Abstract
Neural stem cells with astrocyte-like characteristics exist in the human brain subventricular zone (SVZ), and these cells may give rise to glioblastoma multiforme (GBM). We therefore analyzed MRI features of GBMs in specific relation to the SVZ. We reviewed the preoperative and serial postoperative MR images of 53 patients with newly diagnosed GBM. The spatial relationship of the contrast-enhancing lesion (CEL) with the SVZ and cortex was determined preoperatively. Classification was as follows: group I, CEL contacting SVZ and infiltrating cortex; group II, CEL contacting SVZ but not involving cortex; group III, CEL not contacting SVZ but involving cortex; and group IV, CEL neither contacting SVZ nor infiltrating cortex. Patients with group I GBMs (n = 16) were most likely to have multifocal disease at diagnosis (9 patients, 56%, p = 0.001). In contrast, group IV GBMs (n = 14) were never multifocal. Group II (n = 14) and group III (n = 9) GBMs were multifocal in 11% and 29% of cases, respectively. Group I GBMs always had tumor recurrences noncontiguous with the initial lesion(s), while group IV GBM recurrences were always bordering the primary lesion. Group I GBMs may be most related to SVZ stem cells; these tumors were in intimate contact with the SVZ, were most likely to be multifocal at diagnosis, and recurred at great distances to the initial lesion(s). In contrast, group IV GBMs were always solitary lesions; these may arise from non-SVZ, white matter glial progenitors. Our MRI-based classification of GBMs may further our understanding of GBM histogenesis and help predict tumor recurrence pattern.
Keywords: glioblastoma multiforme (GBM), MRI, neural stem cell, subventricular zone (SVZ), tumor stem cell
Recent studies demonstrate that glioblastoma multiforme (GBM) contains a subpopulation of cancer cells with stem cell characteristics, including self-renewal and multipotentiality; these cancer stem cells can propagate tumors in vivo.1–4 The adult human brain harbors astrocyte-like neural stem cells within the subventricular zone (SVZ), which is located just under the ependyma of the brain ventricles5,6 (Fig. 1A). In animal models, gliomas—including GBM—can be induced from SVZ cells.7–9 These accumulating data have thus given rebirth to the notion that human gliomas may arise from SVZ neural stem cells (reviewed in Sanai et al.10), an idea that was first suggested in the first half of the 20th century.11–13
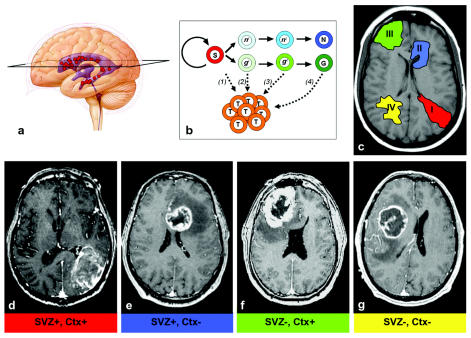
Fig. 1.
(A) Neural stem cells (red circles) are found in the walls of the adult brain ventricles (blue). An axial section (black trapezoid) is indicated on this cartoon, representing the approximate level of section of the MR images shown. (B) Multiple hypotheses of brain tumor origin: subventricular zone (SVZ) stem cells (S, red) normally self-renew and produce progenitor cells (light green, glial [g]; light blue, neuronal [n]), which then progress through a developmental lineage (e.g., g1 → g2 …) and differentiate into mature neurons (N, dark blue) or glia (G, dark green). g1 and n1 cells are highly migratory progenitors, directly descendent of SVZ stem cells. g2 and n2 cells represent less migratory local progenitors. Theories include the following: (1) stem cells become dysregulated and give rise to glioma cells (T, orange); (2) highly migratory progenitor cells are the tumor-initiating cells; (3) local progenitors with limited migration give rise to the tumor; and (4) mature glial cells dedifferentiate to produce tumor cells. (C) Schematic of the different groups of glioblastoma multiforme (GBM) lesions based on MRI patterns. (D–G) Axial postcontrast T1-weighted MR images of representative examples for each group (I–IV) in this classification system. (D) For this group I GBM, note that the contrast-enhancing lesion (CEL) extends from the atrium SVZ to the pia. Interestingly, despite the large size, this GBM exerts relatively little mass effect. (E) Group II: SVZ-contacting tumor that does not involve the cortex. (F) Group III: The CEL is invading the cortex, reaching the pia, but does not touch the SVZ. (G) Group IV: These lesions “respect” both the SVZ and cortex; the CEL mass tends to displace brain matter rather than directly invade the cortex and reach the ventricle wall.
