Abstract
Vascular endothelial growth factor (VEGF) is one of the key regulators of tumor neoangiogenesis. It acts through two types of high-affinity tyrosine kinase receptors (VEGF receptor-1 [VEGFR-1]/fms–related tyrosine kinase 1 [Flt-1] and VEGFR-2/kinase domain receptor [KDR]) expressed on endothelial cells. VEGFRs have also been detected on cancer cells, suggesting a possible autocrine effect of VEGF on their growth. We studied the expression of VEGF, VEGFR-1, and VEGFR-2 in human medulloblastoma cell lines (DAOY, D283Med, and D341Med) and investigated the possible autocrine mechanisms of VEGF on medulloblastoma cell proliferation. Reverse transcriptase PCR analysis showed the presence of VEGF and VEGFR mRNAs in all cell lines studied. Of the three VEGF isoforms, VEGF121 and VEGF189 were detected by Western blot analysis in all three medulloblastoma cell lines, whereas VEGF165 was identified only in DAOY cells. Medulloblastoma cell lines expressed both VEGFR-1 and VEGFR-2. We also demonstrated expression of VEGF and its receptors in medulloblastoma tumor specimens. Exogenous VEGFR-2 inhibitor reduced the VEGF-dependent cell proliferation of DAOY and D283Med cells. In DAOY cells, VEGF165 induced phosphorylation of VEGFR-2/KDR and of downstream proteins in the signal transduction pathway. These data suggest a possible autocrine role for VEGF in medulloblastoma growth. Targeting VEGF signaling may represent a new therapeutic option in the treatment of medulloblastoma.
Keywords: medulloblastoma, VEGF, VEGFRs
Medulloblastoma is the most common childhood malignant brain tumor. Despite recent progress in prognosis, thanks to refinements of conventional craniospinal radiotherapy and systemic chemotherapy, a third of these children die of their disease, with survivors having significant long-term side effects of treatment.1,2 Thus, the need to investigate the biology of this cancer to develop more effective and less toxic therapies is imperative. Neoangiogenesis, the formation of new blood vessels from existing vasculature, seems to be a critical factor for tumor growth and progression and a prerequisite for metastases.3 Indeed, the quantitative assessment of angiogenesis has been proposed as an additional criterion to be incorporated into the anaplasia grading system for childhood medulloblastoma.4 A variety of angiogenic factors have been implicated, individually or as a whole, in the neoangiogenesis of medulloblastoma, highlighting the complexity of the biological processes regulating tumor neovascularization.5 The activation, proliferation, and migration of the endothelial cells, starting from preexisting vascular structures, are regulated by specific growth factors secreted by tumor cells and by cellular components of the surrounding microenvironment.
Vascular endothelial growth factor (VEGF) is one of the most powerful mitogens for endothelial cells in CNS tumors.6,7 Generated by mechanisms of alternative splicing of the same gene, different isoforms of VEGF exist; among these, the 121– and 165–amino acid soluble isoforms are the most abundant. The different isoforms bind to two tyrosine kinase receptors (fms-related tyrosine kinase 1 [Flt-1] or VEGF receptor 1 [VEGFR-1], and kinase domain receptor [KDR] or VEGFR-2), and upon binding, the receptor is phosphorylated, thus triggering the intracellular transduction signal pathway. Differences exist in the function and signaling properties of these two receptors. VEGFR-2 is primarily responsible for the angiogenic and vascular permeability effects of VEGF, whereas VEGFR-1 seems to have a role in sequestering VEGF (“decoy receptor”) and thus in regulating its interaction with VEGFR-2.8 Although VEGFRs were initially found on endothelial cells, recent studies have shown that tumor cells of different origins also express VEGFRs, suggesting that VEGF may act as an autocrine signal.9,10 The presence of an autocrine loop resulting in increased proliferation has been demonstrated in different cell lines (melanoma, prostate carcinoma, leukemia, and rhabdomyosarcoma),11–14 although an antiproliferative effect has also been occasionally observed in other tumor models.15 Starting from these considerations, we investigated the role of VEGF/VEGFR signaling on medulloblastoma cell growth. Here, we report that VEGF and VEGFRs are expressed in medulloblastoma cell lines and tumor specimens and that exogenous VEGF promotes cell growth in vitro.
Materials and Methods
Reagents
Antihuman VEGF (sc-152), VEGFR-1 (sc-316), and VEGFR-2 (sc-504) rabbit polyclonal antibodies were from Santa Cruz Biotechnology (Heidelberg, Germany); antiphosphotyrosine (PY-20) monoclonal antibody was from Transduction Laboratories (Lexington, KY, USA). EnVision+ System horseradish peroxidase was purchased from DAKO (Milan, Italy), human VEGF165 from Sigma-Aldrich (Milan, Italy), and VEGF inhibitor VI from Calbiochem (Darmstadt, Germany). Cell culture media were purchased from Euroclone (Milan, Italy). Endothelial cell growth medium was from PromoCell (Heidelberg, Germany), protein assay from Bio-Rad (Milan, Italy), Complete Protease Inhibitor Cocktail Tablets from Roche Diagnostic (Milan, Italy), SuperSignal West Pico Chemiluminescent Substrate from Pierce (Rockford, IL, USA), and TRIzol reagent and SuperScript II reverse transcriptase from Invitrogen (Milan, Italy). All primers were purchased from Sigma-Genosys (Haverhill, UK). All remaining reagents were from Amersham-Pharmacia Biotech (Milan, Italy).
