Abstract
Tumor recurrence is the major clinical complication in meningiomas, and its prediction in histologically benign/grade I tumors remains a challenge. In this study, we analyzed the prognostic value of specific chromosomal abnormalities and the genetic heterogeneity of the tumor, together with other clinicobiological disease features, for predicting early relapses in histologically benign/grade I meningiomas. A total of 149 consecutive histologically benign/grade I meningiomas in patients who underwent complete tumor resection were prospectively analyzed. Using interphase fluorescence in situ hybridization, we studied the prognostic impact of the abnormalities detected for 11 different chromosomes, together with other relevant clinicobiological and histopathological characteristics of the disease, on recurrence-free survival (RFS) at 2.5, 5, and 10 years. From the prognostic point of view, losses of chromosomes 9, 10, 14, and 18 and del(1p36) were associated with a shorter RFS at 2.5, 5, and 10 years. Similarly, histologically benign/grade I meningiomas showing coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone displayed a higher frequency of early relapses. In fact, coexistence of −14 and del(1p36) in the ancestral tumor cell clone, together with tumor size, represented the best combination of independent prognostic factors for the identification of those patients with a high risk of an early relapse. Our results indicate that patients with large histologically benign/grade I meningiomas carrying monosomy 14 and del(1p36) in their ancestral tumor cell clone have a high probability of relapsing early after diagnostic surgery. These findings suggest the need for closer follow-up in this small group of patients.
Keywords: ancestral tumor cell clone, benign/grade I meningiomas, chromosomal abnormalities, cytogenetics, del(1p36), early recurrence, monosomy 14, prognostic factors
Tumor recurrence is the major clinical complication of meningiomas, occurring in between 10%–15% and 25%–37% of patients undergoing curative surgery after a 5- to 10-year follow-up period, respectively. Although relapses may occur 10 or more years after complete tumor resection, in a high proportion of cases they develop in the first years following curative surgery. Among other disease features, such as patient age, tumor location, extent of tumor resection, and proliferation-associated markers,1–5 tumor histology has long proven to be a powerful independent prognostic factor for recurrence-free survival (RFS). Accordingly, while only a minor proportion of all histologically benign/grade I meningiomas relapse, most atypical and anaplastic tumors do recur in the first years after complete surgical removal of the lesion.6–20 However, in absolute numbers, the majority of the relapses correspond to histologically benign meningiomas, even among those cases showing recurrence early after diagnosis. In line with this, more recent studies have shown that specific genetic abnormalities such as del(1p36) and monosomy 14 alone or combined in the ancestral tumor cell clone as assessed by fluorescence in situ hybridization (FISH) in association with clinicopathological disease features such as tumor size and/or patient age15,21–31 can be of further help in predicting outcome, independent of tumor histology. Despite this, prediction of those relapses occurring among histologically benign tumors in the first years following diagnostic surgery still remains a major challenge.
In the present study, we prospectively analyzed the prognostic value of specific chromosomal abnormalities and the genetic heterogeneity of the tumor, together with other clinicobiological disease features, for predicting early relapses in histologically benign/grade I meningiomas. Our results, based on a series of 149 patients who underwent complete tumor resection, show that most relapses occurring during the first 2.5 years after surgery correspond to large tumors in which del(1p36) and monosomy 14 coexist in the ancestral tumor cell clone, providing a new scoring system for the stratification of histologically benign tumors at diagnosis, according to risk of early relapse.
Materials and Methods
Patients
A total of 149 patients with histologically benign/grade I meningiomas (43 males, 106 females; mean age, 59 ± 14 years; range, 13–84 years) from a series of 171 consecutive patients diagnosed with meningioma at the Neurosurgery Service of the University Hospital of Salamanca were prospectively included in this study. In all cases, histological diagnosis and classification were performed by an experienced neuropathologist according to WHO criteria32 and confirmed by a second independent experienced neuropathologist. A total of 22 cases were not entered into the study: eight were excluded due to incomplete resection of the tumor, and 14 corresponded to grade II and grade III tumors. In all other cases, a complete surgical resection of the tumor was performed according to the Simpson’s criteria33 (grade 1, 4%; grade 2, 35%; grade 3, 61%).
According to tumor localization, most (n = 134; 90%) meningiomas corresponded to intracranial tumors, and only 15 (10%) were spinal meningiomas. According to their intracranial localization, the former tumors were distributed as follows: convexity, 27%; parasagittal and tentorial, 33%; ventricular, 2%; and cranial base, 38%. The great majority of the tumors corresponded to meningotheliomatous meningiomas (78%), 12% were psammomatous tumors, 9% were transitional, and 1% were fibroblastic meningiomas. Following diagnostic surgery, none of the patients received any additional antitumor-directed therapy. Tumor size was reported as the longest diameter of the tumor mass on MR or CT images.
Median follow-up at the moment of closing this study was 73 months (range, 6–241 months). Follow-up studies were performed according to a standardized clinicobiological protocol, including MRI techniques performed 3 months after surgery and every 12 months thereafter; whenever clinical signs and/or symptoms were noted and a relapse was suspected, additional MRI studies were performed. In recurrent meningiomas, histological diagnosis was confirmed after tumor resection, and all recurrent cases showed histological behavior identical to that of the corresponding meningiomas studied at diagnosis.
