Thin filaments | (Ono et al., 1999) |
| unc-78 | AIP1 | Anti-UNC-78 | Absent | This study | Thin filaments | (Mohri and Ono, 2003) |
| ajm-1 | AJM-1 | MH27 | Absent | This study | Absent (adherens junction in the hypodermis) | (Hresko et al., 1994) |
| let-805 | Myotactin | MH1 | Absent | This study | Absent (hemidesmosomes in the hypodermis | (Hresko et al., 1999) |
MS, myoepithelial sheath.
BWM, body wall muscle.
Caenorhabditis elegans has five actin genes. It is not known which actin gene is expressed in the myoepithelial sheath.
Fig. 6.
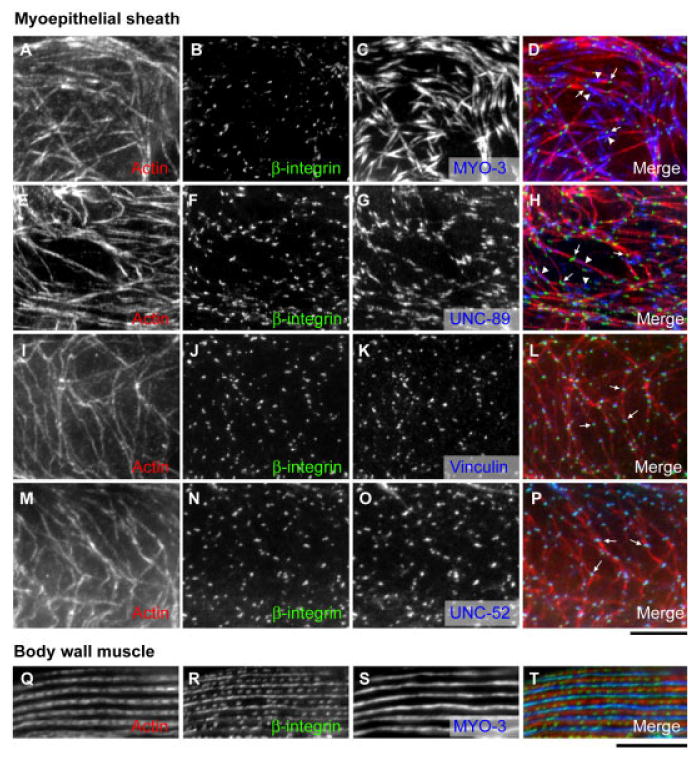
Accumulation of PAT-3 β-integrin and other cell attachment proteins to the dense bodies but not the M-spots. A–C,E–G,I–K,M–O: The proximal myoepithelial sheath was triply stained for actin (A,E,I,M), PAT-3 β-integrin (B,F,J,N), and MYO-3 myosin heavy chain (C), UNC-89 (G), DEB-1 vinculin (K), or UNC-52 perlecan (O). D,H,L,P: Merged images of actin (red), PAT-3 β-integrin (green), and other proteins (blue) are shown. In the merged images, arrows indicate representative spots of PAT-3 β-integrin where segregation from MYO-3 (arrowheads in D) or UNC-89 (arrowheads in H) or colocalization with DEB-1 vinculin (L) or UNC-52 perlecan (P) is clearly seen. Q–T: The body wall muscle was triply stained for actin (Q), PAT-3 β-integrin (R), and MYO-3 myosin heavy chain (S) under the same conditions for A–D, and a merged image actin (red), PAT-3 β-integrin (green), and MYO-3 (blue) is shown in T. Scale bars = 10 μm.
Moreover, other components of the dense bodies, DEB-1 vinculin (Fig. 6I–L, arrows in L) and UNC-52 perlecan (Fig. 6M–P, arrows in P), showed nearly identical localization patterns with PAT-3 β-integrin at the dense bodies. Vinculin is a component of the dense bodies but not M-lines in the body wall muscle (Francis and Waterston, 1985; Barstead and Waterston, 1989), while UNC-52 localizes to both dense bodies and M-lines in the body wall muscle (Francis and Waterston, 1991; Rogalski et al., 1993). These observations suggest that the contractile apparatuses of the myoepithelial sheath are attached to the plasma membrane only at the dense bodies or that the thick filaments are attached to the membrane by an unknown mechanism. Thus, the gonadal myoepithelial sheath and the body wall muscle share common cell attachment proteins, but they are organized in different manners.
Other components
We also examined distribution of AJM-1, a component of adherens junctions (Koppen et al., 2001), myotactin, a component of hemidesmosomes (Hresko et al., 1999), UNC-78 actin-interacting protein 1 (Ono, 2001), and UNC-60B actin depolymerizing factor/ cofilin (Ono et al., 1999, 2003). However, these proteins were detected only in the spermatheca but not in the myoepithelial sheath (our unpublished data).
