Abstract
Indications for the use of radiotherapy in the management of a variety of benign intracranial neoplastic and nonneoplastic pathologies are increasing. Although the short-term risks are minimal, the long-term risks of radiation-induced de novo secondary neoplasms or malignant progression of the primary benign tumor need to be considered. There are currently 19 reported cases of tumors linked with stereotactic radiotherapy/radiosurgery, to which we add our second institutional experience of a patient who succumbed to a glioblastoma multiforme (GBM) after stereotactic radiotherapy for an acoustic neuroma (AN). Review of these 20 cases revealed 10 de novo secondary tumors, of which eight were malignant, with six being malignant gliomas. The majority of the cases (14 of 20) involved AN, with most being in patients with neurofibromatosis-2 (NF2; 8 of 14), reflecting the large numbers and long-term use of radiotherapy for AN. Accelerated growth of the primary benign AN, some 2 to 6 years after focused radiotherapy, was found in six of eight NF2 patients, with pathological verification of a malignant nerve sheath tumor documented in most. The exact carcinogenic risk after radiotherapy is unknown but likely extremely low. However, the risk is not zero and requires discussion with the patient, with specific consideration in young patients and those with a cancer predisposition.
Keywords: acoustic neuroma, neurofibromatosis-2, radiation-induced GBM, radiosurgery
Focused radiotherapy in the form of stereotactic radiosurgery (SRS) or stereotactic fractionated radiotherapy (SFRT) has become a primary or adjunct treatment modality for an increasing number of benign intracranial pathologies. SRS is defined as the delivery of a single large dose of radiation to an intracranial target utilizing stereotactic guidance. SFRT is a technique where, using stereotactic guidance and a relocatable frame, repeated small doses of radiation are delivered to an intracranial target. The number of fractions may vary from 5 to 30, and this type of treatment is delivered most often using the linear accelerator. The indications and uses of either approach have a large overlap, but generally SFRT is preferred if the target is adjacent to a critical normal structure or is larger than 3 cm.
The minimal up-front risks of focused radiotherapy, especially compared to surgery, are highly attractive. The long-term benign risks of radiation-induced neuropathy, vasculopathy, and CNS necrosis are acknowledged. These risks are usually minimal and often delayed. However, the potential long-term carcinogenic risks of focused radiotherapy, especially those associated with development of secondary de novo malignant tumors or malignant conversion of a benign primary, are of concern. Although a significant literature exists on carcinogenic risks of conventional radiation,1–9 similar risks after focused radiotherapy have been considered unlikely by many who regularly use this treatment modality. Cited reasons why the carcinogenic risks are theoretically low after focused versus conventional radiotherapy include that the small irradiated perilesional normal structures receive extremely low-dose scatter radiation, and that the targeted lesion would receive such a high radiation dose that it is cytotoxic, rather than allowing survival of mutagenized and potentially transformed cells.10
However, as focused radiotherapy enters its fourth decade, with increased use and increase in potential indications, the comment by Heros and Korosue11 regarding the carcinogenic risks of focused radiotherapy for arteriovenous malformations (AVMs), that “this should always be a source of concern when radiation is used to treat conditions compatible with prolonged survival,” is bearing true. Our review of the literature yielded 19 cases of malignant progression or secondary radiation-induced tumors after focused radiotherapy for benign intracranial pathologies (Table 1). The vast majority of these tumors occurred in patients being treated for acoustic neuromas (AN), with most of the secondary tumors being malignant in nature. This report represents our second single-institutional experience of a patient who succumbed to a glioblastoma multiforme (GBM), which fulfills all of Cahan’s criteria for radiation-induced tumors,12 after focused radiotherapy for an AN. Adapted criteria from Cahan’s original report include the following: the new tumor must arise within the previously radiated tissue, must be histologically distinct from the prior tumor, and must arise at least several years following the prior course of radiotherapy. The first patient developed GBM after SRS,13 whereas the patient described here received SFRT. We present this case to highlight the need of judicious and individualized decision toward use of focused radiotherapy, in consultation with the patient.
Table 1.
