Abstract
Objective:
Previous studies from our laboratory and others found that NO is a potent inducer of heme oxygenase-1 (HO-1) gene transcription in vascular smooth muscle cells (SMC), however, the mechanism responsible for the induction of HO-1 gene expression has not been elucidated. In the present study, we determined the signaling pathway responsible for the induction of HO-1 and its biological significance.
Methods:
Cultured rat aortic SMC were exposed to nitrosative stress by treating cells with various NO donors or with inflammatory cytokines.
Results:
Nitrosative stress stimulated an increase in HO-1 mRNA expression and promoter activity in vascular SMC. However, mutation of the antioxidant response element (ARE) in the HO-1 promoter or overexpression of a dominant-negative mutant of NF-E2-related factor-2 (Nrf2) abrogated the activation by NO. Electromobility shift assays using an ARE probe detected a complex that was significantly increased in intensity by NO. In addition, the migration of this complex was retarded by using an antibody directed against Nrf2. NO also increased Nrf2 mRNA expression, total and nuclear Nrf2 levels, and the binding of Nrf2 to the HO-1 promoter. Finally, treatment of SMC with NO stimulated apoptosis that was increased by HO-1 inhibition.
Conclusions:
These results demonstrate that nitrosative stress induces HO-1 gene transcription through the activation of the Nrf2/ARE complex to counteract NO-induced apoptosis of vascular SMC. The capacity of nitrosative stress to activate Nrf2 and stimulate HO-1 gene transcription may represent a critical adaptive response to maintain cell viability at sites of vascular inflammation and atherosclerosis.
INTRODUCTION
Nitric oxide (NO) is a well characterized signaling molecule that mediates numerous physiological effects. The release of NO by vascular cells plays a key role in regulating blood flow by inhibiting vascular tone, smooth muscle cell (SMC) proliferation, matrix deposition, platelet aggregration, and leukocyte adhesion (1). In addition, NO is an important modulator of cell survivor functioning in either a pro-apoptotic or anti-apoptotic fashion depending on concentration, delivery method, cellular redox status, and cell type (2). The pro-apoptotic activity of NO is mainly mediated via the loss of mitochondrial potential, release of cytochrome c, and activation of caspases (2-4). The protective pathway is not as clearly defined but involves scavenging of reactive radical species, direct S-nitrosation and inhibition of caspases, and the upregulation of cytoprotective genes (2,5). In this respect, numerous studies have documented that NO is a potent inducer of heme oxygenase-1 (HO-1) in vascular cells (6-8). HO-1 catalyzes the oxidative degradation of heme to iron, biliverdin, and carbon monoxide (9). Induction of HO-1 in the vasculature provides an important cellular defense mechanism against cytokine and oxidant stress-mediated cytotoxicity (6,10). Several potential mechanisms account for the beneficial actions of HO-1, including the catabolism of the pro-oxidant heme to the antioxidant bile pigments biliverdin and bilirubin, the coordinate induction of ferritin which chelates free iron, and the liberation of carbon monoxide which exerts anti-inflammatory and anti-apoptotic effects (11,12).
The majority of studies suggest that the induction of HO-1 by NO donors occurs predominantly via transcriptional mechanisms (7,8,13), though increases in HO-1 mRNA stability have also been noted (8,14). Nuclear run-on studies have confirmed that de novo transcription is largely responsible for the NO-mediated activation of the HO-1 gene in vascular SMCs (7,8). However, the transcription factors and the regulatory regions that are responsible for the induction of the HO-1 gene have not been elucidated. The transcription factor Nrf2, which interacts with the antioxidant responsive elements (AREs), has recently emerged as a major player in the transcriptional activation of HO-1 (15). Activation of Nrf2 is regulated by the cytosolic protein Keap1 that negatively modulates the nuclear translocation of Nrf2 and facilitates degradation of Nrf2 via the proteasome (16,17). Upon activation, Nrf2 enters the nucleus where it binds to the ARE in the HO-1 promoter to trigger gene expression. Nrf2 has recently been reported to regulate the induction of HO-1 in response to various forms of cellular stress, including hemodynamic, oxidative, and endoplasmic reticulum stress (18-20). Moreover, fibroblasts and lung tissue from Nrf2-deficient animals express reduced levels of HO-1 (21,22), further implicating Nrf2 in the induction of HO-1.
In the present study we examined the role of Nrf2 in regulating NO-mediated HO-1 expression in vascular SMC. We now report that NO stimulates HO-1 gene expression in SMC via the activation of the Nrf2/ARE complex. The mobilization of Nrf2 by NO is independent of the mitogen-activated protein kinase (MAPK) or phosphatidyinositol-3-kinase (PI3K) pathways, but is dependent on oxidative stress. In addition, we show that HO-1 functions in an adaptive manner to promote cell survival during periods of nitrosative stress.
MATERIALS AND METHODS
Materials
Phenylmethylsulfonyl fluoride, aprotinin, leupeptin, and pepstatin A were from Roche Applied Science (Indianapolis, IN); zinc protoporphyrin-IX was from Frontier Scientific Porphyrin Products (Logan, Utah); Spermine NONOate (SNN), Sin-1, Angeli's salt, S-nitroso-N-acetyl-penicillamine (SNAP), and sodium nitroprusside (SNP) were from Calbiochem (La Jolla, CA); interleukin-1β was purchased from R&D Systems (Minneapolis, MN); tumor necrosis factor-α and interferon-γ were from Genzyme (Boston, MA); GAPDH and 18S cDNA were from Ambion (Austin, TX); Nrf2 and lamin B1 polyclonal antibodies were from Santa Cruz (Santa Cruz, CA); antibodies against phospho-ERK, phospho-JNK, phospho-p38 MAPK, or phospho-Akt were from Cell Signaling (Beverley, MA). [α-32P]dCTP (3000 Ci/mmol), [γ-32P] (3000 Ci/mmol), and Gene Screen Plus membranes were from PerkinElmer Life Sciences (Boston, MA). All other agents were purchased from Sigma (St. Louis, MO).