Roles of the Myofibrillar Components in Ovulation
To determine the roles of the structural components of the myoepithelial sheath in ovulation, they were knocked down by RNA interference (RNAi) and the resultant phenotypes in ovulation were examined. When ovulation fails, a mature oocyte remains in the proximal ovary, repeats DNA synthesis without cell division, and becomes endomitotic (Iwasaki et al., 1996; McCarter et al., 1997, 1999). This phenotype is designated as Emo (endomytotic oocytes in the proximal ovary) and is characterized by intense staining by 4′6-diamidino-2-phenylindole, dihydrochloride (DAPI) of overly amplified nuclear DNA in endomitotic oocytes (Fig. 7C, arrows; Iwasaki et al., 1996). The ovulation process is also regulated by signals from sperm and oocytes. Therefore, to distinguish the effects of RNAi in the somatic gonads from those in the germ cells, we compared the RNAi phenotypes in wild-type and rrf-1 backgrounds. The rrf-1 mutants are defective with RNAi in the somatic cells but not in the germ cells (Sijen et al., 2001). Thus, if function of an RNAi target is important in the somatic gonad (either sheath contraction [McCarter et al., 1997] or spermathecal dilation [Clandinin et al., 1998; Aono et al., 2004]), ovulation will be defective in wild-type but normal in the rrf-1 background.
Fig. 7.
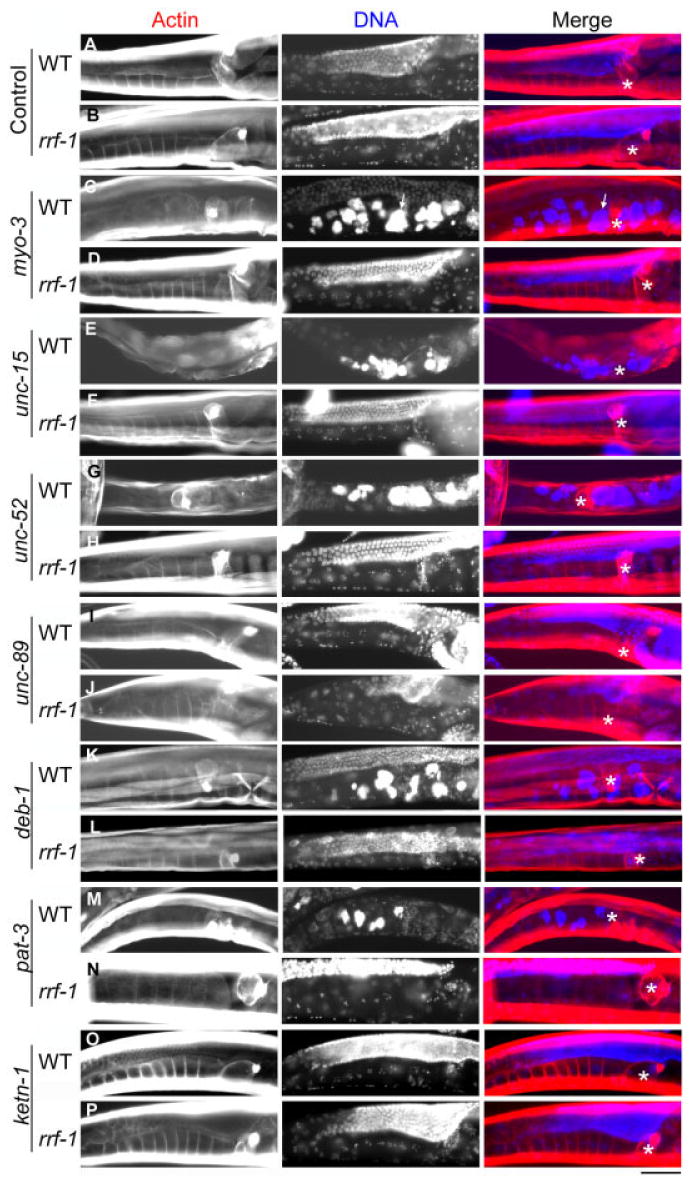
Effects of RNA interference (RNAi) knockdown of the structural proteins on ovulation. A–P: Wild-type (A,C,E,G,I,K,M,O) or an rrf-1 strain (B,D,F,H,J,L,N,P) was treated with RNAi for control (vector with no insert, A,B), myo-3 (C,D), unc-15 (E,F), unc-52 (G,H), unc-89 (I,J), deb-1 (K,L), pat-3 (M,N), or ketn-1 (O,P). Worms were fixed and stained with tetramethylrhodamine-phalloidin and 4′6-diamidino-2-phenylindole, dihydrochloride (DAPI) to visualize actin and DNA, respectively, and merged images are shown in red (actin) and blue (DNA). Positions of the spermatheca are indicated by asterisks. Defective ovulation was characterized by the presence of endomitotic oocytes (arrows in C) that have intense DNA staining (Emo phenotype). Scale bar = 50 μm.
