Abstract
Increased neurotoxicity and poor long-term neurocognitive outcome of preschool children treated for brain tumors have led to innovative therapeutic strategies in order to delay or avoid the use of craniospinal radiation and to improve survival. Because these protocols are relatively new, few data exist regarding cognitive outcome. We conducted a twin case–control study to investigate neurocognitive and behavioral outcome in a preschool patient who was 16 months old at diagnosis of medulloblastoma and was treated with surgery, chemotherapy, stem cell transplant, and focal radiation to the tumor bed. Stability and change over two assessments were compared for the patient and her nonaffected twin for standardized measures of cognitive function and experimental measures of parent–child interaction, social competence, and goal-directed play. A striking finding was improvement in intelligence, receptive language, and visual-motor functioning in the affected twin from 12 months to 24 months after treatment. Improvement in ratings of parent–child interaction and social competence for the affected twin was also evident. These findings are notable compared with the potentially devastating impact of craniospinal tumor, and this study is among the first to document the relative benefit of focal radiation in sparing cognitive function, albeit in a single case study.
Keywords: focal radiotherapy, medulloblastoma, neurocognitive outcome, preschool children
The majority of brain tumors in preschool children are aggressive neoplasms with a propensity for widespread neuraxis dissemination, which often require radiotherapy for control.1–3 However, young children with brain tumors are vulnerable to significant neurotoxicity and intellectual impairment after treatment with craniospinal radiation,1,4–9 and attempts have been made to avoid this therapy.1,8,9 Contemporary treatment studies for preschool children increasingly rely on primary postoperative chemotherapy intended to delay or eliminate the need for radiation.10–13 Although improved intellectual outcome has been documented for survivors treated without radiation,7,12 protocols have demonstrated variable efficacy for disease control when treatment involved chemotherapy only, particularly for children with postoperative residual and/or disseminated disease. Hence, some protocols have included second-look surgery, high-dose chemotherapy, stem cell transplant, and focal radiation therapy in an effort to improve treatment efficacy while minimizing adverse neurocognitive late effects for patients with residual tumor or disease progression.11 Few data exist regarding the impact of focal radiation on cognitive function: In a single study, full-scale IQ was 1 standard deviation below normative means in a group treated with radiation to the posterior fossa.14 Serial and comprehensive neurocognitive evaluation is critical in this vulnerable group of young patients to evaluate the potential benefit of such new strategies in sparing cognitive function.
Comparing the cognitive functioning of twins with overlapping genetic backgrounds and comparable socioeconomic, prenatal, perinatal, and family history, but discordant for a brain tumor, can provide important preliminary information on neurocognitive late effects of focal radiation.15 Twin case–control studies have documented poor spine and upper and lower limb growth, increased levels of thyroid-stimulating hormone, and deteriorated intellectual ability in twins treated with craniospinal radiation relative to their nonaffected twin.16–18 The goal of the present twin case–control study was to serially investigate neurocognitive and behavioral outcome in a preschool girl diagnosed with medulloblastoma compared to her nonaffected twin sister, after treatment with a protocol including focal radiotherapy. If focal radiation has an adverse impact on early neurocognitive functioning, then over time the affected twin treated should demonstrate poorer performance in cognitive and behavioral function relative to her nonaffected twin.
Materials and Methods
We saw the dizygotic same-sex preschool twins for neurocognitive and behavioral assessment on two occasions: (1) 21 months after diagnosis of a posterior fossa medulloblastoma for the affected twin (and 12 months after the completion of focal posterior fossa radiation) and (2) 12 months later. The twins were 16 months of age at the time of diagnosis. The Wechsler Preschool and Primary Scale of Intelligence–Revised (WPPSI-R) was used to assess intelligence. Receptive and expressive language ability was evaluated using the Peabody Picture Vocabulary Test—Third Edition (PPVT-III) and the Preschool Language Scale (PLS). The Beery Visual-Motor Integration Test (VMI) was used to evaluate visual-motor integration. Further, well-validated and reliable experimental tests of parent–child interaction, social competency, and goal-directed play were used.19 For these videotaped observational measures, children engaged in a number of tasks with their mother, with the examiner, or by themselves, and relevant behavior was recorded. All the experimental measures predict later cognitive and social skills in young children at biological risk over and above the contributions of intelligence measures.19
Case History
Affected Twin Case History
The affected twin was born at 36 weeks by induced vaginal delivery and weighed 3 pounds 11 ounces. Because of her low birth weight, she received neonatal intensive care unit intervention, including respiratory support and tube feeding. She remained hospitalized for 2 weeks after birth. Her early developmental motor milestones (i.e., sitting alone, crawling, and walking) were all met at the expected ages; however, her early language development was delayed prior to tumor diagnosis.
