Abstract
Clinical laboratories are moving towards global standardisation to produce equivalent test results across space and time. Standardisation allows use of evidence-based medicine, eliminates the need of method-specific reference intervals, decision levels and cut-offs, and can be achieved by application of metrological principles. For example, in vitro diagnostics (IVD) manufacturers can make kit calibrators traceable to internationally recognised reference materials and reference methods.
The first step towards standardisation is to identify appropriate reference materials and methods. This has been undertaken by a new international consortium, the Joint Committee for Traceability in Laboratory Medicine (JCTLM), formed in 2002. It brings together experts representing the clinical laboratory profession, government agencies, and manufacturers, to promote international comparability, reliability, and equivalence of measurement results in clinical laboratories for the purpose of improving healthcare. Through the efforts of the JCTLM, manufacturers are able to assign values to kit calibrators with consistency using appropriate higher order reference materials and methods, and traceability flowcharts, according to ISO Standards to ensure accuracy of test results and to promote assay performance harmonisation. Users of assay kits can assess suitability of calibrators on the basis of acceptable reference materials and/or methods identified by the JCTLM. The JCTLM exemplifies the dynamic nature of clinical laboratory medicine, the inherent spirit of cooperation among professionals in this scientific field, and the international desire to strive for the highest level of clinical laboratory practice for the benefit of patients.
Introduction
Clinical laboratories exist to provide medical information for patient care. Day in and day out, the immediate product of laboratories are test results that are interpreted by health care providers in light of the clinical presentation of each patient and in relation to the results of previous and future values for the same measurand (analyte) and the values for other measurands. Both medical care providers and patients expect high quality service from every clinical laboratory. The most important gauge of quality is that the test results are accurate and suitable for medical practice. In fact, the various customers of clinical laboratories expect (i.e. take for granted) that all test results produced by all laboratories at all times are accurate and clinically meaningful. So ingrained is this perception that medical errors, originating in the laboratory or any other medical service, may be the subject of front-page news, depending on how egregious the mistake. Clinical laboratories naturally pride themselves on providing the highest quality service feasible. Ideally, if two or more laboratories at any location in the world tested the same patient specimen, equivalent values would be reported. Global clinical laboratory practice has not yet reached this level of performance, but significant progress is currently being made in this direction. The JCTLM is a significant part of the movement towards assay standardisation and global harmonisation in the clinical laboratory community.
Clinical Need for Standardisation
It is undeniable that the clinical laboratory is experiencing globalisation. Physical geography has not changed; the world is still round and considerable time and distance separates people. But yet it can be argued that the world is “shrinking”or “flattening” when referring to the “virtual world” that has been created by personal computers, the Internet, mobile phones, and all of the other means by which individuals, organisations, and nations now communicate on a real time basis with each other. Additionally, economies are more dependent on multiple countries to market their products. As a result, people travel more, work in different countries and may find themselves seeking health care in different locations. In addition, the need to “standardise” medical practice, including clinical laboratory practice, has increased in importance. The implications of such globalisation are:
An individual patient and/or his physician may find himself with a test result obtained in one region or country and needing to compare that result with another result obtained in a different location. Without standardisation, the differences between the two results may be uninterpretable.
Standardised clinical practice guidelines, in many cases, dictate actions or treatments when a test result is either greater or less than a given medical decision level. These levels are assumed to be independent of the methodology used to obtain the result. For example, further action may be taken when a cholesterol value is greater than 5.2 mmol/L or a prostate specific antigen (PSA) result exceeds 4.0 μg/L no matter what method is used to generate the value. In the case of cholesterol, the methods are sufficiently standardised to assure that reliable decisions are made. For PSA, however, that is not the case.
Other examples of measurands which may not be sufficiently harmonised are creatinine, and HbA1c. These measurands are well-characterised compounds and current efforts to standardise results are underway.
