Abstract
The standardisation of measurements is of high priority in laboratory medicine, its purpose being to achieve closer comparability of results obtained using routine measurement procedures. At present, there is international cooperation in developing reference measurement systems (reference methods, reference materials, and reference laboratory networks) for analytes of clinical significance. These reference systems will reduce, wherever possible, measurement uncertainty and promote the comparability of results. The implementation of measurement traceability through the reference system provides one of the most important tools that supports the standardisation process in laboratory medicine. It aims to achieve result comparability regardless of the measurement procedure (test kit) and the clinical laboratory where analyses are carried out. The aim of this review is to discuss some concepts related to the achievement of standardisation by the implementation of a metrologically-correct measurement system and to provide some examples that illustrate the complexity of this approach and the impact of these activities on patient care.
Background
The primary goal of laboratory medicine is to provide information that is useful to assist medical decision-making, allowing optimal healthcare.1 This can only be obtained by generating reliable analytical results on patient samples. Meaningful measurements are indeed essential for the diagnosis, monitoring, and treatment of diseases, and for risk assessment of individuals. Inadequate laboratory performance may have extensive consequences for practical medicine, healthcare systems, and, last but not least, for the patient. Poor-quality results may actually lead to incorrect interpretation by the clinician, impairing the patient’s situation.
Foremost among the laboratory’s problems is the poor comparability of analytical results that originate from different laboratories using different methods. Even today considerable differences can still be observed in the results obtained using different measurement procedures for the same analyte.2–4 Analytical systems may give results that are specific for a particular method or instrument so that different results may be obtained for a given analyte depending on the assay and platform used by the laboratory. Such differences may cloud interpretations of reported data, creating problems for both clinicians and laboratory communities.
In 2002, the Institute for Reference Materials and Measurements (IRMM) of the European Union (EU) surveyed 950 global laboratories in an International Measurement Evaluation Program (IMEP) for the measurement of the most common biochemical constituents in human serum.5 As an example of the between-laboratory variation, results for γ-glutamyltransferase, one of the most commonly employed biomarkers in hepatology, showed biases of −60% to +30% at a serum catalytic activity of 35 U/L, a value close to the upper reference limit. It is easy to argue that, on the basis of this large variation, many of the study participating laboratories would have misclassified patients at this critical decision level.
Most importantly, the lack of result comparability between different assays prevents use of common reference intervals or decision limits for a particular analyte, thus creating confusion among clinicians when interpreting results.6 A typical case is that of cardiac troponin I measurement.7 Standardisation of troponin I laboratory measurements would ensure the interchangeability of results over time and space and significantly contribute to improvements in healthcare by allowing results of clinical studies undertaken in different locations or times to be universally applied.8 This would enable an effective application of evidence-based medicine and use of guidelines established by scientific or professional bodies which often advocate use of specific decision limits for diagnosis and therapeutic intervention. An example showing the benefits of standardisation is cholesterol testing in the evaluation of cardiovascular risk. Data from the U.S. Government Accounting Office have shown that the marked improvement in the accuracy of its measurement in the last 40 years has saved ~100 million dollars per year in treatment costs with a parallel significant reduction of untreated “false-negative” individuals who are at increased risk.