Cell Culture
Human medulloblastoma cell lines DAOY, D283Med, and D341Med were grown in complete culture medium (RPMI medium supplemented with 10% fetal bovine serum [FBS], 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin). The human fibrosarcoma cell line HT1080 was maintained in Dulbecco’s modified Eagle medium supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Human umbilical endothelial vein cells (HUVEC) were grown in endothelial cell growth medium supplemented with SupplementMix (PromoCell) and 8% FBS.
Western Blot Analysis
Subconfluent cells were lysed in 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 1% Triton plus 1× Complete Protease Inhibitor. Thirty micrograms of total protein was resuspended in sample buffer, heated at 95°C for 5 min, and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Subsequently, proteins were electrotransferred overnight at 4°C to nitrocellulose membranes. The membranes were then exposed to primary antibodies, washed in Tris-buffered saline/0.05% Tween 20, and incubated with a secondary peroxidase-conjugated antibody at a 1:4,000 dilution. Signals were detected by SuperSignal West Pico Chemiluminescent Substrate, following the manufacturer’s instructions.
RNA Extraction and Reverse Transcriptase PCR Analysis
Total RNA was extracted from cell monolayers using the TRIzol reagent following the manufacturer’s instructions. Briefly, 3 μg of total RNA from each sample was reverse transcribed into cDNA at 37°C for 1 h using 500 ng oligo(dT)15–18 and 200 U SuperScript II Reverse Transcriptase in a final volume of 20 μl. The cDNA solution was diluted 1:10, and 10 μl was subsequently amplified using 5 pmol of the sense and antisense primers previously reported.16 The cycling parameters for the PCR reaction were denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. To determine the range of PCR cycles over which amplification was linear for each target molecule, we performed preliminary linear range-finding experiments. Amplification was performed for 24 cycles for GAPDH, 30 cycles for VEGF, or 40 cycles for VEGFR-1 and VEGFR-2. Expression of GAPDH was used as an internal control to normalize the target gene levels by densitometry.
Aliquots (10 μl) of the PCR reactions were electrophoresed through a 7% acrylamide gel in 1× Tris/borate/EDTA buffer; the gels were silver stained and stored following air drying. The densitometric value of each amplicon band was quantified using a Kodak Digital Image Station 440 CF and Kodak 1D Image Analysis software (Eastman Kodak, Rochester, NY, USA).
Proliferation Assay
Medulloblastoma cells in mid-log phase were seeded in 96-well culture plates (100 μl/well) in RPMI medium containing 10% FBS for 8 h and then cultured for 16 h in RPMI medium containing 1% FBS (for D283Med cells), 2% FBS (for D341Med), or 0.2% FBS (for DAOY). Subsequently, the cells were treated with increasing concentrations of VEGF165 (0, 10, 30, 50 ng/ml) with or without 80 μM VEGF inhibitor for 72 h. At the end of the treatment, 10 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml in phosphate-buffered saline) was added to each well, and cells were incubated for 4 h at 37°C. The cells were then centrifuged at 500g for 5 min and lysed, and the precipitates were dissolved in 150 μl of dimethyl sulfoxide. The cell number was evaluated by measuring optical density at 540 nm on a microtiter plate reader.
Immunoprecipitation of VEGFR-2
For VEGFR-2 phosphorylation analysis, 1 × 106 DAOY cells were seeded in 100-mm culture dishes in RPMI medium containing 10% FBS for 8 h, cultured overnight in RPMI medium containing 1% FBS, and then cultured in serum-free medium for 2 h. Cells were treated for 0, 1, 2.5, and 5 min with VEGF165 (200 ng/ml) and then suspended for 1 h at 4°C in 0.5 ml buffer A (20 mM Tris-HCl [pH 7.5], 10% glycerol, 1 mM EDTA, 150 mM NaCl, 1 mM sodium orthovanadate, and protease inhibitor cocktail) containing 1% Nonidet P-40. After centrifugation, equal amounts of supernatants were supplemented with 0.5 ml buffer A, without Nonidet P-40, and immunoprecipitation was performed overnight in the presence of anti–VEGFR-2 antibody. Immunocomplexes were washed three times in 50 mM Tris-HCl pH 7.5, 1 mM orthovanadate, and protease inhibitor cocktail and subjected to Western blot analysis by immunostaining with antiphosphotyrosine monoclonal antibody and anti–VEGFR-2 polyclonal antibody.