Cell Culture
Vascular SMC were isolated by elastase and collagenase digestion of rat thoracic aortas and were cultured serially in minimum essential media supplemented with 10% serum, 2 mM L-glutamine, 5 mM Tes, 5 mM HEPES, 100 U/ml penicillin and 100 U/ml streptomycin (7,10). The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
mRNA Analysis
Total RNA (30 μg) was fractionated by electrophoresis, blot transferred to Gene Screen Plus membranes and prehybridized for 4 h at 68°C in rapid hybridization buffer (Amersham, Boston, MA). Membranes were hybridized overnight at 68°C in hybridization buffer containing [32P]DNA probes (1 × 108 cpm) for HO-1, Nrf2, or 18 S rRNA. DNA probes were generated by RT-PCR and labeled with [α-32P]dCTP using a random priming kit (7,23). Following hybridization, membranes were washed and then exposed to x-ray film. HO-1 and Nrf2 mRNA levels were quantified by scanning densitometry and normalized with respect to 18 S rRNA.
HO-1 Promoter Analysis
The sequences for the wild-type E1 enhancer coupled to a minimum HO-1 promoter (E1) as well as the mutant E1 enhancer (M739) that had its three antioxidant responsive element (ARE) core sequences mutated have been previously described (24). These promoter/luciferase constructs (1 μg/ml), pCMVβgal (1 μg/ml), and a plasmid expressing a dominant-negative Nrf2 mutant that had it transactivation domain deleted (24) were transfected into SMC using lipofectamine, cultured for an additional 24 hours, and then treated with various NO donors. Cells were then collected, lysed, and luciferase activity determined, as previously described (20).
Electromobility Shift Assay (EMSA)
A double stranded consensus ARE containing the sequence 5'TTTATGCTGTGTCATGGTT-3' (core ARE sequence is underlined) was end-labeled with [γ-32P]ATP and incubated with nuclear protein (3 μg) for 30 min at room temperature in a reaction mixture containing 20 mM Hepes (pH 7.9), 0.1 mM EDTA, 40 mM KCl, 1 mM MgCl2, 0.5 mM DTT, 10% glycerol, and 1 μg poly(dI-dc). DNA-protein complexes were resolved by electrophoresis in 5% polyacrylamide gels run at 220 volts for 3 h. In antibody supershift assays, 1 μg of Nrf2 or Nrf1 antibody was added to the reaction mixture and incubated for 3 h at 4°C prior to the addition of the probe.
Nrf2 Analysis
For detection of Nrf2 in total cell lysates, SMC were lysed in electrophoresis buffer (125 mM Tris [pH 6.8], 12.5% glycerol, 2% SDS, and 0.1% bromophenol blue), proteins resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. Blots were blocked in PBS and non-fat milk (5%) and then incubated with antibodies against Nrf2 (1:100) or β-actin (1:200) for one hour. Membranes were then washed in PBS, incubated with horseradish peroxidase-conjugated goat anti-rabbit (1:7,500) or goat anti-mouse antibody (1:5,000) and incubated with commercial chemoluminescence reagents (Amersham Biosciences, Boston, MA). For nuclear Nrf2 detection, nuclear fractions were obtained as previously described (20) and Nrf2 and lamin B1 levels determined by western blotting using the appropriate antibodies (1:1000), as described above.
Finally, cellular Nrf2 levels were also determined by immunofluorescence. SMC were grown on glass coverslips and following treatment, cells were fixed with cold acetone at 4°C for 5 min, washed twice with PBS, and incubated with blocking buffer (3% BSA, 5% goat serum, and 0.1% Triton X-100 in PBS) for 30 min at room temperature. After a brief wash with PBS, cells were incubated with an antibody against Nrf2 (1:100 dilution) in blocking buffer for one hour at room temperature. Coverslips were washed and incubated with AlexaFluor®488 goat anti-rabbit IgG (1:1000) in blocking buffer for 45 min at room temperature. Coverslips were then washed, mounted on glass slides, and images obtained with a Bio-Rad Radiance 2000 confocal system coupled to an inverted IX70 inverted microscope and a digital camera.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed using the ChIP assay kit from Upstate Cell Signaling Solutions (Charlottesville, VA). Briefly, proteins and DNA were cross-linked with formaldehyde and cells lysed in SDS-lysis buffer and then sonicated. Sheared chromatin was immunocleared with protein agarose-A and a portion of the precleared chromatin was stored and labeled as “input DNA”. The remaining chromatin was immunoprecipated with IgG (control) or Nrf2 antibodies, and immunoprecipitates washed sequentially with wash buffers. Protein-DNA complexes were eluted from the antibody with elution buffer (1% SDS, 0.1 M NaHCO3) and formaldehyde cross-links reversed by addition of NaCl (5 M) and heating at 65°C for 4 h. DNA was purified and PCR performed using a primer pair that spanned the mouse HO-1 E1 enhancer. The primers used were: E1 forward, 5'-AAGAGCTCCACCCCCACCCA-3' and reverse, 5'-GGGCTAGCATGCGAAGTGAG-3'. A 1.5% agarose gel with ethidium bromide was used to separate and examine the PCR products.
MAPK and PI3K Activation
MAPK and PI3K activity were determined by western blotting using phospho-specific antibodies (1:1000) (10).
NO synthesis
NO synthesis was assessed by measuring the accumulation of nitrite, the stable oxidation product of NO, in the culture media, as we previously reported (7).
Cell Viability
Cell viability was assessed by measuring the uptake of trypan blue while apoptosis was monitored by measuring DNA laddering via agarose gel electrophoresis, as we have previously described (10).
Statistics
Results are expressed as the means ± SEM. Statistical analysis was performed with the use of a Student's two-tailed t test (n=3-5), or with an analysis of variance when more than two treatment groups were compared (n=6). P <0.05 was considered statistically significant.
