Abstract
In mammals, the circadian oscillators that drive daily behavioral and endocrine rhythms are located in the hypothalamic suprachiasmatic nucleus (SCN). While the SCN is anatomically well-situated to receive and transmit temporal cues to the rest of the brain and periphery, there are many holes in our understanding of how this temporal regulation occurs. Unanswered questions include how cell autonomous circadian oscillations within the SCN remain synchronized to each other as well as communicate temporal information to downstream targets. In recent years, it has become clear that neuropeptides are critically involved in circadian timekeeping. One such neuropeptide, vasoactive intestinal peptide (VIP), defines a cell population within the SCN and is likely used as a signaling molecule by these neurons. Converging lines of evidence suggest that the loss of VIP or its receptor has a major influence on the ability of the SCN neurons to generate circadian oscillations as well as synchronize these cellular oscillations. VIP, acting through the VPAC2 receptor, exerts these effects in the SCN by activating intracellular signaling pathways and, consequently, modulating synaptic transmission and intrinsic membrane currents. Anatomical evidence suggests that these VIP expressing neurons connect both directly and indirectly to endocrine and other output targets. Striking similarities exist between the role of VIP in mammals and the role of Pigment Dispersing Factor (PDF), a functionally related neuropeptide, in the Drosophila circadian system. Work in both mammals and insects suggests that further research into neuropeptide function is necessary to understand how circadian oscillators work as a coordinated system to impose a temporal structure on physiological processes within the organism.
I. Introduction
The outputs of endocrine systems, as measured at the level of secreted hormones, are rarely static through time. Some of this temporal variation is due to episodic challenges or changes in the environment that require physiological responses. Other changes are quite predictable as many hormones exhibit daily rhythms in synthesis and secretion. These rhythms are driven by endogenous timing systems referred to as circadian oscillators. When appropriately synchronized to the environment, these circadian oscillators provide temporal structure to an organism’s physiological processes and allow organisms to anticipate predictable changes in the environment. This temporal organization is important for many behavioral and endocrine outputs, including reproductive functions. In mammals, the part of the nervous system responsible for most circadian behavior can be localized to a pair of structures in the hypothalamus known as the suprachiasmatic nucleus (SCN). Early lesion studies (Moore and Eichler, 1972; Stephan and Zucker, 1972) provided the first information localizing circadian clock function in mammals by establishing that the SCN is necessary for the expression of certain behavioral and endocrine rhythms. Subsequent studies showed that SCN cells are rhythmic both in vivo and in vitro, these cells begin to oscillate in utero, they show circadian cycles of metabolism, and transplantation of fetal SCN restores rhythmicity in SCN-lesioned animals (see Weaver, 1998; van Esseveldt et al., 2000 for reviews). Other circadian oscillators, for instance in the liver, have also been discovered, and an interplay between the SCN and these peripheral oscillators govern physiological, behavioral, and endocrine rhythms. Despite the existence of other circadian oscillators, the clock within the SCN is still considered the “master” oscillator that coordinates daily rhythms in the mammalian body (see Reppert and Weaver, 2002).
The SCN is a bilaterally paired nucleus made up of tightly compacted, small-diameter neurons just lateral to the third ventricle atop the optic chiasm (van den Pol, 1980). Anatomical studies generally support the division of the SCN into at least two subdivisions including a ventrolateral (core) and dorsomedial (shell) (Morin et al., 2006; Fig. 1). The ventrolateral neurons are thought to act as an integrator of external input, receiving information from three major pathways: the retino-hypothalamic tract (RHT), the geniculohypothalamic tract (GHT) from the intergeniculate leaflet of the thalamus (IGL) and from the raphe nuclei (Morin and Allen, 2005). RHT neurons transmit light information to the SCN while GHT and raphe inputs are thought to modulate light information and transmit non-photic signals. The ventrolateral neurons must integrate the environmental information and relay this information to the rest of the SCN. In contrast, neurons of the dorsomedial shell appear to generate the most robust circadian oscillations at least at the level of gene expression (e.g. Hamada et al., 2001; Yan and Okamura, 2002; Nakamura et al., 2005). Most afferent projections of this area come from other areas in the hypothalamus, basal forebrain, brainstem, and limbic cortex (Gooley and Saper, 2005). The fact that many core projections terminate on shell neurons further supports the idea that interplay between these two centers is responsible for the output of circadian information from the SCN (Antle and Silver, 2005). The outputs of the SCN largely travel to other hypothalamic regions including the subparaventricular zone (SPZ) and the dorsomedial nucleus (Abrahamson and Moore, 2001; Deurveilher and Semba, 2005). These hypothalamic relay nuclei send projections throughout the central nervous system and endocrine system, providing multiple pathways by which the SCN can convey temporal information to the brain and body (Deuveilher and Semba, 2005; Kalsbeek and Buijs, 2002; Kalsbeek et al., 2006; Fig. 1). In some cases, the anatomy of the circuits responsible for specific outputs is beginning to be understood. For example, neurons within the dorsal SPZ appear to be particularly important for circadian rhythms of sleep and waking while ventral SPZ neurons appear to be particularly important for rhythmic feeding and adrenal functions (Saper et al., 2005).
Figure 1.

illustrates a simplified model of a circuit within the SCN. Light signals are detected and transmitted through neurons in the retina directly to the SCN via the retinohypothalamic tract (RHT). These neurons use glutamate (Glut) and PACAP as transmitters. The neurons in the ventral region of the SCN receive most of this retinal information along with information from the raphe nuclei, carried by serotonin (5HT), and the intergeniculate leaflet of the thalamus (IGL), carried by neuropeptide Y (NPY). These ventral neurons, in turn, use GABA and VIP to communicate with other neurons within the different SCN cell populations, including the vasopressin (VP) expressing neurons of the dorsal SCN. Neural outputs from the SCN can arise from the ventral or dorsal cell populations, but most projections are to a few key relay nuclei within the hypothalamus. While there are a few reported direct connections from the SCN to some neuroendocrine outputs, strong evidence exists for an indirect endocrine output pathway that utilizes these hypothalamic relay nuclei and in some cases, the autonomic nervous system as well. 3V, third ventricle; OC, optic chiasm; CRH, corticotrophin-releasing hormone; TRH, thyrotropin-releasing hormone; GnRH, gonadotropin releasing hormone; DMH, dorsomedial nucleus of the hypothalamus; SPZ, subparaventricular zone; PVN, paraventricular nucleus of the hypothalamus; MPOA, medial preoptic area; PNS, parasympathetic nervous system; SNS, sympathetic nervous system.
Despite recent advances in the field, a detailed understanding of how temporal information is communicated both to and from the SCN is not understood. It is known that there are endogenously oscillating neurons within the SCN that may act as pacemakers, and a great deal has been uncovered regarding the molecular basis of rhythm generation in individual SCN neurons. However, these individual pacemaking neurons, when separated in a dish, have different phases (Welsh et al., 1995). Therefore, in a non-dissociated SCN, there must be some agent or mechanism involved in synchronizing these neurons to provide a robust population signal from the SCN. This synchronization would seem important for the spread of timekeeping cues across SCN neurons as well as for the presentation of a clear output signal from the SCN.