Treatment with λ Protein Phosphatase and Two-dimensional Gel Electrophoresis
To validate the phosphorylation of VEGFR-2 by VEGF165, we performed dephosphorylation experiments using the broad specificity λ protein phosphatase (λPPase).17 DAOY cells were cultured and treated with VEGF165 (200 ng/ml) as above and finally suspended for 1 h at 4°C in buffer A without sodium orthovanadate. One hundred microliters of cellular lysate, corresponding to 100 μg of protein, was added to 60 μl of 20 mM MnCl2 and 60 μl of λPPase buffer, and the solution was then brought to a final volume of 600 μl with deionized water. The mixture was divided into two aliquots, and 1,000 units of λPPase was added to one of the aliquots. After 45 min of incubation, phosphatase activity was stopped and the samples were acetone-precipitated at −20°C.
Acetone-precipitated proteins were solubilized in focusing buffer (9 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate [CHAPS], 65 mM dithiothreitol, 0.5% carrier ampholytes [pH 3–10]) for 30 min. Then, 125 μl of the solubilized sample was rehydrated and simultaneously loaded on the immobilized pH gradient (IPG) strip (Bio-Rad ReadyStrip IPG Strips; 7 cm, pH 3–10) at 50 V for 12 h. The voltage was increased to 4,000 V and focused for a total of 25,000 V/h using the Protean IEF Cell System (Bio-Rad). Immediately after being focused, IPG strips were equilibrated in 6 M urea, 2% SDS, 0.375 mM Tris [pH 8.8], 20% glycerol, and 2% dithiothreitol for 10 min and subsequently in 6 M urea, 2% SDS, 0.375 mM Tris [pH 8.8], 20% glycerol, and 2.5% iodoacetamide for 10 min. The second-dimension separation was run on 8% SDS-PAGE using the Mini Protean 3 Cell (Bio-Rad) at 25 mA per gel. After electrophoresis, gels were subjected to Western blot analysis by immunostaining with antiphosphotyrosine and anti–VEGFR-2 antibodies.
Immunohistochemical Analysis of Tumor Specimens
For immunohistochemical analysis of tumor specimens, we studied 13 cases of classic medulloblastoma in children treated at the Department of Pediatrics, Division of Hematology-Oncology, University-Hospital of Padua. Paraffin-embedded tumor sections were deparaffinized and placed in a plastic jar with 10 mM citrate buffer (pH 6.0). The solution was then heated in a water bath and boiled for 30 min. After cooling, the slides were incubated with the primary rabbit antibodies. Anti-VEGF, anti–VEGFR-1/Flt-1, and anti–VEGFR-2/KDR antibodies were used at a dilution of 1:100. Isotype-specific rabbit immunoglobulin G was used as a negative control. Slides were then washed, and the signal was detected by EnVision+ System horseradish peroxidase according to the manufacturer’s instructions. The slides were counterstained with hematoxylin. The degree of positive staining for VEGF and VEGFRs was determined by using a semiquantitative staining index (SI) in which SI = I × D, with intensity (I) and distribution (D) each scored on a scale of 1 to 4.4 Samples with SI ⩽ 4 were considered weakly positive, whereas those with SI > 4 were regarded as strongly positive.
Results
Medulloblastoma Cell Lines Express VEGF, VEGFR-1, and VEGFR-2
Because of the well-established paracrine role of VEGF in endothelial cells and the potential autocrine effect on cancer cells, we studied VEGF and its receptors in medulloblastoma cell lines. By means of alternative splicing, six different isoforms can be generated from the human VEGF gene, and the 121-, 165-, and 189-amino acid molecules are the three most frequently expressed.
We analyzed the mRNA and the protein expression of VEGF, VEGFR-1, and VEGFR-2 in three different medulloblastoma cell lines (DAOY, D283Med, and D341Med).
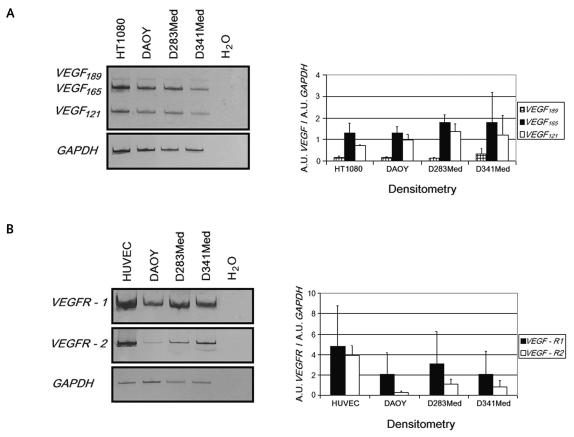
Reverse transcriptase PCR (RT-PCR) analysis indicated that VEGF mRNA was expressed in all medulloblastoma cell lines (Fig. 1A). Using a previously published primer set, we determined the expression of three isoforms of VEGF. In particular, in all medulloblastoma cell lines assayed, VEGF165 and VEGF121 amplicons were detectable (407 and 275 base pairs, respectively), whereas the VEGF189 signal (479 base pairs) was weak. VEGFR-1 and VEGFR-2 mRNAs were also expressed in all cell lines. We found that the VEGFR-1 RT-PCR signal was stronger than that of VEGFR-2 and comparable with that of the HUVECs used as a positive control (Fig. 1B). These data were confirmed by densitometric analysis and normalization against GAPDH expression level (Fig. 1A, B).