Many other assays for key analytes, such as the cardiac markers troponin I and B-type natriuretic peptide (BNP), often produce patently non-equivalent test results.1–4 Even a cursory review of proficiency testing (PT) survey and external quality assurance (EQA) results demonstrate that reported values can differ by orders of magnitude and are highly method specific. PT samples, of course, may exhibit matrix effects with some assays leading to discordant results, but even comparison of fresh patient specimens confirms that radically different values may be generated for the same measurand by tests from different manufacturers. In the case of critical cardiac markers such as troponin and BNP, the lack of assay standardisation is not only inconvenient but a potential source of medical misinterpretation that could lead to patient safety issues. Due to the lack of international method standardisation, various assays require method-specific reference intervals, or “cut-offs”,as with some cardiac markers. This means that physicians must interpret results relative to the appropriate medical decision level for a specific assay. A patient specimen obviously contains a finite amount of any given analyte, but due to the fact that different assays exhibit non-equivalent analytical responses, a spectrum of different values may result from testing of the same specimen by different methods. This increases the likelihood that the result may be misinterpreted or that the results will be discordant with a previous value.
As previously mentioned, PSA, one of the most common tumour markers, provides another example of the lack of standardisation and the potential for very real impact on patient care. Currently, two sources of calibration are in common use for PSA. One is based on the calibration scheme that produces results consistent with the first PSA assay on the market. The second is calibrated using the World Health Organization International Reference Preparation (IRP 96/670).5 Not only are different results produced for the same patient specimen if tested by assays using different calibration schemes, but clinical interpretation varies, with adverse consequences for patients. It was found that 19% of patients were candidates for prostatic biopsy if analysed by a method based on the first calibration scheme but were not if tested by an assay based on the second calibration scheme, both sets of results having been assessed against the traditional 4.0 μg/L cut-off. Without even knowing which result is “right,” the reader can understand that either biopsies may be performed when they are not warranted, based on the PSA value, or biopsies will not be indicated when they should be performed. Either scenario is obviously undesirable and can result in an adverse patient outcome.
The above examples (troponin, BNP and PSA) concern relatively complex, esoteric measurands for which physicochemical analytical methods are not available and that are tested using immunoassays. But calibration based on reference materials and methods is critical even for very simple elemental analytes, such as calcium.6 As recently reported, a manufacturer announced a restandardisation of its calcium calibrators, based on atomic absorption.7 However, one laboratory found that this resulted in a 5.4% change in value assignment and exceeded the desirable total error for calcium of 2.4%. Further investigation comparing field method values to the reference method, inductively coupled plasma-mass spectrometry, and the Standard Reference Material (SRM) for calcium developed by the National Institute of Standards and Technology, NIST SRM 956b, demonstrated that the change in calcium kit calibrator values should have been much less. The repercussions of overestimation of calcium values are large. It has been estimated that an analytical bias of only +/− 0.10 mmol/L for calcium could increase the cost of patient care by approximately $150 million annually in the United States.6 In 2002 the Institute for Reference Materials and Measurements (IRMM) conducted a study of the comparability of clinical laboratory test results worldwide. Their objective was to determine the level of comparability for 20 analytes including calcium.8 A graph in the IRMM report represents the average calcium results obtained from 983 international laboratories. From this graph, it is apparent many laboratories would have been reporting patient calcium results with a bias greater than 0.10 mmol/L.
There are a variety of reasons why assays for the same measurand yield different values. The simple fact is that not all assays are created equal. The simple, small molecular weight analytes, such as the electrolytes and glucose, tend to exhibit a high degree of standardisation. This is because they can be tested using well-developed, robust physicochemical methods based on transferable, reproducible first principles, and pure reference materials are available. The more esoteric analytes, such as those measured by immunoassays, are more challenging and exhibit greater discordance. There are several factors preventing the standardisation of such analytes including:
Use of different calibrators by manufacturers because an internationally recognised reference material or reference measurement procedure is not available;
Comparison of assays to different predicate devices (competitor assays) or the use of inadequately qualified reference measurement procedures;
Use of antibodies by manufacturers that recognise different antigens or epitopes present on the same analyte; and
Use of different capture or detection antibodies by manufacturers in two-step immunoassays for the same analyte.
Sometimes, differences among assays are due to chance but often the differences are intentional. Manufacturers are restricted by patents from producing tests that are too similar to other manufacturers, or manufacturers purposely seek to set their assays apart from those of competitors. Whether intentional or not, many assays produce patient test results that are not comparable and clearly not interchangeable.
In summary, the Holy Grail for clinical laboratory medicine is the ability to generate the correct and “true” patient test result on any specimen tested in any laboratory around the world regardless of the specific analytical system used. The patient test results provided by all clinical laboratories should be accurate and meet medical interpretive needs, and the performance of the analytical systems that produce clinical laboratory results should be comparable over space and time.