Interphase FISH Studies
In all cases, freshly frozen tumor samples obtained at diagnostic surgery were used for interphase FISH (iFISH) analysis of numerical abnormalities of chromosomes 1p, 1q, 7, 9, 10, 11, 14, 15, 17, 18, 22, X, and Y according to techniques that have been previously described in detail.15,34,35 Based on the iFISH patterns, the presence of one or more tumor cell clones was defined as previously described.36 To define intratumoral clonal evolution in those tumors with multiple subclones, we assumed that karyotypic abnormalities shared by all subclones represented the earliest changes, whereas the latter cytogenetic changes would be present in only some of the tumor cell clones. In all those cases in which two or more cell clones were present by iFISH, an ancestral tumoral cell clone could be identified as that carrying only those karyotype abnormalities common to all tumor cells.
Seventy cases (47%) showed the presence of a single tumor cell clone, with 55 of these cases (79%) consisting of either diploid tumor cells with no abnormalities for any of the chromosomes studied (n = 46; 66%) or neoplastic cells carrying monosomy 22 (n = 9; 13%) as the sole cytogenetic alteration; in the other 15 patients (21%), different chromosomal abnormalities were detected. In turn, 79 cases (53%) showed two or more tumor cell clones by iFISH. In these cases, the ancestral tumor cell clone frequently showed losses of one or more chromosomes (n = 72; 92%); these corresponding to −22 alone (32 cases; 41%) or associated with other chromosome losses (n = 21; 27%), loss of chromosome 1p36 (n = 21; 27%), monosomy 14 (n = 11; 14%), and nulisomy Y in males (n = 5; 6%). Numerical abnormalities of chromosomes 7 (3%), 9 (4%), 10 (7%), 11 (1%), 15 (3%), 17 (5%), 18 (3%), and X (7%) were detected in a minority of cases.
Statistical Methods
For all continuous variables included in the study, mean values and their standard deviation and range were calculated using SPSS (version 11.0; SPSS Inc., Chicago, IL, USA); for categorical variables, frequencies were used. To establish the statistical significance of the differences observed between groups, the Student t-test and Mann-Whitney U-test were used for continuous variables; for qualitative variables, the chi-square test was applied (cross-tab; SPSS). RFS curves were plotted according to the method of Kaplan and Meier, and the one-sided log-rank test was used to establish the statistical significance of the differences observed between curves (survival; SPSS). Multivariate analysis of prognostic factors for RFS was performed using the Cox stepwise regression model (regression; SPSS). In this part of the study, only those variables showing a significant association with RFS in the univariate analysis were included. Values of p lower than 0.05 were considered statistically significant.
Results
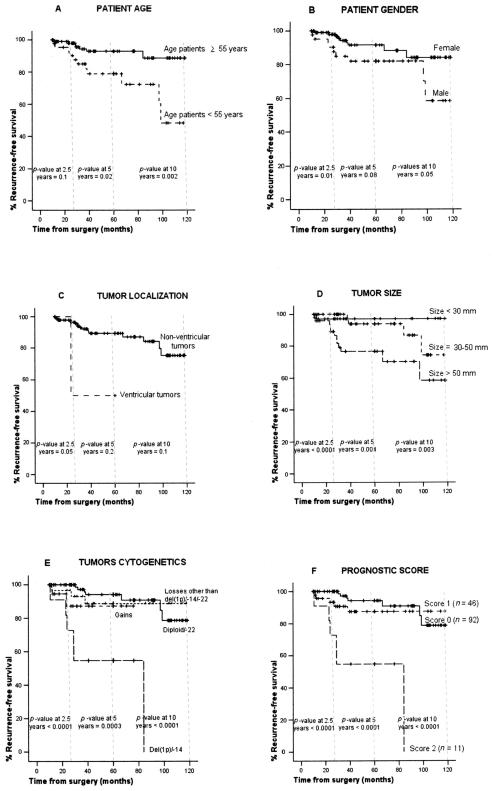
Overall, at the moment of closing this study, 20 of the 149 cases studied had relapsed, with nine of the relapses occurring during the first 30 months after diagnostic surgery (median time to recurrence, 30 months; Table 1); among the other 11 relapses, four occurred between months 39 and 60 after diagnostic surgery, five between months 61 and 120, and the other two recurrences were diagnosed at months 122 and 241 of follow-up. From the prognostic point of view, losses of chromosomes 9 (p = 0.01), 18 (p = 0.005), 10 (p < 0.0001), and 14 (p = 0.001) and del(1p36) (p = 0.008) were associated with a shorter RFS at 2.5 years, the latter three also showing a significant impact on RFS at both 5 and 10 years (Table 2). Similarly, histologically benign/grade I meningiomas showing coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone displayed a higher frequency of relapses during the first 30 months after diagnostic surgery (p < 0.0001) as well as at 5 and 10 years (p = 0.0003 and p < 0.0001, respectively; Table 2, Fig. 1E). Other clinical features of the disease showing an adverse impact on the occurrence of tumor relapses included (1) male gender (p = 0.01), ventricular tumors (p = 0.05), and tumor size > 50 mm in diameter (p < 0.0001) at 2.5 years; (2) younger patient age (p = 0.02) and tumor size > 50 mm (p = 0.004) at 5 years; and (3) younger patient age (p = 0.002), male gender (p = 0.05), and tumor size > 50 mm (p = 0.003) at 10 years (Table 2, Fig. 1A–D).
Table 1.