Reported cases of de novo secondary or malignant progression of primary tumor, after focused radiotherapy
| Case Type and No. | Reference | SRS or Age/Sex | Primary SRT | Years to Tumor/Lesion | Secondary Secondary | Tumor |
|---|---|---|---|---|---|---|
| De Novo Secondary Malignant Tumor | ||||||
| 1 | Yu et al. 200022 | 57/F | RS-GK | Meningioma | 7 | GBM |
| 2 | Shamisa et al. 200113 | 57/F | RS-GK | Acoustic neuroma (NF2−) | 7.5 | GBM |
| 3 | Kaido et al. 200117 | 14/M | RS-GK | Arteriovenous malformation | 6.25 | GBM |
| 4 | Salvati et al., 1992, 20037,8 | 79/F | RS-GK | Cavernous angioma | 13 | GBM |
| 5 | Baser et al. 200029 | N/A | RS-?? | Acoustic neuroma (NF2+) | N/A | Malignant ependymoma |
| 6 | Baser et al. 200029 | N/A | RS-?? | Acoustic neuroma (NF2+) | N/A | Malignant meningioma |
| 7 | Sanno et al. 200423 | 56/F | RS-GK | Meningioma | 5 | Malignant osteosarcoma |
| De Novo Secondary Benign Tumor | ||||||
| 8 | Loeffler et al. 200310 | 41/M | Proton RS | Pituitary | 16 | Meningioma |
| 9 | Loeffler et al. 200310 | 53/M | Proton RS | Pituitary | 19 | Acoustic neuroma |
| Malignant Progression of Primary Tumor: Verified Pathology | ||||||
| 10 | Shin et al. 200219 | 26/F | RS-GK | Acoustic neuroma (NF2−) | 6 | Malignant nerve sheath tumor |
| 11 | Baser et al. 200029 | N/A | RS-?? | Acoustic neuroma (NF2+) | N/A | Malignant nerve sheath tumor |
| 12 | Baser et al. 200029 | N/A | RS-?? | Acoustic neuroma (NF2+) | N/A | Malignant nerve sheath tumor |
| 13 | Baser et al. 200029 | N/A | RS-?? | Acoustic neuroma (NF2+) | N/A | Malignant nerve sheath tumor |
| 14 | Bari et al. 200214 | 28/F | RS-GK | Acoustic neuroma (NF2+) | 4 | Malignant nerve sheath tumor |
| 15 | Shin et al.19 | 26/F | RS-?? | Acoustic neuroma (NF2−) | 3 | Malignant nerve sheath tumor |
| 16 | Comey et al. 199815 | 50/M | RS-GK | Acoustic neuroma (NF2−) | 5 | Triton |
| 17 | Thomsen et al. 200020 | 19/F | RS-GK | Acoustic neuroma (NF2+) | 6 | Meningiosarcoma |
| Malignant Progression of Primary Tumor: Unverified Pathology | ||||||
| 18 | McEvoy and Kitchen 200318 | 22/M | RS-GK | Acoustic neuroma (NF2+) | 2 | Rapid growth |
| 19 | Hanabusa et al. 200116 | 57/F | RS-GK | Acoustic neuroma (NF2−) | 0.5 | Rapid growth |
Abbreviations: SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy; GBM, glioblastoma multiforme, RS-GK, radiosurgery–Gamma Knife; NF2, neurofibromatosis-2; RS-??, type of radiosurgery not stated; N/A, data not available.
Case History
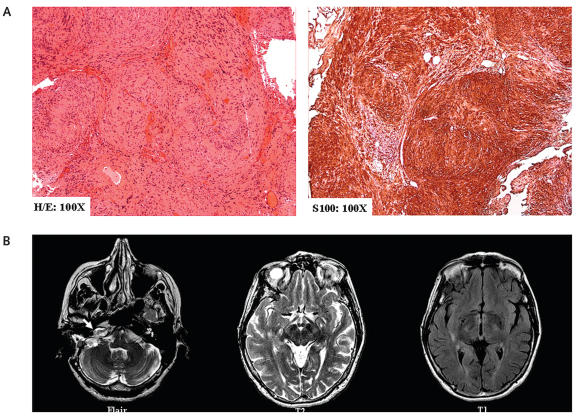
A 60-year-old previously healthy woman, without any significant family history of cancer, presented with progressive right sensorineural hearing loss. She was diagnosed with a 3-cm right cerebellopontine angle mass, consistent with the diagnosis of AN. A translabyrinthine radical but subtotal resection was undertaken, with a small residual amount of the schwannoma (Fig. 1A) left in the anterior portion of the porus acousticus to minimize risk of seventh cranial nerve palsy. Follow-up imaging did not show any residual schwannoma until approximately 2 years postsurgery, when a 1.8-cm recurrent AN was detected on MRI. Although the recurrent AN remained stable on follow-up MRI for approximately an additional 2 years, the patient subsequently insisted upon active treatment and underwent SFRT with 25 fractions for a total dose of 50 Gy. Follow-up MRI for the next approximately 4 years did not reveal any alteration in the recurrent AN, or any other intracranial lesion (Fig. 1B).