GBM is a pathologically and clinically heterogeneous disease. While some GBMs may arise from transformed SVZ stem cells, other gliomas may be initiated by neoplastic transformation of non-SVZ progenitor cells or mature glial cells that have undergone dedifferentiation14 (Fig. 1B). Identifying a subset of GBMs that are related to tumor stem cells in the SVZ would have significant therapeutic implications:15 stem-cell–derived GBM may need treatment that attends to both the primary lesion(s) and the SVZ. To better understand the histogenetic and clinical heterogeneity of GBM, we developed an MRI-based classification of GBMs based on their involvement of the SVZ and cortex.
We identified a subset of GBMs that are more likely to be SVZ stem cell – related based on the spatial relationship of the tumor to the SVZ, involvement of cortex, multifocality, and recurrence pattern. Another GBM subset is more likely related to non-SVZ progenitor cells or dedifferentiated glial cells. This study provides MRI evidence suggesting a relationship between a subset of GBMs and tumor stem cells in the SVZ.
Materials and Methods
We studied 53 consecutive patients with newly diagnosed GBM who underwent preoperative, immediately postoperative (within 48 h after surgery), and subsequent follow-up MR images as a part of a brain tumor imaging research study (SPORE project grant). Informed consent for participation in this study was obtained from each patient in accordance to institutional protocol. The medical records of all 53 patients were reviewed.
All patients underwent the same preoperative MRI protocol, which consisted of a three-plane localizer sequence (8.5/1.6 ms [TR/TE]), an axial fluid-attenuated inversion-recovery (FLAIR) sequence (10,000/148/2,200 [TR/TE/TI]), an axial fast spin-echo T2-weighted sequence (3,000/102, echo train length 16, matrix 256 × 196), axial diffusion-weighted imaging (10,000/99, b = 1,000 s/mm2), and a postcontrast three-dimensional spoiled gradient-recalled acquisition in the steady state (SPGR; 34/8) T1-weighted sequence. Postoperatively, we obtained additional postcontrast imaging in the coronal and axial planes.
A neuroradiologist (S.C.) and MRI research assistant (M.C.M.) reviewed each tumor for the following on preoperative imaging: distance of contrast-enhancing lesion (CEL) to nearest ventricle, infiltration of CEL into cortex, volume of CEL, and anatomical location of the primary (largest volume) CEL. Multifocal disease at initial diagnosis was recognized by the presence of CELs or nonenhancing FLAIR lesions not typical of nonspecific white matter disease and suspicious for tumor that were noncontiguous with the primary CEL. Distances and volumes were measured with qBrain (Medis Medical Imaging, Leesburg, VA, USA). Tumor recurrence, determined based on contrast-enhanced T1-weighted MR images, was defined as a new or progressive increase in CEL, within the initial surgical resection site and/or in a remote location. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). The likelihood ratio test of independence was used to evaluate the incidence rate of multifocal GBM among groups. Assuming there is a natural ordering in severity among groups (group I is more severe than group II, group II is more severe than group III, etc.), the Cochran-Armitage trend test was used to assess the association between the incidence rate and group order. To assess the contribution of individual variables (SVZ contact, cortical involvement, patient age, and tumor volume) to the incidence of multifocal GBM, we performed both univariate and multivariate analyses, as described in Results.
Results
GBMs were classified preoperatively into four groups by the spatial relationship of the CEL with the SVZ and cortex as follows: group I, CEL contacting SVZ and infiltrating cortex; group II, CEL contacting SVZ but not involving cortex; group III, CEL not contacting SVZ but involving cortex; and group IV, CEL neither contacting SVZ nor infiltrating cortex (Fig. 1C–G). Table 1 lists patient demographics and tumor MRI characteristics.
Table 1.