Clinical, histopathological, and cytogenetic characteristics of those meningioma patients showing relapse within the first 30 months after complete tumor resection at diagnostic surgery
| Case No. | Age | Gender | Tumor Location | Histological Subtype | iFISH Findings at Diagnosis | Time from Diagnosis to Relapse (Months) |
|---|---|---|---|---|---|---|
| 1 | 13 | Female | Cranial base | Meningotheliomatous | −1p, −9, −10, −22/TT | 12 |
| 2 | 60 | Male | Parasagittal | Meningotheliomatous | −1p, −10, −14q | 29 |
| 3 | 46 | Male | Parasagittal | Meningotheliomatous | −1p, −14q, −22 | 24 |
| 4 | 43 | Female | Ventricular | Meningotheliomatous | +7 | 23 |
| 5 | 65 | Male | Tentorial | Meningotheliomatous | −Y | 12 |
| 6 | 63 | Male | Convexity | Meningotheliomatous | −1p, −10, −14q, −22, −Y | 23 |
| 7 | 43 | Male | Convexity | Meningotheliomatous | −1p, −14q, −10/+15 | 11 |
| 8 | 60 | Female | Convexity | Meningotheliomatous | −1p, −14q, −22, +9 | 22 |
| 9 | 50 | Male | Parasagittal | Meningotheliomatous | −22, −14q | 27 |
Abbreviations: iFISH, interphase fluorescence in situ hybridization; TT, tetraploidy.
Table 2.
Prognostic impact of the cytogenetic characteristics of histologically benign/grade I meningiomas on early recurrence-free survival (n = 149)
| Prognostic Factors for Recurrence-Free Survival
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 75% Recurrence-Free Survival
|
Univariate Analysis (p Value)
|
Multivariate Analysis (p Value)
|
||||||||||
| Variable | Group | No. Patients (%) | % Recurrences from Total Cases | 30 m | 60 m | 120 m | 30 m | 60 m | 120 m | 30 m | 60 m | 120 m |
| Chromosome 1p36 | Diploid | 92 (62) | 3 | NR | NR | NR | 0.008 | 0.009 | 0.01 | 0.06 | 0.05 | 0.05 |
| Losses | 32 (23) | 18 | NR | NR | 84 m | |||||||
| Gains | 22 (15) | 0 | NR | NR | 97 m | |||||||
| Chromosome 1q | Diploid | 108 (73) | 6 | NR | NR | NR | 0.9 | 0.8 | 0.8 | |||
| Losses | 1 (1) | 0 | NR | NR | NR | |||||||
| Gains | 39 (26) | 5 | NR | NR | NR | |||||||
| Chromosome 7 | Diploid | 97 (73) | 6 | NR | NR | 98 m | 0.7 | 0.4 | 0.6 | |||
| Losses | 0 (0) | 0 | NR | NR | NR | |||||||
| Gains | 36 (27) | 8 | NR | NR | NR | |||||||
| Chromosome 9 | Diploid | 107 (72) | 6 | NR | NR | NR | 0.01 | 0.8 | 0.07 | 0.7 | 0.3 | 0.4 |
| Losses | 2 (1) | 50 | 12 m | 12 m | 12 m | |||||||
| Gains | 40 (27) | 5 | NR | NR | 97 m | |||||||
| Chromosome 10 | Diploid | 104 (70) | 6 | NR | NR | NR | <0.0001 | 0.0001 | 0.0002 | 0.05 | 0.004 | 0.01 |
| Losses | 5 (3) | 60 | 23 m | 23 m | 23 m | |||||||
| Gains | 39 (27) | 0 | NR | NR | 97 m | |||||||
| Chromosome 11 | Diploid | 42 (76) | 7 | NR | NR | NR | 0.3 | 0.6 | 0.2 | |||
| Losses | 0 (0) | 0 | NR | NR | NR | |||||||
| Gains | 13 (24) | 15 | NR | NR | 97 m | |||||||
| Chromosome 14 | Diploid | 97 (65) | 3 | NR | NR | NR | 0.001 | 0.03 | 0.04 | 0.06 | 0.5 | 0.4 |
| Losses | 19 (13) | 26 | 29 m | 29 m | 29 m | |||||||
| Gains | 32 (22) | 3 | NR | NR | 97 m | |||||||
| Chromosome 15 | Diploid | 106 (71) | 6 | NR | NR | NR | 0.8 | 0.6 | 0.6 | |||
| Losses | 0 (0) | 0 | NR | NR | NR | |||||||
| Gains | 43 (29) | 7 | NR | NR | 97 m | |||||||
| Chromosome 17 | Diploid | 106 (71) | 7 | NR | NR | NR | 0.8 | 0.9 | 0.7 | |||
| Losses | 1 (1) | 0 | NR | NR | NR | |||||||
| Gains | 42 (28) | 5 | NR | NR | 97 m | |||||||
| Chromosome 18 | Diploid | 66 (67) | 6 | NR | NR | 84 m | 0.005 | 0.06 | 0.08 | 0.05 | 0.9 | 0.8 |
| Losses | 5 (5) | 40 | 23 m | 23 m | 29 m | |||||||
| Gains | 28 (28) | 0 | NR | NR | NR | |||||||
| Chromosome 22 | Diploid | 59 (40) | 5 | NR | NR | 98 m | 0.6 | 0.7 | 0.8 | |||
| Losses | 82 (55) | 6 | NR | NR | NR | |||||||
| Gains | 8 (5) | 13 | 29 m | 29 m | NR | |||||||
| Chromosome X | Diploid | 103 (70) | 7 | NR | NR | NR | 0.7 | 0.7 | 0.9 | |||
| Losses | 17 (12) | 6 | NR | NR | 97 m | |||||||
| Gains | 27 (18) | 4 | NR | NR | NR | |||||||
| Chromosome Y | Diploid | 26 (58) | 9 | NR | NR | 84 m | 0.9 | 0.7 | 0.8 | |||
| Losses | 9 (20) | 12 | NR | NR | NR | |||||||
| Gains | 10 (22) | 11 | NR | 38 m | 38 m | |||||||
| No. of tumor cell clones | 1 clone | 67 (45) | 4 | NR | NR | NR | 0.3 | 0.5 | 0.6 | |||
| ⩾2 clones | 82 (55) | 8 | NR | NR | 97 m | |||||||
| Karyotype of the ancestral tumor cell clone | Diploid | 50 (36) | 0 | NR | NR | NR | <0.0001 | 0.0003 | <0.0001 | 0.001 | 0.04 | 0.02 |
| −22 | 42 (28) | 0 | NR | NR | NR | |||||||
| −1p/−14q | 11 (7) | 46 | 23 m | 24 m | 24 m | |||||||
| Chromosome losses other than −22 and −1p/−14q | 28 (18) | 7 | NR | NR | NR | |||||||
| Chromosome gains | 18 (11) | 11 | NR | NR | NR | |||||||
Abbreviations: NR, not reached; m, months.