Calibration Traceability and Standardisation
One mechanism to improve the standardisation of laboratory test results is to assure that the methods are traceable to higher order reference materials and methods. The principles of traceability of test procedures have been available in the analytical community for several years. Several guidelines have been prepared describing both the advantages and the science underpinning traceability.9, 10 Three important features of traceability are:
A traceable method will have an unbroken chain from a specific reference material and/or method to the final result;
An associated measurement uncertainty (an estimate of the variation of the result) will be included; and
The methods will be validated and, where possible, the commutability of each reference material in the unbroken chain will be demonstrated.
Traceable methods have several advantages. Many that apply to the clinical laboratory are listed in Table 1.
Table 1.
Advantages of traceability of results in the clinical laboratory.
|
Method comparisons against reference measurement procedures and validation of improved comparability through proficiency testing/external quality assurance (PT/EQA) schemes deserve special mention. While it seems logical to expect an IVD medical device to provide performance at least equivalent to a device already available on-market, it is not always certain that the quality of the predicate device is adequate or representative of the “gold standard”. Equivalent performance between devices may not be adequate to ensure that they are “fit for purpose” and meet clinical needs. A better basis for comparison of routine “field tests” is a benchmark analytical reference measurement system that has been accepted as providing scientific “truth”. That is, a system that represents the best analytical capability available at present.
PT/EQA surveys were originally intended as educational exercises to allow laboratories to compare themselves to their peers. While still useful for this purpose, PT surveys now serve a regulatory purpose in many countries. There is general agreement that target values for PT samples should be determined on the basis of reference methods and reference materials.11 This is the case for samples provided by some PT survey providers, but “peer group” grading is still common. The peer group approach allows a laboratory to gauge whether its performance is comparable to other labs using the same method/analyser. However, peer group comparison leaves open the question of the absolute accuracy of a test method as there can be multiple “true values” (i.e. each peer group mean value represents a relative “true value”). Comparison to the “true value” as determined by a reference measurement procedure allows both an absolute and relative performance yardstick. Although uniform calibrator traceability by IVD manufacturers is no guarantee of equivalent assay performance, it definitely promotes assay standardisation and minimises the difference between method peer groups.
The In Vitro Diagnostics Directive and Traceability
The In Vitro Diagnostics Directive (IVDD) in Europe represents a major step forward in promoting assay standardisation and global harmonisation in the clinical laboratory community.12 The IVDD became effective on 7 December 2003, and applies to all manufacturers who provide calibrators for IVD devices in the European Union. Compliance with the IVDD allows manufacturers to affix the “CE” mark on their products as an assurance of quality. Without the CE mark, IVD devices cannot be sold in the European Community. The IVDD requires manufacturers to provide information about the traceability of their calibrators when requested, including estimates of uncertainty for the values assigned as appropriate. This entails constructing “traceability chains” that begin with reference materials and/ or reference methods of the highest metrological order and to document an unbroken link from the reference materials and/ or methods to the kit calibrators used in clinical laboratories. The IVDD requires that calibrator traceability information be made available for Competent Authorities, Notified Bodies, and users of IVD devices, but it does not describe how to construct a suitable traceability chain. Instead, it points to ISO 17511 (In vitro diagnostic devices - Measurement of quantities in biological samples - metrological traceability of values assigned to calibrators and control materials).13 ISO 17511 describes the elements of calibrator traceability chains. Another useful document for those who are interested in calibrator traceability is CLSI X5-R.14 This is a report that provides guidance to IVD manufacturers in meeting the requirements of ISO 17511.
ISO 17511
ISO 17511 is based on metrology, the science of measurement, and provides a very useful roadmap for calibrator traceability. The Figure illustrates a generic calibrator traceability flowchart, with an unbroken chain linking the materials and methods of the highest metrological order to the manufacturer’s kit calibrators. The metrological scheme described in ISO 17511 assumes that appropriate reference materials and reference measurement procedures exist for measurands. This is not always the case. In fact, five different scenarios exist:
Figure.