Previous studies have shown that unc-54 myosin heavy chain (Rose et al., 1997), lev-11 tropomyosin (Ono and Ono, 2004), pat-10 troponin C (Ono and Ono, 2004), and mup-2 troponin T (Myers et al., 1996) are required for ovulation. Here, we found that the Emo phenotype (22–51%; n = 100) was induced by RNAi treatments in wild-type background for myo-3 myosin heavy chain, unc-15 paramyosin, pat-3 β-integrin, deb-1 vinculin, or unc-52 perlecan (Fig. 7C,E,G,K; Table 3). However, RNAi of unc-89 or ketn-1 Ce-kettin did not significantly increase the percentages of the Emo phenotype (Fig. 7I,O; Table 3). Only background levels of the Emo phenotype (0–5%) were detected in the rrf-1 background after RNAi of myo-3, unc-15, unc-52, or deb-1 (Table 3), suggesting strongly that these genes function in the somatic cells. pat-3(RNAi) caused 99% P0 sterility with the Emo phenotype (n = 100) in wild-type, but it is significantly reduced in rrf-1 (Fig. 6M,N; Table 3). A slightly higher rate of the Emo phenotype (10%) by pat-3(RNAi) in rrf-1 than the background level may indicate a possible role of pat-3 in the germline or the gut, which is a known source of yolk proteins (Kimble and Sharrock, 1983; Grant and Hirsh, 1999). RNAi of unc-89 did not cause the Emo phenotype. Instead, the unc-89(RNAi) animals, in wild-type but not rrf-1 background, were defective in egg laying and accumulated embryos in the uterus (our unpublished data), suggesting that UNC-89 is functionally important in the vulval and/or uterine muscle. Together, these results indicate that the structural components of the contractile apparatuses in the myoepithelial sheath, except for UNC-89 and Ce-kettin, are essential for ovulation. However, we cannot rule out the possibilities that UNC-89 and Ce-kettin are also essential for ovulation, because the gene products might not have been knocked down by the RNAi treatments sufficiently to cause phenotypes.
TABLE 3. Effects of RNAi Treatments on Ovulation.
| RNAi treatment (generation observed) | % Emo phenotype (n = 100) | |
|
| ||
| Wild-type | rrf-1 | |
|
| ||
| Control (F1) | 1 | 3 |
| myo-3 (F1) | 51 | 3 |
| unc-15(F1) | 22 | 5 |
| unc-52 (F1) | 30 | 1 |
| unc-89 (F1) | 0 | 3 |
| deb-1 (F1) | 30 | 0 |
| ketn-1 (F1) | 0 | 3 |
| Control (P0) | 0 | 0 |
| pat-3 (P0) | 99 | 10 |
Discussion
In this study, we characterized organization and function of nonstriated contractile apparatuses in the C. elegans gonadal myoepithelial sheath. Based on our results, we propose a model for the organization of the contractile apparatuses in the myoepithelial sheath (Fig. 8A). The thin filaments are anchored to the plasma membrane at the dense bodies where integrin is accumulated. The dense bodies lack α-actinin. Therefore, vinculin, talin, or other actin-binding proteins may connect actin with integrin. The thick filaments have M-spots but do not have integrin-based attachments with the plasma membrane. An alternative model (Fig. 8B) is that cytoplasmic actin-anchoring structures, possibly containing α-actinin–like molecules, links the thin filaments to the integrin-based structure and that the thick filaments are attached to the membrane through non–integrin-based structures. Whether the thick filaments in the myoepithelial sheath are physically connected with the plasma membrane is unknown. Previous ultrastructural studies on the somatic gonad did not reveal such connections (Hirsh et al., 1976; Strome, 1986; Hall et al., 1999).
Fig. 8.
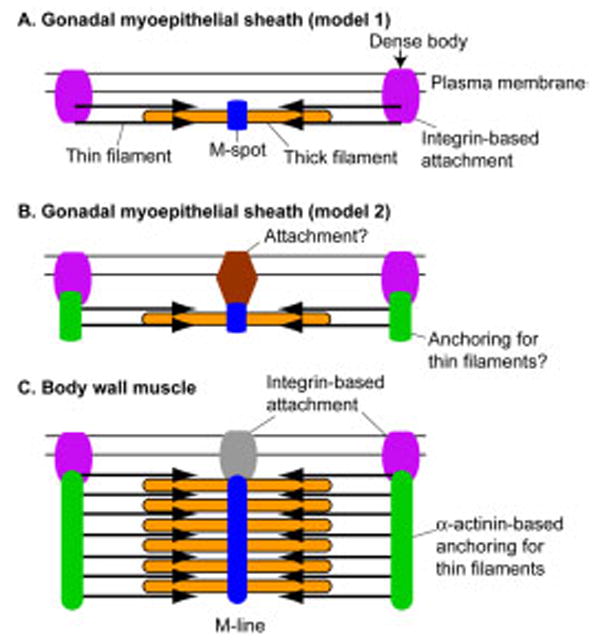
Models of the organization of structural components of the contractile apparatuses in the myoepithelial sheath. A: Model 1 shows that the thin filaments are attached to the plasma membrane at the integrin-based structures, while the thick filaments lack a physical link with the membrane. B: Model 2 shows that the thin filaments are connected to the integrin-based structures through unknown anchoring components, whereas the thick filaments are attached to the membrane with unknown attachment structures. C: An established model of the structure of the contractile apparatuses of the body wall muscle (Waterston, 1988; Moerman and Fire, 1997). Arrows of the thin filaments are pointing toward the pointed ends of the actin filaments.