Fig. 1.
Recurrence-free survival (RFS) at 30, 60, and 120 months of histologically benign/grade I meningioma tumors (n = 149), according to patient age (A), gender (B), tumor location (C), tumor size (D), and the cytogenetic abnormalities detected in the ancestral tumor cell clone defined as the tumor cell clone carrying only those chromosomal abnormalities common to all tumor cells in the sample (E: diploid/−22; losses other than −22 or −1p/−14; gains; −1p/−14). F shows RFS curves at 30, 60, and 120 months according to the proposed prognostic score for predicting early relapses in histologically benign/grade I meningiomas. In this prognostic score, two variables were considered with a prognostic score of either 0 or 1: tumor size (score 0 for tumors < 50 mm, score 1 for tumors > 50 mm) and the presence (score 1) or absence (score 0) of both monosomy 14 and del(1p36) in the ancestral tumor cell clone.
Multivariate analysis of prognostic factors for RFS at 30 months showed that tumor size (p = 0.008) together with coexistence of both del(1p36) and monosomy 14 in the ancestral tumor cell clone (p = 0.001) was the best combination of independent parameters for predicting RFS early after diagnosis in histologically benign/grade I meningiomas. Interestingly, at both 5 and 10 years of follow-up, in addition to tumor size and the karyotype of the ancestral tumor cell clone, patient age (p = 0.008 and p = 0.001, respectively) and abnormalities of chromosome 10 (p = 0.004 and p = 0.01, respectively) also proved to have independent prognostic value for RFS in benign/grade I meningiomas (Tables 2 and 3). Based on these results, a prognostic score was built. Patients carrying tumors larger than 50 mm as well as those carrying both del(1p36) and monosomy 14 as the earliest detectable chromosome lesions were assigned a score of 1 each. Based on this score, three different groups of patients at distinct risk of early recurrence were identified: a good prognostic category that included most cases (n = 92), with a score of 0 and a recurrence risk at 30, 60, and 120 months of 0%, 5%, and 8%, respectively; an intermediate group (n = 46) with a score of 1 and a relapse risk of 7%, 11%, and 11% at 2.5, 5, and 10 years, respectively; and a poor prognostic category, with only 11 cases, showing a score of 2 and risk of relapse at 2.5, 5, and 10 years of 45%, 45%, and 45%, respectively (p < 0.0001; Fig. 1F). Although none of the other variables analyzed significantly improved prediction of RFS at 2.5 years in the multivariate study, other cytogenetic abnormalities involving losses of chromosomes 10 and 18 together with tumor location showed a predictive value close to statistical significance (p ≥ 0.05 and ≤ 0.1).
Table 3.
Prognostic impact of the clinicobiological characteristics of histologically benign/grade I meningiomas on early recurrence-free survival (n = 149)
| Prognostic Factors for Recurrence-Free Survival
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 75% Recurrence-Free Survival
|
Univariate Analysis (p Value)
|
Multivariate Analysis (p Value)
|
||||||||||
| Variable | Group | No. Patients (%) | % Recurrences from Total Cases | 30 m | 60 m | 120 m | 30 m | 60 m | 120 m | 30 m | 60 m | 120 m |
| Age (years) | <55 | 47 (32) | 11 | NR | NR | 66 m | 0.1 | 0.02 | 0.002 | 0.008 | 0.001 | |
| ⩾55 | 102 (68) | 4 | NR | NR | NR | |||||||
| Sex | Male | 43 (29) | 14 | NR | NR | 31 m | 0.01 | 0.08 | 0.05 | 0.4 | 0.2 | 0.6 |
| Female | 106 (71) | 3 | NR | NR | NR | |||||||
| Tumor location | Convexity | 36 (24) | 8 | NR | NR | 97 m | 0.05 | 0.2 | 0.1 | 0.5 | 0.7 | 0.9 |
| Parasagittal or tentorial | 44 (29) | 9 | NR | NR | NR | |||||||
| Ventricular | 3 (2) | 43 | 23 m | 23 m | 23 m | |||||||
| Cranial base | 51 (34) | 2 | NR | NR | 41 m | |||||||
| Spinal | 15 (10) | 0 | NR | NR | NR | |||||||
| Histologic subtype | Meningotheliomatous | 107 (78) | 9 | NR | NR | NR | 0.2 | 0.2 | 0.3 | |||
| Psammomatous | 17 (12) | 0 | NR | NR | NR | |||||||
| Transitional | 13 (9) | 0 | NR | NR | NR | |||||||
| Fibroblastic | 2 (1) | 0 | ||||||||||
| Tumor size | <30 mm | 36 (24) | 6 | NR | NR | NR | <0.0001 | 0.004 | 0.003 | 0.008 | 0.01 | 0.003 |
| 30–50 mm | 64 (43) | 10 | NR | NR | 98 m | |||||||
| >50 mm | 49 (33) | 25 | NR | NR | 97 m | |||||||
| Simpson’s grade | 1 | 6 (4) | 17 | NR | NR | NR | 0.4 | 0.7 | 0.3 | |||
| 2 | 52 (35) | 4 | NR | NR | NR | |||||||
| 3 | 91 (61) | 7 | NR | NR | 97 m | |||||||
| DNA ploidy status | DNA diploid | 126 (84) | 5 | NR | NR | NR | 0.4 | 0.9 | 0.6 | |||
| DNA aneuploid | 23 (16) | 9 | NR | NR | 84 m | |||||||
| % S phase + G2M tumor cells | ⩽1 | 43 (29) | 2 | NR | NR | NR | 0.8 | 0.8 | 0.8 | |||
| >1 | 106 (71) | 5 | NR | NR | 97 m | |||||||
Abbreviations: NR, not reached; m, months.