The recognition that it is the standardisation of results requiring improvement in laboratory medicine has raised questions about what contributes to the lack of standardisation.9,10 It was recognised that an insufficient calibration approach, i.e. the lack of result traceability to certified standards, is the major cause. Consequently, an international agreement on the need to improve standardisation through the implementation of metrologically-correct measurement systems has been reached.11 The importance of the metrological principles has been described in two documents of the International Organization for Standardization (ISO), the ISO 17511 and the ISO 18153.12,13 In these documents, the traceability to internationally recognised and accepted reference materials and measurements is considered the key element in assuring the accuracy and comparability of clinical laboratory measurements. The EU directive on in vitro diagnostic (IVD) devices supports these ISO standards and requests application of the standards for all IVD reagents used within the EU, the aim being to ensure that the use of IVDs do not compromise the health and safety of patients, users, and third parties and, to attain the performance levels attributed to them by their manufacturer.14 This European legislation has, however, an obvious worldwide impact. From a practical perspective, diagnostic manufacturers must ensure that the analytical systems they market have been calibrated against available certificate reference materials and reference measurement procedures and that repeatability and reproducibility of their internal calibration procedures are quantified and documented.15
The Concept of the Reference Measurement System
Reference materials and measurement procedures
In order to achieve standardisation, an approach is required that provides reliable transfer of the measurement values from the highest hierarchical level to methods which are routinely used in the clinical laboratories. Such a structure is presented by the reference measurement system, based on the concepts of metrological traceability and a hierarchy of analytical measurement procedures.11 Key elements of the system are the reference measurement procedure and reference materials. The reference procedure is used to assign a certified value to a given reference material. Once the appropriate reference material is certified, this material and the manufacturer’s testing procedure can be used by industry to assign values to commercial calibrators. Clinical laboratories use routine procedures with validated calibrators, both from commercial sources, to measure human samples. In this way, the obtained value will be traceable to the reference procedure and materials, and the standardisation of measurement, that is, the process of realising traceability, will be reached.16
It should however be noted that the practical implementation of the reference system concept cannot compensate for poor precision and lack of analytical specificity of commercial assays. Furthermore, the above statements are only true if the reference materials used to transfer trueness to the field methods are commutable. Commutability is the ability of a reference or calibrator material to show interassay properties similar to those of human samples.17 In practical terms, the numerical ratio between the results determined by a given routine and a reference procedure found for the reference material must be the same as the average ratio found for patients’ samples. Only commutable materials can be used by industry for direct calibration of commercial methods, to ensure there is an unbroken traceability chain. It is well known that purification procedures sometimes used in the preparation of reference materials may result in non-commutability of these materials compared with native samples.18 Pure compounds prepared by recombinant techniques may also have altered structures with the consequence that the materials have a high probability of matrix effects.19
A solution to the commutability problems is the preparation and use of secondary reference materials as an intermediate step in the traceability chain.20 In their preparation, human serum (or defibrinated plasma) is the preferred base matrix, the effects of the natural variation between donors being minimised by using pooled collections from a number of individuals.21 However, although matrix-based materials are desirable as they are more likely to behave in a similar fashion to human samples, this does not a priori eliminate the non-commutability problems.22 Thus, “patient-like” reference materials should be used for calibration of commercial methods only if their commutability has been proven experimentally.8 If commutable reference materials suitable for direct use in the field method calibration are lacking, the only possible alternative for establishing traceability to a reference measurement procedure is for IVD manufacturers to split human fresh samples with a laboratory performing the reference measurement procedure. Calibration of the commercial system is in accordance with correlation results obtained using the value-assigned samples.23
Definition of the Measurand
In addition to reference procedures and materials, essential elements of a comprehensive reference measurement system also include the definition of the measurand in regards to the intended clinical use and the reference laboratories that may collaborate in a network (Table 1).24,25 The main responsibility of reference laboratories is to assign target values to reference materials, using the reference measurement procedures. In addition, as reported above, they may assist commercial companies in the validation of routine procedures through direct comparison of a routine analytical system with the reference procedure, using a number of appropriately selected, native human samples.26 Finally, reference laboratories may be regarded as a concerted means of supporting External Quality Assessment Schemes (EQAS) by setting up reference methods for their control materials in the post-market surveillance of clinical laboratory performance. Endorsement of this practice, however, is only possible if the commutability of quality control materials in the reference method and the routine procedures, for which they will be used, has been validated.27
Table 1.
Components of a working reference measurement system.
|
As stated before, a detailed definition of the quantity to be measured, i.e. the “measurand”, constitutes an indispensable part of any analytical reference system.28 In laboratory medicine, many hundreds of different analytes are measured or determined. With regard to the implementation of traceability, it is, however, important to differentiate between:
analytes which are well defined chemical entities and are traceable to International System (SI) units, called type A quantities, and
analytes which are rather heterogeneous in human samples and are not directly traceable to SI units, called type B quantities (Table 2).