At 16 months of age, the affected twin presented with a 3-week history of intermittent vomiting and irritability, progressive ataxia, and lethargy. A CT scan demonstrated a midline posterior fossa mass with marked dilation of the lateral ventricles and compression of the fourth ventricle. The tumor was incompletely resected: there was tumor invasion of the brainstem along the inferior margins of the fourth ventricle bilaterally that precluded total resection. An external ventricular drain was first inserted, and 11 days later a left ventriculoperitoneal shunt was placed to address hydrocephalus. Pathological evaluation was consistent with medulloblastoma, with tumor cytogenetics abnormal with hypertriploidy and cerebrospinal fluid positive for tumor cells. Based on these findings and combined with the presence of residual tumor, she was treated with MOPP chemotherapy (mustard IV, vincristine, procarbazine, and prednisone). Subsequent imaging revealed response to therapy, with a small residual in the area of the foramen of Luschka remaining.
In order to avoid/delay craniospinal radiation, she was treated with high-dose chemotherapy (intensification with busulfan/thiotepa) followed by autologous stem cell rescue at 20 months of age and then with 5,400 cGy/30 daily fractions via focal stereotactic intensity-modulated radiation therapy (IMRT) to the surgical cavity plus a 1.5-cm margin (Fig. 1). Although the affected twin lost many of her developmental motor milestones during treatment, she recovered all of them by 6 months posttreatment. Follow-up MRI showed no evidence of residual or recurrent disease. Both Italian and English were spoken in the home. The affected twin was 37 months old at the time of the first neurocognitive assessment and was 49 months old at second assessment. Thus, she was evaluated approximately 12 and 24 months after completion of radiotherapy.
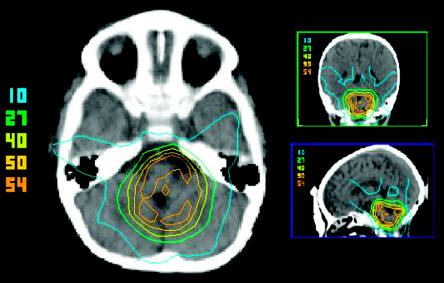
Fig. 1.
Planning CT distribution in axial, coronal, and sagittal planes for the affected twin. The dose values from the center to the periphery represent the volumes receiving 54, 50, 40, 27, and 10 Gy, respectively, in all three planes.
Nonaffected Twin Case History
The nonaffected twin was born at 36 weeks by induced vaginal delivery and weighed 4 pounds 14 ounces. She was monitored in hospital for 3 weeks after birth due to her relatively low birth weight. The nonaffected twin’s early developmental motor milestones (i.e., sitting alone, crawling, and walking) were mildly delayed; she sat at 8 months and walked at 15–18 months. She demonstrated significant speech-language delay and has been seen for developmental assessment, which documented significant expressive language delay with significant delay in sound production abilities, as well as gross motor delay. She had a tongue tie clipped at 30 months of age. Recent ophthalmological and hearing examination was normal. She was 37 and 49 months at the time of the first and second assessments, respectively.