Discussion
Until now, reports about the prognosis of meningioma patients have mainly focused on the 5- to 10-year RFS. From a practical point of view, this does not favor the use of prognostic scoring systems established already at diagnosis to help in deciding on closer follow-up of those patients at a higher risk of relapse after relatively long remission periods (e.g., 5–10 years). Because of this, in practice, a closer follow-up of meningioma patients who underwent curative surgery is frequently restricted to histologically atypical and anaplastic tumors.14,35,37,38 Despite this, it is well known that, in absolute numbers, most relapses occurring in meningioma patients correspond to histologically benign/grade I tumors,19,39–41 as also found in our series, where 20 of 27 recurrent tumors corresponded to histologically benign/grade I meningiomas (data not shown). Previous studies have shown that the use of specific cytogenetic markers such as del(1p36) and monosomy 14, either in the whole tumor cell population including the ancestral tumor cell clone or in some tumor cells, in combination with tumor size and age, is of great help in the prognostic stratification of histologically benign/grade I meningiomas.15,21–31 However, little information exists about the prognostic factors for predicting early relapses among histologically benign/grade I tumors. In the present study, we searched for the best combination of prognostic factors for predicting early relapses among histologically benign/grade I meningiomas. RFS at 2.5 years was chosen as an end point of interest since during this early period around half of all relapses occurred, their frequency clearly decreasing thereafter. We have previously shown that age together with monosomy 14 was the best combination of variables for predicting RFS in histologically benign meningiomas after a median follow-up of more than 5 years.15 Further studies from our group and others indicate that tumor size could also be of additional prognostic value in this regard. In the present study, monosomy 14 and tumor size showed a significant association with the occurrence of early relapses in histologically benign/grade I meningiomas, while age appeared to be informative only after longer follow-up periods. Other clinical and cytogenetic variables showing a prognostic impact on the 30-month RFS included patient gender, tumor location and size, del(1p36), and losses of chromosomes 9, 10, and 18. In fact, all these clinical and cytogenetic variables have been identified as potentially associated with RFS once histologically benign and malignant meningiomas are considered together.10,16,23,24,29,42–44
Until now, it has been well established that meningiomas are cytogenetically heterogeneous tumors both at the inter- and intratumoral cell level.45–49 In recent years, multicolor iFISH approaches have been systematically applied to the study of large series of meningioma patients, as in the present study, and hypothetical models of intratumoral clonal evolution have been proposed based on the cytogenetically different tumor cell clones detected.50 Despite this, no study has been reported so far in which the prognostic impact of the number of tumor cell clones, or the intratumoral pathways of clonal evolution, has been analyzed in histologically benign meningiomas. In the present study, we show that, while the number of cytogenetically identified clones in a tumor had no significant impact on predicting early RFS in histologically benign meningiomas, coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone was a powerful adverse prognostic factor for early relapses in these patients. In fact, together with tumor size, it represented the best combination of independent prognostic factors for the identification of a relatively small group of patients with an early relapse risk of around 45%, in contrast to a larger group of individuals that showed an extremely low frequency (2%) of recurrences during the first 2.5 years after diagnostic surgery. Interestingly, coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone is not restricted to histologically benign/grade I meningiomas but is also observed at significantly higher frequencies among histologically atypical and anaplastic meningiomas (25% vs. 8% in the present series), supporting the more aggressive behavior of meningiomas carrying these cytogenetic features.
Until now, several genes have been identified at chromosome 1p32 to 1p36 that could help to explain the more aggressive behavior of meningioma tumors carrying del(1p36), including the COKN2C (p18INK4C), p73, RAD45L, and alkaline phosphatase genes.51–53 Despite this, the potential role of these genes as tumor suppressor genes in the pathogenesis of meningiomas remains unknown. In turn, regarding chromosome 14, no tumor suppressor genes have been clearly identified as potentially involved in determining the clinical behavior of meningiomas, either at the most frequently deleted regions (from 14q21 to 14q32) or at other parts of chromosome 14.54,55 However, Lusis et al.56 recently suggested that the NDRG2 gene localized at 14q11.2 could be involved in tumor progression since it is under-expressed in anaplastic meningiomas due to methylation. In addition, it should be noted that recent results36 based on both cytogenetic and histological data indicate that development of recurrent meningiomas after complete tumor resection could be frequently due to regrowth of the primary tumor and rarely associated with emergence of an unrelated meningioma. In line with this, in the present study, all recurrent tumors displayed histological behavior identical to that of their corresponding diagnostic meningiomas.