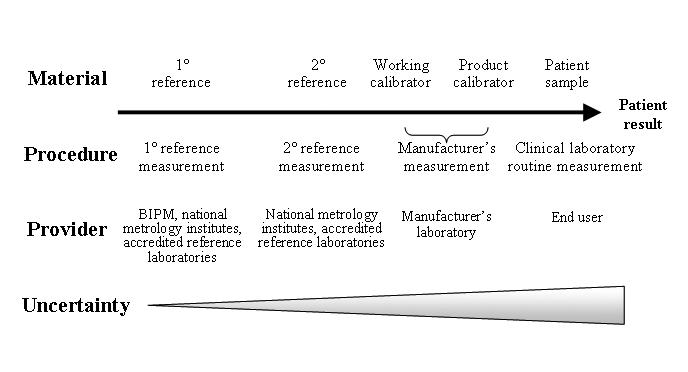
A generic calibrator traceability flowchart. Based on ISO 17511:2003 Fig 4.2.2.
Reference material and reference measurement procedure are both available and traceable to SI units (the ideal; e.g. glucose, creatinine);
Reference material and reference measurement procedure are both available but not traceable to SI;
Reference material is not available, but a reference measurement procedure is available (e.g. coagulation factors);
Reference material is available, but a reference measurement procedure is not available (e.g. specific plasma proteins such as transferrin, immunoglobulins); and
Neither a reference material nor reference measurement procedure is available (e.g. some tumour markers).
In the last situation, manufacturers must prepare calibrators from the best options available, increasing the chances that kit calibrators will be traceable to very different starting materials and unique to a given manufacturer’s assay.
Caveats about the IVDD and Metrological Traceability
The IVDD does not identify internationally recognised reference materials and measurement procedures. Manufacturers are expected to select the appropriate reference systems to anchor their calibrators, judiciously choosing from those that are available. Nor does the IVDD require that new or improved reference measurement systems be developed for calibrator traceability and be applied by manufacturers. Naturally, it is expected that improved reference materials and methods will be developed over time resulting in changes to calibrator traceability and improved accuracy and standardisation of assays. The reality is that, if the materials and methods used to develop the unbroken chain are not well designed, the traceability scheme may not provide the expected improvement in comparability of results. Metrology allows for multiple traceability schemes and leaves it up to the user to develop and validate the calibration scheme that is technically best for the manufacturer’s product(s). The problem then becomes, how accurate are the materials, methods and laboratories being used to assign values to the manufacturer’s working calibrators. This is where using materials and methods of a “higher metrological” order to construct an unbroken link to kit calibrators, should help in generating results that are close to “the true value.” However, unless the materials and methods are of an appropriately higher order, two or more “metrologically legitimate” traceability chains can be constructed for some measurands and each can produce “a true value” that may or may not be equivalent or even quantitatively close, but nevertheless are considered to be metrologically acceptable. Of course, what is desired is a traceability chain that produces “the true value”-a value that is generated by a sufficiently accurate measurement and that represents “scientific truth” (or as close to absolute scientific truth as current technology allows) as opposed to multiple forms of “relative truth” (results that are valid according to the traceability chains on which they are based, but not quantitatively equivalent). While the IVDD is a great leap forward for global harmonisation of clinical laboratory practice, manufacturers and users of IVD devices must consciously strive to ensure metrological consistency by the use of internationally recognised and accepted reference systems.
Another complication is that reference materials need to be “commutable.” That is, they should produce an analytical response with a reference measurement procedure and with routine field methods mimicking that of fresh patient specimens. All methods should generate results that are in close agreement when used to test a candidate reference material. For example, a specification for commutability might be that test results agree within a range representing 95% of the patient’s results in a comparison study. Fresh patient specimens (healthy and diseased individuals) should be tested by field methods in addition to the reference material to demonstrate that the reference material mimics the analytical response of patient specimens.
The Joint Committee for Traceability in Laboratory Medicine
It is clear that the key to assay standardisation and the global harmonisation of clinical laboratory test results rests with the practical application of metrological concepts and the use of internationally accepted reference materials and methods. But who is the arbiter of traceability in the clinical laboratory field? As noted by Stenman: “Although quite a few organisations deal with standardisation, it is not clear who is responsible for what. Because standardisation is an international rather than a national or regional problem, it is desirable that one international organisation should be responsible for the coordination of various standardisation projects.”15 In Stenman’s view, “Currently, most of the standardisation problems are taken care of by assay manufacturers, and this situation will not change soon.” Indeed, the premise of the IVDD is that manufacturers will play the primary role in traceability. Manufacturers are the logical leaders of the traceability/global standardisation movement, but how will they know which reference materials and methods they should use to anchor their assays? Of necessity, the JCTLM was created.