One key to understanding how neurons in the SCN communicate temporal information may lie within the different neuropeptides expressed in the SCN. Cell populations within the SCN express distinct neuropeptides (Fig. 1). For example, many neurons within the ventrolateral SCN express the neuropeptide VIP while many neurons in the dorsomedial shell express vasopressin. In the hamster, there is also good evidence for a third population of calbindin positive cells that may function to “gate” the photic response of the SCN population (Antle and Silver, 2005). Over the last few years, a number of studies have examined the role of neuropeptides in cellular communication and function of the circadian system. Nevertheless, it is probably fair to say that we are still in the early stages of understanding the physiological roles played by these peptides in the SCN or in the nervous system in general. We believe that the neuropeptide VIP: 1) acts as a major synchronizing agent among SCN neurons, 2) modulates the molecular oscillations within individual oscillators and 3) synchronizes SCN neurons with light cues. In this review, we seek to provide a summary of some of the field’s recent progress in understanding the role of VIP in the circadian system of mammals. Utilizing data from anatomical, genetic, and physiological studies, we will propose a model of how VIP communicates temporal cues through the SCN. As part of this summary, we will also compare the role of VIP in mammals to the role of Pigment Dispersing Factor (PDF), a functionally related neuropeptide, in insect circadian systems.
II. Evidence that VIP and VPAC2R are highly expressed in the SCN
VIP is a neuropeptide in the secretin superfamily (reviewed in Vaudry et al., 2000), which includes structurally similar pituitary adenylate cyclase-activating polypeptide (PACAP), glucagon and growth hormone-releasing hormone. Comparative studies have revealed members of the secretin superfamily expressed in the oldest vertebrates, jawless fish (Irwin et al., 1999). In mammals, VIP is expressed in specific subpopulations of neurons in the brain and peripheral nervous system as well as other tissues in the body. Anatomical organization of VIP and its receptors provided early indications that this peptide may be important for circadian function. Studies in the rat revealed that the SCN had large amounts of VIP- and VIP-receptor-containing neurons (Besson et al., 1986; Roberts et al., 1980; Vertongen et al., 1998). These VIP-neurons are primarily located in the ventrolateral aspect of the nucleus (Abrahamson and Moore, 2001; Ibata et al., 1989). Neurons in this region receive retinal input from the RHT and express both VIP and GABA (e.g. Buijs et al., 1995). At least one study in rats has directly demonstrated the termination of retinal afferents on VIP expressing cells (Tanaka et al., 1993). Thus, the VIP expressing cells in the ventrolateral SCN directly receive photic information via the RHT. These retino-recipient cells must then convey this environmental information to the rest of the SCN (Fig. 1).
VIP binding sites are found early in vertebrate brain phylogeny with binding described in the anuran, reptilian, avian, and mammalian brains (Dietl et al., 1990). Two receptors, encoded by distinct genes, bind VIP with high affinity: VPAC1R and VPAC2R (Ishihara et al., 1992; Lutz et al., 1993; Usdin et al., 1994). No known receptors specifically bind VIP without binding PACAP. VPAC1R and VPAC2R exhibit widespread expression in the brain and mediate most or all of the biological actions of these peptides. The SCN may be the most abundant site of expression of the VPAC2R in the central nervous system (Sheward et al., 1995; Usdin et al., 1994; Cagampang et al., 1998; Vertongen et al., 1998). In the mouse SCN, it has been estimated that VPAC2R are co-expressed with ≈30% of VIP expressing and ≈50% of the vasopressin expressing neurons (Kallo et al., 2004b). Clearly, the VIP signal does not have to travel far to influence SCN neurons in both the ventrolateral and dorsomedial cell populations. The presence of the VPAC2R in the ventrolateral region also suggests that VIP signals may “feed back” to regulate VIP secreting cells (Fig. 1).
While not highly studied, there is some evidence that the levels of VIP and its receptor vary with a circadian oscillation. For example, measurements of VIP release from rat SCN slice cultures reveal circadian oscillations that continue for a number of cycles in constant conditions (Shinohara et al., 2000). Furthermore, in the mouse SCN, the levels of VIP mRNA show a circadian rhythm (Dardente et al., 2004). Similarly, the mRNA coding for the VPAC2R varies with a daily cycle in rodents (Shinohara et al., 1999; Kallo et al., 2004a). The functional significance of these rhythms is not yet known. For example, it may be that the rhythms in peptide levels are responsible for driving outputs from the circadian system. Alternatively, the presence (but not the rhythm) of the peptide and its receptor may be sufficient to fulfill the functions of this signaling system.
As described above, VIP is expressed in a subpopulation of cells within the SCN. Our starting assumption is that these neurons are using this peptide to communicate with specific post-synaptic targets. For example, the depolarization of the VIP expressing neurons by light causes the release of VIP and co-transmitters including GABA, and these neurotransmitters alter the membrane properties of the next set of neurons in the circuit via activation of VPAC2R. This assumption has not been directly tested within the SCN and we do not yet know if VIP directly mediates cell-to-cell communication within the SCN. At this point, it is equally plausible that VIP functions more as a paracrine signal acting at sites more distant then just the adjacent postsynaptic neurons.
III. Genetic models and the exploration of the functional roles of VIP and VPAC2R in the circadian system
Recently, transgenic mouse models have provided additional tools to understand the role of VIP and the VPAC2R in the circadian system in vivo (see Shen et al., 2000; Harmar et al., 2002; Colwell et al., 2003). The development of these models has had a major impact on research in this area. Figures 2 and 3 illustrate some of the phenotypes expressed by the VIP- and VPAC2R-deficient mice. We would like to start by raising a couple of points that we feel are important to keep in mind when interpreting the experiments with these transgenic models. First, it is possible that the loss of PACAP signaling could contribute to the circadian phenotype associated with Vipr2 −/− mice. The VPAC2R also recognizes the peptide PACAP with the same affinity as VIP. Analysis of PACAP-deficient mice suggests that PACAP is not important for the generation of rhythmicity, and these mice primarily present abnormalities in the light response of the circadian system (Colwell et al., 2004). Still, the loss of the PACAP signal could contribute to the more severe phenotype seen with the Vipr2 −/− mice when compared to the Vip −/− mice. This brings us to a point about the VIP-deficient mice. The VIP precursor polypeptide contains sequences encoding the peptide histidine-isoleucine (PHI, Linder et al., 1987). The construct used in making the VIP-deficient mice also eliminates PHI and the loss of PHI could contribute to some of the phenotype associated with the Vip −/− mice. The mRNA coding for VIP and PHI are not differentially localized (Linder et al., 1987) and no putative PHI receptors have been described in the mammalian nervous system. Until more information emerges, we are inclined to view the circadian phenotypes as being solely due to the loss of VIP. These caveats point to the advantages our field has enjoyed in having the ability to analyze and compare mice missing either VIP or VPAC2R.