Fig. 1.
Expression analysis. Reverse transcriptase PCR analysis was performed on total RNA as described in Materials and Methods. Expression of GAPDH was used as an internal control to normalize the target gene levels through densitometry. H2O was a negative control to exclude potential contamination. Human fibrosarcoma cell line HT1080 and human umbilical endothelial vein cell (HUVEC) lines were used as positive controls. (A) Left, amplification products from VEGF189, VEGF165, and VEGF121 cDNAs (479, 407, and 275 base pairs, respectively). Right, measurement of relative gene expression levels after densitometric analysis and GAPDH normalization. (B) VEGFR-1 and VEGFR-2 cDNA expression (left) and relative quantification (right). Values are expressed in arbitrary units (A.U.) as means ± SD from three independent experiments.
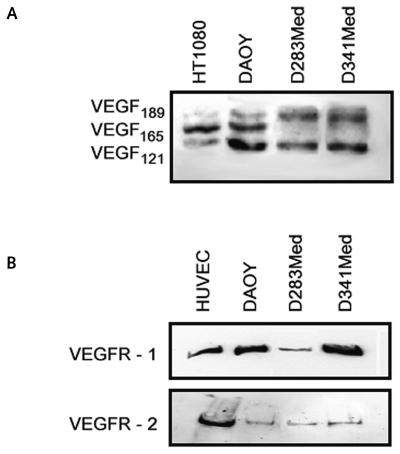
Most of these findings are in agreement with the results of Western blot analysis. Fig. 2A shows that all the cell lines expressed VEGF189 and VEGF121 isoforms, whereas only DAOY cells showed a clear expression of the VEGF165 isoform. In addition, DAOY, D283Med, and D341Med cells expressed both VEGFR-1 and VEGFR-2 protein at the membrane level. VEGFR-2 had a low expression in all cell lines, whereas the VEGFR-1 signal was weak only in D283Med cells (Fig. 2B).
Fig. 2.
Expression analysis. Western blot analysis of vascular endothelial growth factor (VEGF) (A) and VEGF receptors (B) was performed on total cell lysates. Human fibrosarcoma cell line HT1080 and human umbilical endothelial vein cell (HUVEC) lines were used as positive controls. Three independent experiments were performed, and similar expression patterns were obtained.
Proliferative Response to Exogenous VEGF165
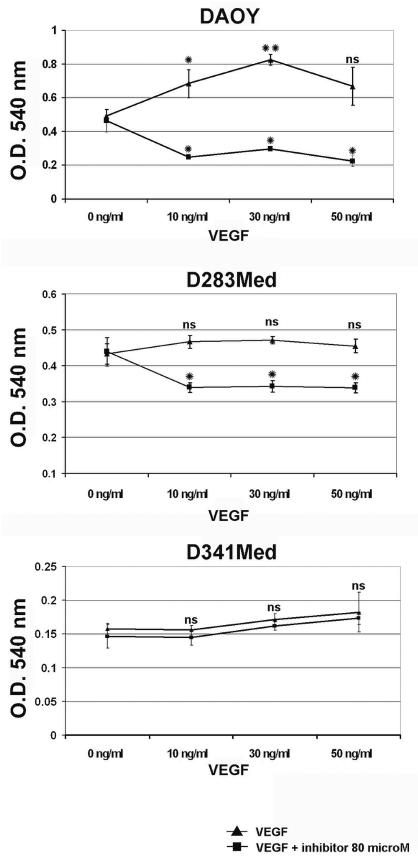
To further investigate the biological significance of the coexpression of VEGF and its receptors, medulloblastoma cells were cultured with increasing amounts (0, 10, 30, and 50 ng/ml) of VEGF165. As shown in Fig. 3, in DAOY cells a statistically significant increase of cell proliferation (+39% compared with control conditions) was seen with 10 ng/ml VEGF165 treatment (p < 0.05) and with 30 ng/ml (+67% with p < 0.005). Proliferation of D283Med cells seemed to be less influenced by VEGF addition (increase of 6%–9% compared with control). Cotreatment with the VEGF inhibitor (80 μM) abolished these proliferative stimuli, as expected, resulting in a significant decrease in cell growth with respect to the control (−23% for D283Med, p < 0.05, and −36 to −52% for DAOY, p < 0.05; Fig. 3). In contrast, proliferation of D341Med cells seemed to be VEGF independent, because no difference was seen with or without the addition of VEGF inhibitor.
Fig. 3.
