Abstract
We evaluated the visual outcome of a cohort of children with neurofibromatosis type 1 (NF1) and optic pathway glioma (OPG) treated according to standardized therapeutic guidelines. The study population consisted of all consecutive patients with NF1 and OPG referred to a specialized pediatric neuro-oncology program between 1994 and 2004. Treatment was instituted only in cases of progressive disease or clinical deterioration. Treatment modalities were chemotherapy (based on vincristine/carboplatin) for children younger than 5 years and radiotherapy for all others. Ten boys and 10 girls (seven with a positive family history) entered the trial (median age at diagnosis of OPG, 29 months). At a median follow-up time of 78 months, seven patients had been treated with chemotherapy only, four with radiotherapy, and four with chemotherapy plus radiotherapy. Five patients were observed only. Currently, 18 are alive and two have died. Eight patients were treated for progressive visual loss in the face of stable disease, five for tumor volume increase without visual deterioration, and two for symptomatic tumor volume increase. At referral, six children had a visual acuity (VA) of <30% in both eyes; eight children had 100% VA bilaterally. At referral, the visual field (VF) could be assessed in three children: One had VF loss in both eyes, one had VF loss in one eye, and one had normal VF. At last follow-up, eight children had VA <20% in both eyes; only two children had 100% VA in both eyes. Among 11 children who had some visual function, three had VF loss in one eye and three in both eyes, and five had an intact VF. Contrast and color sensitivity were abnormal in seven and six patients, respectively. Thirteen children fell into the WHO hypovision category. In summary, among the 15 children treated, one had a definitive and two a mild improvement in VA. In conclusion, the visual outcome of this selected cohort of NF1 patients with OPG is unsatisfactory. A critical reappraisal of the therapeutic strategy adopted is needed.
Keywords: chemotherapy, neurofibromatosis type 1, optic pathway glioma, radiotherapy, visual function
The prognosis quod vitam of children with neurofibromatosis type 1 (NF1) and optic pathway glioma (OPG) is almost uniformly favorable, with few, if any, fatal outcomes.1 Furthermore, it is generally assumed that most cases of OPG have a very indolent course and that those few tumors that do grow have a self-limiting growth potential confined to the first decade of life.1–3 However, valid prognostic evaluation regarding the visual outcome of these patients is still difficult.4,5
With the advent of prospective cooperative clinical research on childhood low-grade glioma and of a more systematic approach to the children affected by this neoplasm, it is becoming more evident that the population of children with NF1 and OPG is quite heterogeneous and that not all of them have a favorable functional outcome. There is a strong need to determine the effect of the present therapeutic guidelines and treatment modalities on the functional outcome of these patients.4,6,7 It is commonly agreed that some form of oncologic treatment is recommended only for those NF1 patients with OPG who manifest unequivocal signs of tumor progression and/or deteriorating clinical conditions, notably, decreased visual function even with no change in tumor volume.1,6,8 Chemotherapy, especially for young children, and, less frequently, radiotherapy for the older children are the treatment modalities used.
Here we address the issue of the visual outcome of NF1 children affected by OPG by reporting the experience of a single institutional series of these patients referred, followed, and accurately evaluated, especially in response to therapy, by a dedicated pediatric neuro-oncology team.
Patients and Methods
The study was approved by the Institutional Ethics Committee at the University Hospital of Padua, and written consent to enter the trial was obtained from the parents or legal guardians before patients were enrolled in the trial.
The study population consisted of all consecutive children with NF1 and OPG referred to the Pediatric Neuro-oncology Program of the Department of Pediatrics of the University Hospital of Padua between January 1994 and December 2005. Patients were followed and treated according to the diagnostic and therapeutic guidelines of the prospective cooperative trial of the International Consortium on Childhood Low Grade Glioma (LGG1) of the International Society of Pediatric Oncology (SIOP).