Table 2.
Traceability and analyte classification.
Type A analytes:
|
Type B analytes:
|
Type A Analytes
Type A analytes represent a small number of well defined compounds (approximately 65), which belong to “classical” clinical chemistry including electrolytes (e.g. sodium), minerals (e.g. calcium), metabolic products (e.g. cholesterol, glucose, creatinine, etc.), steroid hormones, and vitamins. Test results for these measurements are nowadays expressed in terms of moles per litre, which represents the accepted system of SI units. However, for many hundreds of measurable quantities, designated as type B analytes, e.g. all proteins and glycoproteins – usually measured by some kind of immunochemical techniques – test results are not expressed in terms of SI units, but in terms of arbitrary units, e.g. WHO international units or mass units of a preparation belonging to a manufacturer.
For type A analytes, reference materials containing the analyte as a pure compound can usually be prepared and reference measurement procedures which specifically measure the analyte and are independent of routine analytical principles can be developed. Consequently, for many of these analytes, reference systems are already available.29 An example of a reference measurement system for type A analytes is that for creatinine in blood serum (Figure 1).30 Creatinine is a chemical substance whose entity can be unequivocally defined as a single species. The unit for the measurement of the amount-of-substance concentration of creatinine is mol/L and gravimetry can therefore be used for the value assignment of a primary reference material prepared with the pure substance. The reference measurement procedure for creatinine, applying the isotope dilution-mass spectrometry (IDMS) principle, is directly calibrated against this primary reference material. Using this reference procedure, reference laboratories working under well-defined performance conditions are able to assign values to commutable secondary reference material. Manufacturers then may use this material for calibration of a routine method, leading to traceable results for the end user’s routine method.
Figure 1.
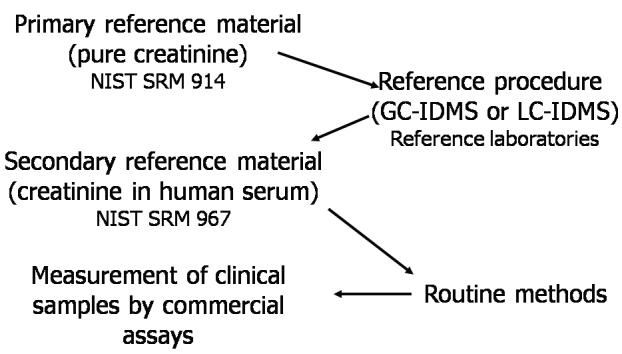
The reference measurement system for serum creatinine. Adapted from ref. 30. NIST, National Institute of Standards and Technology; SRM, standard reference material; GC-IDMS, gas chromatography-isotope dilution mass spectrometry; LC-IDMS, liquid chromatography-isotope dilution mass spectrometry.
Type B Analytes
For type B analytes, the implementation of standardisation is in general more difficult. Much scientific work still has to be done before reference measurement systems for these analytes can be established. Since they are heterogeneous and their composition in human body fluids varies, all reference materials for type B analytes are, by definition, only surrogates for the analytes measured in patient samples. While such materials may resemble to some extent the typical heterogeneous mixture of the analyte present in the human fluids, they often may represent only an “average” condition.24,31 Furthermore, for type B analytes, reference measurement procedures independent of routinely employed analytical principles are currently lacking in the majority of cases.32 Thus, the value assignment of candidate secondary reference materials is frequently problematic.33 As a consequence, manufacturers prepare their own calibrators, that are often not available to other manufacturers, and assign values to the selected preparation on a mass basis. This can lead to a disagreement between results from different commercial assays.