Effects of vascular endothelial growth factor (VEGF) on medulloblastoma cell growth. Proliferation of DAOY, D283Med, and D341Med human medulloblastoma cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay after 72 h of VEGF165 treatment (0, 10, 30, 50 ng/ml) with (squares) or without (triangles) the addition of VEGF inhibitor (80 μM). The maximal proliferative effect was seen using 30 ng/ml for DAOY cells (+67%) and D283Med (+9%). No significant differences in proliferation could be detected for the D341Med cell line. Proliferative response to VEGF was expressed as an increase in optical density (O.D.) with respect to the effect obtained with control. Values are means ± SD of triplicate experiments repeated twice. Student’s t-test: *p < 0.05; **p < 0.005. Abbreviation: ns, not significant.
VEGF165 Induces VEGFR-2 Phosphorylation
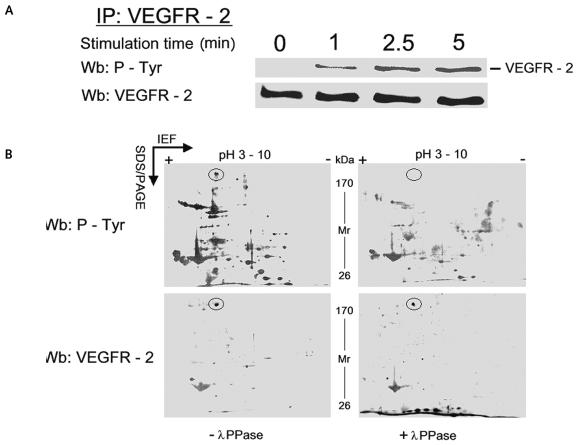
To provide further evidence that VEGFR-2 was stimulated by VEGF165, we evaluated the tyrosine phosphorylation rate of VEGFR-2, which was immunoprecipitated from DAOY cells lysed at various time intervals after VEGF stimulation. VEGFR-2–containing immunocomplexes were resolved on SDS-PAGE and identified with antiphosphotyrosine antibody. These experiments showed that, upon VEGF165 treatment, VEGFR-2 was Tyr-phosphorylated, reaching its maximum after 5 min (Fig. 4A).
Fig. 4.
Tyr-phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) in VEGF165-stimulated human medulloblastoma DAOY cells. (A) Cells lysed at 0, 1, 2.5, and 5 min were immunoprecipitated with anti–VEGFR-2 antibody, and the blots of the immunocomplexes were immunostained with antiphosphotyrosine antibody and reprobed with anti–VEGFR-2. (B) Two-dimensional gel electrophoresis of DAOY cell lysates treated with VEGF165. The pH scale is marked horizontally; the protein molecular weight range is indicated vertically. Cell lysates were treated with (right) or without (left) λ protein phosphatase (λPPase). The VEGFR-2 spot is circled. The molecular weight and isoelectric point of VEGFR-2 are 195–235 kDa and 5.6, respectively. The analysis indicated the shift of the VEGFR-2 protein from a more acidic to less acidic form. The figure illustrates a representative experiment of at least three separate experiments. Abbreviations: IEF, isoelectric focusing; Wb, Western blot.
To confirm the phosphorylation of VEGFR-2 by VEGF165, we used the broad specificity λPPase in dephosphorylation experiments. After 5 min of treatment with VEGF, DAOY cell lysates were incubated with or without λPPase and resolved on two-dimensional electrophoresis (2DE). In the absence of λPPase treatment, Western blot analysis of the 2DE map showed the overlay between the spot with anti–VEGFR-2 antibody and antiphosphotyrosine antibody (Fig. 4B). With λPPase, the immunostaining of tyrosine phosphorylation was abolished, demonstrating the specificity of the reaction. Moreover, the 2DE map showed that, in DAOY cells, VEGF165 induced tyrosine phosphorylation not only of VEGFR-2/KDR but also of other downstream proteins in the signal transduction pathway (Fig. 4B).
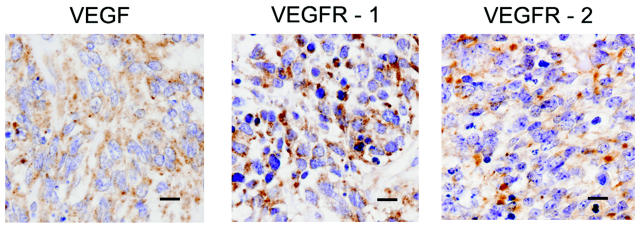
Expression of VEGF, VEGFR-1, and VEGFR-2 in Medulloblastoma Tumor Specimens
Tumor specimens from 13 cases of classic pediatric medulloblastoma were carefully reviewed, and we could exclude the presence of large cell areas, anaplasia, or extensive nodularity, all characteristics associated with different survival. Immunohistochemical study of the medulloblastoma biopsy specimens was performed to determine expression of VEGF, VEGFR-1, and VEGFR-2 in primary medulloblastoma. Representative examples of the immunohistochemical analysis for each target are shown in Fig. 5. Cells of our medulloblastoma cases showed an intense and diffuse cytoplasmic expression of VEGF. VEGFR-1 expression was lower and more heterogeneous than that of VEGF, but with several cells reacting intensively. VEGFR-2 expression was lower than the expression of VEGF or VEGFR-1. All targets showed a stronger staining in the interfollicular areas than in the follicular areas (data not shown). A positive reaction for VEGF and its receptors was also seen around the endothelia of tumor-supplying vessels (data not shown). Semiquantitative analysis of VEGF and VEGFR expression was also performed and generally showed moderate to high expression levels (Table 1).