Fig. 1.
(A) Hematoxylin and eosin (H/E) and S100 immunohistochemistry demonstrated classical features of benign schwannoma at initial translabyrinthine resection. A small amount of tumor was left carpeted on the seventh cranial nerve. (B) Two years after translabyrinthine resection: axial T2- and fluid-attenuated inversion recovery–weighted images demonstrated an approximately 1.8-cm recurrent right acoustic neuroma (white arrow). There were no T2 or T1 abnormalities in the adjacent right temporal lobe.
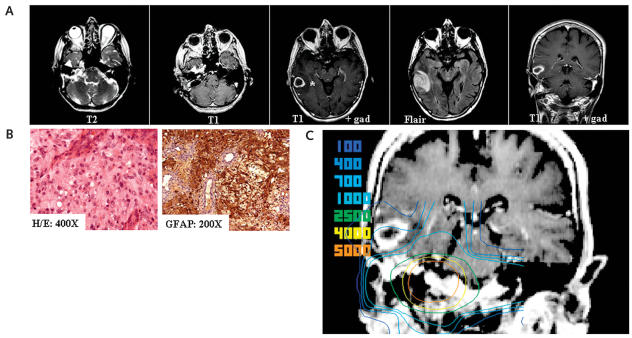
About 9 years postdiagnosis of AN (5 years after SFRT), the patient was diagnosed with breast carcinoma. Routine metastatic surveillance MRI of the brain revealed a new 1.5-cm asymptomatic right temporal lobe lesion on fluid-attenuated inversion recovery images. Repeat MRI in 2 weeks demonstrated progression of the lesion, leading to excision biopsy and pathological verification of a GBM (Fig. 2A,B).
Fig. 2.
(A) Nine years after translabyrinthine resection (5 years after stereotactic radiotherapy [SRT]): T2 and T1 gadolinium-enhanced axial MR images demonstrated the unchanged recurrent, approximately 1.8-cm acoustic neuroma (white arrowhead). However, there was a new right temporal ring-enhancing lesion (*) with perilesional edema (T1 plus gadolinium [gad] and fluid-attenuated inversion recovery (Flair)–weighted axial and coronal MR images). (B) Hematoxylin and eosin and glial fibrillary acidic protein (GFAP) immunohistochemistry of right temporal lesion, demonstrating features of a glioblastoma multiforme (GBM). (C) Fusion of SRT isodose curves to T1 gadolinium-enhanced coronal MR image of the de novo GBM, approximately 5 years after SRT. GBM volume, 4.82 cm3; minimum dose, 145 cGy; maximum dose, 694 cGy; mean dose, 446 cGy.
The MRI demonstrating the occurrence of the glioblastoma was image fused with the original MRI on which the patient’s radiation distribution was calculated for the treatment of her AN. The glioblastoma was contoured, and a dose volume histogram was plotted that demonstrated the dose distribution in the region where the glioblastoma arose. The mean radiation dose received to the right temporal lobe from her stereotactic radiotherapy, at the site of the de novo GBM, was 4.46 Gy (minimum dose, 1.45 Gy; maximum dose, 6.94 Gy; Fig. 2C). The patient went on to receive additional conventional radiation to the right temporal lobe (30 Gy in 10 fractions) but succumbed to her GBM approximately 4 months after surgery, almost 10 years after initial diagnosis of her AN.