Myofibrils in the myoepithelial sheath and the body wall muscle are not only different in their organization but also in their size. The myoepithelial sheath cells are ∼1 μm thick and the thin and thick filaments are loosely arranged in a meshwork pattern (Hall et al., 1999). In contrast, the body wall muscle cells are 2- to 3-μm thick and the myofibrils are densely packed within 1–1.5 μm from the plasma membrane to transmit contractile forces to the hypodermis and the cuticles (Fig. 8C; Waterston et al., 1980; Francis and Waterston, 1985). Therefore, the body wall muscle may require very rigid attachment structures for both thin and thick filaments. These muscles are also different in their manners of contraction. The myoepithelial sheath needs to be flexible in changing their cell shape to support transport of oocytes. Thus, nonstriated filamentous organization and lack of integrin-based links between the thick filaments and the membrane might be beneficial for such a function. On the other hand, the body wall muscle always contracts in the longitudinal direction, and tight arrangement of sarcomeres in a uniform direction will be very efficient to coordinate contractile forces.
pat-3 is the single gene for β-integrin in C. elegans and is essential for assembling the adhesion structures in body wall muscle (Francis and Waterston, 1985; Williams and Waterston, 1994; Gettner et al., 1995). PAT-3 β-integrin is expressed in a variety of muscle and nonmuscle cells and also involved in cell migration, gonadal morphogenesis, and axon guidance (Lee et al., 2001, 2005; Poinat et al., 2002; Xu et al., 2006). Localization of PAT-3 β-integrin to the dense bodies and the M-lines in body wall muscle and cell attachment structures in several other cell types has been reported previously (Francis and Waterston, 1985; Gettner et al., 1995). However, this study is the first to describe intracellular localization of PAT-3 β-integrin in the myoepithelial sheath, supporting previous observations that PAT-3 β-integrin is required for ovulation (Lee et al., 2001; Xu et al., 2005, 2006). Limited localization of PAT-3 β-integrin to the dense bodies in the myoepithelial sheath is a remarkable difference from its localization to both dense bodies and M-lines in the body wall muscle. Integrin plays critical roles in mediating extracellular signals to trigger several intracellular signaling events, including reorganization of the actin cytoskeleton (Brakebusch and Fassler, 2003; Wozniak et al., 2004; Lo, 2006). Thus, the difference in the integrin localization between striated and nonstriated muscles suggests that integrin has different functions in these muscles. One possible role for integrin might be to regulate the morphogenetic processes of nonstriated or striated myofibrils by sending signals to different cytoskeletal regulators. Currently, little is known about the mechanism of morphogenesis of the myoepithelial sheath, and further studies may reveal how integrin might be involved in this process.
RNA interference of the major contractile proteins in the myoepithelial sheath caused defective ovulation. However, RNAi of myo-3, unc-15, unc-52, or deb-1 resulted in 22–51% sterility, which is weaker than nearly 100% sterility caused by RNAi of pat-3 (this study), pat-10 troponin C, or lev-11 tropomyosin (Ono and Ono, 2004). This finding is likely due to incomplete knockdown of these genes by the RNAi treatments, as strong loss-of-function or null mutation of deb-1, myo-3, or unc-52 results in embryonic lethality (Waterston, 1989; Barstead and Waterston, 1991; Rogalski et al., 1995). Nonetheless, these weak RNAi phenotypes allowed us to characterize the ovulation defects without having severe embryonic defects. We also did not observe severe disorganization of the actin filament structures in the myoepithelial sheath even after the RNAi treatments, suggesting that a mild decrease in the amounts of these contractile proteins might be sufficient to impair sheath contraction but insufficient to cause severe morphogenetic defects.
Our study also suggests that the C. elegans myoepithelial sheath might be a good model to study structure and function of smooth muscle, myoepithelial cells, and myofibroblasts, which have nonstriated contractile apparatuses. To date, a genetic model for cytoskeletal assembly in such nonstriated contractile cells has not been developed. Smooth muscles are present in both vertebrates and invertebrates and play a variety of functions (Somlyo and Somlyo, 1994). Myoepithelial cells are often found in the outer layer of exocrine glands and have characteristics of both smooth muscle and epithelial cells (Lazard et al., 1993; Foschini et al., 2000; Furuse et al., 2005). Myofibroblasts are present in many organs and express several sarcomeric proteins (Mayer and Leinwand, 1997; Walker et al., 2001; Rice and Leinwand, 2003). Regulatory mechanisms for contraction is also various. Vertebrate smooth muscles are regulated by a myosin-linked system (Marston, 1995), whereas ascidian smooth body wall muscle (Endo and Obinata, 1981) and C. elegans myoepithelial sheath (Ono and Ono, 2004) are regulated by an actin-linked troponin-tropomyosin system. However, nonstriated organization of the contractile apparatuses is the common feature of all smooth muscles, myoepithelial cells, and myofibroblasts, and little is known about the mechanism by which nonstriated myofibrils are assembled during development. Thus, C. elegans might be a suitable model to investigate this fundamental mechanism. We preliminarily found that known regulators of actin dynamics in the body wall muscle, including unc-60B actin depolymerizing factor/cofilin (Ono et al., 1999, 2003) and unc-78 actin-interacting protein 1 (Ono, 2001), are not required for assembly of nonstriated myofibrils in the myoepithelial sheath (our unpublished data). Although we previously reported that unc-60B might be involved in ovulation antagonistically to lev-11 tropomyosin (Ono and Ono, 2004), we have recently shown that the observed genetic interaction between unc-60B and lev-11 was due to a background mutation that affected the efficiency of RNAi of lev-11 but not due to a direct effect of the unc-60B mutation (Yu and Ono, 2006). These data suggest that nonstriated and striated muscles in C. elegans use different sets of genes to regulate actin organization.