RESULTS
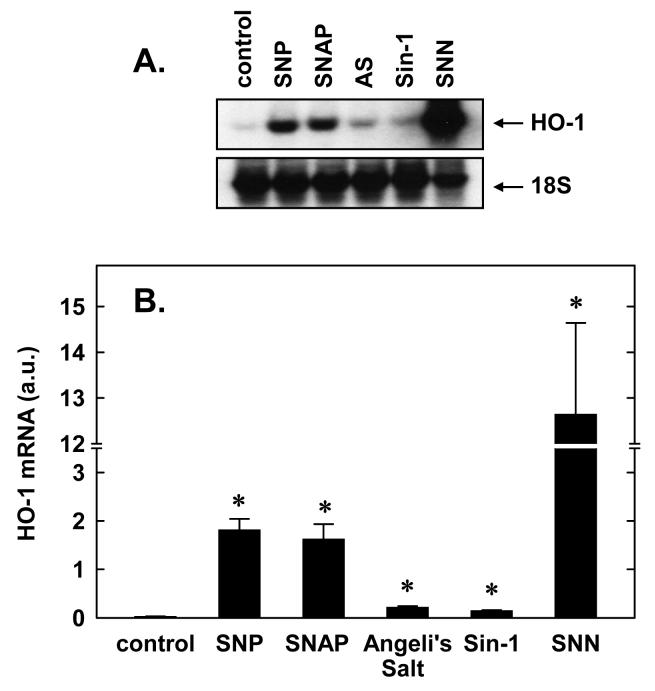
Treatment of vascular SMC with several different NO donors stimulated the expression of HO-1 mRNA (Figure 1). The induction of HO-1 does not appear to depend on the redox state of NO. SNAP and SNN which release NO in physiological buffers markedly enhanced HO-1 expression. Similarly, SNP, an iron nitrosyl complex that liberates NO+, induced HO-1 expression. In addition, Sin-1 which releases stoichiometric amounts of NO and superoxide and promotes the formation of peroxynitrite, stimulated the accumulation of HO-1 mRNA. Interestingly, Angeli's salt, a spontaneous generator of NO−, also induced the expression of HO-1.
1. NO donors stimulate HO-1 mRNA expression in vascular SMC.
A. SMC were exposed to various NO donors (1.0 mM for 24 hour), including S-nitroso-N-acetylpenicillamine (SNAP), sodium nitroprusside (SNP), Sin-1, Angeli's salt (AS), and spermine NONOate (SNN), and HO-1 mRNA determined by northern blotting. B. Quantification of HO-1 mRNA in arbitrary units (a.u.) by scanning densitometry. Results are means ± SEM of 3 separate experiments. *Statistically significant increase in HO-1 mRNA expression.
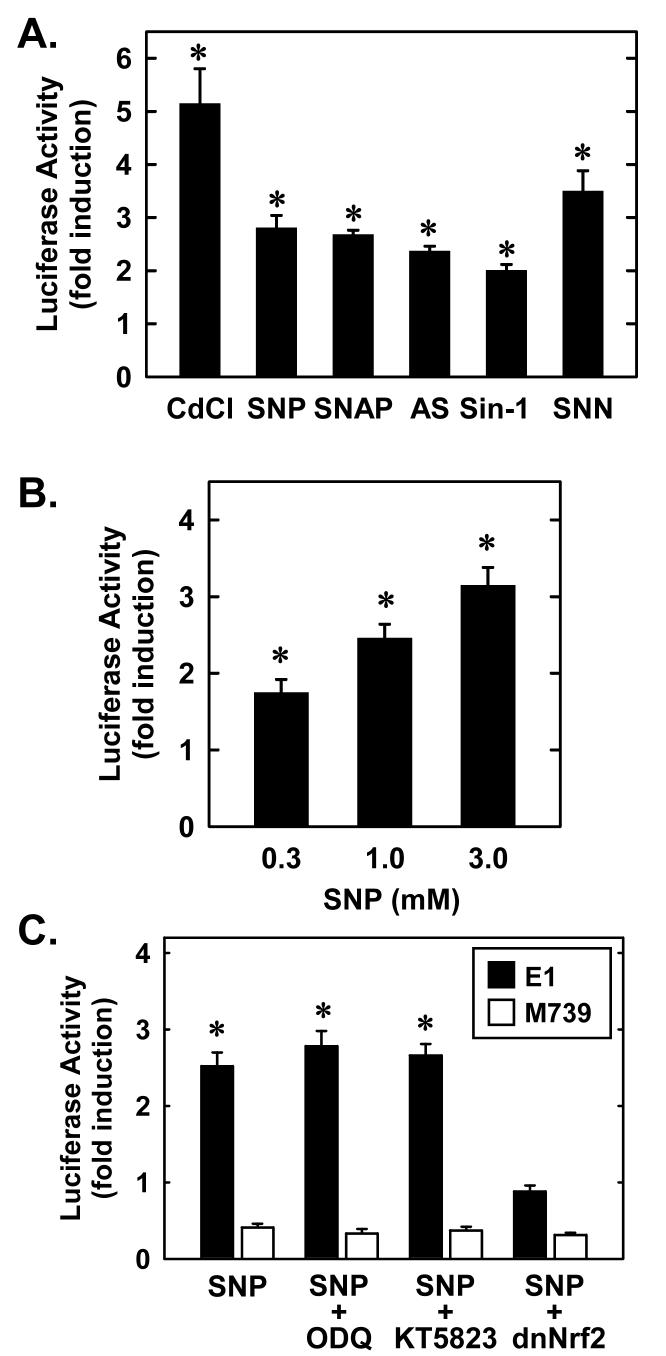
To determine whether increases in HO-1 expression in response to NO involves transcriptional activation of the gene, SMC were transiently transfected with a HO-1 promoter construct and promoter activity monitored. Cadmium, an established activator of the HO-1 promoter (24) induced an approximate 5-fold increase in promoter activity (Figure 2A). All the NO donors stimulated between a 2 and 4-fold increase in HO-1 promoter activity (Figure 2A.). Furthermore, the induction of HO-1 promoter activity by SNP was concentration-dependent (Figure 2B). Interestingly, mutation of the AREs attenuated basal activity and abolished the response to SNP (Figure 2C). These results indicate that NO activates HO-1 transcription via the ARE. Since the transcription factor Nrf2 binds and activates the ARE, the role of Nrf2 was investigated. For this, we employed a dominant-negative mutant that had its activation domain deleted. Transfection of SMC with the dominant negative mutant blocked the induction of HO-1 by SNP (Figure 2C). In contrast, the signaling molecule cGMP was unlikely to contribute to NO-stimulated HO-1 promoter activity since the soluble guanylate cyclase inhibitor, ODQ, or the protein kinase G blocker, KT5823, had no effect on the induction of HO-1 promoter activity (Figure 2C). Experiments done with SNAP yielded similar results (data not shown).