The JCTLM was formed in 2002 and is an international consortium sponsored by the Bureau International des Poids et Mesures (BIPM, or the International Bureaus of Weights and Measures), the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), and the International Laboratory Accreditation Cooperation (ILAC), bringing together government agencies, the clinical laboratory profession, and industry. The goal of the JCTLM is to support worldwide comparability, reliability, and equivalence of measurement results in clinical laboratories for the purpose of improving healthcare.16 The information provided by the JCTLM is intended for the IVD industry and any individual or organisation dealing with traceability of clinical laboratory medicine purposes. This includes the suppliers of PT/EQA programs.
Among the stakeholders invited to apply for JCTLM membership are: intergovernmental organisations; national governmental organisations such as national metrology institutes (NMIs); and any international, regional, and national non-governmental organisations with technical competency in the field. Member organisations may send representatives to JCTLM meetings, have access to the documents created by the JCTLM, participate in their working groups by nominating members to them, and submit written statements concerning matters under consideration by the JCTLM.
The JCTLM consists of two working groups. Working Group 1 is responsible for identifying internationally accepted reference measurement procedures and reference materials. Working Group 2 is responsible for identifying reference laboratories that provide internationally accepted reference measurement procedures to be used for the value assignment of calibrators and the validation of commutability of calibrators and other reference materials.
Both working groups utilise a series of review teams to provide the resources and expertise to identify acceptable reference materials, methods and laboratories. These review teams encompass a range of analytes as listed below.
Blood Gases
Blood Groupings
Coagulation Factors
Drugs
Electrolytes
Enzymes
Metabolites-Substrates
Microbiology Serology
Non-Electrolyte Metals
Non-Peptide Hormones
Nucleic Acids
Proteins
Quality Systems
Vitamins
Each of these specialised subgroups is headed by a Review Team Leader and typically consists of four to eight Team Members. Team Members are drawn from all around the world, chosen for their expertise, and represent NMIs, governmental regulatory agencies, IVD manufacturers, and individuals working in the clinical laboratory professions (e.g. academia, hospitals, accreditation bodies). Significant attempts are made to assure that the balance of government agencies, IVD manufacturers and clinical laboratory personnel is maintained, along with a geographic balance. Any person with the required expertise and the desire to participate in the JCTLM’s activities is encouraged to volunteer; application and disclosure of interests forms are available from JCTLM website.17
At the onset, the JCTLM recognised that it required a transparent process based on internationally agreed upon standards to identify what methods, materials and laboratories adhere to the definition of “higher order”. To meet this need, the JCTLM abides by two main principles:
1. Both working groups are guided by the following ISO standards
Mainly ISO 17511: In vitro diagnostic medical devices – Measurement of quantities in biological samples – Metrological traceability of values assigned to calibrators and control materials;
ISO 15193: Measurement of quantities in samples of biological origin – presentation of reference measurement procedures;
ISO 15194: Measurement of quantities in samples of biological origin – description of reference materials;
ISO 15195: Laboratory medicine – Requirements for reference measurement laboratories; and
ISO 18153: In vitro diagnostic medical devices – Measurement quantities in samples of biological origin – Metrological traceability of values for catalytic concentration of enzymes assigned to calibrators and control materials.
These provide the internationally agreed upon requirements for what constitutes higher-order reference materials and methods.
2. JCTLM Quality Manuals describe in detail the activities of the working groups and review teams. These quality manuals can be found and downloaded from the JCTLM web pages.18, 19 These quality manuals provide the transparency of the decision-making processes used by making the public aware of the practices and procedures used to make the decisions.
There is also an Implementation Protocols Team under Working Group 1. This team’s mission is to address the use of reference materials, reference measurement procedures, and reference laboratories by the IVD industry. Specific tasks include:
Suggesting a realistic timeline for manufacturers to re-standardise assays using traceability to appropriate reference materials and methods;
Recommending efficient means to make the JCTLM information available to the industry (along with changes as they occur);
Providing a mechanism for reviewing and approving new candidate reference materials and methods as they become available; and
Making available an effective forum for communication of traceability and standardisation information among the members of the IVD manufacturing community.