Only those children with unresectable, progressive disease or presenting with severe symptoms unequivocally correlated to tumor growth (e.g., reduced visual acuity [VA] or an increase in visual field [VF] defects) were eligible for oncology therapy, consisting of systemic chemotherapy for patients younger than 5 years and radiotherapy for the older children. For all other patients, only an accurate follow-up, including periodic clinical and neuroradiologic evaluations, was planned. The chemotherapy regimen was based on the combination of carboplatin and vincristine. The treatment plan included a 10-week initial phase and continuation therapy from week 11 to week 53. During the initial 10-week period, patients received carboplatin 550 mg/m2 in a 1-h intravenous infusion on day 1 of weeks 1, 4, 7, and 10 concurrently with vincristine 1.5 mg/m2 intravenously (maximum, 2 mg) on day 1 of weeks 1–10. Subsequently, in cases of objective radiographic response or of stable clinical and neuroradiologic disease, carboplatin and vincristine were to be administered at the same dosage at 28-day intervals up to week 53. External conventional radiotherapy of 54.0 Gy administered via the fractionated guided stereotactic technique was used at a daily dose of 1.8 Gy, five times per week. For treatment planning, all patients were immobilized using an individual mask fixation system consisting of a Gill-Thomas-Cosman frame to ensure an overall accuracy of 1–2 mm. Treatment planning was performed using a three-dimensional treatment planning system (Voxelplan; DKFZ, Heidelberg, Germany). The clinical target volume comprised the tumor visible on the CT and MRI scans plus a 5-mm safety margin to ensure the inclusion of microscopic spread along the typical pathways following the optic pathway structures. The planning target volume was defined as the clinical target volume plus a 2-mm safety margin for possible patient misalignment. The entire procedure was performed under sedation for young uncooperative children.8,9
The diagnosis of NF1 was formulated according to criteria elaborated by the U.S. National Institutes of Health,10 while the diagnosis of OPG was based on unequivocal MRI results as outlined in the SIOP-LGG1 protocol, which defines OPG as “a tumour clearly arising from the optic nerve, tract and chiasm especially if the patient is affected by NF1, which appears iso-intense in the T1 and hyper intense in the T2 ones.”
Histologic confirmation of the diagnosis of OPG was not required. At diagnosis and periodically during treatment and follow-up, children underwent regular physical and neurologic examinations; auxologic, endocrinologic, and ophthalmologic assessments; and contrast-enhanced head MRI. These were performed at 6-month intervals during therapy and for at least the first 2 years after therapy. If not otherwise indicated, a battery of hormonal tests, including free triiodothyronine, free thyroxine, thyroid-stimulating hormone, cortisol, follicle-stimulating hormone, and leutinizing hormone, was also performed on a yearly basis.
Neuroradiologic response was assessed according to SIOP criteria. A complete response was defined as no evidence of disease, a partial response as a reduction in tumor size of more than 50%, and stable disease as the absence of tumor progression. In the particular evaluation of response used for low-grade glioma, we added the category of volume reduction, defined as less than 50% reduction of residual tumor size as measured by the product of the diameter in the plane and the largest tumor extension, with the perpendicular one measured on the same scan. Stable disease was considered a positive response, and chemotherapy was therefore continued.8
For this group of patients, the ophthalmologic assessment was always performed by the same pediatric ophthalmologist (M.L.P.), who is a constitutive member of the neuro-oncology team. The visual assessment included, ideally at all times and any age, fundoscopic examination and evaluation of the VA, the VF, and the contrast and color sensitivity.
VA was evaluated with Teller Acuity Cards in children from 0 to 36 months of age, with LH or E test cards in children from 36 to 72 months, and with the Snellen acuity test in children older than 6 years. VA, expressed in degrees/centimeter, was converted to Snellen acuity using the normograms of the procedure manual; these values were then normalized for the specific age of the children. To have comparable data for VA throughout the different pediatric ages, VA of each child is expressed in terms of the percentage of the normal VA for that specific age interval. In the text, WHO categories used to express and quantify hypovision are used to express visual function, as follows: category 1, VA between 1/10 and 3/10, VF between 20° and 60°; category 2, VA between 1/20 and 1/10, VF between 10° and 20°; category 3, VA between 1/50 and 1/20, VF between 5° and 10°; category 4, VA less than 1/50, VF less than 5°; category 5, no light perception.
Visual evoked potentials were not part of the regular follow-up of these children because they were not included in the diagnostic workup of the SIOP-LGG1 trial. Conventional contrast-enhanced head MRI sequences were used to evaluate the disease status. The series of individual head MRIs were reviewed by one of the authors (M.C.), who was not informed of the clinical status of the patients. OPG extension was also expressed according to the Dodge system,11 which classifies OPG as follows: Dodge 1, tumor confined to the optic nerve only; Dodge 2, tumor involving the chiasm with or without optic nerve involvement; Dodge 3, tumor extending along the optic pathway to optic tracts and/or involving the hypothalamus.