Patient demographics and MRI characteristics of glioblastoma multiforme (GBM) in groups I–IV
| Parameter | Group I (SVZ+, Ctx+) | Group II (SVZ+, Ctx−) | Group III (SVZ−, Ctx+) | Group IV (SVZ−, Ctx−) |
|---|---|---|---|---|
| Patients | ||||
| No. (%) | 16 (30) | 9 (17) | 14 (26) | 14 (26) |
| Sex | ||||
| Male | 11 | 8 | 10 | 9 |
| Female | 5 | 1 | 4 | 5 |
| Age (years, mean ± SD) | 59.8 ± 13.7 | 64.9 ± 14.3 | 54.6 ± 10.3 | 54.0 ± 14.7 |
| MRI data | ||||
| No. patients with multifocal GBM (%)* | 9 (56) | 1 (11) | 4 (29) | 0 (0) |
| CEL volume (cc, mean ± SD) | 36.5 ± 26.5 | 14.9 ± 11.3 | 20.6 ± 16.6 | 18.2 ± 14.7 |
| CEL distance to nearest ventricle (cm, mean ± SD) | 0 | 0 | 0.93 ± 1.89 | 0.76 ± 0.97 |
Abbreviations: SVZ, subventricular zone; Ctx, cortex; CEL, contrast-enhancing lesion.
p = 0.001, likelihood ratio test of independence.
Multifocal GBM is suggestive of a more invasive and migratory tumor phenotype, a feature more common to stem-cell–derived cancer.16 We therefore examined the incidence of multifocal GBM among the four groups defined above. Patients with group I GBMs had the highest frequency of multifocal disease (Fig. 2A–C) at initial diagnosis (9 of 16 patients, 56%). In contrast, patients with group IV tumors did not have multifocal disease. Patients with group II and III GBMs had multifocal disease in 11% and 29% of cases, respectively. There was a significant difference in the incidence of multifocal GBM among the four groups (p = 0.001, likelihood ratio test of independence). In addition, the results of Cochran-Armitage exact trend test indicated that the incidence of multifocal GBM was highest for group I and lowest for group IV (two-sided test, p = 0.002).
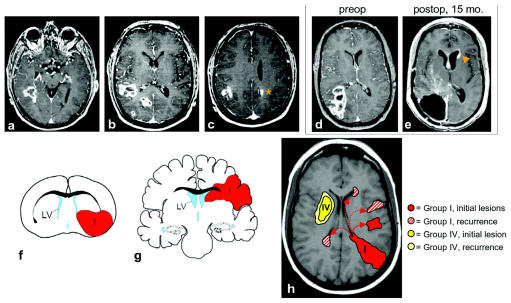
Fig. 2.
(A–C) Axial postcontrast T1-weighted MR images of the same patient showing an example of group I multifocal glioblastoma multiforme (GBM). The contrast-enhancing lesion (CEL) contacts the posterior temporal horn subventricular zone (SVZ) in A. Rostrally, there are at least three separate lesions in B and two in C, including one in the contralateral hemisphere (orange asterisk). (D and E) Recurrence of group I GBM in distant SVZ location. (D) Preoperative axial postcontrast T1-weighted MR images of a patient with group I GBM. This patient did not have multifocal disease preoperatively. (E) Fifteen months postoperatively, there was an enhancing mass lesion in the SVZ of the contralateral frontal horn (orange arrowhead) consistent with tumor recurrence. In addition, there is subependymal enhancement bilaterally. No abnormal subarachnoid enhancement was found in this or other images of this patient. (F–H) Integration of GBM cell-of-origin data from animal models with human MRI data. (F) P53−, NF1− SVZ astrocytes give rise to group I- and group II-like GBMs in the mouse. This coronal schematic shows an example of a group I tumor (red) extending from the SVZ to the pia. (G) A schematic of human group I and II GBMs in a similar coronal plane. (H) Model of group I and group IV GBM subtypes. At diagnosis, group I GBMs were often multifocal (9 of 16, 56%), possibly from the migration of tumor-initiating cells from either the SVZ or tumor mass. After resection, group I GBM always recurred at locations noncontiguous with the initial lesion(s). Distant, recurrent lesions may arise from tumor cells migrating from the SVZ through the parenchyma (solid arrow) or along the SVZ itself (dotted arrows). The cell of origin of group I GBMs may reside in or near the SVZ, and tumor cells derived from SVZ neural stem cells may have a greater migratory potential than those of group IV. Group IV GBMs occurred only as solitary lesions, and group IV GBM recurrences were always contiguous with the resection cavity. Group IV GBMs may arise from non-SVZ progenitor cells that have a limited migration potential.