2. NO donors stimulate HO-1 promoter activity in vascular SMC.
A. HO-1 promoter activity was determined in SMC exposed to various NO donors (1.0 mM for 24 hour), including S-nitroso-N-acetyl-penicillamine (SNAP), sodium nitroprusside (SNP), Sin-1, Angeli's salt (AS), and spermine NONOate (SNN), or cadmium (Cd; 10 μM for 24 hour) B. Treatment of SMC with SNP (0.3-3 mM for 24 hour) stimulates a concentration-dependent increase in HO-1 promoter activity. C. SMC were transfected with the mouse HO-1 promoter construct (E1) or a mutated mouse HO-1 promoter construct (M739) and treated with SNP (3 mM for 24 hour) in the presence or absence of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 30 μM) or KT5823 (20 μM). In some experiments, a dominant-negative Nrf2 (dnNrf2; 1 ng/ml) construct was cotransfected into SMC. Results are expressed as the means ± SEM of 3-5 separate experiments. *Statistically significant increase in HO-1 promoter activity.
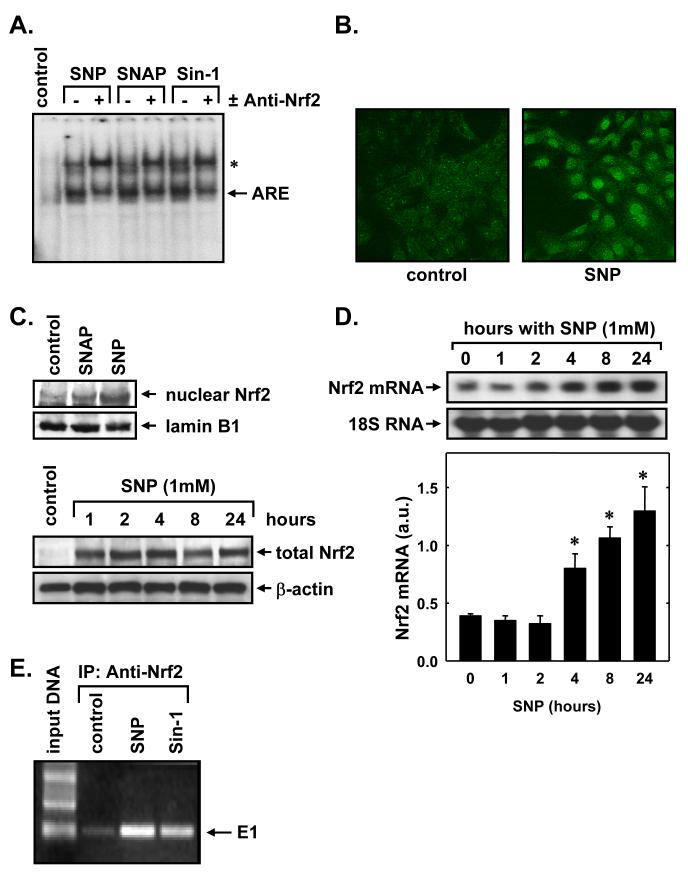
EMSA using an ARE probe corresponding to the mouse HO-1 promoter detected a complex that was significantly increased in intensity by the NO donors, SNP, SNAP, and Sin-1 (Figure 3A). Since the transcription factor Nrf2 appears critical for ARE-mediated gene expression (Figure 2C), we explored whether NO stimulated the binding of Nrf2 to the ARE. Indeed, supershift EMSA reactions using an antibody directed against Nrf2 retarded the migration of the ARE complex, indicating the presence of Nrf2 in the ARE-nuclear protein complex (Figure 3A). NO donors also stimulated the expression and nuclear localization of Nrf2. Immunofluorescence experiments demonstrate weak, diffuse staining of Nrf2 in control, untreated SMC, however, SNP-treatment results in a marked increase in nuclear Nrf2 staining (Figure 3B). The nuclear accumulation of Nrf2 by NO donors was also corroborated by western blotting (Figure 3C). Interestingly, NO also induces a rapid and prolonged elevation in total Nrf2 protein in vascular SMC (Figure 3C). An increase in Nrf2 protein was observed following one hour of NO exposure and this persisted for 24 hours. In addition, NO stimulated a delayed increase in Nrf2 mRNA with a significant increase in Nrf2 message observed after 4 hours of SNP-treatment (Figure 3D). Interestingly, the induction of Nrf2 protein by NO preceded the increase in Nrf2 mRNA, suggesting that NO stimulates Nrf2 expression via both transcriptional and posttranscriptional mechanisms. To further assess the involvement of Nrf2 in HO-1 transcription, we determined the binding of Nrf2 to the HO-1 enhancer E1 in its native chromatin environment. ChIP assays with an antibody directed against Nrf2 revealed the binding of Nrf2 to the E1 enhancer and this binding was substantially enriched after treatment with SNP or SNAP (Figure 3E).
3. NO donors stimulate Nrf2 activation and binding to the HO-1 promoter in vascular SMC.
A. EMSA using an ARE probe and nuclear extracts from control SMC or SMC exposed to sodium nitroprusside (SNP; 1 mM), S-nitroso-N-acetyl-penicillamine (SNAP; 1 mM), or Sin-1 (1 mM), for 4 hours. Supershift assays were performed using an antibody directed against Nrf2. The complex induced by NO is marked with an arrow and the supershift complex is indicated with an asterisk. B. Cellular localization of Nrf2. SMC were treated with SNP (1 mM) for 4 hours and the localization of Nrf2 determined by confocal laser microscopy. C. Western blots of total and nuclear Nrf2 protein following treatment of SMC with SNP (1 mM) or SNAP (1mM). D. Northern blot of Nrf2 mRNA expression following treatment of SMC with SNP (1 mM). HO-1 mRNA levels in arbitrary units (a.u.) were quantified by scanning densitometry. Results are means ± SEM of 3 separate experiments. *Statistically significant increase in HO-1 mRNA expression. E. ChIP assay demonstrating Nrf2 binding to the HO-1 E1 enhancer following treatment of SMC with SNP (1 mM) or SNAP (1 mM) for 4 hours. Gel shows PCR product of E1. Similar findings were observed in 4 separate experiments.