Fig. 5.
Immunohistochemical detection of vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) in medulloblastoma specimens. Formalin-fixed, paraffin-embedded medulloblastoma specimens were reacted with specific polyclonal antibodies against VEGF, VEGFR-1, or VEGFR-2. Immunohistochemical analysis was performed as described in Materials and Methods and demonstrated a diffuse cytoplasmic positivity for all of the targets. Scale bars = 10 μm.
Table 1.
Immunohistochemical scoring in 13 patients with medulloblastoma
| VEGF
|
VEGFR-1
|
VEGFR-2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age (Years) | D | I | SI | D | I | SI | D | I | SI |
| 1 | M | 3 | 3 | 4 | 12 | 2 | 4 | 8 | 2 | 2 | 4 |
| 2 | M | 7 | 3 | 4 | 12 | 3 | 3 | 9 | 2 | 3 | 6 |
| 3 | F | 14 | 3 | 4 | 12 | 3 | 3 | 9 | 2 | 2 | 4 |
| 4 | F | 7 | 4 | 3 | 12 | 3 | 4 | 12 | 2 | 2 | 4 |
| 5 | M | 10 | 4 | 3 | 12 | 3 | 3 | 9 | 2 | 2 | 4 |
| 6 | F | 10 | 3 | 3 | 9 | 2 | 2 | 4 | 2 | 3 | 6 |
| 7 | F | 3 | 3 | 4 | 12 | 2 | 2 | 4 | 2 | 2 | 4 |
| 8 | M | 11 | 3 | 4 | 12 | 3 | 4 | 12 | 2 | 2 | 4 |
| 9 | M | 13 | 3 | 3 | 9 | 3 | 3 | 9 | 2 | 2 | 4 |
| 10 | M | 5 | 4 | 4 | 16 | 3 | 4 | 12 | 2 | 3 | 6 |
| 11 | F | 11 | 4 | 4 | 16 | 2 | 2 | 4 | 2 | 2 | 4 |
| 12 | F | 15 | 4 | 3 | 12 | 3 | 3 | 9 | 2 | 3 | 6 |
| 13 | M | 8 | 4 | 3 | 12 | 3 | 4 | 12 | 2 | 2 | 4 |
Abbreviations: VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; I, intensity; D, distribution; SI, staining index = I × D—tissues with SI ⩽ 4 were considered weakly positive, and tissues with SI > 4 were considered strongly positive; M, male; F, female.
Discussion
Initially identified as a vascular permeability factor secreted by tumor cells,18 VEGF was later isolated and cloned as a 45-kDa glycoprotein with proangiogenic activity on endothelial cells.19 VEGF plays a pivotal role in embryonic hematopoiesis and vasculogenesis as well as in several physiological and pathological conditions, including tumor vascularization.20 High levels of VEGF have been associated with tumor progression, distant metastases, and lower survival in different cancer models.7 Moreover, several studies have demonstrated the presence of VEGFRs in tumor cell lines of different origins and the activity of VEGF as a mitogen/survival factor for tumor cells in cases of hypoxia, chemotherapy, and radiotherapy,15,21,22 suggesting that an autocrine loop for VEGF may exist in some cancers.9,10,23 Because of the limited data available regarding the expression and activity of the VEGF/VEGFRs system in medulloblastoma cells, we investigated the expression of VEGF and VEGFRs and tested whether they were functional.
Our study showed that the medulloblastoma cell lines DAOY, D283Med, and D341Med expressed VEGF and its cognate receptors (VEGFR-1 and VEGFR-2). In particular, Western blot analysis showed that the DAOY cells expressed the three splice variants VEGF121, VEGF165, and VEGF189, whereas the D283Med and D341Med cell lines expressed only VEGF121 and VEGF189. Such differences may be related to the diverse phenotypes reported for these cell lines: DAOY cells possess a more “glial” protein expression pattern, whereas D283Med and D341Med cells have more “neuronal” features.24 VEGF165 represents the predominant isoform and is frequently overexpressed in several human tumors where its activity exerts the highest angiogenesis stimulation.25 With regard to VEGFRs, our study showed that the medulloblastoma cell lines under investigation possessed both VEGFR-1 and VEGFR-2, the first being more abundant than the latter. All these cells expressed low levels of VEGFR-2 compared with endothelial cells (HUVEC), whereas VEGFR-1 had a lower expression only in D283Med. To our knowledge, this is the first report showing that medulloblastoma cells express VEGFRs. Generally, endothelial cells express both VEGFRs, whereas a preferential expression of VEGFR-1 has been reported in tumor cell lines and biopsy specimens.26 With regard to VEGF165 and VEGFR-1, protein expression levels did not strictly correlate with mRNA levels. Of note, recent study found a significant correlation between mRNA and protein expression in only 17% of 165 proteins examined in a series of lung adenocarcinomas.27 Moreover, that study also demonstrated that the expression of individual isoforms of the same protein might not correlate with mRNA, suggesting that different posttranslational control mechanisms exist.