As many type B analytes are very important parameters in the medical field, such as in oncology, endocrinology, and virology, the establishment of a reference measurement system for these analytes is urgently needed. For these analytes in particular, the traceability model emphasises the importance of the definition of the measurand. For complex substances the definition may not be so clear because of their potential intrinsic or acquired heterogeneity. One way to circumvent the issue of heterogeneity of type B analytes is to define the measurand as a unique, invariant part of the molecule that is common to all components of the mixture present in blood. Methods used for the development of commercial assays should, without distinction, recognise this common part with a consequent increase in the homogeneity of assay reactivity. Using this approach, a number of significant efforts have recently been initiated to standardise measurement results for type B analytes.
An excellent example is the IFCC project for standardisation 34 According to of measurement of haemoglobin A1c (HbA1c). the decision made by the IFCC HbA1c Working Group, HbA1c is defined as haemoglobin molecules having in common a glycated amino-terminal hexapeptide on the haemoglobin β-chain. The rationale was that this quantity is biochemically in human well characterised, is the major form of HbA1c blood, and most of the commercial tests claim to measure only this form. Two equivalent reference measurement procedures specifically measuring this hexapeptide were then developed, using either a combination of high-pressure liquid chromatography (HPLC) and electron-spray mass spectrometry or, alternatively, a two-dimensional approach using HPLC and capillary electrophoresis.35 Finally, secondary values reference materials have been prepared and their HbA1c certified by a network of reference laboratories, allowing the establishment of a complete reference measurement system (Figure 2).36
Figure 2.
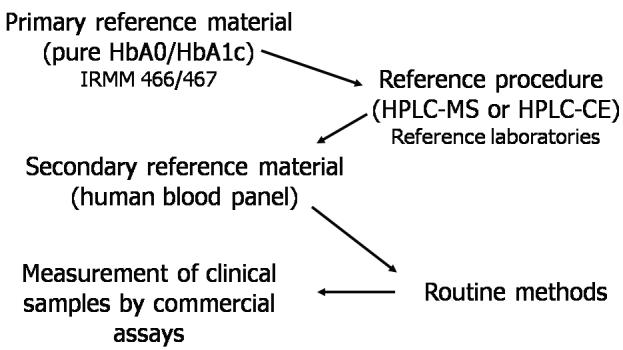
The reference measurement system for haemoglobin A1c (HbA1c). Hb, haemoglobin; IRMM, Institute for Reference Materials and Measurements; HPLC-MS, high-pressure liquid chromatography-mass spectrometry; HPLC-CE, highpressure liquid chromatography-capillary electrophoresis.
A special class of analytes is the enzymes, defined in terms of the so-called “catalytic amount” which is the amount of an agreed-upon substrate converted to product in an agreed-upon measurement system. Theoretically, enzymes defined by substrate conversion do not belong to the SI category of analytes, even if the definition of “katal” may suggest so.37 Rather the measurand may well be part of a family and, in some cases, may be totally or partially unknown.24 Hence, the problems of mixture analysis and unknown enzyme entities, typical of type B analytes, may also apply for enzymes defined by substrate conversion.
Compared with other analytes, the numerical results of catalytic activity measurements depend entirely on the experimental conditions under which the measurements are made.13 In the standardisation of enzyme assays, therefore, a reference measurement procedure, which defines the conditions under which a given enzyme activity is measured, occupies the highest level of the traceability chain whereas this is the primary reference material for non-enzyme analytes.38 Complete reference measurement systems, comprising reference measurement procedures, reference materials, and reference laboratories, are currently available for alanine aminotransferase, creatine kinase, lactate dehydrogenase, γ-glutamyltransferase, and amylase. For aspartate aminotransferase certification of the reference material using the IFCC reference procedure is ongoing. Reference systems for alkaline phosphatase and pancreatic lipase are also under discussion.