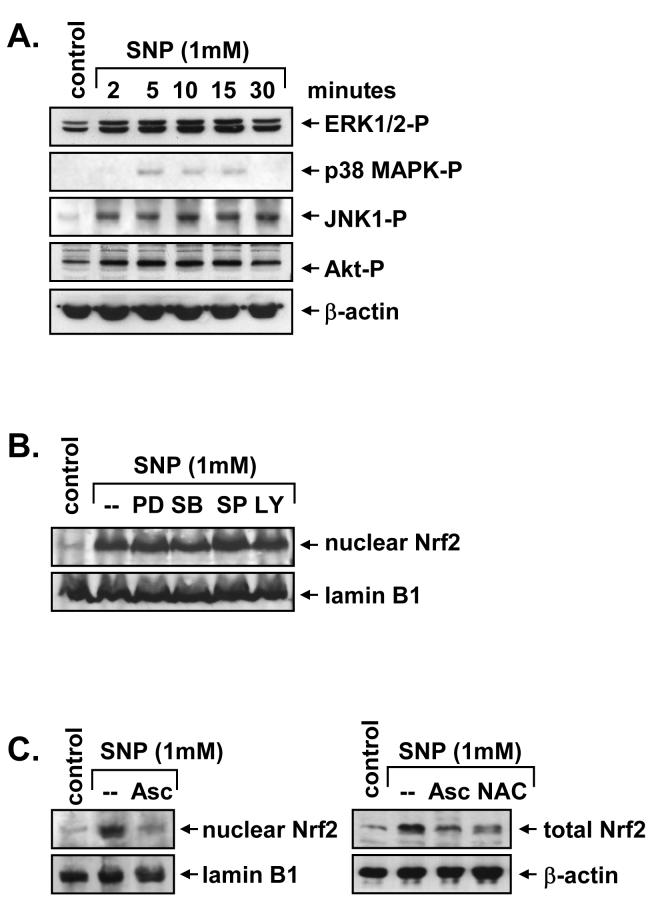
In subsequent experiments, we determined the upstream signaling pathway that mediates the activation of Nrf2. Since MAPK and PI3K have been implicated in the activation of Nrf2 (25), the involvement of these proteins was examined. Treatment of vascular SMC with SNP resulted in the rapid activation of ERK1/2, JNK1, p38 MAPK, and PI3K, as reflected by the phosphorylation of Akt (Figure 4A). Activation of these proteins was observed within 5 minutes, and with the exception of p38 MAPK, persisted for 30 minutes following SNP exposure. However, pretreatment of vascular SMC with the ERK inhibitor, U0126, the p38 MAPK inhibitor, SB203580, the JNK inhibitor, SP600125, or the PI3K inhibitor, LY294002, had no effect on SNP-mediated nuclear Nrf2 accumulation (Figure 4B). The effectiveness of the pharmacological inhibitors for their respective enzymes was confirmed in SNP-treated SMC (data not shown). Alternatively, since oxidative stress is a well characterized activator of Nrf2 (17,25) and inducer of HO-1 (26) the role of reactive oxygen was also investigated. Interestingly, pretreatment of SMC with the antioxidants, ascorbate and N-acetyl-L-cysteine, completely abrogated the increase in both nuclear and total Nrf2 evoked by SNP (Figure 4C).
4. Regulation of NO-mediated Nrf2 activation in vascular SMC.
A. NO stimulates MAPK and PI3K activation. SMC were treated with sodium nitroprusside (SNP; 1 mM) and the activation of ERK1/2, p38 MAPK, JNK1 and Akt monitored by western blotting using phospho-specific antibodies. B. Effect of MAPK and PI3K inhibitors on NO-mediated Nrf2 activation. SMC were pretreated with U0126 (30 μM), SB203580 (30 μM), SP600125 (20 μM) or LY294002 (10 μM) for 2 hours and then treated with SNP (1 mM) for 4 hours. C. Antioxidants inhibit NO-mediated Nrf2 activation. SMC were pretreated with ascorbate (Asc; 1 mM) or N-acetyl-L-cysteine (NAC; 10 mM) for 2 hours and then treated with SNP (1 mM) for 4 hours. Similar findings were observed in 3 separate experiments.
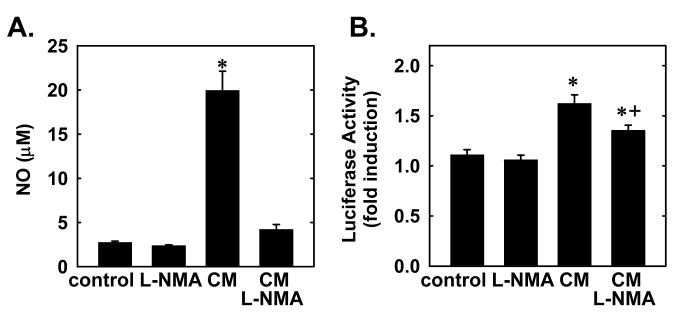
We next determined whether endogenously derived NO also induces HO-1 promoter activity. Treatment of vascular SMC with a cytokine mixture consisting of interleukin-1β (10 ng/ml), tumor necrosis factor-α (20 ng/ml), or interferon-γ (200 U/ml) resulted in a significant increase in NO synthesis and this was associated with an approximate 2-fold increase in HO-1 promoter activity (Figure 5). Interestingly, the NO synthase inhibitor methyl-L-arginine (1 mM) blocked the cytokine-mediated synthesis of NO and, in part, the increase HO-1 promoter activity (Figure 5).
5. Endogenously derived NO stimulates HO-1 promoter activity in vascular SMC.
A. NO synthesis following treatment of SMC with a cytokine mixture (CM) consisting of interleukin-1β (10 ng/ml), tumor necrosis factor-α (20 ng/ml), or interferon-γ (200 U/ml) for 24 hours in the presence or absence of methyl-L-arginine (L-NMA; 1 mM). B. HO-1 promoter activity following treatment of SMC with the CM for 24 hours in the presence and absence of L-NMA (1 mM). Results are expressed as the means ± SEM of 6 separate experiments. *Statistically significant increase in NO synthesis or HO-1 promoter activity.