Our data further suggest that VEGFRs are functional in medulloblastoma cells, because the addition of exogenous VEGF or treatment with VEGF inhibitor caused an increase or a reduction, respectively, in cell proliferation in DAOY and D283Med cells. These observations are consistent with a previous study that demonstrated a similar increase in cell proliferation in the presence of VEGF165 in the DEV cell line derived from a human cerebellar primitive neuroectodermal tumor/medulloblastoma tumor.28 VEGF addition to D283Med cells seemed to influence cell proliferation at a lower degree, suggesting a role for VEGF as a survival factor. To evaluate the functional effect of these findings, we challenged medulloblastoma cells with exogenous VEGF in the presence of a peptide blocking the VEGF interaction with VEGFR-2, which is the major mediator of VEGF signaling.25,29 Under these conditions, proliferative stimuli were abolished, signifying that this effect was dependent on the binding of VEGF165 to VEGFR-2 and therefore that VEGFR-2 is the receptor transducing the VEGF signal in these cell lines. In both DAOY and D283Med cells, the presence of VEGF inhibitor resulted in significantly lower cell proliferation compared with the control, thus suggesting that endogenous VEGF is present and can be inhibited. The reasons for little or no effects of VEGF165 on VEGFR-positive D341Med cells remain to be elucidated, since the presence of VEGF inhibitor did not influence the proliferation rate. Other authors have reported that stimulation or presence of VEGFRs may have functions other than proliferation, for example, migration, adhesion, and invasiveness.30,31
Because previous analysis indicated that DAOY cells were the most responsive to VEGF165, we further investigated the role of VEGFR-2 signaling in this cell line. In particular, we determined whether VEGF165 caused tyrosine phosphorylation of its receptor and of a number of other intracellular proteins. Extracts from VEGF165-treated DAOY cells were analyzed by Western blot and two-dimensional gel electrophoresis. After immunoprecipitation, a clear increase in the phosphorylation of VEGFR-2 was observed at 1, 2.5, and 5 min. Furthermore, the 2DE map highlighted the overlay between the spot corresponding to VEGFR-2 and phosphotyrosine, whose signal specificity was confirmed by λPPase treatment. It is important to note that VEGFR-1 did not appear to be activated, because no phosphotyrosine signal corresponding to this receptor was seen in the 2DE map. We think these results supply a direct measure of VEGFR-2 activity following VEGF165 stimulation and strongly support the hypothesis that VEGF can act as an autocrine mitogen/survival factor for the tumor cells themselves.
Immunohistochemical analysis of the medulloblastoma specimens supported an autocrine role of VEGF in vivo, given the concomitant expression of VEGF and its cognate receptors in medulloblastoma cells. A moderate to high level of expression of VEGF and VEGFRs was in fact observed in all of the tumors studied (Table 1). Although VEGF expression in human surgical medulloblastoma specimens has been previously reported,4 this is the first time that concomitant expression of VEGF and VEGFRs has been demonstrated in medulloblastoma, suggesting that an autocrine effect of VEGF might also occur in vivo.
In summary, our results not only revealed new cellular and molecular aspects of the VEGF/VEGFR system in medulloblastoma cells but also have possible implications for the development of novel therapies for this neoplasm. Indeed, most of the kinase-targeted antitumor drugs under clinical evaluation have VEGF/VEGFR as a target.32 The study and development of molecules that specifically inhibit VEGF signaling, thus blocking tumor angiogenesis and tumor cell growth, is warranted in medulloblastoma.
Acknowledgments
This work was supported by the Ministero dell ’Università e della Ricerca (MIUR) (Cofin 2002, grant 2002062915_005). M.L.S. was supported by the Giaime Fiumanò fellowship from Associazione Italiana per la Ricerca sul Cancro (AIRC). We are grateful to Dr. Vincenza Guzzardo (Department of Oncological Sciences, Pathology Section, University of Padua) for her technical contribution on immunohistochemistry. Further support was obtained from Fondazione Città della Speranza.