Results
Standardized test scores for each twin are shown in Table 1. At the initial assessment, the FSIQ (full-scale intelligence quotient), VIQ (verbal intelligence quotient), and PIQ (performance intelligence quotient) for both twins were within the borderline, low-average, and borderline ranges, respectively (Table 1). Both twins demonstrated better VIQ than PIQ, with a significant difference between these indices for the nonaffected twin (p < 0.05). By the second assessment, the FSIQ for the affected twin had increased by 12 points and fell within the low average range; this increase was substantially greater than the SEM for the WPPSI-R FSIQ (Table 1). The VIQ for the affected twin was significantly higher than the PIQ (p < 0.05), although both indices were improved relative to the first assessment: VIQ was 17 points higher and fell within the average range, and PIQ was 9 points higher and fell within the low-average range. Both increases were substantially greater than the SEM for the test, indicating that improvement was not due to random fluctuations in performance over time. All IQ scores for the nonaffected twin remained stable at the second assessment, falling within the borderline to low-average range; although decreases in IQ scores were evident, these were very close to the SEM of the test and likely reflected random fluctuation in scores rather than true declines (Table 1).
Table 1.
IQ, language function, and visual-motor functioning scores for the affected and nonaffected twin at the first and second assessment (normative means = 100, SD = 15)
| Standardized Test | First Assessment | Second Assessment | Difference | Test SEM |
|---|---|---|---|---|
| WPPSI-R FSIQ | 2.82 | |||
| Affected twin | 74 | 88 | +12 | — |
| Nonaffected twin | 80 | 75 | −5 | — |
| WPPSI-R VIQ | 3.15 | |||
| Affected twin | 80 | 97 | +17 | — |
| Nonaffected twin | 88 | 84 | −4 | — |
| WPPSI-R PIQ | 4.10 | |||
| Affected twin | 72 | 81 | +9 | — |
| Nonaffected twin | 76 | 70 | −6 | — |
| PPVT-III—receptive vocabulary | 3.17 | |||
| Affected twin | 88 | 92 | +4 | — |
| Nonaffected twin | 97 | 111 | +14 | — |
| PLS—receptive language | ||||
| Affected twin | 81 | 98 | +17 | — |
| Nonaffected twin | 90 | 90 | 0 | — |
| PLS—expressive language | ||||
| Affected twin | 65 | 79 | +14 | — |
| Nonaffected twin | 73 | 70 | −3 | — |
| VMI—visual motor integration | 4 | |||
| Affected twin | 90 | 99 | +9 | — |
| Nonaffected twin | 83 | 92 | +9 | — |
Abbreviations: WPPSI-R, Wechsler Preschool and Primary Scale of Intelligence–Revised; FSIQ, full-scale intelligence quotient; VIQ, verbal intelligence quotient; PIQ, performance intelligence quotient; PPVT-III, Peabody Picture Vocabulary Test—Third Edition; PLS, Preschool Language Scale; VMI, Beery Visual-Motor Integration Test.
Variable performance in language functioning was observed for both twins (Table 1). Based on the PLS, the twins demonstrated very poor to borderline expressive language function across both assessments. The PLS index of receptive language was significantly higher than the expressive language index for both twins. The nonaffected twin demonstrated stable low-average performance on receptive language tasks across both assessments. Consistent with the pattern observed for intellectual measures, the affected twin demonstrated improvement in expressive and receptive language from the first to the second assessment. Both twins demonstrated average receptive vocabulary (PPVT-III). The nonaffected twin’s scores were higher than the affected twin’s and showed substantial improvement in relative functioning from the first to the second assessment. Visual-motor integration (VMI) improved for both twins across assessments (Table 1), from low-average to average ability. The affected twin demonstrated higher test scores compared to her sister at both time points.