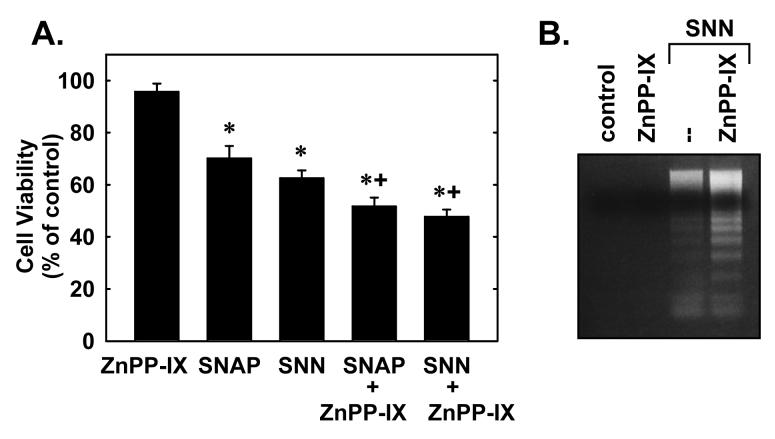
Finally, the functional role of HO-1 during nitrosative stress was examined. Treatment of SMC with SNAP or SNN induced rounding and blebbing of SMC and this was associated with a significant decrease in cell viability (Figure 6A) and pronounced DNA fragmentation (Figure 6B). Interestingly, the addition of the HO inhibitor zinc protoporphyrin-IX enhanced SNAP or SNN-mediated cell death and DNA laddering (Figure 6A and B). In the absence of NO however, zinc protoporphryin-IX failed to stimulate cell death or DNA fragmentation.
6. HO-1 promotes vascular SMC survival during nitrosative stress.
A. Cell viability following treatment of SMC with S-nitroso-N-acetyl-penicillamine (SNAP; 1mM) or spermine NONOate (SNN; 1 mM) for 48 hours in the presence or absence of zinc protoporphyrin-IX (ZnPP-IX; 10 μM). Results are expressed as the means ± SEM of 6 separate experiments. *Statistically significant decrease in cell viability. †Statistically significant effect of ZnPP-IX. B. DNA fragmentation following treatment of SMC with SNN (1 mM) for 48 hours in the presence or absence of ZnPP-IX (10 μM). Similar findings were observed in 3 separate experiments.
DISCUSSION
In the present study, we characterized the signaling pathway by which NO stimulates HO-1 gene transcription in vascular SMC. We found that diverse reactive nitrogen species stimulate HO-1 mRNA expression and promoter activity by inducing the formation of the Nrf2/ARE complex. The activation of Nrf2 is independent of the MAPK or PI3K pathways but is dependent on oxidative stress. In addition, we show that the induction of HO-1 by NO functions in an autocrine manner to limit SMC apoptosis. These findings implicate the Nrf2-HO-1 signaling cascade as a crucial modulator of vascular cell survival during periods of nitrosative stress.
Treatment of vascular SMC with a number of structurally dissimilar NO donors stimulate HO-1 mRNA expression; however, the magnitude of HO-1 induction is quite different with SNP, SNAP, and SNN being the more potent inducers. Interestingly, this contrasts with the modest, but comparable, stimulation of HO-1 promoter activity (2 to 4-fold) evoked by all the NO donors, suggesting additional mechanisms by which some NO donors may regulate HO-1 expression. In this respect, SNN has also been shown to increase HO-1 mRNA stability in several cell types, including vascular SMC (8,14) Furthermore, some NO donors may induce HO-1 mRNA expression via a NO-independent pathway that may exert posttranscriptional effects. In particular, SNP-mediated stimulation of HO-1 expression and activity is partially antagonized by the iron chelator deferoxamine, suggesting a role for this metal in regulating HO-1 expression (6,27). Our finding that nitrosative stress stimulates the murine HO-1 promoter in vascular SMC is consistent with most, but not all, studies demonstrating that NO activates the human HO-1 promoter (28-31).
Although the induction of HO-1 by NO is widely appreciated, the signaling pathways leading to the transcriptional activation of HO-1 have not been fully characterized. Since the activation of soluble guanylate cyclase and the consequent formation of cGMP mediate many of the biological actions of NO, we initially determined the involvement of this pathway. However, treatment of SMC with the selective inhibitor of soluble guanylate cyclase, ODQ, or with the protein kinase G inhibitor, KT5823, did not prevent the induction of HO-1 promoter activity by NO. Failure of these inhibitors of the cGMP pathway to block HO-1 gene transcription is in-line with previous work showing that lipophilic cGMP analogues are unable to stimulate HO-1 expression in vascular cells (6-8). Instead, we found that activation of HO-1 transcription by nitrosative stress occurs via the Nrf2/ARE complex since mutation of the AREs abrogates the stimulation of HO-1 promoter activity by NO. Furthermore, NO stimulates Nrf2 expression and the translocation of Nrf2 from the cytosol to the nucleus. Moreover, EMSA and ChIP assays detected increased Nrf2 binding to the HO-1 ARE and E1 enhancer, respectively, following NO exposure. Finally, a role for Nrf2 in the induction of HO-1 promoter activity is also demonstrated by the ability of a dominant-negative mutant of Nrf2 to abolish the activation of HO-1 promoter activity in response to NO.
Our finding that NO stimulates the activation of Nrf2 in vascular SMC extends recent studies showing that NO induces the nuclear translocation of Nrf2 in endothelial, neuroblastoma, and cardiac cells (32-34). Since MAPK and PI3K have been reported to activate Nrf2, the role of these kinases in regulating Nrf2 activation was investigated. However, pharmacological inhibition of either the MAPK or PI3K pathway had no effect on the mobilization of Nrf2 by NO. This contrasts with the findings in vascular endothelium, where NO-mediated Nrf2 activation is partially dependent on MAPK activation, suggesting that MAPK may activate Nrf2 in a cell-specific manner (32). Interestingly, we found that the antioxidant-reducing agents ascorbate and N-acetyl-L-cysteine, are capable of blocking the activation of Nrf2 by NO in SMC. The observation that antioxidants prevent the activation of Nrf2 by NO in this study is consistent with previous reports showing that antioxidants suppress NO-mediated HO-1 gene expression in vascular cells (8,32,35). The mechanism by which oxidative stress activates Nrf2 is not completely known but several cysteine residues in Keap1 are capable of undergoing redox-dependent alterations that may result in the liberation of Nrf2 and/or the inhibition of Keap1-dependent ubiquitination and degradation of Nrf2 (16,17).