Experimental Procedures
Nematode Strains
Wild-type C. elegans strain N2 and an RNAi-defective strain NL2098 rrf-1(pk1417) (Sijen et al., 2001) were obtained from the Caenorhabditis Genetics Center (Minneapolis, MN). Nematodes were grown under standard conditions at 20°C (Brenner, 1974).
Antibodies and Fluorescent Probes
Anti-actin mouse monoclonal antibody (C4) was purchased from MP Biomedicals. Anti-actin rabbit polyclonal antibody (AAN01) was purchased from Cytoskeleton, Inc. Anti-myoA (5-6; Miller et al., 1983) and anti-paramyosin (5-23; Ardizzi and Epstein, 1987) mouse monoclonal antibodies were provided by Dr. Henry Epstein (University of Texas Medical Branch, Galveston, TX). Anti–PAT-3 β-integrin (MH25; Francis and Waterston, 1985), anti–DEB-1 vinculin (MH24; Francis and Waterston, 1985), anti–UNC-52 perlecan (MH2; Francis and Waterston, 1991) monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). Anti–UNC-89 monoclonal antibody (MH42) (Benian et al., 1996) was provided by Drs. Pamela Hoppe (Western Michigan University, Kalamazoo, MI) and Robert Waterston (University of Washington, Seattle, WA). Tetramethylrhodamine-phalloidin and DAPI were purchased from Sigma-Aldrich. All Alexa dye-conjugated secondary antibodies and Zenon Mouse IgG2a Labeling Reagents were purchased from Invitrogen/Molecular Probes. Cy3-conjugated donkey anti-rabbit IgG and anti-mouse IgG were purchased from Jackson ImmunoResearch Laboratories.
Fluorescence Microscopy
The gonads were dissected by cutting adult hermaphrodites at the level of pharynx (Fig. 1)–on poly-lysine–coated slides as described previously (Rose et al., 1997). These samples were fixed by an optimal method and stained with antibodies as listed in Table 1. When the host animals of the primary antibodies were different, they were mixed and reacted with the samples simultaneously, and followed by treatments with appropriate fluorescently labeled secondary antibodies (Table 1). We also performed differential labeling of two mouse monoclonal antibodies and one rabbit antibody by using secondary antibodies that distinguish IgG isotypes and host species as described previously (Ono et al., 2006). Briefly, the samples were first treated with a mixture of mouse IgG1 antibody and rabbit antibody, and then with secondary antibodies (nonspecific for IgG isotypes). They were washed with phosphate buffered saline (PBS) and blocked with 0.1 mg/ml mouse IgG (Rockland Immunochemicals) in 1% bovine serum albumin in PBS for 10 min, and reacted with a mouse IgG2a antibody. Then, it was visualized by Zenon Alexa Fluor Mouse IgG2a Labeling Reagents (Invitrogen/Molecular Probes) (Table 1).
Samples were viewed by epifluorescence using a Nikon Eclipse TE2000 inverted microscope with a ×40 or ×60 CFI Plan Fluor objective. Images were captured by a SPOT RT Monochrome CCD camera (Diagnostic Instruments) and processed by the IPLab imaging software (Scanalytics, Inc.) and Adobe Photoshop 6.0.
RNA Interference Experiments
Namatodes were treated with RNAi by feeding Escherichia coli–expressing double-stranded RNA (Timmons and Fire, 1998; Timmons et al., 2001). Except for pat-3(RNAi), L4 larvae were treated with RNAi, and phenotypes were characterized in their F1 generation as described previously (Ono and Ono, 2002). pat-3(RNAi) caused strong P0 sterility. Therefore, L1 larvae were treated with pat-3(RNAi), and phenotypes were characterized when they became adults. RNAi clones were obtained from the C. elegans RNAi library from Geneservice (Cambridge, United Kingdom), and the clone numbers are as follows: myo-3, V-8003; unc-15, I-3N01; unc-52, II-9A20; unc-89, I-1B22; pat-3, III-1P02; and ketn-1, V-2F01. Because an RNAi clone for deb-1 was not available from the library, we made a construct by cloning a 1.0-kb genomic fragment of the deb-1 gene into an RNAi vector plasmid L4440 (provided by Dr. Andrew Fire, Stanford University) and transformed E. coli HT115(DE3) with the recombinant plasmid. Control experiments were performed with E. coli HT115(DE3) that was transformed with L4440 with no insert. Staining of RNAi-treated worms with tetramethylrhodamine-phalloidin and DAPI was performed as described previously (Ono, 2001).
Acknowledgments
The authors thank Dr. Takashi Obinata for helpful discussion, Drs. Henry Epstein, Pamela Hoppe, and Robert Waterston for antibodies used in this study, and Dr. Andrew Fire for the RNAi vector. Monoclonal antibodies MH2, MH24, MH25 were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa (Department of Biological Sciences, Iowa City, IA 52242). Some C. elegans strains were provided by the Caenorhabditis Genetics Center, which is supported by the National Institutes of Health National Center for Research Resources. S.O. was supported by a grant from the National Institutes of Health.