References
- 1.Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol. 2004;5:209–218. doi: 10.1016/S1470-2045(04)01424-X. [DOI] [PubMed] [Google Scholar]
- 2.Rood BR, Macdonald TJ, Packer RJ. Current treatment of medulloblastoma: recent advances and future challenges. Semin Oncol. 2004;31:666–675. doi: 10.1053/j.seminoncol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 4.Ozer E, Sarialioglu F, Cetingoz R, et al. Prognostic significance of anaplasia and angiogenesis in childhood medulloblastoma: a Pediatric Oncology Group study. Pathol Res Pract. 2004;200:501–509. doi: 10.1016/j.prp.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Huber H, Eggert A, Janss AJ, et al. Angiogenic profile of childhood primitive neuroectodermal brain tumours/medulloblastomas. Eur J Cancer. 2001;37:2064–2072. doi: 10.1016/s0959-8049(01)00225-8. [DOI] [PubMed] [Google Scholar]
- 6.Bjerkvig R, Lund-Johansen M, Edvardsen K. Tumor cell invasion and angiogenesis in the central nervous system. Curr Opin Oncol. 1997;9:223–229. doi: 10.1097/00001622-199709030-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumour growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy WT, Richter L, Frutiger Y, Grogan TM. Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res. 1999;59:728–733. [PubMed] [Google Scholar]
- 10.Masood R, Cai J, Zheng T, Smith DL, Hinton DR, Gill PS. Vascular endothelial growth factor (VEGF) is an autocrine factor for VEGF receptor-positive human tumours. Blood. 2001;98:1904–1913. doi: 10.1182/blood.v98.6.1904. [DOI] [PubMed] [Google Scholar]
- 11.Lacal PM, Failla CM, Pagani E, et al. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J Invest Dermatol. 2000;115:1000–1007. doi: 10.1046/j.1523-1747.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer FA, Miller LJ, Lindquist R, et al. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54:567–572. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- 13.Dias S, Hattori K, Zhu Z, et al. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest. 2000;106:511–521. doi: 10.1172/JCI8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gee MF, Tsuchida R, Eichler-Jonsson C, Das B, Baruchel S, Malkin D. Vascular endothelial growth factor acts in an autocrine manner in rhabdomyosarcoma cell lines and can be inhibited with all-trans-retinoic acid. Oncogene. 2005;24:8025–8037. doi: 10.1038/sj.onc.1208939. [DOI] [PubMed] [Google Scholar]
- 15.Steiner H-H, Karcher S, Muller MM, Nalbantis E, Kunze S, Herold-Mende C. Autocrine pathways of the vascular endothelial growth factor (VEGF) in glioblastoma multiform: clinical relevance of radiation-induced increase of VEGF levels. J Neurooncol. 2004;66:129–138. doi: 10.1023/b:neon.0000013495.08168.8f. [DOI] [PubMed] [Google Scholar]
- 16.Onisto M, Slongo ML, Gregnanin L, Gastaldi T, Carli M, Rosolen A. Expression and activity of vascular endothelial growth factor and metalloproteinases in alveolar and embryonal rhabdomyosarcoma cell lines. Int J Oncol. 2005;27:791–798. [PubMed] [Google Scholar]
- 17.Yamagata A, Kristensen DB, Takeda Y, et al. Mapping of phosphorylated proteins on two-dimensional polyacrylamide gels using protein phosphatase. Proteomics. 2002;2:1267–1276. doi: 10.1002/1615-9861(200209)2:9<1267::AID-PROT1267>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumour cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 19.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 20.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dias S, Hattori K, Heissig B, et al. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signalling pathways is essential to inducing long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci USA. 2001;98:10857–10862. doi: 10.1073/pnas.191117498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the anti-tumour effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 23.Huh JI, Calvo A, Stafford J, et al. Inhibition of VEGF receptors significantly impairs mammary cancer growth in C3(1)/Tag transgenic mice through antiangiogenic and non-antiangiogenic mechanisms. Oncogene. 2005;24:790–800. doi: 10.1038/sj.onc.1208221. [DOI] [PubMed] [Google Scholar]
- 24.He X, Skapek SX, Wikstrand CJ, et al. Phenotypic analysis of four human medulloblastoma cell lines and transplantable xenografts. J Neuropath Exp Neurol. 1989;48:48–68. doi: 10.1097/00005072-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 26.Herold-Mende C, Steiner HH, Andl T, et al. Expression and functional significance of vascular endothelial growth factor receptors in human tumour cells. Lab Invest. 1999;79:1573–1582. [PubMed] [Google Scholar]
- 27.Chen G, Gharib TG, Huang C-C, et al. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1:304–313. doi: 10.1074/mcp.m200008-mcp200. [DOI] [PubMed] [Google Scholar]
- 28.Bagnard D, Vaillant C, Khuth S-T, et al. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binétruy-Tournaire R, Demangel C, Malavaud B, et al. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J. 2000;19:1525–1533. doi: 10.1093/emboj/19.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price DJ, Miralem T, Jiang S, Steinberg R, Avraham H. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signalling of breast cancer cells. Cell Growth Differ. 2001;12:129–135. [PubMed] [Google Scholar]
- 31.Wey JS, Fan F, Gray MJ, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–438. doi: 10.1002/cncr.21145. [DOI] [PubMed] [Google Scholar]
- 32.Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006;441:457–462. doi: 10.1038/nature04874. [DOI] [PubMed] [Google Scholar]