The self-appointed tasks of the JCTLM are daunting. It is estimated that clinical laboratories perform analyses for about 400 1000 different measurands. Only about 10% of these measurands are what the JCTLM describes as type A (JCTLM List 1) analytes, those for which well-recognised reference materials and methods exist and can be traced to the SI unit. Some of the measurands have either a reference measurement procedure or a reference material but not both. Most of the measurands which have references that meet the criteria of “higher order” are the common, routine clinical chemistry tests, such as the electrolytes (Na, K, Cl), glucose and cholesterol. These are examples of type A analytes. But about 80% of analytes fall into the type B (JCTLM List 2) analytes category. Type B analytes consist of the more esoteric tests, such as coagulation factors, tests for nuclear materials, and immunoassays, (including those for hormones, cardiac markers, tumour markers, vitamins, and viral markers). In the clinical laboratory about 80% of total lab tests represent type A analytes and about 20% represent type B analytes. To date, the JCTLM has identified reference materials and/or methods for about 120 150 analytes. The fact of the matter is that many analytes lack higher order reference materials, reference measurement procedures, or both. The goal of the JCTLM is not to provide reference materials and methods, only to qualify candidate materials and methods as acceptable for traceability purposes and make this information available to the global clinical laboratory community. Knowing that a reference material method or laboratory service is not available, or does not meet the ISO criterion of a higher order reference is also useful information for manufacturers and clinical laboratories.
Limitations of the JCTLM Process
Naturally, the JCTLM faces several challenges. Like many similar professional organisations, it depends on volunteers and their expertise. Many laboratory professionals are actively engaged in JCTLM activities, and certainly the organisations to which these individuals belong (NMIs, governmental regulatory agencies, medical and graduate schools and other academic facilities, medical centres, hospitals, and manufacturers) support this involvement. But the reality is that JCTLM participation is for most of the volunteers an “extracurricular activity”, effort that is meaningful and necessary, but which typically is not part of the “day job” and may not be a top priority for employers. A real concern is whether companies, government bodies, medical centres, and other employers will support the volunteers’ efforts and allow them to contribute time and effort. Another potential weakness is that the NMIs and other institutions that typically provide the necessary reference materials and methods may have limited resources to maintain the necessary metrological infrastructure to meet the needs of the global clinical laboratory community. It is not unusual for NMIs to be subject to budget constraints from their respective governments. Even while recognising the logic of traceability, standardisation, and global harmonisation efforts, many stakeholders may have difficulties allocating the human and other resources to support the JCTLM.
In addition, the JCTLM operates by consensus. Obtaining consensus among the members may, in some cases, be easy, but in others it may require considerable discussion. After all, if agreement on internationally accepted reference materials and methods were simple, there would have been no need to form the JCTLM in the first place. Furthermore, there is not a fixed JCTLM budget. The participating organisations are expected to fund activities on a “pay as you go” basis.
Issues for Manufacturers
As noted previously, the primary onus is on the manufacturers to drive traceability, and they face some major challenges. While the JCTLM will identify appropriate references when they are nominated and available, for many analytes they simply do not exist. The manufacturers must then decide how to best “anchor” their assays, i.e. make them traceable, given the available options. Reference materials may be available for some measurands, but not others. For example, a serum/ plasma reference material may exist for an analyte, but a urine or whole blood based reference material may not exist for the same analyte. In some cases, as for the enzymes, reference preparations may be available, but they may not be human sourced materials and may consist of a single isoenzyme although two or more isoenzymes are present in a patient specimen. Even when very pure reference materials can be obtained, they may not be commutable, thus limiting their effectiveness and the degree of agreement between methods they can provide. Reference measurement procedures also pose challenges as they are quite demanding and require a high level of discipline. For example, isotope dilution gas chromatography/mass spectrometry reference methods are typically available from only a handful of reference laboratories and are not readily set up in manufacturers’ facilities. There are many issues faced by manufacturers when developing new assays and the main ones are listed below.