The contribution of individual variables (SVZ contact, cortical involvement, patient age, and tumor volume) was investigated by both univariate and multivariate analysis. Univariate analysis showed that only SVZ contact (p = 0.04) and cortical involvement (p = 0.01) are significantly associated with multifocality. Multivariate analysis was conducted using a stepwise selection with 0.1 significance level to enter the model and 0.1 significance level for staying in the model, and the resulting model included both SVZ contact and cortical involvement.
Follow-up MRI was available for 11 of 16 group I patients (follow-up period of 5–23 months) and 13 of 14 group IV GBM patients (follow-up period of 3–38 months). For the 11 evaluable group I GBM patients, tumors recurred in all cases, and CELs were always found in locations noncontiguous with the primary lesion(s), in addition to recurrence at the resection cavity (Fig. 2D,E). Nine of these 11 patients had CELs in distant SVZ locations (contralateral in two cases), and two had CELs in noncontiguous brain parenchyma (contralateral in one case). For the 13 evaluable group IV GBM patients, 10 had tumor recurrence contiguous with the resection cavity, and none had any evidence of noncontiguous tumor.
Discussion
Our results show that GBMs contacting the SVZ and involving the cortex (group I) were most likely to be multifocal at the time of initial diagnosis and were more likely to have recurrent tumor at locations distant to the initial lesion. GBMs not involving either the SVZ or the cortex (group IV) were not multifocal at diagnosis and always recurred within 2 cm of the resection margin.
Group I GBMs may be most closely related to SVZ neural stem cells. The physical association of malignant glioma with the SVZ has led neuropathologists to suggest that these tumors arise from this region of the brain.11–13 This hypothesis is supported by the results of animal model experiments involving viral or carcinogen transformation of SVZ cells and resultant tumor formation.10 Recently, Zhu et al.9 demonstrated that p53 inactivation cooperates with NF1 loss to induce GBM formation from mouse SVZ astrocytes; intriguingly, these SVZ-derived tumors usually contact the SVZ and infiltrate the cortex like human group I GBMs (Fig. 2F,G).
The multifocality at diagnosis and recurrence pattern of group I GBMs suggest that the cell of origin of this tumor type is highly migratory and invasive (see Fig. 2H). Neural stem cells express matrix metalloproteinases (MMPs)—proteolytic enzymes implicated in tumor cell metastasis—and although the adult brain as a whole does not express MMPs, gliomas overexpress certain MMPs, leading to increased invasiveness (reviewed in Yong et al.17). It is possible that gliomas arising from stem cells maintain neural stem cell MMP expression. The SVZ region, or niche, may also be highly permissive for both tumor growth and cellular migration, allowing tumor stem cells and their progeny to rapidly proliferate and migrate under the ependyma.
In this study, group IV GBMs occurred as solitary lesions and recurred only around the resection cavity of the initial tumor, suggesting that these tumor cells are less migratory than their group I counterparts. Group IV GBMs were not intimately associated with the SVZ (and often displaced the SVZ away with their mass), suggesting that these tumors arise from non-SVZ progenitor cells. Progenitor cells expressing the chondroitin sulfate proteoglycan NG2 (neuronal/glial 2) are found throughout adult human brain.18 In the mouse, NG2+ progenitor cells from non-SVZ regions have a very limited migration potential, whereas NG2+ cells from the SVZ migrate long distances.19
The distinguishing characteristic between group I and group II tumors in this study is cortical invasion. What might account for the presence or absence of this phenotype? In their analysis of asymptomatic mice with early GBMs arising from p53−, NF1− astrocytes, Zhu et al.9 demonstrate that not all SVZ-associated gliomas infiltrate the cortex. Perhaps the difference between the two classifications is simply a temporal consequence: Some human group II GBMs are simply group I GBMs that have yet to reach the cortex at the time of imaging. Alternatively, it is possible that some group II GBMs are derived from later-lineage cells with less migration/invasion potential.