References
- Aono S, Legouis R, Hoose WA, Kemphues KJ. PAR-3 is required for epithelial cell polarity in the distal spermatheca of C. elegans. Development. 2004;131:2865–2874. doi: 10.1242/dev.01146. [DOI] [PubMed] [Google Scholar]
- Ardizzi JP, Epstein HF. Immunochemical localization of myosin heavy chain isoforms and paramyosin in developmentally and structurally diverse muscle cell types of the nematode Caenorhabditis elegans. J Cell Biol. 1987;105:2763–2770. doi: 10.1083/jcb.105.6.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstead RJ, Waterston RH. The basal component of the nematode dense-body is vinculin. J Biol Chem. 1989;264:10177–10185. [PubMed] [Google Scholar]
- Barstead RJ, Waterston RH. Vinculin is essential for muscle function in the nematode. J Cell Biol. 1991;114:715–724. doi: 10.1083/jcb.114.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benian GM, Tinley TL, Tang X, Borodovsky M. The Caenorhabditis elegans gene unc-89, required for muscle M-line assembly, encodes a giant modular protein composed of Ig and signal transduction domains. J Cell Biol. 1996;132:835–848. doi: 10.1083/jcb.132.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324–2333. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, DeModena JA, Sternberg PW. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- Corrigan C, Subramanian R, Miller MA. Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation. Development. 2005;132:5225–5237. doi: 10.1242/dev.02083. [DOI] [PubMed] [Google Scholar]
- Cram EJ, Clark SG, Schwarzbauer JE. Talin loss-of-function uncovers roles in cell contractility and migration in C. elegans. J Cell Sci. 2003;116:3871–3878. doi: 10.1242/jcs.00705. [DOI] [PubMed] [Google Scholar]
- Deitiker PR, Epstein HF. Thick filament substructures in Caenorhabditis elegans: evidence for two populations of paramyosin. J Cell Biol. 1993;123:303–311. doi: 10.1083/jcb.123.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Obinata T. Troponin and its components from ascidian smooth muscle. J Biochem. 1981;89:1599–1608. doi: 10.1093/oxfordjournals.jbchem.a133355. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Miller DM, Ortiz I, Berliner GC. Myosin and paramyosin are organized about a newly identified core structure. J Cell Biol. 1985;100:904–915. doi: 10.1083/jcb.100.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein HF, Berliner GC, Casey DL, Ortiz I. Purified thick filaments from the nematode Caenorhabditis elegans: evidence for multiple proteins associated with core structures. J Cell Biol. 1988;106:1985–1995. doi: 10.1083/jcb.106.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foschini MP, Scarpellini F, Gown AM, Eusebi V. Differential expression of myoepithelial markers in salivary, sweat and mammary glands. Int J Surg Pathol. 2000;8:29–37. doi: 10.1177/106689690000800108. [DOI] [PubMed] [Google Scholar]
- Francis GR, Waterston RH. Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J Cell Biol. 1985;101:1532–1549. doi: 10.1083/jcb.101.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R, Waterston RH. Muscle cell attachment in Caenorhabditis elegans. J Cell Biol. 1991;114:465–479. doi: 10.1083/jcb.114.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse C, Sousa SO, Nunes FD, Magalhaes MH, Araujo VC. Myoepithelial cell markers in salivary gland neoplasms. Int J Surg Pathol. 2005;13:57–65. doi: 10.1177/106689690501300108. [DOI] [PubMed] [Google Scholar]
- Gettner SN, Kenyon C, Reichardt LF. Characterization of βpat-3 heterodimers, a family of essential integrin receptors in C. elegans. J Cell Biol. 1995;129:1127–1141. doi: 10.1083/jcb.129.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Winfrey VP, Blaeuer G, Hoffman LH, Furuta T, Rose KL, Hobert O, Greenstein D. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol. 1999;212:101–123. doi: 10.1006/dbio.1999.9356. [DOI] [PubMed] [Google Scholar]
- Harris HE, Epstein HF. Myosin and paramyosin of Caenorhabditis elegans: biochemical and structural properties of wild-type and mutant proteins. Cell. 1977;10:709–719. doi: 10.1016/0092-8674(77)90105-2. [DOI] [PubMed] [Google Scholar]
- Harris HE, Tso MY, Epstein HF. Actin and myosin-linked calcium regulation in the nematode Caenorhabditis elegans. Biochemical and structural properties of native filaments and purified proteins. Biochemistry. 1977;16:859–865. doi: 10.1021/bi00624a008. [DOI] [PubMed] [Google Scholar]
- Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- Hresko MC, Williams BD, Waterston RH. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J Cell Biol. 1994;124:491–506. doi: 10.1083/jcb.124.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko MC, Schriefer LA, Shrimankar P, Waterston RH. Myotactin, a novel hypodermal protein involved in muscle-cell adhesion in Caenorhabditis elegans. J Cell Biol. 1999;146:659–672. doi: 10.1083/jcb.146.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EJ, Greenstein D. The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev Dyn. 2000;218:2–22. doi: 10.1002/(SICI)1097-0177(200005)218:1<2::AID-DVDY2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, McCarter J, Francis R, Schedl T. emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J Cell Biol. 1996;134:699–714. doi: 10.1083/jcb.134.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Sharrock WJ. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev Biol. 1983;96:189–196. doi: 10.1016/0012-1606(83)90322-6. [DOI] [PubMed] [Google Scholar]