For the purpose of this study, the patient’s ophthalmologic status was evaluated at the time of referral to the pediatric neuro-oncology program, at the end of therapy, and at last follow-up.
Results
Study Population
During the study period, 25 children with NF1 and OPG were referred to the Neuro-Oncology Program at the Division of Hematology Oncology of the Department of Pediatrics of the University Hospital of Padua, Italy, and then followed and treated according to the SIOP-LGG1 protocol. Five children who were lost to follow-up very early during the initial clinical course were excluded from the present analysis. Thus, 20 children with NF1 and OPG constituted the study population and are the subjects of this report. The relevant clinical characteristics of these patients are reported in Table 1. The mean patient age at the time of OPG diagnosis was 40 months (range, 16 months to 9 years 7 months). None of the children was younger than 1 year at the time of OPG diagnosis. For only six children, the date of referral coincided with the data of diagnosis of OPG. The others were referred to us after OPG diagnosis, for possible tumor progression and/or visual deterioration. Seven children (35%) presented with some visual symptoms, most often a decrease in VA and/or squint.
Table 1.
Clinical characteristics of the 20 children with neurofibromatosis type 1 (NF1) and optic pathway glioma (OPG)
| Characteristics | Number (%) |
|---|---|
| Gender distribution (male vs. female) | 10 vs. 10 |
| Cases of familial NF1 | 7 (35%) |
| Age at the time of NF1 diagnosis | Mean, 18.2 months; median, 6.0 months; range, 1 month to 4 years 2 months |
| Age at the time of OPG diagnosis | Mean, 40 months; median, 29 months; range, 16 months to 9 years 7 months |
| Clinical presentation [n (%)] | |
| Visual symptoms at diagnosis | 7 (35%) |
| Other symptoms at diagnosis | 3 (15%) |
| Asymptomatic | 10 (50%) |
| Therapeutic decision at the time of referral [n (%)] | |
| Observation only | 15 (75%) |
| Chemotherapy | 5 (25%) |
| Overall management during the study period [n (%)] | |
| Observation only | 5 (25%) |
| Chemotherapy only | 7 (35%) |
| Radiotherapy only | 4 (20%) |
| Chemotherapy + radiotherapy | 4 (20%) |
| Time to treatment | Mean, 2 years; range, 7 months to 7 years 5 months |
| Follow-up time | Mean, 6 years 9 months; median, 6 years 4 months; range, 5 months to 20 years |
Patient Functional Status at Time of Referral
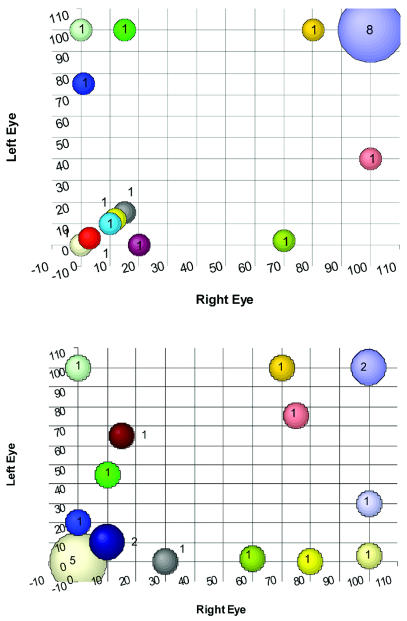
The VA of the 20 children at the time of referral is presented in Table 2 and shown graphically in Fig. 1A. At the time of referral, six children had a VA of less than 30% in both eyes, while at the other extreme, eight children had 100% VA bilaterally. Due to the young age of this population, the VF could be assessed reliably in only three children; the VF was restricted in both eyes in one patient and in only one eye in the second, whereas the third patient had a normal VF. Six of the children fell into the hypovision categories according to the WHO classification system of the visual function, and one child had no light perception in either eye at the time of the referral (which coincided with the start of therapy), whereas another had no light perception in only one eye.
Table 2.