Zhu et al.9 additionally describe mice with gross GBM lesions noncontiguous with the SVZ; in these mice, microscopic cellular abnormalities are present in the SVZ. This pattern of microscopic SVZ cellular abnormality and distant GBM mass lesion(s) is suggestive of a mechanism in which SVZ tumor stem cells produce migratory tumorigenic daughter cells; these daughter cells take up residence in locations distant from the SVZ, where they then proliferate to form a mass. This migratory tumor progenitor cell hypothesis15 may account for the MRI findings of group III tumors: SVZ cellular abnormalities may be below the threshold of detection by MRI, but the distant mass lesions arising from migratory tumorigenic daughter cells are easily apparent on typical MRI sequences. Postmortem analysis of patients with group III GBM would be useful to investigate this hypothesis.
Our results do not directly address whether GBMs can arise from the SVZ. It is likely that for GBM there will be different cells of origin, each with different clinical and pathological presentations. Because it will be difficult to directly determine the cell of origin in humans, it is important to draw parallels between animal model data and actual human disease. Our findings suggest a testable hypothesis for animal models of glioma, that tumors from NG2+ SVZ cells are more invasive and migratory than tumors induced from NG2+ non-SVZ progenitors. Group I GBM may be enriched for CD133+ tumor stem cells in comparison to group IV GBM. It will also be valuable to compare the genomic profile of tumors in the different GBM groups to understand their different biological behaviors (e.g., cortical infiltration between group I and II) on a molecular level, as well as to make comparisons to human neural stem cells.
There is great interest in classifying GBMs for prognosis. Currently, age and KPS are the two most significant prognostic factors.20 Gene expression profiling holds promise for GBM classification;21 however, transcriptional profiling is not performed routinely. In contrast, nearly all patients with GBM are diagnosed and followed with MRI. Classifying GBMs based on their MRI features may allow a practical yet powerful tool for the assessment of patient prognosis and treatment decisions.
Despite advances in surgery, radiotherapy, chemotherapy, and immunotherapy, the median survival for GBM has remained between 9 and 12 months.22 The potential of SVZ neural stem cells to divide asymmetrically combined with the migratory potential of SVZ-derived neural progenitors may contribute to the treatment-resistant nature of GBM.15 Based on our preliminary results, it is possible that patients who present with group I GBM lesions will benefit from radiation therapy that includes distant SVZ locations. Perhaps group IV GBM lesions would be the most amenable to regional therapy (e.g., convection-enhanced delivery of immunotoxins), given that this group of tumors recurs only locally. Follow-up studies will be required to conclusively address the clinical value of MRI for assessing GBM SVZ and/or cortical involvement, but the preliminary results presented here appear promising for this type of tumor analysis.
References
- 1.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 2.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 5.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 6.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 7.Lantos PL, Cox DJ. The origin of experimental brain tumours: a sequential study. Experientia. 1976;32:1467–1468. doi: 10.1007/BF01937439. [DOI] [PubMed] [Google Scholar]
- 8.Vick NA, Lin MJ, Bigner DD. The role of the subependymal plate in glial tumorigenesis. Acta Neuropathol (Berl) 1977;40:63–71. doi: 10.1007/BF00688574. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Guignard F, Zhao D, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 11.Smyth GE, Stern K. Tumors of the thalamus: a clinico-pathological study. Brain. 1938;61:339–374. [Google Scholar]
- 12.Globus JH, Kuhlenbeck H. Tumors of the striatothalamic and related regions: their probable source of origin and more common forms. Arch Pathol. 1942;34:674–734. [Google Scholar]
- 13.Globus JH, Kuhlenbeck H. The subependymal plate (matrix) and its relationship to brain tumors of the ependymal type. J Neuropath Exp Neurol. 1944;3:1–35. [Google Scholar]
- 14.Holland EC. Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet. 2001;2:120–129. doi: 10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- 15.Berger F, Gay E, Pelletier L, Tropel P, Wion D. Development of gliomas: potential role of asymmetrical cell division of neural stem cells. Lancet Oncol. 2004;5:511–514. doi: 10.1016/S1470-2045(04)01531-1. [DOI] [PubMed] [Google Scholar]
- 16.Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002;3:508–513. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 17.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro-Oncology. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Y, Diehn M, Watson N, et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A. 2005;102:5814–5819. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]