- Koppen M, Simske JS, Sims PA, Firestein BL, Hall DH, Radice AD, Rongo C, Hardin JD. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol. 2001;3:983–991. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- Kosinski M, McDonald K, Schwartz J, Yamamoto I, Greenstein D. C. elegans sperm bud vesicles to deliver a meiotic maturation signal to distant oocytes. Development. 2005;132:3357–3369. doi: 10.1242/dev.01916. [DOI] [PubMed] [Google Scholar]
- Lazard D, Sastre X, Frid MG, Glukhova MA, Thiery JP, Koteliansky VE. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci U S A. 1993;90:999–1003. doi: 10.1073/pnas.90.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Cram EJ, Shen B, Schwarzbauer JE. Roles for βpat-3 integrins in development and function of Caenorhabditis elegans muscles and gonads. J Biol Chem. 2001;276:36404–36410. doi: 10.1074/jbc.M105795200. [DOI] [PubMed] [Google Scholar]
- Lee M, Shen B, Schwarzbauer JE, Ahn J, Kwon J. Connections between integrins and Rac GTPase pathways control gonad formation and function in C. elegans. Biochim Biophys Acta. 2005;1723:248–255. doi: 10.1016/j.bbagen.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Lo SH. Focal adhesions: what's new inside. Dev Biol. 2006;294:280–291. doi: 10.1016/j.ydbio.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Marston S. Ca2+-dependent protein switches in actomyosin based contractile systems. Int J Biochem Cell Biol. 1995;27:97–108. doi: 10.1016/1357-2725(94)00080-u. [DOI] [PubMed] [Google Scholar]
- Mayer DC, Leinwand LA. Sarcomeric gene expression and contractility in myofibroblasts. J Cell Biol. 1997;139:1477–1484. doi: 10.1083/jcb.139.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev Biol. 1997;181:121–143. doi: 10.1006/dbio.1996.8429. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- Miller DM, Ortiz I, Berliner GC, Epstein HF. Differential localization of two myosins within nematode thick filaments. Cell. 1983;34:477–490. doi: 10.1016/0092-8674(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 2003;17:187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman DG, Fire A. Muscle: structure, function, and development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Plainview, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 417–470. [PubMed] [Google Scholar]
- Mohri K, Ono S. Actin filament disassembling activity of Caenorhabditis elegans actin-interacting protein 1 (UNC-78) is dependent on filament binding by a specific ADF/cofilin isoform. J Cell Sci. 2003;116:4107–4118. doi: 10.1242/jcs.00717. [DOI] [PubMed] [Google Scholar]
- Myers CD, Goh PY, Allen TS, Bucher EA, Bogaert T. Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. J Cell Biol. 1996;132:1061–1077. doi: 10.1083/jcb.132.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KR, Fazzio RT, Mellem JE, Espelt MV, Strange K, Beckerle MC, Maricq AV. The Rho/Rac-family guanine nucleotide exchange factor VAV-1 regulates rhythmic behaviors in C. elegans. Cell. 2005;123:119–132. doi: 10.1016/j.cell.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Ono S. The Caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments. J Cell Biol. 2001;152:1313–1319. doi: 10.1083/jcb.152.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Ono K. Tropomyosin inhibits ADF/cofilin-dependent actin filament dynamics. J Cell Biol. 2002;156:1065–1076. doi: 10.1083/jcb.200110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Ono S. Tropomyosin and troponin are required for ovarian contraction in the Caenorhabditis elegans reproductive system. Mol Biol Cell. 2004;15:2782–2793. doi: 10.1091/mbc.E04-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Baillie DL, Benian GM. UNC-60B, an ADF/cofilin family protein, is required for proper assembly of actin into myofibrils in Caenorhabditis elegans body wall muscle. J Cell Biol. 1999;145:491–502. doi: 10.1083/jcb.145.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Parast M, Alberico C, Benian GM, Ono S. Specific requirement for two ADF/cofilin isoforms in distinct actin-dependent processes in Caenorhabditis elegans. J Cell Sci. 2003;116:2073–2085. doi: 10.1242/jcs.00421. [DOI] [PubMed] [Google Scholar]
- Ono S, Mohri K, Ono K. Molecular and biochemical characterization of kettin in Caenorhabditis elegans. J Muscle Res Cell Motil. 2005;26:449–454. doi: 10.1007/s10974-005-9028-3. [DOI] [PubMed] [Google Scholar]
- Ono K, Yu R, Mohri K, Ono S. Caenorhabditis elegans kettin, a large immunoglobulin-like repeat protein, binds to filamentous actin and provides mechanical stability to the contractile apparatuses in body wall muscle. Mol Biol Cell. 2006;17:2722–2734. doi: 10.1091/mbc.E06-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinat P, De Arcangelis A, Sookhareea S, Zhu X, Hedgecock EM, Labouesse M, Georges-Labouesse E. A conserved interaction between β1 integrin/PAT-3 and Nck-interacting kinase/MIG-15 that mediates commissural axon navigation in C. elegans. Curr Biol. 2002;12:622–631. doi: 10.1016/s0960-9822(02)00764-9. [DOI] [PubMed] [Google Scholar]