Figure 2.
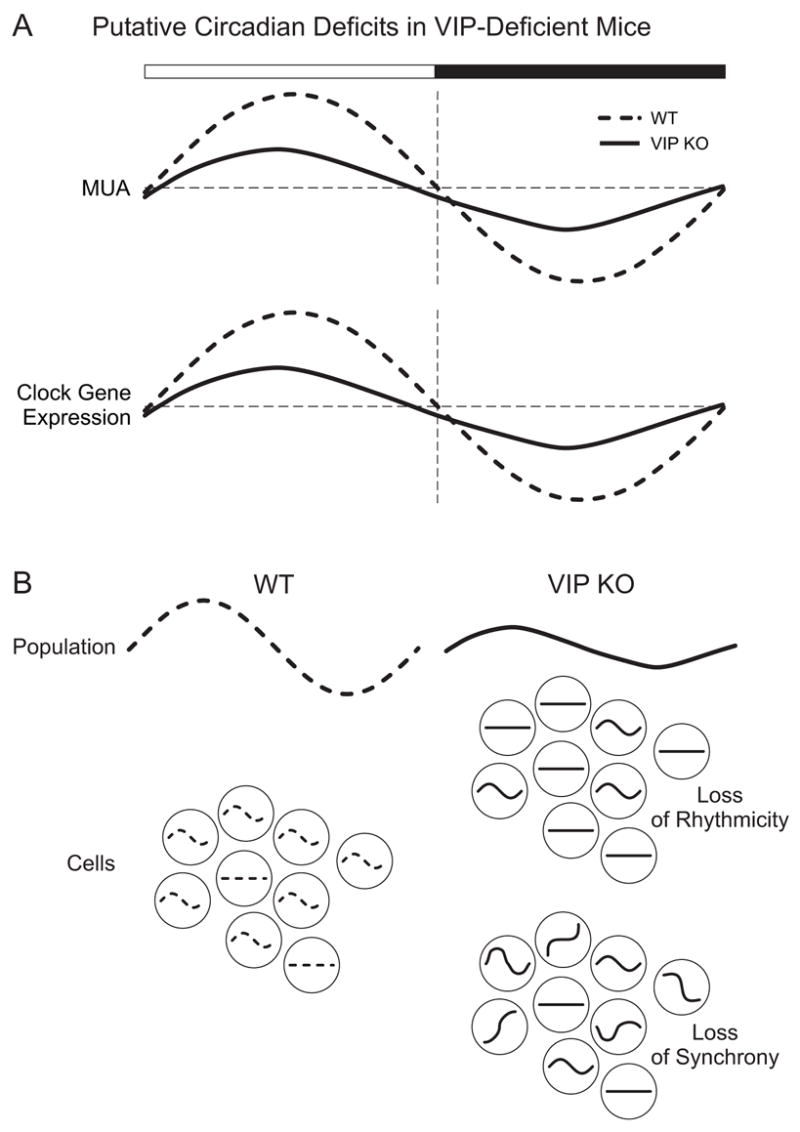
illustrates the putative deficits in the SCN of VIP-deficient mice. A) Based on the published results with Vipr2 −/− mice (Harmar et al, 2002) as well as our own unpublished data, we expect that the rhythms in extracellularly recorded multi-unit activity (MUA) and clock gene expression will be reduced in the mutant mice. B) In this schematic, we seek to illustrate two explanations for the loss of rhythms in the SCN cell population of mutant (solid lines) compared to WT (dashed lines) mice. The sine waves represent rhythmic cells while the straight line represents arrhythmic cells. One possibility is that the single cell oscillators may lose their ability to generate oscillations (proposed by Harmar et al., 2002). Alternatively, the single cell oscillators may lose their synchrony (proposed by Colwell et al., 2003). There is evidence that suggests that both factors are at work in the Vip −/− and Vipr2 −/− mice (Aton et al., 2005; Maywood et al., 2006; Brown et al., 2007).
Figure 3.

illustrates the key behavioral deficits exhibited by the VIP-deficient mice (see Colwell et al., 2003). The black bars represent the daily wheel running activity with successive days plotted one on top of the other. WT mice (left panel) exhibit stable activity patterns in an LD cycle. When released into DD on day 8, the mice exhibit a period of ≈23.5 hrs. Exposure to a brief light pulse at CT 16 (asterisk) will reset the phase of the activity cycle. VIP KO mice (right panel) in a LD cycle are entrained though activity onset is variable compared to controls. When placed into DD, the mice start their activity from a phase 8 to 10 hrs earlier compared to WT. The period of the activity rhythm is ≈22.5 hrs. Many, but not all of the Vip −/− mice, eventually become arrhythmic in DD. The Vipr2 −/− mice exhibit a similar phenotype (see Harmar et al., 2002). Furthermore, VIP KO mice fail to shift their behavioral rhythms in response to a phase-delaying light pulse during early subjective night, as indicated by the yellow asterisk.
Evidence that VIP and VPAC2R are critical for the generation of coherent circadian oscillations in behavior
All of the VIP- and VPAC2R-deficient mice exhibit disruptions in their ability to express a coherent circadian rhythm in constant conditions (Harmar et al., 2002; Colwell et al., 2003; Fig. 3). In many cases, the transgenic mice exhibit wheel-running behavior that is arrhythmic on the circadian time scale. The remainder of the mutant mice express a rhythm with a significantly shortened period that lacks coherence and statistical power due to variability in activity onset and expansion of the duration of wheel running activity. The extent of the arrhythmic phenotype varies from animal to animal and between transgenic models, with the VPAC2R knockout (Vipr2−/−) mice exhibiting the most disrupted rhythms. Recent evidence suggests that one explanation for the variability in phenotype in the Vipr2−/− is that gastrin-releasing peptide (GRP) has some ability to compensate for the loss of the VPAC2R (Brown et al., 2005). The instability of the circadian system of VIP- and VPAC2R-deficient mice becomes even more apparent when the system is challenged. For example, when placed on a skeleton photoperiod consisting of two light pulses per cycle, the VIP-deficient animals exhibit two discrete activity bouts instead of one activity bout seen in WT controls. Finally, the over-expression of the VPAC2 receptor results in mice expressing a daily rhythm of locomotor activity with a shortened period (Shen et al., 2000). Together, these data indicate that VIP and VPAC2R are critical for the generation of behavioral rhythmicity in mice.