Visual acuity of the study population at time of referral and of last follow-up
| VA at Referral
|
VA at Last Follow-Up
|
||||||
|---|---|---|---|---|---|---|---|
| Patient | Age (Years, Months) | Dodge Tumor Site | Right Eye (%a) | Left Eye (%a) | Treatment Strategy | Right Eye (%) | Left Eye (%) |
| 1 | 1, 3 | 2 | 3/10 (100%) | 3/10 (100%) | Observation | 9/10 (100%) | 8.5/10 (100%) |
| 2 | 8, 0 | 3 | 7/10 (70%) | 1/50 (2%) | Observation | 6/10 (60%) | 0/10 (2%) |
| 3 | 3, 5 | 2 | 1/50 (3%) | 1/50 (3%) | Observation | 0/10 (0%) | 0/10 (0%) |
| 4 | 1, 6 | 3 | 3/10 (100%) | 3/10 (100%) | Observation | 10/10 (100%) | 3/10 (30%) |
| 5 | 5, 2 | 3 | 1/10 (10%) | 1/10 (10%) | Observation | 1/10 (10%) | 1/10 (10%) |
| 6 | 1, 7 | 3 | 3/10 (100%) | 3/10 (100%) | CT | 3/10 (75%) | 3/10 (75%) |
| 7 | 1, 7 | 3 | NLP | NLP | CT | NLP | NLP |
| 8 | 4, 2 | 1 | 8/10 (80%) | 10/10 (100%) | CT | 7/10 (70%) | 10/10 (100%) |
| 9 | 1, 10 | 3 | 3/10 (100%) | 3/10 (100%) | CT | 0/10 (0%) | 0/10 (0%) |
| 10 | 2, 0 | 3 | 8/10 (100%) | 8/10 (100%) | CT | 1.5/10 (15%) | 6.5/10 (65%) |
| 11 | 2, 6 | 2 | 4/10 (100%) | 4/10 (100%) | CT | 10/10 (100%) | 0/10 (3%) |
| 12 | 2, 3 | 3 | 1/20 (12%) | 1/20 (12%) | CT | 0/10 (0%) | 0/10 (0%) |
| 13 | 3, 8 | 2 | 10/10 (100%) | 4/10 (40%) | RT | 8/10 (80%) | 0/10 (0%) |
| 14 | 8, 8 | 2 | 8/10 (100%) | 8/10 (100%) | RT | 10/10 (100%) | 10/10 (100%) |
| 15 | 4, 1 | 3 | 1/10 (15%) | 6–7/10 (100%) | RT | 1/10 (10%) | 4.5/10 (45%) |
| 16 | 9, 7 | 3 | NLP | 10/10 (100%) | RT | NLP | 10/10 (100%) |
| 17 | 4, 0 | 2 | 1/10 (15%) | 1/10 (15%) | CT + RT | 3/10 (30%) | 0/10 (0%) |
| 18 | 2, 2 | 2 | 1/10 (20%) | 1/10 (20%) | CT + RT | 0.5/10 (5%) | 0.5/10 (5%) |
| 19 | 2, 2 | 3 | 4/10 (100%) | 4/10 (100%) | CT + RT + RT | 1/10 (10%) | 1/10 (10%) |
| 20 | 2, 0 | 3 | 1/200 (1%) | 3/10 (75%) | CT + CT + RT | 0/10 (0%) | 2/10 (20%) |
Abbreviations: VA, visual acuity; CT, chemotherapy; NLP, no light perception; RT, radiation therapy.
Percent adjusted for age.
Fig. 1.
Visual acuity of the study population at the time of referral (A) and at the last follow-up (B). Numbers attached to the colored circles indicate how many patients had that combination of visual acuity in the left and right eye respectively.
Tumor Status at Time of Referral
OPG tumor “stage” according to the Dodge system at the time of referral is shown in Table 2. Only one child had a tumor confined to one optic nerve (Dodge 1), whereas in seven children (35%) the tumor extended to the chiasm with or without optic nerve involvement (Dodge 2). In 12 (60%) of the 20 patients, the tumor extended further posteriorly, along the optic tracts (Dodge 3); only four (33%) of these 12 children had some visual disturbances at the time of OPG diagnosis. In all but two patients, contrast-enhanced head MRI showed a variable grade of contrast enhancement in the tumor. Two patients presented with hypothalamic-chiasmatic tumors with cystic formations. Typical T2 hyperintense nonneoplastic lesions (unidentified bright objects) were seen in 14 patients (70%) in different regions of the brain parenchyma.