Discussion
This report is the tenth case of a de novo tumor and sixth malignant glioma after SRS or SFRT (Table 1).13–23 An additional case report of a de novo anaplastic astrocytoma occurring 64 months after radiosurgery for metastatic melanoma was not included,24 because we restricted this review to cases where the primary indication for focused radiotherapy was a benign CNS pathology. Like our previous institutional report of a GBM after SRS for AN,13 the present case also fulfills all of Cahan’s criteria of radiation-induced secondary tumor.12 In view of the lack of systematic reporting of all possible radiation-induced malignancies following prior focused radiotherapy, and the lack of knowledge of the total number of patients treated with either SRS or SFRT, it is currently impossible to make an accurate assessment of the magnitude of the risk of radiation-induced malignancy in these populations.
The radiation dose to the right temporal lobe site of the GBM was low (mean, 4.46 Gy; Fig. 2C); however, the minimal carcinogenic dose to the brain, if one exists at all, has not been firmly established. Radiation doses as low as 1 Gy have been associated with an increased relative risk of a secondary tumor of 1.57–8.75, a figure that increases to 18.4 after an interval of 20–25 years.25 In support, long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for taenia capitis estimated the mean radiation dose delivered to the brain to be 1.5 Gy.6 Experimental evidence also suggests that low nonlethal-dose radiation is more carcinogenic than higher dose radiation. Nondividing primary human fibroblasts were not able to repair DNA double-strand breaks (DSBs) resulting from very low radiation (1 mGy) as efficiently as those resulting from higher radiation doses.26 Normally, these cells harboring DNA DSBs do not proliferate due to G1 cell-cycle arrest, secondary to p53 induction by the low-dose radiation. However, additional genetic alterations or environmental proliferative signals, which result in escape from G1 cell-cycle arrest, would make these cells receiving low- dose radiation and harboring DNA DSBs more prone to transformation.27,28
Among the 20 cases, it is not surprising that 14 of the primary tumors were ANs (Table 1). Cumulative data published on patients treated with the Gamma Knife until December 2005 for North and South America at the Leksell Gamma Knife Society Website (www.elekta.com/site_dbase.php?form_link_id=1090859) indicate that approximately 30% of the indications have been for benign tumors, with ANs accounting for almost one-third of these cases (~9,000 ANs treated by Gamma Knife). This represents only the Americas and does not include the rest of the world and, of course, does not include patients treated with linear accelerator technology. Of most interest, it is striking that 8 of 14 of these cases of AN, with carcinogenic complications associated with focused radiotherapy, were in the setting of neurofibromatosis-2 (NF2), a tumor predisposition syndrome that contributes to less than 5% of all ANs. Although de novo malignant gliomas did arise in these NF2 ANs, in six of eight cases there was progression to a malignant nerve sheath tumor, which was verified at second surgery in five cases and presumed in one case by rapid radiological growth of the AN, which led to the patient’s death. The risk for malignant progression in NF2 ANs resulting from radiosurgery has not been formally examined; however, a survey accompanying the report of three cases of malignant nerve sheath tumors in NF2 ANs, which had initially been managed by focused radiotherapy29 (Table 1), is of interest. The survey estimated the incidence of malignant nerve sheath tumors in nonradiated NF2 AN to be less than 0.5%, compared to a 6% incidence in NF2 ANs that had received focused radiotherapy, suggesting a 12-fold increased radiotherapy-linked risk.29 Similarly, a suggestion of increased susceptibility to radiation-induced carcinogenesis and loss of the NF2 gene was suggested by Plowman et al.30 In strict adherence to Cahan’s criteria,12 one may argue that the existence of a primary AN precludes the involvement of radiation induction in these cases of a malignant nerve sheath tumor; however, progression to malignant nerve sheath tumor occurs extremely rarely, if ever, in sporadic or NF2-associated peripheral schwannoma or AN.21 This is in contrast to plexiform neurofibromas in neurofibromatosis-1 (NF1) patients, where sporadic malignant progression is well recognized, with an approximately 10%–15% lifetime risk.31
The pathological alterations associated with AN following focused radiotherapy, although reported, are not clear.32–34 The requirement for surgery is not usually due to malignant transformation, but rather necessitated by continued growth, peritumoral swelling, and increased cyst formation, all resulting in local mass effect on the brainstem and/or hydrocephalus.32 Accompanying pathological differences compared to nonradiated ANs, other than those expected from the radiation effects on the tumor microvasculature, are scant.32 Limited studies have suggested an increase in the proliferative index with accompanying microsatellite instability in a minority of these postradiosurgery ANs,33,34 but not enough to diagnose conversion to a malignant nerve sheath tumor. Lack of precise clinical-pathological-molecular data makes the need for additional evidence from in vitro and in vivo models paramount. Unfortunately, these preclinical experiments have not been thoroughly undertaken for NF2, unlike the much more common NF1 syndrome. Deletion of both copies of the Nf2 or Nf1 gene in mice is embryonic lethal, whereas heterozygous mice for both genes are viable but develop tumors other than those associated with the respective syndromes at a higher rate than do normal control mice.35 Carcinogenic susceptibility with Nf2 heterozygous mice is suggested by double transgenics of Nf2 heterozygous and p53 mutant mice, which do develop malignant tumors of neural crest origin.36 Susceptibility to radiation- and chemotherapy-induced carcinogenesis has been demonstrated in Nf1 heterozygotes, in keeping with clinical observations,37 but similar, much-needed studies are lacking with the Nf2 heterozygous mice or NF2 null schwannomas or derivative Schwann cell cultures.