The International Cooperation
Since the development of metrologically sound reference measurement systems is a complicated and expensive process, it is clear that the objective of improving standardisation in Laboratory Medicine will only be achieved if the problems are dealt with not on a national level but through international cooperation. In order to avoid confusion and waste of resources, the development and establishment of different national or regional analytical reference systems are not acceptable. International consensus and agreement should be reached by all major players in the field. This was the reason for the creation of the Joint Committee for Traceability in Laboratory Medicine (JCTLM), established under the auspices of the International Bureau of Weights and Measures (BIPM), the IFCC, and the International Laboratory Accreditation Cooperation (ILAC).39 In addition to these international and intergovernmental organisations concerned with measurements in Laboratory Medicine, metrology, and health, other JCTLM key stakeholders are represented by the principal producers of IVD reference materials; the IVD industry associations from Europe, Japan and the US; regulatory bodies from Europe, Japan and the US; standards writing bodies, and accreditation and quality assessment organisations.
Since April 2004, a list of higher order reference materials and reference methods for analytes measured in Laboratory Medicine, identified by a thorough review process for conformity with appropriate ISO standards, is publicly available in a database at the BIPM website.40 JCTLM is also publishing the initial list of reference laboratories that fulfil the established selection criteria and are able to deliver a reference measurement service. Using these validated reference measurement systems industry can assign traceable values to commercial calibrators. Clinical laboratories, which will use routine procedures and these validated calibrators to measure human patient specimens, may finally obtain comparable results. Then, the traceability requirement, as formulated by the IVD directive of the EU and in the corresponding ISO standards, can finally be implemented in practice (Figure 3).
Figure 3.
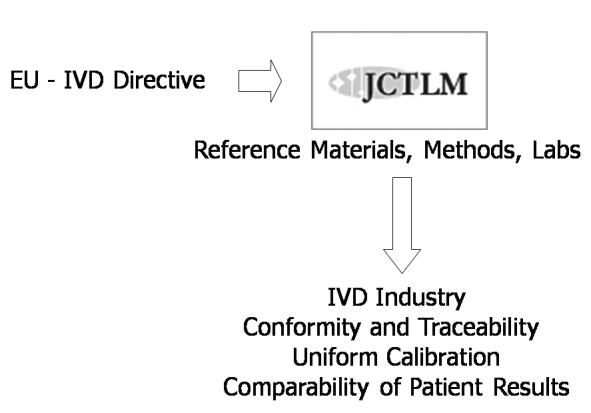
The Joint Committee for Traceability in Laboratory Medicine (JCTLM): the link between the implementation of the in vitro diagnostic (IVD) directive of the European Union (EU) and standardisation in laboratory medicine.
Metrological vs. “Clinical” Traceability
As soon as a new reference measurement system is adopted and implemented, clinical validation of the correctly calibrated routine methods (the IVD products sold onto the market) should take place. In specific cases, in order to maintain the value of clinical experience, correlation of measurement results obtained with the new calibration to results of measurements obtained with the previous calibration should be established. Adjusting the decision-making criteria is of major importance since, even if from a metrological point of view the routine method was biased, clinicians can still reach correct clinical decisions if the decision-making criteria they apply incorporate the same bias. In contrast, they could arrive at incorrect clinical decisions if patient results are true with regard to the reference system, but the decision-making criteria are only valid by using the previous calibration for the test.
Using HbA1c as an example, reliable, linear relationships between results traceable to the IFCC reference system and previous routine methods were demonstrated allowing the conversion of analytical and clinical data from one system to another.41 In practice it is therefore possible to translate target values generated in previous landmark clinical studies, using methods not traced to the IFCC system, in order to maintain the clinical experience. In addition, use of the SI unit as the unit of measurement for HbA1c, namely “mmol/mol” can targets to avoid confusion in the recalculation of the old HbA1c the new IFCC standardised results if clinical laboratories wish results traceable to the IFCC reference to implement HbA1c system (Table 3). Other advantages of this approach include a results positive impact of a change of scale of reported HbA1c thereby allowing clinicians and diabetic patients to better HbA1c changes (currently they may perceive understand HbA1c small changes – although linked to large health effects as – unimportant), and the increased potential for future use of as a diagnostic tool.
Table 3.