Treatment Strategy
At the time of referral, four patients were treated because of a medical history of unequivocal progressive visual loss in the face of a stable head MRI, and one because of evidence of tumor volume growth despite stable visual function. The other 15 patients were candidates for clinical observation only. During the observation period, four children were treated because of visual loss in the face of stable tumor volume, four others for tumor volume increase in the face of stable vision, and two for tumor progression associated with visual deterioration. These patients were treated after a mean follow-up time of 2 years from date of referral (range, 7 months to 7 years 5 months). In summary, 15 patients (75%) were candidates for some form of intervention at diagnosis or during follow-up, and of these, eight were treated because of a visual function deterioration not associated with MRI evidence of tumor progression.
Initial treatment was chemotherapy in 11 patients and radiotherapy in 4 patients, per protocol guidelines based on patient age. During follow-up, four children initially treated with chemotherapy had further treatment. Three of these four children underwent radiotherapy because of further progressive visual loss in two patients (at 2 and 5 years from the end of therapy) and because of tumor enlargement in one patient, documented 6 months after the start of chemotherapy. This latter child developed a brainstem pilocytic astrocytoma for which he underwent surgery and then further local radiotherapy, 5 years after the end of radiotherapy. The tumor in this patient developed in an area not previously irradiated. The fourth child initially underwent unsuccessful primary chemotherapy with tumor volume enlargement. Due to his young age, he was then treated with a different chemotherapy regimen, and afterward, because of further tumor progression and visual deterioration, with radiotherapy. The median age of the cohort of children who underwent radiotherapy was 9 years 1 month.
Patients Status at Time of Last Follow-Up
At the last follow-up, 18 children were alive with stable disease after a mean follow-up time from the diagnosis of OPG of 6 years 9 months (median, 6 years 4 months; range, 5 months to 18 years). As previously described, one child developed a pilocytic astrocytoma of the spinal-medullary junction and died of local progressive disease not directly related to the OPG, despite different forms of treatment, at 12 years from diagnosis of the OPG. One other child who also was found to be affected by a metachromatic leukoencephalopathy died of progressive brain deterioration 10 years after a diagnosis of OPG. This latter patient was never treated with chemotherapy or radiotherapy. These two patients are included in the summary of the study population’s visual function, taking into consideration their last available clinical assessment, because they did not die of progressive OPG.
The VA of these 20 children at the time of the last follow-up is described in Table 2 and shown graphically in Fig. 1B. Eight children had VA of less than 20% in both eyes; only two children, at the end of the study period, had 100% VA in both eyes. In the other 11 children who had some visual function remaining, the VF was assessed. Among these 11 children, three had reduced VF in one eye and three in both eyes, whereas five children had an intact VF. These children were also assessed for contrast and color sensitivity, which were abnormal in seven and six patients, respectively. In summary, of 15 patients who have been treated, only one patient has had a definitive improvement in terms of VA after radiotherapy alone (Table 2, patient 14), while two others have had a mild improvement in one eye but a deterioration in the other (Table 2, patients 11 and 17). Among the five children who were observed, VA improved in both eyes of patient 1 and in one eye of patient 3.
According to the WHO classification system for expression of hypovision, 13 children fell into the hypovision category. Six of them already had hypovision at the time of starting therapy; however, two of them moved from category 5 to category 1. The relevant clinical characteristics and the treatment received by these 13 patients are reported in Table 3.
Table 3.
Visual function outcome and tumor volume in response to treatment
| Visual Function
|
|||
|---|---|---|---|
| Tumor Volume | Improved | Stable | Worse |
| Reduced | 0 | 3 | 0 |
| Stable | 1 | 2 | 5 |
| Increased | 0 | 0 | 4 |
In terms of tumor volume reduction, 14 patients had stable disease, three patients had some tumor volume reduction, and three had progressive disease. The visual outcome of these patients in relationship to the tumor volume response to the treatment received is summarized in Table 4. The five children who only were observed during this study period always had stable disease and visual function.
Table 4.