Although lacking definitive clinical or substantial preclinical data, the available data are highly supportive of caution in the use of focused radiotherapy in management of NF2 ANs. In addition to their germline tumor predisposition, NF2-associated ANs occur at a much earlier age, thereby cumulating the radiation carcinogenic risks over a longer period of time. NF2 patients do pose a difficult multifaceted management challenge, not only from their bilateral ANs and other multiple tumors, but also from critical issues regarding hearing and other cranial nerve preservation. One is therefore tempted to justify managing these NF2 ANs with focused radiotherapy, due to minimal short-term risks. However, we should be cognizant of the potential long-term carcinogenic risks, and perhaps observation for those NF2 patients with functional hearing and no clinically significant brainstem compression is prudent. For young NF2 patients with clinically significant mass effect from their AN, most of whom are functionally deaf in that side, microneurosurgical removal, even if subtotal to minimize risks on seventh cranial nerve, may be more desirable to avoid the long-term radiation risks.
In conclusion, our present case and those reported in the literature to date demonstrate that there is a definitive and increasing incidence of de novo malignant gliomas and malignant progression of ANs after focused radiotherapy using either SRS or SFRT for benign intracranial pathologies. This risk is extremely low, given the total number of cases of focused radiotherapy undertaken to date, and certainly should play a small role in our recommendation to the select group of patients where the surgical risks are high or natural life expectancy is relatively short. The primary lesion and associated risks for radiation-induced carcinogenesis are probably factors, but likely this risk is not restricted to ANs, with the increased representation of these cases reflecting our use and experience with focused radiotherapy to date. Although among the 20 cases, two cases involve a primary vascular malformation, reviews by centers with greater experience of treating AVMs with focused radiotherapy are highly suggestive that the risks in these patients may be even smaller than in AN patients.38,39 What the risks are with other benign pathologies, such as trigeminal neuralgia and seizures, needs to be carefully monitored and studied at the very least, as we increase accrual of these cases. Given this uncertainty, we still should practice caution using this treatment modality, especially in young patients and those with tumor predisposition syndromes, because certainly the patient will not benefit by exchanging a benign pathology for a malignant one. One must remember that despite development of instrumentation and refinement of delivery, the final emission of focused radiotherapy is ionizing radiation, a well-recognized carcinogen. Like other management options, it needs to be used judiciously with a thorough risk-benefit discussion with our patients.
Acknowledgments
A.B. was supported by an Arthur and Sonia Labatt Brain Tumor Center research fellowship.