Altogether, these results indicate that, independent of the genes involved, coexistence of del(1p36) and monosomy 14 confers a unique pattern of tissue involvement and/or clonogenic potential to those histologically benign/grade I meningiomas carrying both abnormalities in their ancestral tumor cell clone. Interestingly, this does not appear to be related to the proliferative index of meningioma tumor cells since the percentage of S phase or S + G2 phase cells did not demonstrate a significant prognostic impact at any of the RFS end points analyzed. However, due to the low number of relapses occurring in the first 2.5 years after surgery (n = 9), our results require further confirmation in a longer series of histologically benign/grade I meningiomas.
In summary, in the present study, we show that histologically benign/grade I meningiomas displaying a large tumor size and carrying both monosomy 14 and del(1p36) in their ancestral tumor cell clone have a high probability of relapsing during the first 2.5 years after diagnostic surgery, and they should be followed more closely and/or treated differently from the other cases.
Acknowledgments
This work was partially supported by grant FIS 02/0010 from the Fondo de Investigaciones Sanitarias and RETICC RD06/0020/0035 from the Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo, Madrid, Spain; grants HUS2/03, SA02/02, HUS05A06, and 44-05 from the Junta de Castilla y León, Valladolid, Spain; Fundación MMA, Madrid, Spain; Ministerio de Ciencia y Tecnologia, Programa Ramón y Cajal, Madrid, Spain, and IECSCYL (M.D.T.); and grants F105/00266 (A.B.E.) and CP05/00321 (J.M.S.) from the Ministerio de Sanidad y Consumo, Madrid, Spain.
References
- 1.Fewings PE, Battersby RD, Timperley WR. Long-term follow up of progesterone receptor status in benign meningioma: a prognostic indicator of recurrence? J Neurosurg. 2000;92:401–405. doi: 10.3171/jns.2000.92.3.0401. [DOI] [PubMed] [Google Scholar]
- 2.Hsu DW, Efird JT, Hedley-Whyte ET. MIB-1 (Ki-67) index and transforming growth factor-alpha (TGF alpha) immunoreactivity are significant prognostic predictors for meningiomas. Neuropathol Appl Neurobiol. 1998;24:441–452. doi: 10.1046/j.1365-2990.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- 3.Maes L, Kalala JP, Cornelissen M, de Ridder L. P CNA, Ki-67 and hTERT in residual benign meningiomas. In Vivo. 2006;20:271–275. [PubMed] [Google Scholar]
- 4.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. The prognostic significance of MIB-1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer. 1998;82:2262–2269. [PubMed] [Google Scholar]
- 5.Roser F, Nakamura M, Bellinzona M, Rosahl SK, Ostertag H, Samii M. The prognostic value of progesterone receptor status in meningiomas. J Clin Pathol. 2004;57:1033–1037. doi: 10.1136/jcp.2004.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayerbe J, Lobato RD, de la Cruz J, et al. Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir (Wien) 1999;141:921–932. doi: 10.1007/s007010050398. [DOI] [PubMed] [Google Scholar]
- 7.Baird M, Gallagher PJ. Recurrent intracranial and spinal meningiomas: clinical and histological features. Clin Neuropathol. 1989;8:41–44. [PubMed] [Google Scholar]
- 8.Cerda-Nicolas M, Lopez-Gines C, Perez-Bacete M, Barcia-Salorio JL, Llombart-Bosch A. Histopathological and cytogenetic findings in benign, atypical and anaplastic human meningiomas: a study of 60 tumors. Clin Neuropathol. 2000;19:259–267. [PubMed] [Google Scholar]
- 9.Chan RC, Thompson GB. Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg. 1984;60:52–60. doi: 10.3171/jns.1984.60.1.0052. [DOI] [PubMed] [Google Scholar]
- 10.Christensen D, Laursen H, Klinken L. Prediction of recurrence in meningiomas after surgical treatment. A quantitative approach. Acta Neuropathol (Berl) 1983;61:130–134. doi: 10.1007/BF00697392. [DOI] [PubMed] [Google Scholar]
- 11.Hilbig A, Barbosa-Coutinho LM. Meningiomas. Histopathological aspects and recurrence. Arq Neuropsiquiatr. 1997;55:431–437. doi: 10.1590/s0004-282x1997000300014. [DOI] [PubMed] [Google Scholar]
- 12.Jan M, Bazeze V, Saudeau D, Autret A, Bertrand P, Gouaze A. Outcome of intracranial meningioma in adults. Retrospective study of a medicosurgical series of 161 meningiomas. Neurochirurgie. 1986;32:129–134. [PubMed] [Google Scholar]
- 13.Mahmood A, Qureshi NH, Malik GM. Intracranial meningiomas: analysis of recurrence after surgical treatment. Acta Neurochir (Wien) 1994;126:53–58. doi: 10.1007/BF01476410. [DOI] [PubMed] [Google Scholar]
- 14.Maier H, Ofner D, Hittmair A, Kitz K, Budka H. Classic, atypical, and anaplastic meningioma: three histopathological subtypes of clinical relevance. J Neurosurg. 1992;77:616–623. doi: 10.3171/jns.1992.77.4.0616. [DOI] [PubMed] [Google Scholar]