Reference materials not expressed in SI units (e.g. human chorionic gonadotrophin)
Time required for restandardisation of an assay (e.g. 18–24 months)
Restandardisation when a new reference material or measurement procedure is recognised
Satisfying regulatory requirements of different countries and global regions
Producing assays that will meet the different standards of care and medical practice around the world
Development of internal references when no others exist
Currently manufacturers use the information supplied by the JCTLM to accomplish the following:
Provide calibrator traceability information to clinical laboratories and regulatory bodies;
Restandardise assays, making them traceable to internationally accepted reference materials and methods;
Improve manufacturing procedures to decrease lot to lot variability of calibrators and reagents;
Support professional organisations involved in global standardisation activities (e.g. JCTLM, American Association for Clinical Chemistry, IFCC, Australasian Association of Clinical Biochemists, ISO, CLSI); and
Design assays to meet medically relevant total error, imprecision, and bias goals.
The Future of Global Standardisation in the Clinical Laboratory
It can be expected that the application of metrological concepts in the clinical laboratory will continue in the 21st century, certainly affecting clinical chemistry but impacting the other specialties within the field as well. The obvious, and good, reasons for this trend have previously been explained. There are some caveats that should also be noted. The identification of reference materials and methods by the JCTLM and their use to properly document traceability of calibrators will promote assay standardisation and global harmonisation of patient test results but will not guarantee it. This is especially true for the more esoteric, type B measurands that are analysed using immunoassays. The very nature of many of these measurands makes it difficult for different methods to produce comparable results. Nevertheless, the closer comparability of immunoassays is a worthy and practical goal.
It should be recognised that the application of metrology and traceability alone does not necessarily equate with good clinical laboratory science. The often accepted assumptions expected of metrology are that the method being used is “perfect” in that there will be no matrix effects or individual sample variations and the methods and materials are highly precise giving no bias. As a result, metrology alone will work when the methods are highly specific and part of a more “pure” and refined measurement system but may not be as effective on complex matrices such as the patient samples used in the clinical laboratory. Designing assays that generate equivalent results and values that are as accurate as possible and as close as feasible to absolute scientific “truth” is, however, clearly desirable. However, this does not negate the excellent work that clinical laboratories currently do. The fact is that clinical laboratories have been making vital contributions to medicine for decades even though assays for some measurands may produce widely divergent results and values may be reported in different units (e.g. SI vs conventional units). Clinical laboratories can fulfil the majority of their patient care mission even if every lab throughout the world doesn’t produce equivalent results for the same patient specimen regardless of the measurement system used. Effective and clinically meaningful laboratory operations in support of the practice of medicine trump the rigorous adaptation of metrology to the clinical laboratory. However, global standardisation efforts can contribute to improved patient care and foster closer laboratory-clinician interactions as exemplified by the estimated glomerular filtration rate (eGFR) and standardisation of serum creatinine measurement.
The JCTLM, regulatory bodies, professional societies, and IVD manufacturers all have key roles in promoting calibrator traceability, assay standardisation, and global harmonisation. But individual clinical laboratories also have the responsibility to select field methods that demonstrate metrological traceability and that meet the clinical standards suggested by laboratory medicine practice guidelines. Labs must also continuously assess their routine methods to ensure that they consistently produce accurate, medically useful (“fit for purpose”) results. Thus there is an onus on laboratory directors and clinical laboratory scientists to understand the significance of reference materials and methods and how they should be used to guarantee the quality of assays.
Conclusions
Various imperatives in the global clinical laboratory community, including the medical need for standardisation of assays and the regulatory requirements of the IVDD to document metrological traceability, are currently impacting the practice of clinical laboratory medicine. It is to be expected that the movement to firmly establish proper and internationally accepted traceability chains for assay kit calibrators, allowing them to be linked by an unbroken chain to reference materials and methods of the highest metrological order, will continue indefinitely. This trend in turn will promote assay standardisation and global harmonisation in the clinical laboratories of the 21st century. The pace, completeness, and success of this movement depends on cooperation among a variety of entities, including professional societies, regulatory bodies and other governmental agencies, industry, and individual clinical laboratories. The JCTLM is a new organisation, just formed in 2002, but it can be expected to play a central role in these efforts.
Footnotes
Competing Interests: None declared.