At a cellular level, SCN neurons typically exhibit a daily rhythm of electrical activity with high activity during the day and low activity during the night (Fig. 2). This rhythm is essential for SCN neurons to remain synchronized with each other as well as control rhythmicity in other regions of the body. VIP- and VPAC2R-deficient mice fail to exhibit the midday peak in electrical activity that is characteristic of impulse rhythms from SCN brain slices (Cutler et al., 2003; Brown et al., 2007). Furthermore, the loss of the rhythms in electrical activity is more pronounced in the mice who express the most severe disruption in their rhythms in wheel running activity (Brown et al., 2007). The loss of electrical rhythmicity in the knockouts could be due to a deficit in communication or a deficit in the generation of single cell oscillations. This issue was examined using high-density dispersed cultures of SCN neurons grown on a microelectrode array (Aton et al., 2005). Herzog and colleagues found that both the ability of the population to remain synchronized as well as the ability of single cells to generate oscillations are compromised in mice deficient in VIP or its receptor (Aton et al., 2005). Importantly, in the VIP-deficient mice, the daily administration of a VPAC2R agonist was sufficient to restore robust rhythmicity to the SCN neural population. It is not yet known if the tonic application of the agonist would also be able to “rescue” the rhythmicity in the SCN cultures or whether the phasic application is necessary.
In recent years, the field of circadian rhythms has moved rapidly to identify the rhythmically expressed genes responsible for the generation of circadian rhythms in the SCN. One of the more surprising observations that emerged from the initial description of the Vipr2 −/− mice is that the rhythms in clock gene expression were lost (Harmar et al., 2002; Fig. 2). When the VPAC2R-deficient mice were placed in constant dim red light, expression of mPer1, mPer2, mCry1 and mBmal1 was uniformly low and arrhythmic in the SCN as measured by in situ hybridization, while WT controls exhibited robust rhythms. These observations led the authors to suggest that VIP/VPAC2R signaling is essential for the basic molecular oscillation thought to drive cell-autonomous rhythms (King and Takahashi, 2000; Reppert and Weaver, 2001). Important support for this interpretation comes from a recent study following Per1 gene expression within the SCN using optic imaging (Maywood et al., 2006). In this study, the Vipr2 −/− mice were crossed onto mice carrying either the mPer1::luciferase or mPer1::GFP transgenes. Using these optical reporters, SCN slice cultures were found to exhibit low levels of mPer1 expression without the robust circadian variation seen in the controls. Furthermore, the single cell imaging indicates that fewer cells were rhythmic and that the normal synchrony of mPer1 expression in the SCN was lost in the Vipr2 −/− mice. These findings measuring gene expression (Maywood et al., 2006) echo the results of the analysis of electrical activity (Aton et al., 2005; Brown et al., 2007) with both indicating that VIP/VPAC2R are necessary for the synchrony among the SCN cells as well as the maintenance of rhythmicity within individual SCN neurons (Fig. 2). Unfortunately, comparable data examining rhythmic gene expression in the VIP-deficient mice has not yet become available.
These observations with VIP and VPAC2R bring up an important issue regarding molecular oscillations. While 24-hour transcriptional/translational feedback loops in individual neurons may ultimately control the expression of circadian rhythmicity, agents outside of these loops, such as VIP, are also necessary for normal expression of this rhythmicity. One means by which VIP affects molecular oscillations could be through membrane currents, as a growing body of evidence suggests that membrane excitability and/or synaptic transmission are required for generation of molecular oscillations. In Drosophila, electrically silencing circadian pacemaker neurons with the expression of a non-voltage sensitive potassium (K+) channel blocks behavioral rhythms as well as circadian rhythms in expression of the PER and TIM proteins (Nitabach et al., 2002). This suggests that keeping the cells in an appropriate voltage range may be required for the generation of rhythmicity at the single cell level. Similarly, in the mammalian SCN, membrane hyperpolarization, caused by lowering the extracellular concentration of K+ or blocking calcium (Ca2+) influx in SCN cultures by lowering the extracellular Ca2+ concentration, reversibly abolishes the rhythmic expression of mPer1 and mPER2 (Lundkvist et al., 2005). The blockade of voltage-sensitive Ca2+ channels also abolishes the oscillatory patterns of Per2 and Bmal1 expression in a cell line (SCN2.2) derived from the SCN (Nahm et al., 2005). These results strongly suggest that a transmembrane Ca2+ flux is necessary for sustained molecular rhythmicity in the SCN. Another study measuring mPer1-luc activity within single neurons in a slice (Yamaguchi et al., 2003) supports the view that membrane electrical activity is critical for circadian rhythmicity. Exposing SCN slices to TTX damps Per1 rhythmicity in individual neurons. Furthermore, TTX treatment decreases levels of Per1 and Per2 transcripts and proteins. Together these findings in flies and mammals strongly support the view that the normal function of intrinsic membrane currents is required for rhythmicity in gene expression and raise the possibility that neuropeptides function to regulate these currents.
Evidence that VIP and VPAC2R are critical for the synchronization of circadian oscillations to the environment
The loss of VIP/VPAC2R also appears to alter the synchronization of the circadian oscillator to the environment (Fig. 3). This phenotype is best seen in the experiments in which VIP or VPAC2R knockout animals are released into constant darkness (DD) from an LD cycle. A normally entrained animal will start its activity from a phase predicted from the prior LD cycle. For example, WT mice begin their free-running rhythm within 30 min of the time of lights-off in the prior LD cycle. In contrast, the VIP KO animals start their activity about 8 to 10 hours before the time of the prior lights-off (Colwell et al., 2003). The shorter period of the knockout mice cannot account for this large shift in activity onset, and we postulate that most of this response must be due to an alteration in the processes that couple the oscillator to the environment. This argument is supported by the altered phase angle of entrainment seen in the VIP knockout mice entrained to a single light pulse per cycle. Similarly, the VPAC2R knockout mice also exhibit an extremely large advance in activity onset after release into DD (Harmar et al., 2002). Further supporting a role for VPAC2R in photic signaling, overexpression of the VPAC2R results in mice which entrain more quickly to a phase shift compared to controls (Shen et al., 2000).
Thus, these data from the transgenic models are consistent with the suggestion that VIP is required for normal light-induced synchronization of the circadian system. This proposition is supported by previous behavioral pharmacology studies in which application of VIP alone (Piggins et al., 1995) or in combination with other peptides (Albers et al., 1991) can mimic the phase shifting effects of light. Application of VIP also phase shifts the circadian rhythm of vasopressin release (Watanabe et al., 2000) and neural activity (Reed et al., 2001) measured in vitro. Finally, a study reports that VIP induces rPer1 and rPer2 gene expression during the subjective night (Nielsen et al., 2002). The normal light induction of these Per genes is absent in the Vipr2 −/− mice (Harmar et al., 2002), which also show a lack of phase gating in their response to light (Hughes et al., 2004). Together, these observations suggest that the VIP/VPAC2R signaling pathway is critical for coupling of the oscillator to the environment. Given the anatomical localization of VIP in the retino-recipient SCN neurons (Fig. 1), the simplest explanation is that the loss of VIP prevents the spread of photic information from the ventral neurons to the rest of the SCN circuit.