Observational measures were videotaped and scored by two coders who were blind to the medical status of the twins. Consistent with the pattern observed for the standardized tests, ratings of the affected twin’s behavior in the tasks of social interaction and competence improved across assessment sessions. Ratings of the quality of social play interactions for both parent and affected twin increased from the first to second assessment. The affected twin was rated as being more responsive to and socially engaged with her mother and more engaged with the toys she was playing with and her general environment. Ratings of the mother’s interactions corresponded with this pattern, with increased levels of warmth, supportive actions, and responsiveness noted for the second assessment relative to the first. Both child and parent ratings remained the same for the nonaffected twin: the quality of interactions between the nonaffected twin and the mother were rated as higher than those with the affected twin during the first assessment, but with the improvement noted for the affected twin, ratings were essentially the same for both twins at the second assessment. The affected twin also showed improved social competence across assessments, with increased use and complexity of language and in cooperation and initiative from the first to the second assessment sessions. No increases in ratings of social competence were evident for the nonaffected twin. Finally, for independent exploratory play with novel toys, the affected twin and the nonaffected twin demonstrated similar frequencies of functional play during the first assessment. However, at the second assessment, the nonaffected twin showed increases in her level of play and engagement, and exploration of toys and her play was more advanced in terms of problem solving. In contrast, functional play in the affected twin decreased. These results are consistent with the parent report that with age the affected twin became more cautious and less likely to spontaneously engage in new activities without maternal support.
Discussion
This case study is the first to examine serial neurocognitive and behavioral outcome after treatment with focal radiation for medulloblastoma in a preschool child, relative to her twin sister. A striking finding was improvement in intelligence, language, and visual-motor functioning in the affected twin from 12 months to 24 months after treatment with focal radiation. The affected twin, for the most part, demonstrated higher standard scores than her unaffected sister, who demonstrated a normal rather than accelerated pace of development over the same time period. Further, improved functioning was also evident in ratings of parent–child interaction and social competence for the affected twin. The affected twin’s improvement likely reflected normalization of functioning after illness as her medical condition improved and she experienced increased opportunities that stimulated her behavioral and cognitive development.
Our findings are notable compared with the very poor intellectual outcome typically associated with craniospinal radiation in infants and young children. Focal posterior fossa radiation still encompasses substantial amounts of the cerebral hemispheres, including occipital and parietal lobes, thalamus, and diencephalon, and has been associated with relatively poor intellectual outcome.14 In the present case study, the affected twin was treated with IMRT, which yielded volumes restricted primarily to the tumor bed and involved dose modulation, sparing cerebral tissue from a significant amount of radiation. Hence, IMRT may be especially critical in preserving cognitive function and may be useful for addressing concerns regarding cognitive outcome in this vulnerable population.
Further, we found that measures of preschool children’s social and behavioral functioning, including parent–child interaction, social competence, and goal-directed play, are useful for demonstrating the social consequences of disease and are sensitive to improvement with normalization of function after treatment. Hence, these measures may be useful in examining outcome in larger samples of preschool patients. This would yield ecologically valid information, may be useful in predicting future cognitive and social functioning,19 and can provide important information for developing home-based interventions for young children. Comprehensive assessment of multiple areas is important because subtle deficits may emerge for patients treated with focal radiation that are not detected using only standardized cognitive tests, despite intact intellectual functioning.
Finally, our findings must be considered in the context of previous slight developmental delays for both twins, and their very poor expressive language, which may reflect the bilingual language environment in which the twins reside or may be a consequence of their birth history and low birth weight. However, the twins’ history reflects the reality that children with a range of pre-morbid backgrounds are treated for brain tumors, and the impact of treatment manifests within the context of their broader developmental functioning. Further, because of the case study design, we report qualitative descriptions of change over time. Certainly, the potential benefits of focal radiation require evaluation in larger patient series using quantitative analyses. Despite these constraints, our preliminary findings of improvement in intellectual, language, visual-motor, parent–child interaction, and social competence over time after treatment with focal posterior fossa IMRT are unique, and this study is the first to document minimal deleterious effects after treatment, albeit in a single case study.
Footnotes
Portions of these data were presented at the Eleventh International Symposium on Pediatric Neuro-Oncology, Boston, June 2004.