- 15.Maillo A, Orfao A, Sayagues JM, et al. New classification scheme for the prognostic stratification of meningioma on the basis of chromosome 14 abnormalities, patient age, and tumor histopathology. J Clin Oncol. 2003;21:3285–3295. doi: 10.1200/JCO.2003.07.156. [DOI] [PubMed] [Google Scholar]
- 16.Mathiesen T, Lindquist C, Kihlstrom L, Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39:2–7. doi: 10.1097/00006123-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 17.McLean CA, Jolley D, Cukier E, Giles G, Gonzales MF. Atypical and malignant meningiomas: importance of micronecrosis as a prognostic indicator. Histopathology. 1993;23:349–353. doi: 10.1111/j.1365-2559.1993.tb01218.x. [DOI] [PubMed] [Google Scholar]
- 18.Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- 19.Simoca I, Olarescu AA, Jipescu I, Lisievici M. Postoperative outcome of intracranial meningiomas; long-term prognosis. Rom J Neurol Psychiatry. 1994;32:237–251. [PubMed] [Google Scholar]
- 20.Stafford SL, Perry A, Suman VJ, et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc. 1998;73:936–942. doi: 10.4065/73.10.936. [DOI] [PubMed] [Google Scholar]
- 21.Bello MJ, de Campos JM, Kusak ME, et al. Allelic loss at 1p is associated with tumor progression of meningiomas. Genes Chromosomes Cancer. 1994;9:296–298. doi: 10.1002/gcc.2870090411. [DOI] [PubMed] [Google Scholar]
- 22.Cai DX, Banerjee R, Scheithauer BW, Lohse CM, Kleinschmidt-DeMasters BK, Perry A. Chromosome 1p and 14q FISH analysis in clinicopathologic subsets of meningioma: diagnostic and prognostic implications. J Neuropathol Exp Neurol. 2001;60:628–636. doi: 10.1093/jnen/60.6.628. [DOI] [PubMed] [Google Scholar]
- 23.Lamszus K, Kluwe L, Matschke J, Meissner H, Laas R, Westphal M. Allelic losses at 1p, 9q, 10q, 14q, and 22q in the progression of aggressive meningiomas and undifferentiated meningeal sarcomas. Cancer Genet Cytogenet. 1999;110:103–110. doi: 10.1016/s0165-4608(98)00209-x. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Gines C, Cerda-Nicolas M, Gil-Benso R, et al. Association of loss of 1p and alterations of chromosome 14 in meningioma progression. Cancer Genet Cytogenet. 2004;148:123–128. doi: 10.1016/s0165-4608(03)00279-6. [DOI] [PubMed] [Google Scholar]
- 25.Menon AG, Rutter JL, von Sattel JP, et al. Frequent loss of chromosome 14 in atypical and malignant meningioma: identification of a putative “tumor progression” locus. Oncogene. 1997;14:611–616. doi: 10.1038/sj.onc.1200853. [DOI] [PubMed] [Google Scholar]
- 26.Muller P, Henn W, Niedermayer I, et al. Deletion of chromosome 1p and loss of expression of alkaline phosphatase indicate progression of meningiomas. Clin Cancer Res. 1999;5:3569–3577. [PubMed] [Google Scholar]
- 27.Murakami M, Hashimoto N, Takahashi Y, Hosokawa Y, Inazawa J, Mineura K. A consistent region of deletion on 1p36 in meningiomas: identification and relation to malignant progression. Cancer Genet Cytogenet. 2003;140:99–106. doi: 10.1016/s0165-4608(02)00653-2. [DOI] [PubMed] [Google Scholar]
- 28.Schneider BF, Shashi V, Kapherr C, Golden WL. Loss of chromosomes 22 and 14 in the malignant progression of meningiomas. A comparative study of fluorescence in situ hybridization (FISH) and standard cytogenetic analysis. Cancer Genet Cytogenet. 1995;85:101–104. doi: 10.1016/0165-4608(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 29.Simon M, von Deimling A, Larson JJ, et al. Allelic losses on chromosomes 14, 10, and 1 in atypical and malignant meningiomas: a genetic model of meningioma progression. Cancer Res. 1995;55:4696–4701. [PubMed] [Google Scholar]
- 30.Tabernero MD, Espinosa AB, Maillo A, et al. Characterization of chromosome 14 abnormalities by interphase in situ hybridization and comparative genomic hybridization in 124 meningiomas: correlation with clinical, histopathologic, and prognostic features. Am J Clin Pathol. 2005;123:744–751. [PubMed] [Google Scholar]
- 31.Tse JY, Ng HK, Lau KM, Lo KW, Poon WS, Huang DP. Loss of heterozygosity of chromosome 14q in low- and high-grade meningiomas. Hum Pathol. 1997;28:779–785. doi: 10.1016/s0046-8177(97)90149-0. [DOI] [PubMed] [Google Scholar]
- 32.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 33.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maillo A, Diaz P, Sayagues JM, et al. Gains of chromosome 22 by fluorescence in situ hybridization in the context of an hyperdiploid karyotype are associated with aggressive clinical features in meningioma patients. Cancer. 2001;92:377–385. doi: 10.1002/1097-0142(20010715)92:2<377::aid-cncr1333>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.Sayagues JM, Tabernero MD, Maillo A, et al. Incidence of numerical chromosome aberrations in meningioma tumors as revealed by fluorescence in situ hybridization using 10 chromosome-specific probes. Cytometry. 2002;50:153–159. doi: 10.1002/cyto.10075. [DOI] [PubMed] [Google Scholar]