IV. Mechanisms that underlie the functional roles of VIP and VPAC2R in the SCN
Evidence that VIP/VPAC2R are positively coupled to the AC/PKA pathway
VPAC2R are G-protein-coupled receptors with characteristic seven transmembrane domains, three extracellular and intracellular loops, an extracellular amino-terminus and intracellular carboxy-terminus (Harmar, 2001). All members of this family can also regulate cyclic AMP (cAMP) concentrations by coupling to adenylyl cyclase (Harmar, 2001). In a number of systems, including in the SCN, VIP binding is followed by adenylyl cyclase activity leading to increases in cyclic AMP (cAMP) and PKA (Meyer-Spasche and Piggins, 2004; Rea, 1990; Vanecek and Watanabe, 1998). Downstream in the PKA and the other intracellular signaling pathways is the phosphorylation of CREB and other transcription factors. Interestingly, the mPer1 promoter has CRE domains (Hida et al., 2000), and thus provides a mechanism for VIP to regulate the molecular clock itself. Indeed, the application of VIP to SCN neurons in the night results in increased expression of Per1 and Per2 (Nielsen et al., 2002). While other signaling pathways may ultimately be shown to be involved; at this point, the simplest model is that the AC/PKA pathway mediates the effects of VIP and VPAC2R activation in the SCN (Fig. 4).
Figure 4.
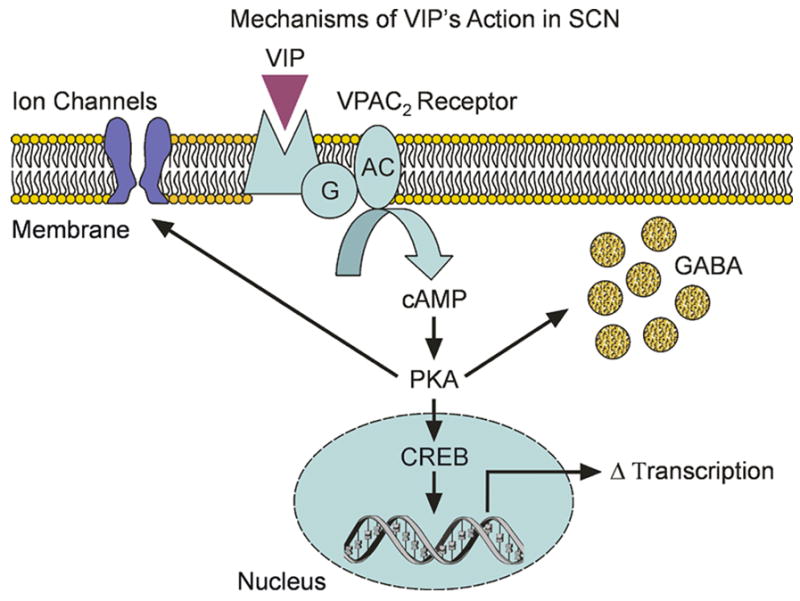
illustrates the possible mechanisms that underlie VIP’s actions in SCN neurons. In all cases, we expect that VIP would activate VPAC2R to stimulate the AC/PKA signaling pathway. This VIP-activated cascade has at least three actions within the SCN including modulation of intrinsic voltage gated channels, presynaptic regulation of GABA release, and alterations in transcription via CREB.
Evidence that VIP/VPAC2R regulates inhibitory synaptic transmission
Understanding the effects of VIP on synaptic transmission can provide clues as to how VIP signaling might synchronize cells within the SCN. It has been established that individual cells within the SCN oscillate with different phases, and application of GABA can synchronize these cellular oscillators (Liu and Reppert, 2000). Electrophysiological experiments characterizing GABAA-mediated spontaneous inhibitory post-synaptic currents (sIPSCs) have found that VIP is a potent modulator of GABA-mediated synaptic transmission within the SCN (Itri and Colwell, 2003; Fig. 4). VIP and VPAC2R agonists both increase sIPSC frequency through a presynaptic mechanism independent from PHI. The effect of VIP is mediated by VPAC2R and is a cAMP/PKA-dependent mechanism (Itri and Colwell, 2003). This VIP regulation of inhibitory synaptic transmission appears to be responsible for driving a circadian rhythm in GABA-mediated synaptic transmission (Itri et al, 2004). Interestingly, our data is consistent with findings that other hypothalamic neuropeptides have the ability to prime vesicle stores for activity-dependent release (Ludwig and Leng, 2006), and this priming could be one mechanism by which VIP affects GABA release. Our studies demonstrate that VIP and VPAC2R modulate GABA-mediated synaptic transmission between SCN neurons and, we speculate, that this mechanism may be important in the loss of synchrony seen in the VIP- and VPAC2R-deficient mice. However, a recent study examining cellular communication among SCN neurons in culture concluded that endogenous GABA controls the amplitude of SCN neuronal rhythms, but unlike VIP, it is not critical for maintaining synchrony among SCN neurons (Aton et al., 2006). More work will be required to understand VIP’s possible role as a regulator of synaptic communication within the SCN circuit.
Evidence that VIP/VPAC2R regulates intrinsic membrane properties of SCN neurons
VIP can regulate intrinsic voltage-sensitive ion channels in other brain regions (e.g. Wang and Aghajanian, 1990; Haug and Storm, 2000; Sun et al., 2003) and is likely to have the same effect in the SCN (Fig. 4). Extracellular recordings indicate that application of VIP alters the firing rate of a subpopulation of SCN neurons with most neurons exhibiting a decreased firing rate, although a few cells are activated (Reed et al., 2002). These VIP-induced changes in firing rate are modulated, but not mediated, by GABAA receptors. Furthermore, the application of VIP or VPAC2R agonist induces an inward current mediated by the closure of K+ channels in SCN neurons (Pakhotin et al., 2006). This type of regulation is more likely to provide an explanation for the VIP-induced excitation than the suppression of firing seen with extracellular recordings. It seems likely that VIP will regulate multiple currents within the SCN neurons. We have found that VIP regulates a fast delayed rectifier K+ current (Itri and Colwell, unpublished). This current is important for the expression of daily rhythms in the frequency of action potentials in SCN neurons (Itri et al., 2005). Interestingly, the net result of the loss of the VPAC2R is that the resting membrane potential of SCN neurons of the mutant mice were significantly hyperpolarized compared to controls (Pakhotin et al., 2006). We speculate that this chronic membrane hyperpolarization may underlie the loss of rhythmicity in firing rate and gene expression seen in SCN neurons from VIP- and VPAC2R-deficient mice. Indeed, depolarization of SCN neurons appears to be able to “rescue” the weak rhythms of gene expression observed in the SCN of VPAC2R-deficient mice (Maywood et al., 2006).