Suggested units and target values for HbA1c when measured with methods traceable to the IFCC reference system. A comparison with the current figures is also given.
| Currenta | IFCC traceable methods | |
|---|---|---|
| Reference interval(non-diabetics) | 4–6% | 20–42 mmol/mol |
| Target for treatment in diabeticsb | <7% | <53 mmol/mol |
| Change of therapy in diabeticsb | >8% | >64 mmol/mol |
refer to methods aligned to the U.S. National Glycohemoglobin Standardization Program.
as recommended by American Diabetes Association.
Other Undefined Issues
Other important issues concerning the implementation of a metrologically-correct approach for result standardisation are still to be defined. Firstly, a clear definition of the clinically allowable error of measurements is required. Since methods with a total error of zero do not exist agreement is required as to what percentage of misclassification of patients is acceptable and whether it is preferable to avoid false positive or false negative classifications. Whereas statistical validation criteria for analytical performance can be easily defined, tolerable deviations for clinical use are often undefined. As highlighted by Thienpont et al., the scientific community has to be aware that the absence of specifications derived from clinical needs for validation of metrologically traceable calibrations might result in a large gray zone with respect to the extent of traceability expected from IVD manufacturers, partially or totally invalidating its theoretical advantages.16
The second important issue relates to the post-market surveillance of the performance of IVD medical devices.2,4 This should be one of the major tasks of our profession through the organisation of appropriate EQAS or proficiency testing. The applicability of the true value concept in EQAS requires, however, the availability of control materials with target values assigned by laboratories using reference methods and that these materials behave exactly as human patient specimens.42 True value assignment to commutable EQAS materials will allow an objective evaluation of the performance of IVD devices, together with an accuracy-based (instead of inferior consensus-group) grading of the competency of participating clinical laboratories.43
Footnotes
Competing Interests: None declared.
References
- 1.Panteghini M. The future of Laboratory Medicine: understanding the new pressures. Clin Biochem Rev. 2004;25:207–15. [PMC free article] [PubMed] [Google Scholar]
- 2.Thienpont LM, Stöckl D, Kratochvila J, Friedecky B, Budina M. Pilot external quality assessment survey for post-market vigilance of in vitro diagnostic medical devices and investigation of trueness of participants’ results. Clin Chem Lab Med. 2003;41:183–6. doi: 10.1515/CCLM.2003.030. [DOI] [PubMed] [Google Scholar]
- 3.Miller WG, Myers GL, Ashwood ER, Kileen AA, Wang E, Thienpont LM, et al. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. 2005;129:297–304. doi: 10.5858/2005-129-297-CMSOTA. [DOI] [PubMed] [Google Scholar]
- 4.Jansen R, Schumann G, Baadenhuijsen H, Franck P, Franzini C, Kruse R, et al. Trueness verication and traceability assessment of results from commercial systems for measurement of six enzyme activities in serum: an international study in the EC4 framework of the Calibration 2000 project. Clin Chim Acta. 2006;368:160–7. doi: 10.1016/j.cca.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 5.Örnemark U, Van Nevel L, Smeyers P, Harper C, Taylor PD. The International Measurement Evaluation Program IMEP-17. Trace and minor constituents in human serum. EUR 20694 EN. Report to participants. Part 2: Methodology and quality speci cations. http://www.irmm.jrc.be/html/interlaboratory_comparisons/imep/imep-17/IMEP17_report_part2.pdf.