- 36.Espinosa AB, Tabernero MD, Maillo A, et al. The cytogenetic relationship between primary and recurrent meningiomas points to the need for new treatment strategies in cases at high risk of relapse. Clin Cancer Res. 2006;12:772–780. doi: 10.1158/1078-0432.CCR-05-1480. [DOI] [PubMed] [Google Scholar]
- 37.Jaaskelainen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233–242. doi: 10.1016/0090-3019(86)90233-8. [DOI] [PubMed] [Google Scholar]
- 38.Mahmood A, Caccamo DV, Tomecek FJ, Malik GM. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. 1993;33:955–963. doi: 10.1227/00006123-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51–56. doi: 10.3171/jns.1983.58.1.0051. [DOI] [PubMed] [Google Scholar]
- 40.Borovich B, Doron Y, Braun J, et al. Recurrence of intracranial meningiomas: the role played by regional multicentricity. Part 2: clinical and radiological aspects. J Neurosurg. 1986;65:168–171. doi: 10.3171/jns.1986.65.2.0168. [DOI] [PubMed] [Google Scholar]
- 41.Yao YT. Clinicopathologic analysis of 615 cases of meningioma with special reference to recurrence. J Formos Med Assoc. 1994;93:145–152. [PubMed] [Google Scholar]
- 42.Gupta PK, Sastry Kolluri VR, Das S, et al. Recurrences in meningioma after surgery. Acta Neurochir (Wien) 1989;100:104–107. doi: 10.1007/BF01403594. [DOI] [PubMed] [Google Scholar]
- 43.Leone PE, Mendiola M, Alonso J, Mino C, Pestana A. Implications of a RAD54L polymorphism (2290C/T) in human meningiomas as a risk factor and/or a genetic marker. BMC Cancer. 2003;3:6. doi: 10.1186/1471-2407-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montriwiwatchai P, Kasantikul V, Taecholarn C. Clinicopathological features predicting recurrence of intracranial meningiomas. J Med Assoc Thai. 1997;80:473–478. [PubMed] [Google Scholar]
- 45.Al Saadi A, Latimer F, Madercic M, Robbins T. Cytogenetic studies of human brain tumors and their clinical significance. II. Meningioma. Cancer Genet Cytogenet. 1987;26:127–141. doi: 10.1016/0165-4608(87)90140-3. [DOI] [PubMed] [Google Scholar]
- 46.Collins VP, Nordenskjold M, Dumanski JP. The molecular genetics of meningiomas. Brain Pathol. 1990;1:19–24. doi: 10.1111/j.1750-3639.1990.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 47.Katsuyama J, Papenhausen PR, Herz F, Gazivoda P, Hirano A, Koss LG. Chromosome abnormalities in meningiomas. Cancer Genet Cytogenet. 1986;22:63–68. doi: 10.1016/0165-4608(86)90138-x. [DOI] [PubMed] [Google Scholar]
- 48.Scholz M, Gottschalk J, Striepecke E, Firsching R, Harders A, Fuzesi L. Intratumorous heterogeneity of chromosome 10 and 17 in meningiomas using non-radioactive in situ hybridization. J Neurosurg Sci. 1996;40:17–23. [PubMed] [Google Scholar]
- 49.Zattara-Cannoni H, Gambarelli D, Dufour H, et al. Contribution of cytogenetics and FISH in the diagnosis of meningiomas. A study of 189 tumors. Ann Genet. 1998;41:164–175. [PubMed] [Google Scholar]
- 50.Sayagues JM, Tabernero MD, Maillo A, et al. Intratumoral patterns of clonal evolution in meningiomas as defined by multicolor interphase fluorescence in situ hybridization (FISH): is there a relationship between histopathologically benign and atypical/anaplastic lesions? J Mol Diagn. 2004;6:316–325. doi: 10.1016/S1525-1578(10)60527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bostrom J, Meyer-Puttlitz B, Wolter M, et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159:661–669. doi: 10.1016/S0002-9440(10)61737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leuraud P, Marie Y, Robin E, et al. Frequent loss of 1p32 region but no mutation of the p18 tumor suppressor gene in meningiomas. J Neurooncol. 2000;50:207–213. doi: 10.1023/a:1006400723490. [DOI] [PubMed] [Google Scholar]
- 53.Zhu J, Guo SZ, Beggs AH, et al. Microsatellite instability analysis of primary human brain tumors. Oncogene. 1996;12:1417–1423. [PubMed] [Google Scholar]
- 54.Leone PE, Bello MJ, de Campos JM, et al. NF2 gene mutations and allelic status of 1p, 14q and 22q in sporadic meningiomas. Oncogene. 1999;18:2231–2239. doi: 10.1038/sj.onc.1202531. [DOI] [PubMed] [Google Scholar]
- 55.Weber RG, Bostrom J, Wolter M, et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci U S A. 1997;94:14719–14724. doi: 10.1073/pnas.94.26.14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lusis EA, Watson MA, Chicoine MR, et al. Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res. 2005;65:7121–7126. doi: 10.1158/0008-5472.CAN-05-0043. [DOI] [PubMed] [Google Scholar]