References
- 1.Panteghini M, Gerhardt W, Apple FS, Dati F, Ravkilde J, Wu AH. Quality speci cations for cardiac troponin assays. Clin Chem Lab Med. 2001;39:175–9. doi: 10.1515/cclm.2001.39.2.175. [DOI] [PubMed] [Google Scholar]
- 2.Apple FS. Standardization of cardiac markers. Scan J Clin Lab Invest Suppl. 2005;240:107–11. doi: 10.1080/00365510500236242. [DOI] [PubMed] [Google Scholar]
- 3.Apple FS, Panteghini M, Ravkilde J, Mair J, Wu AH, Tate J, et al. Quality speci cations for B-type natriuretic peptide assays. Clin Chem. 2005;51:486–93. doi: 10.1373/clinchem.2004.044594. [DOI] [PubMed] [Google Scholar]
- 4.Christenson RH, Duh SH, Apple FS, Bodor GS, Bunk DM, Panteghini M, et al. Toward standardization of cardiac troponin I measurements part II: assessing commutability of candidate reference materials and harmonization of cardiac troponin I assays. Clin Chem. 2006;52:1685–92. doi: 10.1373/clinchem.2006.068437. [DOI] [PubMed] [Google Scholar]
- 5.Link RE, Shariat SF, Nguyen CV, Farr A, Weinberg AD, Morton RA, et al. Variation in prostate speci c antigen results from 2 different assay platforms: clinical impact on 2304 patients undergoing prostate cancer screening. J Urol. 2004;171:2234–8. doi: 10.1097/01.ju.0000127736.86597.e7. [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Standards and Technology. Planning Report 04.1. The impact of calibration error in medical decision making. 2004 http://www.nist.gov/director/prog-ofc/report04-1.pdf.
- 7.Bais R. What information should manufacturers provide on their procedures? Clin Chem. 2006;52:1624–5. doi: 10.1373/clinchem.2006.069773. [DOI] [PubMed] [Google Scholar]
- 8.Van Nevel L, Ornemark U, Smeyers P, Harper C, Taylor PDP. Part 1: International comparability. Retiesweg, Belgium: Institute for Reference Materials and Measurements; IMEP-17 Trace and Minor Constituents in Human Serum EUR 20657 EN Report to Participants. http://www.irmm.jrc.be/html/interlaboratory_comparisons/imep/imep-17/IMEP17_report_part1.pdf. [Google Scholar]
- 9.EURACHEM/CITAC. Traceability in Chemical Measurement. A guide to achieving comparable results in chemical measurement. 2003 http://www.citac.cc/EC_Trace_2003.pdf.
- 10.EURACHEM/CITAC. Quantifying uncertainty in analytical measurement. 2000 http://www.citac.cc/QUAM2000-1.pdf.
- 11.Rej R, Norton-Wenzel CS, Cao Z. Target values and method evaluation in pro ciency testing programs. Clin Chem. 2001;47:2185–6. [PubMed] [Google Scholar]
- 12.Greenberg N. Calibrator traceability: the industry impact of the IVD Directive’s new requirements. IVD Technology. 2001;7:18–27. [Google Scholar]
- 13.ISO 17511:2003. Metrological traceability of values assigned to calibrators and control materials. ISO; Geneva, Switzerland: In vitro diagnostic medical devices -- Measurement of quantities in biological samples. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. CLSI document X5-R. Wayne, PA: CLSI; 2006. Metrological traceability and its implementation. [Google Scholar]
- 15.Stenman UH. Immunoassay standardization: is it possible, who is responsible, who is capable? Clin Chem. 2001;47:815–20. [PubMed] [Google Scholar]
- 16.Mission statement for Joint Committee on Traceability in Laboratory Medicine. [Accessed 29 June 2007]; http://www.bipm.org/cc/JCTLM/Allowed/Workshop/JCTLMMIssion13June.pdf.
- 17.JCTLM: Joint Committee for Traceability in Laboratory Medicine. [Accessed 29 June 2007]; http://www.bipm.org/en/committees/jc/jctlm/
- 18.JCTLM-WG1 Quality Manual. Reference Materials and Reference Procedures. [Accessed 29 June 2007]; http://www.bipm.org/en/committees/jc/jctlm/jctlm-wg1/wg1_quality-manual.html.
- 19.JCTLM-WG2 Procedure Manual. Reference Measurement Services. [Accessed 29 June 2007]; http://www.bipm.org/en/committees/jc/jctlm/jctlm-wg2/wg2_quality-manual.html.