References
- 1.Fouladi M, Gilger E, Kocak M, et al. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J Clin Oncol. 2005;23:7152–7160. doi: 10.1200/JCO.2005.01.214. [DOI] [PubMed] [Google Scholar]
- 2.Packer RJ, Rood BR, MacDonald TJ. Medulloblastoma: present concepts of stratification into risk groups. Pediatr Neurosurg. 2003;39:60–67. doi: 10.1159/000071316. [DOI] [PubMed] [Google Scholar]
- 3.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 4.Chapman CA, Waber DP, Bernstein JH, et al. Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: role of age and perioperative factors. J Child Neurol. 1995;10:209–212. doi: 10.1177/088307389501000308. [DOI] [PubMed] [Google Scholar]
- 5.Copeland DR, deMoor C, Moore BD, III, Ater JL. Neurocognitive development of children after a cerebellar tumor in infancy: a longitudinal study. J Clin Oncol. 1999;17:3476–3486. doi: 10.1200/JCO.1999.17.11.3476. [DOI] [PubMed] [Google Scholar]
- 6.Jakacki RI, Feldman H, Jamison C, Boaz JC, Luerssen TG, Timmerman R. A pilot study of preirradiation chemotherapy and 1800 cGy cranio-spinal irradiation in young children with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;60:531–536. doi: 10.1016/j.ijrobp.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Moore BD, III, Ater JL, Copeland DR. Improved neuropsychological outcome in children with brain tumors diagnosed during infancy and treated without cranial irradiation. J Child Neurol. 1992;7:281–290. doi: 10.1177/088307389200700308. [DOI] [PubMed] [Google Scholar]
- 8.Mulhern RK, Horowitz ME, Kovnar EH, Langston J, Sanford RA, Kun LE. Neurodevelopmental status of infants and young children treated for brain tumors with preirradiation chemotherapy. J Clin Oncol. 1989;7:1660–1666. doi: 10.1200/JCO.1989.7.11.1660. [DOI] [PubMed] [Google Scholar]
- 9.Walter AW, Mulhern RK, Gajjar A, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17:3720–3728. doi: 10.1200/JCO.1999.17.12.3720. [DOI] [PubMed] [Google Scholar]
- 10.Ater JL, van Eys J, Woo SY, Moore B, III, Copeland DR, Bruner J. MOPP chemotherapy without irradiation as primary postsurgical therapy for brain tumors in infants and young children. J Neurooncol. 1997;32:243–252. doi: 10.1023/a:1005744527443. [DOI] [PubMed] [Google Scholar]
- 11.Grill J, Sainte-Rose C, Jouvet A, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6:573–580. doi: 10.1016/S1470-2045(05)70252-7. [DOI] [PubMed] [Google Scholar]
- 12.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 13.White L, Kellie S, Gray E, et al. Postoperative chemotherapy in children less than 4 years of age with malignant brain tumors: promising initial response to a VETOPEC-based regimen. A study of the Australian and New Zealand Children’s Cancer Study Group (ANZCCSG) J Pediatr Hematol Oncol. 1998;20:125–130. doi: 10.1097/00043426-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Grill J, Renaux VK, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45:137–145. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 15.Dennis M, Barnes MA. Neuropsychologic function in same-sex twins discordant for perinatal brain damage. J Dev Behav Pediatr. 1994;15:124–130. [PubMed] [Google Scholar]
- 16.Bodor F, Hakansson CH, Lindgren M. Irradiated cerebellar medulloblastoma in a monozygotic twin. Growth, neurology and chromosomes 13 years after treatment. Acta Radiol Ther Phys Biol. 1974;13:255–265. doi: 10.3109/02841867409129882. [DOI] [PubMed] [Google Scholar]
- 17.Johnston C, Nesbit M, Robison L. Neuropsychological functioning in a long-term survivor of childhood acute lymphocytic leukemia: a case study with comparison to a nonafflicted twin sibling. Dev Neuropsychol. 1986;2:113–123. [Google Scholar]
- 18.Nishiyama K, Funakoshi S, Izumoto S, Ikeda T, Oku Y. Long-term effects of radiation for medulloblastoma on intellectual and physical development. A case report of monozygotic twins. Cancer. 1994;73:2450–2455. doi: 10.1002/1097-0142(19940501)73:9<2450::aid-cncr2820730931>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Landry SH, Smith KE, Swank PR, Miller-Loncar CL. Early maternal and child influences on children’s later independent cognitive and social functioning. Child Dev. 2000;71:358–375. doi: 10.1111/1467-8624.00150. [DOI] [PubMed] [Google Scholar]