V. VIP/VPAC2R involvement in the regulation of circadian outputs
In the past, the field of circadian rhythms has focused more on the basic mechanisms involved in the generation of circadian oscillation than on the mechanisms by which the circadian system regulates other physiological systems, including endocrine functions. In recent years, this focus has been changing to include the study of the output systems that are clearly critical to the development of an integrated view of circadian organization (Kriegsfeld and Silver, 2006). Among other findings, we now have a much better appreciation for the distributed organization of the circadian timing system. Clock genes have been described in neurosecretory cells (Kriegsfeld et al., 2003), the pituitary (von Gall et al., 2002) and peripheral endocrine glands (e.g. Bittman et al., 2003). We assume that these genes drive circadian oscillations in these endocrine cell populations and would normally have access to some type of synchronizing signal from the SCN. In the case of endocrine systems, neural pathways emanating from the SCN appear to be responsible for at least some of this communication. For example, the polysynaptic pathway by which the SCN regulates the secretion of melatonin from the pineal has been particularly well described (Klein, 1985). Anatomical studies have found that bothVIP and vasopressin expressing neurons send projections to a wide range of targets important for neuroendocrine function, mainly through a number of relay nuclei including the SPZ, dorsomedial nucleus of the hypothalamus (DMH), medial preoptic area (MPOA) and paraventricular nucleus of the hypothalamus (PVN) (van den Pol, 1991; Abrahamson and Moore, 2001; Kalsbeek and Buijs, 2002; Kalsbeek et al., 2006; Fig. 1). Among SCN targets are corticotrophin-releasing hormone expressing, thyrotropin-releasing hormone expressing, and gonadotropin releasing hormone expressing neurons of the hypothalamus (Kalsbeek and Buijs, 2002). There has also been evidence to suggest a direct connection between the SCN and the hypothalamic supraoptic nucleus (SON) and arcuate nucleus (ARC) (Cui et al., 1997; Saeb-Parsy et al., 2000). Furthermore, polysynaptic pathways from the SCN targeting the autonomic nervous system also regulate endocrine outputs (Kalsbeek and Buijs 2002; Kalsbeek et al., 2006). Thus there are multiple neural pathways from the SCN that can regulate endocrine activity.
VIP released from SCN neurons may directly affect some of these output targets. VPAC2R is found in the major hypothalamic relay nuclei receiving afferents from the SCN, suggesting that the VIP/VPAC2R signaling system may have important effects outside of the SCN as well (Kallo et al., 2004b). A variety of studies have implicated VIP and VPAC2R in the circadian regulation of gonadotropin-releasing hormone neurons (e.g. Harney et al., 1996; Gerhold and Wise, 2006). There is also evidence to suggest a role for VIP in the temporal regulation of the hypothalamic pituitary adrenal axis (Scarbrough et al., 1996; Buijs et al., 1999), though more studies are required to address this point. In general, we feel that understanding the role of VIP and VPAC2R in the regulation of the endocrine outputs of the circadian system will be an important area of future research.
VI. Parallels between VIP in mammals and PDF in insects
There is evidence that synaptic interactions involving neuropeptides play critical roles in the maintenance and synchronization of circadian rhythms in non-mammalian systems. In Drosophila, the neuropeptide PDF is expressed in a subset of lateral circadian pacemaker neurons implicated in driving locomotor activity rhythms (Helfrich-Forster, 1995). Flies lacking PDF (Renn et al., 1999), or flies in which the PDF expressing subset of clock neurons have been functionally silenced (Nitabach et al., 2002), exhibit parallel and striking deficits in free-running rhythms in behavior. Chronically depolarizing these same neurons also disrupts behavioral rhythms (Nitabach et al., 2006). In constant conditions, the loss of PDF disrupts the ability of flies to sustain molecular oscillations. Furthermore, pacemaker cells lose the ability to regulate the timing of PER and TIM entry into the nucleus, an event that is critical to the generation of rhythms (Nitabach et al., 2002). The dysfunction leads to the dispersal in the phasing of individual cells indicating a loss of synchrony within neuronal subgroups (Lin et al., 2004). The loss of rhythmicity in transcription and protein activity could be due to loss of rhythmicity at the single pacemaker cell (Peng et al., 2003) or due to the loss of synchrony between cell populations (Lin et al., 2004). A recent study reports that the slowpoke mutant displays arrhythmic behavior in constant conditions, which is thought to result from a mishandling of PDF accumulation and/or release (Fernández et al., 2007). In these Drosophila mutants, molecular oscillations in the small ventral lateral cells are intact in constant conditions. However, the dorsal neurons of these mutants, which receive PDF inputs, show desynchronized oscillations of PER and TIM. This recent finding further supports the importance of communication between the different pacemaker cell groups, mediated by PDF in generating synchronized cell oscillations and rhythmic behavior.
The role of PDF in the Drosophila circadian system is still under active investigation. For example, a recent study described a strain that lacked any detectable PDF oscillations, yet displayed normal circadian rhythms indistinguishable from the WT (Kula et al., 2006). The authors suggest that PDF may play only a minor role in the Drosophila circadian system. Alternatively, it may be that the presence of PDF is more functionally important than the rhythms in PDF levels. With the recent identification of the PDF receptor GOP (groom of PDF, also known as HAN or PDFR), the functional role of PDF in the circadian system, its downstream effectors and outputs will surely be clarified (Hyun et al., 2005; Lear et al., 2005; Mertens et al., 2005).
There are many parallels between this emerging story in Drosophila and the work being done with VIP in the mammalian SCN (Table 1). Briefly, the loss of PDF and VIP in free-running conditions results in a decline into behavioral arrhythmicity in a majority of mutants. The presumed molecular basis for the behavioral phenotype is a loss in synchrony between pacemaker cells as well as deficits in the ability of pacemaker cells to generate oscillations. The same behavioral and molecular phenotypes are observed when their respective receptors are knocked-out. Future findings and further characterization of neuropeptidergic signaling in both the mouse and fly circadian system will help us understand how these signaling systems play such an important role in the regulation of neuronal circuitry crucial for behavioral rhythmicity and homeostasis.
Table 1.
Comparison between the Phenotypes expressed by VIP and PDF deficient organisms.