- Rice NA, Leinwand LA. Skeletal myosin heavy chain function in cultured lung myofibroblasts. J Cell Biol. 2003;163:119–129. doi: 10.1083/jcb.200303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski TM, Williams BD, Mullen GP, Moerman DG. Products of the unc-52 gene in Caenorhabditis elegans are homologous to the core protein of the mammalian basement membrane heparan sulfate proteoglycan. Genes Dev. 1993;7:1471–1484. doi: 10.1101/gad.7.8.1471. [DOI] [PubMed] [Google Scholar]
- Rogalski TM, Gilchrist EJ, Mullen GP, Moerman DG. Mutations in the unc-52 gene responsible for body wall muscle defects in adult Caenorhabditis elegans are located in alternatively spliced exons. Genetics. 1995;139:159–169. doi: 10.1093/genetics/139.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KL, Winfrey VP, Hoffman LH, Hall DH, Furuta T, Greenstein D. The POU gene ceh-18 promotes gonadal sheath cell differentiation and function required for meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1997;192:59–77. doi: 10.1006/dbio.1997.8728. [DOI] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Strome S. Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode Caenorhabditis elegans. J Cell Biol. 1986;103:2241–2252. doi: 10.1083/jcb.103.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DD, Tan J. Downregulation of profilin with antisense oligodeoxynucleotides inhibits force development during stimulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2003;285:H1528–H1536. doi: 10.1152/ajpheart.00188.2003. [DOI] [PubMed] [Google Scholar]
- Tang DD, Zhang W, Gunst SJ. The adapter protein CrkII regulates neuronal Wiskott-Aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. J Biol Chem. 2005;280:23380–23389. doi: 10.1074/jbc.M413390200. [DOI] [PubMed] [Google Scholar]
- Terami H, Williams BD, Kitamura S, Sakube Y, Matsumoto S, Doi S, Obinata T, Kagawa H. Genomic organization, expression, and analysis of the troponin C gene pat-10 of Caenorhabditis elegans. J Cell Biol. 1999;146:193–202. doi: 10.1083/jcb.146.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Walker GA, Guerrero IA, Leinwand LA. Myofibroblasts: molecular crossdressers. Curr Top Dev Biol. 2001;51:91–107. doi: 10.1016/s0070-2153(01)51003-0. [DOI] [PubMed] [Google Scholar]
- Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol. 1979;73:304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- Waterston RH. Muscle. In: Wood WB, editor. The Nematode C elegans. Plainview, NY: Cold Spring Harbor Laboratory; 1988. pp. 281–335. [Google Scholar]
- Waterston RH. The minor myosin heavy chain, mhcA, of Caenorhabditis elegans is necessary for the initiation of thick filament assembly. EMBOJ. 1989;8:3429–3436. doi: 10.1002/j.1460-2075.1989.tb08507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston RH, Hirsh D, Lane TR. Dominant mutations affecting muscle structure in Caenorhabditis elegans that map near the actin gene cluster. J Mol Biol. 1984;180:473–496. doi: 10.1016/0022-2836(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Waterston RH, Thomson JN, Brenner S. Mutants with altered muscle structure of Caenorhabditis elegans. Dev Biol. 1980;77:271–302. doi: 10.1016/0012-1606(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Whitten SJ, Miller MA. The role of gap junctions in Caenorhabditis elegans oocyte maturation and fertilization. Dev Biol. 2006;301:432–446. doi: 10.1016/j.ydbio.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Williams BD, Waterston RH. Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J Cell Biol. 1994;124:475–490. doi: 10.1083/jcb.124.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmann A, Ingles J, Mains PE. The Caenorhabditis elegans mel-11 myosin phosphatase regulatory subunit affects tissue contraction in the somatic gonad and the embryonic epidermis and genetically interacts with the Rac signaling pathway. Dev Biol. 1999;209:111–127. doi: 10.1006/dbio.1999.9242. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Xu X, Lee D, Shih HY, Seo S, Ahn J, Lee M. Linking integrin to IP(3) signaling is important for ovulation in Caenorhabditis elegans. FEBS Lett. 2005;579:549–553. doi: 10.1016/j.febslet.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Xu X, Rongali SC, Miles JP, Lee KD, Lee M. pat-4/ILK and unc-112/Mig-2 are required for gonad function in Caenorhabditis elegans. Exp Cell Res. 2006;312:1475–1483. doi: 10.1016/j.yexcr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto I, Kosinski ME, Greenstein D. Start me up: cell signaling and the journey from oocyte to embryo in C. elegans. Dev Dyn. 2006;235:571–585. doi: 10.1002/dvdy.20662. [DOI] [PubMed] [Google Scholar]
- Yin X, Gower NJ, Baylis HA, Strange K. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of myoepithelial sheath cells in Caenorhabditis elegans. Mol Biol Cell. 2004;15:3938–3949. doi: 10.1091/mbc.E04-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Ono S. Dual roles of tropomyosin as an F-actin stabilizer and a regulator of muscle contraction in Caenorhabditis elegans body wall muscle. Cell Motil Cytoskeleton. 2006;63:659– 672. doi: 10.1002/cm.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]