The physiological relevance of NO-mediated stimulation of HO-1 gene transcription is supported by the capacity of endogenously released NO to stimulate HO-1 promoter activity. Treatment of SMC with a cytokine mixture composed of interleukin-1β, tumor necrosis factor-α, and interferon-γ stimulates the expression of inducible NO synthase and a significant increase in NO production (6-8,10) which is associated with a significant rise in HO-1 promoter activity. Although the NO synthase inhibitor, methyl-L-arginine, prevents the cytokine-mediated increase in NO formation, it only partially offsets the increase in HO-1 promoter activity. This is consistent with our previous work (7) showing that NO synthase inhibition partially blocks cytokine-mediated HO-1 protein expression and suggests the presence of an additional NO-insensitive pathway by which cytokines can induce HO-1 gene expression in these cells. In this regard, interleukin-1β has been shown to stimulate HO-1 expression in a NO-independent manner (36).
The induction of HO-1 in vascular cells likely represents an important adaptive response to ameliorate the deleterious effects of nitrosative stress. Consistent with earlier reports (37,38), we found that NO induces apoptosis in vascular SMC. Interestingly, NO-mediated SMC apoptosis is potentiated following the inhibition of HO activity, indicating that the induction of HO-1 by NO promotes cell survival. The cytoprotective action of HO-1 is likely mediated via the release of carbon monoxide since we previously reported that carbon monoxide is the only HO-1 product capable of inhibiting SMC apoptosis (10,20). Consistent with this notion, carbon monoxide has recently been demonstrated to protect against the cytotoxicity evoked by NO in an epithelial cell line while the bile pigments, biliverdin and bilirubin, failed to confer resistance to NO-mediated toxicity (39). Noteably, stable expression of dnNrf2 or knockdown of Nrf2 expression by RNA interference in neuroblastoma cells sensitizes them to NO-induced apoptosis (33). In contrast, overexpression of wild type Nrf2 protects these cells from the cytotoxic action of NO. Thus, Nrf2 may function as a key transcription factor in the cytoprotection of tissues against nitrosative stress.
The ability of NO to induce HO-1 may be of pathophysiological significance. Both inducible NO synthase and HO-1 are induced following balloon angioplasty or in atherosclerotic lesions (40-42). Under these conditions, the expression of HO-1 may serve to protect against the detrimental effects associated with the high output NO synthase enzyme. Given recent work identifying vascular SMC apoptosis as a critical process in mediating plaque vulnerability (43), the capacity of HO-1 to block NO-mediated SMC apoptosis may contribute to plaque stabilization. Consistent with this notion, a recent clinical study found that HO-1 expression is more prevalent in carotid atherosclerotic plaques obtained from asymptomatic patients (44). Furthermore, the antiapoptotic action of HO-1 may also govern lesion size by attenuating the development of the acellular lipid core (45). Thus, the ability of HO-1 to block SMC apoptosis as well as SMC proliferation (46) may play an important role in promoting homeostasis at sites of arterial injury by limiting intimal thickening and plaque size and vulnerability.
In conclusion, the present study demonstrates that NO induces HO-1 gene transcription via the Nrf2/ARE complex in vascular SMC. In addition, it found that the activation of Nrf2 by NO is dependent on oxidative stress and that the induction of HO-1 functions in autocrine fashion to counteract NO-mediated apoptosis.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants HL59976, HL74996, and HL62467.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog. Cardiovasc. Dis. 1995;35:87–104. doi: 10.1016/s0033-0620(05)80001-5. [DOI] [PubMed] [Google Scholar]
- 2.Li C-Q, Wogan GN. Nitric oxide as a modulator of apoptosis. Cancer Lett. 2005;226:1–15. doi: 10.1016/j.canlet.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Brune B, von Knethen A, Sandau KB. Nitric oxide (NO): an effector of apoptosis. Cell Death Differ. 1999;6:969–75. doi: 10.1038/sj.cdd.4400582. [DOI] [PubMed] [Google Scholar]
- 4.Ho WP, Chen TL, Chiu WT, Tai YT, Chen RM. Nitric oxide induces osteoblast apoptosis through a mitochondria-dependent pathway. Ann. NY Acad. Sci. 2005;1042:460–70. doi: 10.1196/annals.1338.039. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y-M, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. 1999;84:253–6. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- 6.Motterlini R, Foresti R, Intaglietta M, Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am. J. Physiol. Heart Circ. Physiol. 1996;270:H107–14. doi: 10.1152/ajpheart.1996.270.1.H107. [DOI] [PubMed] [Google Scholar]
- 7.Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase and carbon monoxide production in vascular smooth muscle cells. Circ. Res. 1997;80:557–64. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- 8.Hartsfield CL, Alam J, Cook JL, Choi AM. Regulation of heme oxygenase-1 gene expression in vascular smooth muscle cells by nitric oxide. Am. J. Physiol. Lung Cell Mol. Physiol. 1997:L980–8. doi: 10.1152/ajplung.1997.273.5.L980. [DOI] [PubMed] [Google Scholar]
- 9.Tenhunen, Marver HS, Schmidt R. The enzymatic conversion of heme to bilirubin by microvascular heme oxygenase. Proc. Natl. Acad. Sci. USA. 1968;61:748–55. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc. Res. 2002;55:396–405. doi: 10.1016/s0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 11.Durante W. Heme oxygenase-1 growth control and its clinical application to vascular disease. J. Cell. Physiol. 2003;195:373–82. doi: 10.1002/jcp.10274. [DOI] [PubMed] [Google Scholar]
- 12.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Rad. Biol. Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Naughton P, Foresti R, Bains SK, Hoque M, Green CJ, Motterlini R. Induction of heme oxygenase 1 by nitrosative stress: a role for nitroxyl anion. J. Biol. Chem. 2002;277:40666–74. doi: 10.1074/jbc.M203863200. [DOI] [PubMed] [Google Scholar]
- 14.Bouton C, Demple B. Nitric oxide-inducible expression of heme oxygenase-1 in human cells: translation-independent stabilization of the mRNA and evidence for direct action of nitric oxide. J. Biol. Chem. 2000;275:32688–93. doi: 10.1074/jbc.275.42.32688. [DOI] [PubMed] [Google Scholar]