| Neuropeptides | VIP | |
|---|---|---|
| Knockouts | ||
| Behavioral Phenotype |
|
|
| Cellular Phenotype |
|
|
| Molecular Phenotype |
|
|
| Receptors | VPAC2 | GOP (groom of PDF) |
| Class |
|
|
| Knockouts |
|
|
| Downstream Targets |
|
|
VII. Summary
In this review, we summarize the evidence that VIP plays distinct and important roles in the generation of circadian rhythms and the regulation of these rhythms by the environment. Specifically, we present evidence that VIP acting through VPAC2R acts to 1) modulate the molecular oscillations within individual oscillators 2) synchronize individually oscillating neurons with each other and 3) synchronize SCN neurons with light cues. We also present recent data describing the mechanisms by which VIP may be acting to produce these diverse actions in SCN neurons including presynaptic regulation of GABA release as well as postsynaptic modulation of intrinsic membrane currents. Finally, we hope to show the relevance of studying VIP in the mammalian circadian system by using it as a model to understand problems of oscillations and coupling in other systems. While we are just beginning to uncover the roles of VIP in the mammalian circadian system, more research will improve our understanding of this and other neuropeptidergic signaling systems in the mammalian brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- Albers HE, Liou SY, Stopa EG, Zoeller RT. Interaction of colocalized neuropeptides: functional significance in the circadian timing system. J Neurosci. 1991;11:846–851. doi: 10.1523/JNEUROSCI.11-03-00846.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci U S A. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse endocrine tissues. Am J Physiol Regul Integr Comp Physiol. 2003;285:R561–569. doi: 10.1152/ajpregu.00783.2002. [DOI] [PubMed] [Google Scholar]
- Besson J, Sarrieau A, Vial M, Marie JC, Rosselin G, Rostene W. Characterization and autoradiographic distribution of vasoactive intestinal peptide binding sites in the rat central nervous system. Brain Res. 1986;398:329–336. doi: 10.1016/0006-8993(86)91493-9. [DOI] [PubMed] [Google Scholar]
- Brown TM, Colwell CS, Waschek JA, Piggins HD. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. J Neurophysiol. 2007;97:2553–2558. doi: 10.1152/jn.01206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Hughes AT, Piggins HD. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC(2) receptor signaling. J Neurosci. 2005;25:11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Hou YX. Colocalization of gamma-aminobutyric acid with vasopressin, vasoactive intestinal peptide, and somatostatin in the rat suprachiasmatic nucleus. J Comp Neurol. 1995;358:343–52. doi: 10.1002/cne.903580304. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Cagampang FRA, Sheward WJ, Harmar AJ, Piggins HD, Coen CW. Circadian changes in the expression of vasoactive intestinal peptide 2 receptor mRNA in the rat suprachiasmatic nuclei. Brain Res Mol Brain Res. 1998;54:108–112. doi: 10.1016/s0169-328x(97)00327-6. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Waschek JA. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1194–R1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- Cui LN, Saeb-Parsy K, Dyball REJ. Neurones in the supraoptic nucleus of the rat are regulated by a projection from the suprachiasmatic nucleus. J Phyisol. 1997;502:149–159. doi: 10.1111/j.1469-7793.1997.149bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD. The mouse VPAC(2) receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur J Neurosci. 2003;17:197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res. 2004;124:143–51. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to the major arousal-promoting cell groups in rat: implications for the circadian control of behavioral state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Dietl MM, Hof PR, Martin JL, Magistretti PJ, Palacios JM. Autoradiographic analysis of the distribution of vasoactive intestinal peptide binding sites in the vertebrate central nervous system: a phylogenetic study. Brain Res. 1990;520:14–26. doi: 10.1016/0006-8993(90)91687-c. [DOI] [PubMed] [Google Scholar]
- Fernández MP, Chu J, Villella A, Atkinson N, Kay SA, Ceriani MF. Impaired clock output by altered connectivity in the circadian network. Proc Natl Acad Sci. 2007;104:5650–5655. doi: 10.1073/pnas.0608260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold LM, Wise PM. Vasoactive intestinal polypeptide regulates dynamic changes in astrocyte morphometry: impact on gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:2197–2202. doi: 10.1210/en.2005-1262. [DOI] [PubMed] [Google Scholar]
- Gooley J, Saper CB. Anatomy of the mammalian circadian system. In: Kryger MH, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. 4. Saunders; 2005. pp. 335–350. [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci. 2001;21:742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ. Family-B G-protein-coupled receptors. Genome Biol. 2001;2(reviews):3013.1–3013.10. doi: 10.1186/gb-2001-2-12-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137:3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- Haug T, Storm JF. Protein kinase A mediates the modulation of the slow Ca2+-dependent K+ current, IsAHP, by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol. 2000;83:2071–2079. doi: 10.1152/jn.2000.83.4.2071. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci USA. 1995;92:612–616. doi: 10.1073/pnas.92.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida A, Koike N, Hirose M, Hattori M, Sakaki Y, Tei H. The human and mouse period1 genes: five well-conserved E-boxes additively contribute to the enhancement of mPer1 transcription. Genomics. 2000;65:224–233. doi: 10.1006/geno.2000.6166. [DOI] [PubMed] [Google Scholar]
- Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J Neurosci. 2004;24:3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S. Drosophila GPCR han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Ibata Y, Takahashi Y, Okamura H, Kawakami F, Terubayashi H, Kubo T, Yanaihara N. Vasoactive intestinal peptide (VIP)-like immunoreactive neurons located in the rat suprachiasmatic nucleus receive a direct retinal projection. Neurosci Lett. 1989;97:1–5. doi: 10.1016/0304-3940(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Irwin DM, Huner O, Youson JH. Lamprey proglucagon and the origin of glucagon-like peptides. Mol Biol Evol. 1999;16:1548–1557. doi: 10.1093/oxfordjournals.molbev.a026067. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Itri J, Colwell CS. Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J Neurophysiol. 2003;90:1589–1597. doi: 10.1152/jn.00332.2003. [DOI] [PubMed] [Google Scholar]
- Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J Neurophysiol. 2004;92:311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier poatassium current is required for circadian neural activity. Nat Neurosci. 2005;8:650–656. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallo I, Kalamatianos T, Piggins HD, Coen CW. Ageing and the diurnal expression of mRNAs for vasoactive intestinal peptide and for the VPAC2 and PAC1 receptors in the suprachiasmatic nucleus of male rats. J Neuroendocrinol. 2004a;16:758–766. doi: 10.1111/j.1365-2826.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- Kallo I, Kalamatianos T, Wiltshire N, Shen S, Sheward WJ, Harmar AJ, Coen CW. Transgenic approach reveals expression of the VPAC2 receptor in phenotypically defined neurons in the mouse suprachiasmatic nucleus and in its efferent target sites. Eur J Neurosci. 2004b;19:2201–2211. doi: 10.1111/j.0953-816X.2004.03335.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FAJL, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- Klein DC. Photoneural regulation of the mammalian pineal gland. Ciba Found Symp. 1985;117:38–56. doi: 10.1002/9780470720981.ch4. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Korets R, Silver R. Expression of the circadian clock gene Period 1 in neuroendocrine cells: an investigation using mice with a Per1::GFP transgene. Eur J Neurosci. 2003;17:212–20. doi: 10.1046/j.1460-9568.2003.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: timing is everything. Horm Behav. 2006;49:557–74. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kula E, Levitan ES, Pyza E, Rosbash M. PDF cycling in the dorsal protocerebrum of the Drosophila brain is not necessary for circadian clock function. J Biol Rhythms. 2006;21:104–117. doi: 10.1177/0748730405285715. [DOI] [PubMed] [Google Scholar]
- Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Barkhem T, Norberg A, Persson H, Schalling M, Hokfelt T, Magnusson G. Structure and expression of the gene encoding the vasoactive intestinal peptide precursor. Proc Natl Acad Sci USA. 1987;84:605–609. doi: 10.1073/pnas.84.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron. 2000;25:123–128. doi: 10.1016/s0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25:7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GKY, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Meyer-Spasche A, Piggins HD. Vasoactive intestinal polypeptide phase-advances the rat suprachiasmatic nuclei circadian pacemaker in vitro via protein kinase A and mitogen-activated protein kinase. Neurosci Lett. 2004;358:91–94. doi: 10.1016/j.neulet.2003.12.114. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system. Brain Res Rev. 2005;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Morin LP, Shivers KY, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience. 2006;137:1285–1297. doi: 10.1016/j.neuroscience.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Nahm SS, Farnell YZ, Griffith W, Earnest DJ. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J Neurosci. 2005;25:9304–9308. doi: 10.1523/JNEUROSCI.2733-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura W, Yamazaki S, Takasu NN, Mishima K, Block GD. Differential response of period 1 within the suprachiasmatic nucleus. J Neurosci. 2005:5481–5487. doi: 10.1523/JNEUROSCI.0889-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HS, Hannibal J, Fahrenkrug J. Vasoactive intestinal polypeptide induces per1 and per2 gene expression in the rat suprachiasmatic nucleus late at night. Eur J Neurosci. 2002;15:570–574. doi: 10.1046/j.0953-816x.2001.01882.x. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free–running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhotin P, Harmar AJ, Verkhratsky A, Piggins H. VIP receptors control excitability of suprachiasmatic nuclei neurones. Pflugers Arch. 2006;452:7–15. doi: 10.1007/s00424-005-0003-z. [DOI] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free running rhythms require intercellular communication. PLoS Biol. 2003;1:32–40. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci. 1995;15:5612–5622. doi: 10.1523/JNEUROSCI.15-08-05612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MA. VIP-stimulated cyclic AMP accumulation in the suprachiasmatic hypothalamus. Brain Res Bull. 1990;25:843–847. doi: 10.1016/0361-9230(90)90179-4. [DOI] [PubMed] [Google Scholar]
- Reed HE, Cutler DJ, Brown TM, Brown J, Coen CW, Piggins HD. Effects of vasoactive intestinal polypeptide on neurones of the rat suprachiasmatic nuclei in vitro. J Neuroendocrinol. 2002;14:639–646. doi: 10.1046/j.1365-2826.2002.00826.x. [DOI] [PubMed] [Google Scholar]
- Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro. Eur J Neurosci. 2001;13:839–843. doi: 10.1046/j.0953-816x.2000.01437.x. [DOI] [PubMed] [Google Scholar]
- Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and abltion of PDF Neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Woodhams PL, Bryant MG, Chow TJ, Bloom SR, Polak JM. VIP in the rat brain: evidence for a major pathway linking the amygdale and hypothalamus via the stria terminals. Histochemistry. 1980;65:103–119. doi: 10.1007/BF00493159. [DOI] [PubMed] [Google Scholar]
- Saeb-Parsy K, Lombardelli S, Khan FZ, McDowall K, Au-Young IT, Dyball RE. Neural connections of hypothalamic neuroendocrine nuclei in the rat. J Neuroendocrinol. 2000;12:635–648. doi: 10.1046/j.1365-2826.2000.00503.x. [DOI] [PubMed] [Google Scholar]
- Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Scarbrough K, Harney JP, Rosewell KL, Wise PM. Acute effects of antisense antagonism of a single peptide neurotransmitter in the circadian clock. Am J Physiol Regul Integr Comp Physiol. 1996;170:R283–288. doi: 10.1152/ajpregu.1996.270.1.R283. [DOI] [PubMed] [Google Scholar]
- Shen S, Spratt C, Sheward WJ, Kallo I, West K, Morrison CF, Coen CW, Marston HM, Harmar AJ. Overexpression of the human VPAC(2) receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc Natl Acad Sci USA. 2000;97:11575–11580. doi: 10.1073/pnas.97.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheward WJ, Lutz EM, Harmar AJ. The distribution of vasoactive intestinal peptide(2) receptor messenger RNA in the rat brain and pituitary gland as assessed by in-situ hybridization. Neuroscience. 1995;67:409–418. doi: 10.1016/0306-4522(95)00048-n. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Kimura F. Temporal profiles of vasoactive intestinal polypeptide precursor mRNA and its receptor mRNA in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1999;63:262–267. doi: 10.1016/s0169-328x(98)00289-7. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Two distinct oscillators in the suprachiasmatic nucleus of rat suprachiasmatic nucleus, in vitro. Proc Natl Acad Sci USA. 1995;92:7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Mitushima D, Kimura F. Effects of gap junction blocker on vasopressin and vasoactive intestinal polypeptide rhythms in the rat suprachiasmatic nucleus in vitro. Neurosci Res. 2000;38:43–47. doi: 10.1016/s0168-0102(00)00141-3. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ, Prince DA, Huguenard JR. Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide activate hyperpolarization-activated cationic current and depolarize thalamocortical neurons in vitro. J Neurosci. 2003;23:2751–2758. doi: 10.1523/JNEUROSCI.23-07-02751.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Ichitani Y, Okamura H, Tanaka Y, Ibata Y. The direct retinal projection to VIP neuronal elements in the rat SCN. Brain Res Bull. 1993;31:637–640. doi: 10.1016/0361-9230(93)90134-w. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Mezey E. 2 Receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat - intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. The suprachiasmatic nucleus: morphological and cytochemical substrates for cellular interaction. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic nucleus: the mind’s clock. Oxford University Press; New York: 1991. pp. 17–50. [Google Scholar]
- van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain Res Rev. 2000;33:34–77. doi: 10.1016/s0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- Vanecek J, Watanabe K. Melatonin inhibits the increase of cyclic AMP in rat suprachiasmatic neurons induced by vasoactive intestinal peptide. Neurosci Lett. 1998;252:21–24. doi: 10.1016/s0304-3940(98)00530-8. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharm Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Vertongen P, Schiffmann SN, Gourlet P, Robberecht P. Autoradiographic visualization of the receptor subclasses for vasoactive intestinal polypeptide (VIP) in rat brain. Ann N Y Acad Sci. 1998;865:412–415. doi: 10.1111/j.1749-6632.1998.tb11206.x. [DOI] [PubMed] [Google Scholar]
- von Gall C, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm-Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci. 2002;5:234–8. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- Wang YY, Aghajanian GK. Excitation of locus coeruleus neurons by vasoactive intestinal peptide: role of a cAMP and protein kinase A. J Neurosci. 1990;10:3335–3343. doi: 10.1523/JNEUROSCI.10-10-03335.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Vanecek J, Yamaoka S. In vitro entrainment of the circadian rhythm of vasopressin-releasing cells in suprachiasmatic nucleus by vasoactive intestinal polypeptide. Brain Res. 2000;877:361–366. doi: 10.1016/s0006-8993(00)02724-4. [DOI] [PubMed] [Google Scholar]
- Weaver DR. The suprachiasmatic nucleus: A 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Yan LL, Okamura H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur J Neurosci. 2002;15:1153–62. doi: 10.1046/j.1460-9568.2002.01955.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]