References
- 1.Anderson JR, Treip CS. Radiation-induced intracranial neoplasms. A report of three possible cases. Cancer. 1984;53:426–429. doi: 10.1002/1097-0142(19840201)53:3<426::aid-cncr2820530310>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein M, Laperriere LN. Radiation-Induced Tumors of the Nervous System. New York: Raven Press; 1991. [Google Scholar]
- 3.Brada M, Ford D, Ashley S, et al. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. Br Med J. 1992;304:1343–1346. doi: 10.1136/bmj.304.6838.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar PP, Good RR, Skultety FM, Leibrock LG, Severson GS. Radiation- induced neoplasms of the brain. Cancer. 1987;59:1274–1282. doi: 10.1002/1097-0142(19870401)59:7<1274::aid-cncr2820590708>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Liwnicz BH, Berger TS, Liwnicz RG, Aron BS. Radiation-associated gliomas: a report of four cases and analysis of postradiation tumors of the central nervous system. Neurosurgery. 1985;17:436–445. doi: 10.1227/00006123-198509000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163:424–432. doi: 10.1667/rr3329. [DOI] [PubMed] [Google Scholar]
- 7.Salvati M, Ciappetta P, Raco A, Capone R, Artico M, Santoro A. Radiation-induced schwannomas of the neuraxis. Report of three cases. Tumori. 1992;78:143–146. doi: 10.1177/030089169207800217. [DOI] [PubMed] [Google Scholar]
- 8.Salvati M, Frati A, Russo N, et al. Radiation-induced gliomas: report of 10 cases and review of the literature. Surg Neurol. 2003;60:60–67. doi: 10.1016/s0090-3019(03)00137-x. [DOI] [PubMed] [Google Scholar]
- 9.Tsang RW, Laperriere NJ, Simpson WJ, Brierley J, Panzarella T, Smyth HS. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer. 1993;72:2227–2233. doi: 10.1002/1097-0142(19931001)72:7<2227::aid-cncr2820720727>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Loeffler JS, Niemierko A, Chapman PH. Second tumors after radiosurgery: tip of the iceberg or a bump in the road? Neurosurgery. 2003;52:1436–1432. doi: 10.1227/01.neu.0000064809.59806.e8. [DOI] [PubMed] [Google Scholar]
- 11.Heros R, Korosue K. Radiation treatment of cerebral arteriovenous malformations. N Engl J Med. 1990;323:127–129. doi: 10.1056/NEJM199007123230211. [DOI] [PubMed] [Google Scholar]
- 12.Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone: report of eleven cases. Cancer. 1998;82:8–34. doi: 10.1002/(sici)1097-0142(19980101)82:1<8::aid-cncr3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Shamisa A, Bance M, Nag S, et al. Glioblastoma multiforme occurring in a patient treated with Gamma Knife surgery. Case report and review of the literature. J Neurosurg. 2001;94:816–821. doi: 10.3171/jns.2001.94.5.0816. [DOI] [PubMed] [Google Scholar]
- 14.Bari ME, Forster DM, Kemeny AA, Walton L, Hardy D, Anderson JR. Malignancy in a vestibular schwannoma. Report of a case with central neurofibromatosis, treated by both stereotactic radiosurgery and surgical excision, with a review of the literature. Br J Neurosurg. 2002;16:284–289. doi: 10.1080/02688690220148888. [DOI] [PubMed] [Google Scholar]
- 15.Comey CH, McLaughlin MR, Jho HD, Martinez AJ, Lunsford LD. Death from a malignant cerebellopontine angle triton tumor despite stereotactic radiosurgery. Case report. J Neurosurg. 1998;89:653–658. doi: 10.3171/jns.1998.89.4.0653. [DOI] [PubMed] [Google Scholar]
- 16.Hanabusa K, Morikawa A, Murata T, Taki W. Acoustic neuroma with malignant transformation. Case report. J Neurosurg. 2001;95:518–521. doi: 10.3171/jns.2001.95.3.0518. [DOI] [PubMed] [Google Scholar]
- 17.Kaido T, Hoshida T, Uranishi R, et al. Radiosurgery-induced brain tumor. Case report. J Neurosurg. 2001;95:710–713. doi: 10.3171/jns.2001.95.4.0710. [DOI] [PubMed] [Google Scholar]
- 18.McEvoy AW, Kitchen ND. Rapid enlargement of a vestibular schwannoma following Gamma Knife treatment. Minim Invasive Neurosurg. 2003;46:254–256. doi: 10.1055/s-2003-42347. [DOI] [PubMed] [Google Scholar]