Relevant clinical characteristics of children of this series included in the WHO hypovision categories
| Status at Last Follow-Up
|
|||||||
|---|---|---|---|---|---|---|---|
| Patient | VA at Referral | Dodge Tumor Site | Treatment | Age at OPG Diagnosis | VA | VF | Hypovision Categorya |
| 1 | RE 1/50 (3%) | 2 | Observation | 3 years 3 months | RE 0/10 | ND | 5 |
| LE 1/50 (3%) | LE 0/10 | ||||||
| 2 | RE 1/10 (10%) | 3 | Observation | 5 years 3 months | RE 1/10 | RE 35° | 1 |
| LE 1/10 (10%) | LE 1/10 | LE 35° | |||||
| 3 | RE NLP (0%) | 3 | CT | 1 year 7 months | RE 0/10 | ND | 5 |
| LE NLP (0%) | LE 0/10 | ||||||
| 4 | RE 3/10 (100%) | 3 | CT | 1 year 10 months | RE 0/10 | ND | 5 |
| LE 3/10 (100%) | LE 0/10 | ||||||
| 5 | RE 1/20 (12%) | 3 | CT | 2 years 5 months | RE 0/10 | ND | 5 |
| LE 1/20 (12%) | LE 0/10 | ||||||
| 6 | RE 1/10 (15%) | 3 | RT | 4 years 1 month | RE 1/10 | RE deficit | 1 |
| LE 6–7/10 (100%) | LE 4.5/10 | ||||||
| 7 | RE 10/10 (100%) | 2 | RT | 3 years 11 months | RE 8/10 | RE 10° | 3 |
| LE 4/10 (40%) | LE 0/10 | LE ND | |||||
| 8 | RE 8/10 (100%) | 2 | RT | 8.8 years | RE 10/10 | RE 30° | 1 |
| LE 8/10 (100%) | LE 10/10 | LE 25° | |||||
| 9 | RE 0/10 (0%) | 3 | RT | 9 years 7 months | RE 0/10 | RE ND | 1 |
| LE 10/10 (100%) | LE 10/10 | LE 60° | |||||
| 10 | RE 1/10 (20%) | 2 | CT + RT | 2 years 2 months | RE 0/10 | ND | 3 |
| LE 0/10 (0%) | LE 0/10 | ||||||
| 11 | RE 1/10 (15%) | 2 | CT + RT | 4 years | RE 3/10 | RE ND | 4 |
| LE 1/10 (15%) | LE 0/10 | LE deficit | |||||
| 12 | RE 1/200 (1%) | 3 | CT + CT + RT | 2 years | RE 0/10 | ND | 1 |
| LE 3/10 (75%) | LE 2/10 | ||||||
| 13 | RE 4/10 (100%) | 3 | CT + RT + RT | 2 years 2 months | RE 1/10 | ND | 2 |
| LE 4/10 (100%) | LE 1/10 | ||||||
Abbreviations: VA, visual acuity; OPG, optic pathway glioma; VF, visual field; RE, right eye; LE, left eye; ND, not determinable; NLP, no light perception; CT, chemotherapy; RT, radiation therapy.
WHO hypovision categories.
Discussion
The experience reported here of a series of 20 NF1 children affected by OPG and followed by a pediatric neuro-oncology program in terms of visual outcome is quite discouraging. At the end of a median follow-up time (from the date of referral) of 6 years 4 months, 13 children (65%) met the WHO definition of hypovision and eight (40%) had VA of less than 20% in both eyes.
Obviously, this population represents a highly selected series of NF1 children with OPG, because usually the most symptomatic patients are the ones referred to pediatric neuro-oncology programs. In addition, most of these children were very young when they started therapy (median age, 40 months, with only three children older than 6 years), the age when the possible growth potential of these tumors is at its peak.
Finally, the proportion of NF1 children in the present series who at the time of referral already had some visual impairment is in the higher part of the range reported in the literature. This highly selected series, however, is also one of the few in the literature in which visual loss and extent of overall visual function deficit have been carefully documented, especially in response to therapy.
In other series, VA loss in children with NF1 and OPG has been reported to vary between 20% and 70%.2,12–14 Reviewing the clinical records of 90 NF1 patients with OPG referred to two pediatric centers, neither housing a neuro-oncology program, King et al.7 found that, at the time of OPG diagnosis, 20 (22%) of these unselected patients had some decrease in VA (<20/20). More recently, Thiagalingam et al.15 retrospectively studied an unselected series of 54 patients with OPG and NF1 and documented that about 31% of them had some visual impairment at the time of OPG diagnosis and only 31.5% underwent treatment.