- 15.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr. Pharm. Des. 2003;9:2499–511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 16.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–91. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang DD, Hannink M. Distinct cysteine residues in keap1 are required for keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–51. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X-L, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells: a novel anti-inflammatory mechanism. J. Biol. Chem. 2003;278:703–11. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 19.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, et al. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004;94:609–16. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Peyton KJ, Ensenat D, Wang H, Schafer AI, Alam J, et al. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle: role in cell survival. J. Biol. Chem. 2005;280:872–7. doi: 10.1074/jbc.M410413200. [DOI] [PubMed] [Google Scholar]
- 21.Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J. Biol. Chem. 2003;278:48021–9. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- 22.Cho Y, Jedlicka AE, Reddy SP, Kensler M, Yamamoto M, Zhang LY, et al. Role of Nrf2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2002;26:175–82. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 23.Peyton KJ, Reyna SV, Chapman GB, Ensenat D, Liu X, Wang H, et al. Heme oxygenase-1-derived carbon monoxide is an inhibitor of vascular smooth muscle cell growth. Blood. 2002;99:4443–8. doi: 10.1182/blood.v99.12.4443. [DOI] [PubMed] [Google Scholar]
- 24.Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, et al. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells: role of p38 kinase and Nrf2 transcription factor. J. Biol. Chem. 2000;275:27694–702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Rad. Biol. Med. 2004;37:433–41. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid. Redox Signal. 2005;7:80–91. doi: 10.1089/ars.2005.7.80. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Tsoy I, Park MK, Lee YS, Lee JH, Seo HG, et al. Iron released by SNP contributes to HO-1 induction via the cAMP-PKA-MAPK pathway in RAW 264.7 cells. Mol. Pharmacol. 2006;69:1633–40. doi: 10.1124/mol.105.020487. [DOI] [PubMed] [Google Scholar]
- 28.Marquis JC, Demple B. Complex genetic response of human cells to sublethal levels of pure nitric oxide. Cancer Res. 1998;58:3435–40. [PubMed] [Google Scholar]
- 29.Hara E, Takahashi K, Takeda K, Nakayama M, Yoshizawa M, Fujita H, et al. Induction of heme oxygenase-1 as a response in sensing the signals evoked by distinct nitric oxide donors. Biochem. Pharmacol. 1999;58:227–36. doi: 10.1016/s0006-2952(99)00097-0. [DOI] [PubMed] [Google Scholar]
- 30.Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am. J. Physiol. Renal Physiol. 2004;286:F425–41. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- 31.Lee B-S, Heo JH, Kim YM, Shim SM, Pae H-O, Kim Y-M, et al. Carbon monoxide mediates heme oxygenase-1 induction via Nrf2 activation in hepatoma cells. Biochem. Biophys. Res. Commun. 2006;343:965–72. doi: 10.1016/j.bbrc.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 32.Buckley BJ, Marshall ZM, Whorton AR. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem. Biophys. Res. Commun. 2003;307:973–9. doi: 10.1016/s0006-291x(03)01308-1. [DOI] [PubMed] [Google Scholar]
- 33.Dhakshinamoorthy S, Porter AG. Nitric oxide-induced transcriptional upregulation of protective genes by Nrf2 via the antioxidant responsive element counteracts apoptosis of neuroblastoma cells. J. Biol. Chem. 2004;279:20096–107. doi: 10.1074/jbc.M312492200. [DOI] [PubMed] [Google Scholar]
- 34.Naughton P, Hoque M, Green CJ, Foresti R, Motterlini R. Interaction of heme with nitroxyl or nitric oxide amplifies heme oxygenase-1 induction: involvement of the transcription factor Nrf2. Cell. Mol. Biol. (Noisy-le-grand) 2002;48:885–94. [PubMed] [Google Scholar]
- 35.Foresti R, Clark JE, Green CJ, Motterlini R. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells: involvement of superoxide and peroxynitrite. J. Biol. Chem. 1997;272:18411–7. doi: 10.1074/jbc.272.29.18411. [DOI] [PubMed] [Google Scholar]
- 36.Yet SF, Pellacani A, Patterson C, Tan L, Folta SC, Foster L, et al. Induction of heme oxygenase-1 expression in vascular smooth muscle cells: a link to endotoxic shock. J. Biol. Chem. 1997;272:4295–301. doi: 10.1074/jbc.272.7.4295. [DOI] [PubMed] [Google Scholar]
- 37.Fukuo K, Hata S, Suhara T, Nakahashi T, Shinto Y, Tsujimoto Y, et al. Nitric oxide induces upregulation of Fas and apoptosis in vascular smooth muscle. Hypertension. 1996;27:823–6. doi: 10.1161/01.hyp.27.3.823. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Z, Francis CE, Welch G, Loscalzo J, Ravid K. Reduced glutathione prevents nitric oxide-induced apoptosis in vascular smooth muscle cells. Biochim. Biophys. Acta. 1997;1359:143–52. doi: 10.1016/s0167-4889(97)00093-1. [DOI] [PubMed] [Google Scholar]
- 39.Reiter TA, Demple B. Carbon monoxide mediates protection against nitric oxide toxicity in Hela cells. Free Rad. Biol. Med. 2005;39:1075–88. doi: 10.1016/j.freeradbiomed.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Tulis DA, Durante W, Peyton KJ, Chapman GB, Evans AJ, Schafer AI. YC-1, a benzyl indazole derivative, stimulates vascular cGMP and inhibits neointima formation. Biochem. Biophys. Res. Commun. 2000;279:646–52. doi: 10.1006/bbrc.2000.3942. [DOI] [PubMed] [Google Scholar]
- 41.Luoma JS, Stralin P, Marklund SL, Hiltunen TP, Sarkioja T, Yla-Herttuala S. Expression and extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins. Arterioscler. Thromb. Vasc. Biol. 1998;18:157–67. doi: 10.1161/01.atv.18.2.157. [DOI] [PubMed] [Google Scholar]
- 42.Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am. J. Pathol. 1998;152:711–720. [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 2006;12:1075–80. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 44.Ameriso SF, Villamil AR, Zedda C, Parodi JC, Garrido S, Sarchi MI, et al. Heme oxygenase-1 is expressed in carotid atherosclerotic plaques infected by Helicobacter pylori and is more prevalent in asymptomatic subjects. Stroke. 2005:1896–90. doi: 10.1161/01.STR.0000177494.43587.9e. [DOI] [PubMed] [Google Scholar]
- 45.Mallat Z, Tedgui A. Apoptosis in the vasculature: mechanisms and functional importance. Br. J. Pharmacol. 2000;130:947–962. doi: 10.1038/sj.bjp.0703407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, et al. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat. Med. 2001;7:693–8. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]