- 19.Shin M, Ueki K, Kurita H, Kirino T. Malignant transformation of a vestibular schwannoma after Gamma Knife radiosurgery. Lancet. 2002;360:309–310. doi: 10.1016/S0140-6736(02)09521-1. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen J, Mirz F, Wetke R, Astrup J, Bojsen-Moller M, Nielsen E. Intracranial sarcoma in a patient with neurofibromatosis type 2 treated with Gamma Knife radiosurgery for vestibular schwannoma. Am J Otol. 2000;21:364–370. doi: 10.1016/s0196-0709(00)80046-0. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson JS, Reid H, Armstrong GR. Malignant transformation of a recurrent vestibular schwannoma. J Clin Pathol. 2004;57:109–110. doi: 10.1136/jcp.57.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu JS, Yong WH, Wilson D, Black KL. Glioblastoma induction after radiosurgery for meningioma. Lancet. 2000;356:1576–1577. doi: 10.1016/S0140-6736(00)03134-2. [DOI] [PubMed] [Google Scholar]
- 23.Sanno N, Hayashi S, Shimura T, Maeda S, Teramoto A. Intracranial osteosarcoma after radiosurgery—case report. Neurol Med Chir (Tokyo) 2004;44:29–32. doi: 10.2176/nmc.44.29. [DOI] [PubMed] [Google Scholar]
- 24.McIver JI, Pollock BE. Radiation-induced tumor after stereotactic radiosurgery and whole brain radiotherapy: case report and literature review. J Neurooncol. 2004;66:301–305. doi: 10.1023/b:neon.0000014497.28981.4b. [DOI] [PubMed] [Google Scholar]
- 25.Muracciole X, Cowen D, Regis J. Radiosurgery and brain radio-induced carcinogenesis: update. Neurochirurgie. 2004;50:414–420. [PubMed] [Google Scholar]
- 26.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc Natl Acad Sci U S A. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–942. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Syljuasen RG, Krolewski B, Little JB. Loss of normal G1 checkpoint control is an early step in carcinogenesis, independent of p53 status. Cancer Res. 1999;59:1008–1014. [PubMed] [Google Scholar]
- 29.Baser ME, Evans DG, Jackler RK, Sujansky E, Rubenstein A. Neurofibromatosis. 2, radiosurgery and malignant nervous system tumours. Br J Cancer. 2000;82:998. doi: 10.1054/bjoc.1999.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plowman PN, Evans DG. Stereotactic radiosurgery XI. Acoustic neuroma therapy and radiation oncogenesis. Br J Neurosurg. 2000;14:93–95. doi: 10.1080/02688690050004480. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335–349. doi: 10.1023/b:neon.0000041891.39474.cb. [DOI] [PubMed] [Google Scholar]
- 32.Kwon Y, Khang SK, Kim CJ, Lee DJ, Lee JK, Kwun BD. Radiologic and histopathologic changes after Gamma Knife radiosurgery for acoustic schwannoma. Stereotact Funct Neurosurg. 1999;72(suppl 1):2–10. doi: 10.1159/000056433. [DOI] [PubMed] [Google Scholar]
- 33.Lee DJ, Maseyesva B, Westra W, Long D, Niparko JK, Califano J. Microsatellite analysis of recurrent vestibular schwannoma (acoustic neuroma) following stereotactic radiosurgery. Otol Neurotol. 2006;27:213–219. doi: 10.1097/01.mao.0000199753.44191.73. [DOI] [PubMed] [Google Scholar]
- 34.Lee F, Linthicum F, Jr, Hung G. Proliferation potential in recurrent acoustic schwannoma following Gamma Knife radiosurgery versus microsurgery. Laryngoscope. 2002;112:948–950. doi: 10.1097/00005537-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Gutmann DH, Giovannini M. Mouse models of neurofibromatosis 1 and 2. Neoplasia. 2002;4:279–290. doi: 10.1038/sj.neo.7900249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robanus-Maandag E, Giovannini M, van der Valk M, et al. Synergy of Nf2 and p53 mutations in development of malignant tumours of neural crest origin. Oncogene. 2004;23:6541–6547. doi: 10.1038/sj.onc.1207858. [DOI] [PubMed] [Google Scholar]
- 37.Meadows AT. A mouse model for studying therapy-induced cancers. Cancer Cell. 2005;8:271–273. doi: 10.1016/j.ccr.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Friedman WA, Bova FJ, Bollampally S, Bradshaw P. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296–298. doi: 10.1227/01.neu.0000043692.51385.91. [DOI] [PubMed] [Google Scholar]
- 39.Pollock BE, Gorman DA, Coffey RJ. Patient outcomes after arteriovenous malformation radiosurgical management: results based on a 5- to 14-year follow-up study. Neurosurgery. 2003;52:1291–1297. doi: 10.1227/01.neu.0000064800.26214.fe. [DOI] [PubMed] [Google Scholar]