The data available in the literature regarding the visual outcome of children with OPG (with or without NF1) followed for a long period of time and variously treated vary substantially. Sutton et al.14 considered a series of children with OPG with and without NF1 treated according to a conservative approach (based on limited surgery); at last follow-up, a mean of 10.9 years after diagnosis, 23 of 28 surviving patients had functional vision in at least one eye and five children were functionally blind. Janss et al.,13 reviewing the treatment outcomes of 46 children younger than 5 years with a diagnosis of OPG (some of whom were already included in Sutton et al.’s series) found that, of the 27 who were evaluated, four were actually blind and one was legally classified as blind (5/27 = 19%). In the group as a whole, 5 patients experienced improvement in vision, 14 remained stable, and 8 had clinical deterioration. Gayre et al.16 investigated retrospectively the visual function in a cohort of 42 children with OPG (55% of whom had NF1) followed from 1970 to 1998, reporting that 25% of them were legally blind, based on VA alone. Their study also pointed out that vision tended to remain stable in the better eye while it tended to decline in the worse eye, despite treatment. Tow et al.17 showed that, in 22 NF1 patients with OPG, 14 (93%) of the 15 untreated tumors were associated with a final VA of 20/40 or better in at least one of the affected eyes. In the previously described series of Thiagalingam et al.,15 25 (46%) of 54 children had less than 6/15 Snellen equivalents of VA at last follow-up, after a median time of 8.6 years; eight children had moderate impairment (<6/15 Snellen equivalent), and 17 (31%) had severe impairment (<6/60 Snellen equivalent) in the worse eye, with 12 of them being blind.
Laithier et al.5 followed a series of 85 children with progressive OPG, with and without NF1 treated with chemotherapy, for a median period of time of 6.5 years. Of the 46 patients who had an accurate visual assessment, 60% had VA less than 3/10 in the better eye and 40% had VA greater than 3/10 in the better eye, with 11 children classified as blind (24%).
It is difficult to draw a coherent picture combining the data derived from these series: the populations are non-homogeneous, only a few studies focus exclusively on NF1 patients, and the modalities of studying or at least of expressing the visual assessments (particularly in very young children) are different. However, the fact clearly emerges that there is a subgroup of children with OPG who are at high risk of progressive deteriorating visual function leading to blindness. It remains to be shown whether the children with NF1 are a relevant part of this high-risk population, as our data seem to indicate.
Obviously, this is a matter of major concern, particularly for children with NF1, not only because of the relevance of this handicap in itself but also because the visual handicap may further and severely aggravate an overall clinical status already potentially compromised by a reduced cognitive capacity and/or other neurologic or physical deficits.
The most striking result from this series is that none of the patients who started therapy had vision improvement, and most actually had deterioration. This fact clearly calls into question the value of modern treatment guidelines to manage children with NF1 and progressive or symptomatic OPG and, more precisely, the role of chemotherapy. Many considerations can be brought forward. Clearly, some of the children of our series began therapy when their vision was already severely compromised. Thus, one could wonder whether, in the face of an already severely compromised visual function, nothing can really be of benefit in the attempt to reverse or at least stop the deterioration of the visual function. This consideration raises the hypothesis of the existence, within the NF1 population of patients affected by OPG, of a subgroup of children at high risk of visual loss who theoretically could benefit from an early therapeutic intervention. Of course, assuming the correctness of this hypothesis, we do not know how to identify this subgroup of children, given the present level of our knowledge. The age of the patients, the type of tumor extension along the optic pathway, and other still unknown biologic and genetic tumor or patient characteristics are suggested to have some prognostic value and could be involved in this hypothesis. In patients with NF1 and OPG, involvement of the optic tracts and other post-chiasmatic structures has been associated with a higher frequency of visual deterioration.12
As stated above, the role of systemic chemotherapy in preserving vision is also called into questioned by the data presented here. Tumor volume reductions in response to therapy have been documented, but no visual improvement has been documented. On the basis of these data, it is tempting to speculate that some NF1 children have severe progressive visual deterioration for reasons that chemotherapy and radiotherapy cannot control, such as, on hypothetical grounds, progressive optic fiber degeneration. At least two children of our series had a dramatic and rapid visual deterioration that resulted in blindness in both of them within 12 months of the first documented VA loss, despite aggressive multimodality treatment (including, in one case, optic foramina enlargement). We believe that there are no convincing data that radiotherapy is more effective than chemotherapy in this population of children in terms of visual function outcome, regardless of the reluctance in recommending this treatment modality in this cohort of children.6,9 In fact, brain irradiation in children with NF1 may further deteriorate an already compromised intellectual function and possibly increase the risk of vascular complications and of second tumor development.