- 6.Klee GG. Clinical interpretation of reference intervals and reference limits. A plea for assay harmonization. Clin Chem Lab Med. 2004;42:752–7. doi: 10.1515/CCLM.2004.127. [DOI] [PubMed] [Google Scholar]
- 7.Panteghini M, Pagani F, Yeo KT, Apple FS, Christenson RH, Dati F, et al. Evaluation of imprecision for cardiac troponin assays at low-range concentrations. Clin Chem. 2004;50:327–32. doi: 10.1373/clinchem.2003.026815. [DOI] [PubMed] [Google Scholar]
- 8.Panteghini M, Forest JC. Standardization in laboratory medicine: new challenges. Clin Chim Acta. 2005;355:1–12. doi: 10.1016/j.cccn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Tietz NW. Accuracy in clinical chemistry -- Does anybody care? Clin Chem. 1994;40:859–61. [PubMed] [Google Scholar]
- 10.Büttner J. The need for accuracy in laboratory medicine. Eur J Clin Chem Clin Biochem. 1995;33:981–8. [PubMed] [Google Scholar]
- 11.Müller MM. Implementation of reference systems in laboratory medicine. Clin Chem. 2000;46:1907–9. [PubMed] [Google Scholar]
- 12.ISO 17511:2003. Metrological traceability of values assigned to calibrators and control materials. ISO; Geneva, Switzerland: In vitro diagnostic medical devices - Measurement of quantities in biological samples. [Google Scholar]
- 13.ISO 18153:2003. Metrological traceability of values for catalytic concentration of enzymes assigned to calibrators and control materials. ISO; Geneva Switzerland: In vitro diagnostic medical devices - Measurement of quantities in biological samples. [Google Scholar]
- 14.Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Official Journal of the European Communities. 1998 Dec 7;L331:1–37. [Google Scholar]
- 15.Dati F. The new European directive on in vitro diagnostics. Clin Chem Lab Med. 2003;41:1289–98. doi: 10.1515/CCLM.2003.196. [DOI] [PubMed] [Google Scholar]
- 16.Thienpont LM, Van Uytfanghe K, Rodriguez Cabaleiro D. Metrological traceability of calibration in the estimation and use of common medical decision-making criteria. Clin Chem Lab Med. 2004;42:842–50. doi: 10.1515/CCLM.2004.138. [DOI] [PubMed] [Google Scholar]
- 17.Franzini C, Ceriotti F. Impact of reference materials on accuracy in clinical chemistry. Clin Biochem. 1998;31:449–57. doi: 10.1016/s0009-9120(98)00054-x. [DOI] [PubMed] [Google Scholar]
- 18.Miller WG, Myers GL, Rej R. Why commutability matters. Clin Chem. 2006;52:553–4. doi: 10.1373/clinchem.2005.063511. [DOI] [PubMed] [Google Scholar]
- 19.Panteghini M, Pagani F. AACC creatine kinase MB (CK-MB) standardization material used as manufacturer’s working calibrator is unable to harmonize CK-MB results between two commercial immunoassays. Clin Chem. 2004;50:1711–2. doi: 10.1373/clinchem.2004.036707. [DOI] [PubMed] [Google Scholar]
- 20.Whicher JT. Secondary reference materials. Clin Biochem. 1998;31:441–6. doi: 10.1016/s0009-9120(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 21.Dati F, Panteghini M, Apple FS, Christenson RH, Mair J, Wu AH. Proposals from IFCC Committee on Standardization of Markers of Cardiac Damage (C-SMCD): strategies and concepts on standardization of cardiac marker assays. Scand J Clin Lab Invest Suppl. 1999;230:113–23. [PubMed] [Google Scholar]
- 22.Miller G. How useful are reference materials? Clin Chem. 1996;42:1733–4. [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. CLSI document X5-R. Wayne, PA: CLSI; 2006. Metrological traceability and its implementation; A report. [Google Scholar]
- 24.Thienpont LM, Van Uytfanghe K, De Leenheer AP. Reference measurement systems in clinical chemistry. Clin Chim Acta. 2002;323:73–87. doi: 10.1016/s0009-8981(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 25.Siekmann L, Doumas BT, Thienpont L, Schumann G. Reference materials and reference measurement systems in laboratory medicine. Networks of Reference Laboratories. Eur J Clin Chem Clin Biochem. 1995;33:1013–7. [PubMed] [Google Scholar]
- 26.Thienpont L, Franzini C, Kratochvila J, Middle J, Ricos C, Siekman L, et al. Analytical quality speci cations for reference methods and operating speci cations for networks of reference laboratories. Eur J Clin Chem Clin Biochem. 1995;33:949–57. [PubMed] [Google Scholar]
- 27.Miller WG. Specimen materials, target values and commutability for external quality assessment (pro ciency testing) schemes. Clin Chim Acta. 2003;327:25–37. doi: 10.1016/s0009-8981(02)00370-4. [DOI] [PubMed] [Google Scholar]
- 28.Lequin RM. Traceability in Laboratory Medicine. Biochim Clin. 2003;27:230–3. [Google Scholar]
- 29.Büttner J. Reference materials and reference methods in laboratory medicine: a challenge to international cooperation. Eur J Clin Chem Clin Biochem. 1994;32:571–7. [PubMed] [Google Scholar]
- 30.Panteghini M, Myers GL, Miller WG, Greenberg N. The importance of metrological traceability on the validity of creatinine measurement as an index of renal function. Clin Chem Lab Med. 2006;44:1287–92. doi: 10.1515/CCLM.2006.234. [DOI] [PubMed] [Google Scholar]
- 31.Panteghini M. Current concepts in standardization of cardiac marker immunoassays. Clin Chem Lab Med. 2004;42:3–8. doi: 10.1515/CCLM.2004.002. [DOI] [PubMed] [Google Scholar]
- 32.Panteghini M. Standardization of cardiac troponin I measurements: the way forward? Clin Chem. 2005;51:1594–7. doi: 10.1373/clinchem.2005.054551. [DOI] [PubMed] [Google Scholar]
- 33.Stenman UH. Immunoassay standardization: is it possible, who is responsible, who is capable? Clin Chem. 2001;47:815–20. [PubMed] [Google Scholar]
- 34.Hoelzel W, Miedema K. Development of a reference system for the international standardization of HbA1c/ glycohemoglobin determinations. J Int Fed Clin Chem. 1996;9:62–7. [PubMed] [Google Scholar]
- 35.Jeppsson JO, Kobold U, Barr J, Finke A, Hoelzel W, Hoshino T, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40:78–89. doi: 10.1515/CCLM.2002.016. [DOI] [PubMed] [Google Scholar]
- 36.Miedema K. Standardization of HbA1c and optimal range of monitoring. Scand J Clin Lab Invest Suppl. 2005;240:61–72. doi: 10.1080/00365510500236143. [DOI] [PubMed] [Google Scholar]
- 37.Siekmann L, Bonora R, Burtis CA, Ceriotti F, Clerc-Renaud P, Ferard G, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degree C. Part 1. The concept of reference procedures for the measurement of catalytic activity concentrations of enzymes. Clin Chem Lab Med. 2002;40:631–4. doi: 10.1515/CCLM.2002.109. [DOI] [PubMed] [Google Scholar]
- 38.Panteghini M, Ceriotti F, Schumann G, Siekmann L. Establishing a reference system in clinical enzymology. Clin Chem Lab Med. 2001;39:795–800. doi: 10.1515/CCLM.2001.131. [DOI] [PubMed] [Google Scholar]
- 39.Müller MM. Traceability in laboratory medicine. Accred Qual Assur. 2003;8:340–5. [Google Scholar]
- 40.Database of higher-order reference materials and reference measurement methods/procedures. [Accessed 26 April 2007]; http://www.bipm.org/en/committees/jc/jctlm/jctlm-db.
- 41.Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50:166–74. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 42.Thienpont LM, Stöckl D, Friedecky B, Kratochvila J, Budina M. Trueness veri cation in European external quality assessment schemes: time to care about the quality of samples. Scand J Clin Lab Invest. 2003;63:195–201. doi: 10.1080/00365510310000349. [DOI] [PubMed] [Google Scholar]
- 43.Baadenhuijsen H, Kuypers A, Weykamp C, Cobbaert C, Jansen R. External Quality Assessment in The Netherlands: time to introduce commutable survey specimens. Lessons from the Dutch “Calibration 2000” project. Clin Chem Lab Med. 2005;43:304–7. doi: 10.1515/CCLM.2005.052. [DOI] [PubMed] [Google Scholar]