The small number in this series of children with NF1 treated for OPG prevents us from reaching any firm conclusions. However, we believe that this study can serve to generate hypotheses and research questions that can be addressed in larger series. Furthermore, the importance of expressing the effect of modern treatment modalities for childhood OPG not only in terms of crude survival rates but also in terms of functional outcome is once again reinforced. Indeed, children with NF1 represent a special population, and clinicians should be aware of the limitations of the present treatment modalities and of the need for a prompt referral to visual rehabilitation for all children with NF1 who present with compromised visual function despite the initiation of therapy.
Acknowledgment
The research was supported in part by the Foundation Città della Speranza. The authors thank Giovanni Scarzello, Department of Radiation Oncology, University Hospital of Padua, for his significant input to this research.
References
- 1.Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol. 1997;41:143–149. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 2.Listernick R, Darling C, Greenwald M, Strauss L, Charrow J. Optic pathway tumors in children: the effect of neurofibromatosis type 1 on clinical manifestation and natural history. J Pediatr. 1995;127:718–722. doi: 10.1016/s0022-3476(95)70159-1. [DOI] [PubMed] [Google Scholar]
- 3.Rosser T, Packer RJ. Intracranial neoplasms in children with neurofibromatosis 1. J Child Neurol. 2002;17:630–637. doi: 10.1177/088307380201700815. [DOI] [PubMed] [Google Scholar]
- 4.Garvey M, Packer RJ. An integrated approach to the treatment of chiasmatic-hypothalamic gliomas. J Neurooncol. 1996;28:167–183. doi: 10.1007/BF00250197. [DOI] [PubMed] [Google Scholar]
- 5.Laithier V, Grill J, Le Deley M, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy—results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:4572–4578. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 6.Gnekow AK, Kortmann RD, Pietsch T, Emser A. Low grade chiasmatic-hypothalamic glioma—carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy—report from the Multicenter Treatment Study for Children and Adolescents with a Low Grade Glioma—HIT-LGG 1996—of the Society of Pediatric Oncology and Hematology (GPOH) Klin Padiatr. 2004;216:331–342. doi: 10.1055/s-2004-832355. [DOI] [PubMed] [Google Scholar]
- 7.King A, Listernick R, Charrow J, Piersall L, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet. 2003;122A:95–99. doi: 10.1002/ajmg.a.20211. [DOI] [PubMed] [Google Scholar]
- 8.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20:4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 9.Kortmann RD, Timmermann B, Taylor RE, et al. Current and future strategies in radiotherapy of childhood low-grade glioma of the brain. Part II: treatment-related late toxicity. Strahlenther Onkol. 2003;179:585–597. doi: 10.1007/s00066-003-8104-0. [DOI] [PubMed] [Google Scholar]
- 10.De Bella K, Szudek J, Friedman JM. Use of the National Institutes of Health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105:608–614. doi: 10.1542/peds.105.3.608. [DOI] [PubMed] [Google Scholar]
- 11.Dodge HJ, Love J, Craig W, et al. Gliomas of the optic nerves. AMA Arch Neurol Psychiatry. 1958;79:607–621. doi: 10.1001/archneurpsyc.1958.02340060003001. [DOI] [PubMed] [Google Scholar]
- 12.Balcer LJ, Liu GT, Heller G, et al. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131:442–445. doi: 10.1016/s0002-9394(00)00852-7. [DOI] [PubMed] [Google Scholar]
- 13.Janss AJ, Grundy R, Cnaan A, et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer. 1995;75:1051–1059. doi: 10.1002/1097-0142(19950215)75:4<1051::aid-cncr2820750423>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Sutton LN, Molloy PT, Sernyak H, et al. Long-term outcome of hypothalamic/chiasmatic astrocytomas in children treated with conservative surgery. J Neurosurg. 1995;83:583–589. doi: 10.3171/jns.1995.83.4.0583. [DOI] [PubMed] [Google Scholar]
- 15.Thiagalingam S, Flaherty M, Billson F, North K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology. 2004;111:568–577. doi: 10.1016/j.ophtha.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Gayre GS, Scott IU, Feuer W, Saunders TG, Siatkowski RM. Long-term visual outcome in patients with anterior visual pathway gliomas. J Neuroophthalmol. 2001;21:1–7. doi: 10.1097/00041327-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28:262–270. doi: 10.1016/s0887-8994(02)00628-8. [DOI] [PubMed] [Google Scholar]